Introduction
Genetic alterations accumulating in cancer cells
give rise to novel tumor-associated antigens, which serve as key
hallmarks of tumor progression (1).
As a result, oncology research has shifted towards precision
oncology, where treatment strategies are customized to target
specific molecular abnormalities in individual patients with
different cancer types (2,3).
Hyperlipidemia is a well-recognized, independent
risk factor for atherosclerotic diseases such as stroke and
myocardial infarction. It has also been linked to several other
major disorders, including cancer (4). Tumor metabolic reprogramming refers to
a series of metabolic shifts caused by structural and functional
changes in key oncogenes and tumor suppressors, enabling cancer
cells to sustain growth and adapt to unfavorable microenvironments
(5). Among these metabolic
adaptations, cholesterol metabolism serves a fundamental role.
Cancer cells rely heavily on cholesterol for uncontrolled
proliferation and survival, and they can reprogram cholesterol
homeostasis by increasing uptake, dysregulating biosynthetic
pathways, reducing efflux and accumulating cholesterol in lipid
droplets to regulate critical signaling pathways (6).
Emerging evidence underscores the pivotal role of
cholesterol in tumor initiation, progression and metastasis.
Several studies have reported a positive association between
elevated serum cholesterol levels and increased cancer risk and
aggressiveness (7). High
circulating cholesterol levels have also been associated with an
increased risk of developing prostate cancer, whereas
cholesterol-lowering interventions have been suggested to confer
protective effects (8). Moreover,
cholesterol has been implicated in rectal cancer progression, where
excessive cholesterol promotes the degradation of squalene
epoxidase, a rate-limiting enzyme in cholesterol biosynthesis.
Notably, reduced squalene epoxidase expression has been associated
with invasive colorectal cancer (CRC) (9). In breast cancer (BRCA), cholesterol
contributes to metastasis through its oxidative metabolite,
27-hydroxycholesterol, which is induced by a high-fat diet.
Notably, eliminating or inhibiting the enzymes responsible for this
metabolite markedly reduces cancer metastasis in animal models
(10). Furthermore,
3β-hydroxysteroid Δ24-reductase (DHCR24) promotes lymphangiogenesis
and lymph node metastasis in bladder cancer (BLCA), both in
vitro and in vivo (11).
Cholesterol synthesis is mainly carried out through
the Bloch pathway, and DHCR24 is the key enzyme in its final step,
in humans and hamsters (12). Our
previous research in a mouse model demonstrated that a high-fat
diet induces brain injury and neuronal apoptosis by downregulating
DHCR24 (13). Furthermore,
suppressing DHCR24 expression has been reported to inhibit the
proliferation and metastasis of multiple cancer cell types. For
example, the anticancer peptide Q7 downregulates DHCR24, thereby
disrupting lipid raft formation and subsequently inhibiting the AKT
signaling pathway in human endometrial cancer cells, leading to
reduced tumorigenicity, proliferation and migration (14). Similarly, DHCR24 is essential for
cholesterol biosynthesis and lipid raft formation, and its
inhibition by Genkwadaphnin has been reported to effectively
suppress the growth and invasion of hepatocellular carcinoma cells
(15). Additionally, methotrexate
resistance in gestational trophoblastic neoplasia cells has been
associated with DHCR24-mediated cholesterol biosynthesis (16).
Despite its crucial role in cholesterol metabolism
and the growing body of evidence suggesting that DHCR24 inhibition
suppresses tumor proliferation and metastasis, a comprehensive
pan-cancer analysis of DHCR24 is lacking. Therefore, the present
study aimed to perform an extensive pan-cancer analysis of DHCR24
and experimentally elucidate its oncogenic role and underlying
mechanisms in BLCA.
Materials and methods
Tumor immune estimation resource
(TIMER)
The DHCR24 expression profile and the abundance of
immune infiltrates in pan-cancer were analyzed using the TIMER
database (https://cistrome.shinyapps.io/timer/). The gene
expression levels are represented as log2 [transcripts
per million (TPM)] values (17).
DHCR24 expression pattern in human
pan-cancer
The difference in the expression of DHCR24 between
tumor and adjacent normal tissues was assessed for the different
tumors or specific tumor subtypes of The Cancer Genome Atlas (TCGA)
project (https://www.cancer.gov/ccg/research/genome-sequencing/tcga/studied-cancers).
R version 4.1.2 (The R Foundation) was used for analysis, and
specific R package, including limma 3.64.0 (https://bioinf.wehi.edu.au/limma/) and ggplot2 3.5.2
(https://ggplot2.tidyverse.org) were
utilized. Additionally, violin plots were generated for DHCR24
expression levels in different pathological stages (stages I–IV) of
all TCGA tumors via the ‘Pathological Stage Plot’ module of
GEPIA2.0 (http://gepia2.cancer-pku.cn/). The log2
(TPM+1) transformed expression data were applied for the box or
violin plots.
Prognostic analysis
The ‘Survival Map’ module of GEPIA2.0 (http://gepia2.cancer-pku.cn/) was used to obtain the
overall survival (OS) and disease-free survival (DFS) significance
map data of DHCR24 across all TCGA tumors. Cutoff-high (50%) and
-low (50%) values were used as the expression thresholds for
splitting the high- and low-expression cohorts. The log-rank test
was used in the hypothesis test, and the survival plots were also
obtained using the ‘Survival Analysis’ module of GEPIA2.0
(http://gepia2.cancer-pku.cn/). Moreover,
to assess whether the acquired genes were associated with the
survival of patients with BLCA, univariate and multifactorial
proportional hazards model (COX) analyses were performed.
Subsequently, the survival of patients with cancer was predicted
and further assessed using a nomogram and receiver operating
characteristic (ROC) curve analysis.
Differential gene enrichment
analysis
Co-expression analysis of the differential genes
obtained from the analysis in BLCA was performed. Subsequently,
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) enrichment analysis, as well as gene set enrichment analysis
were performed on the differential genes.
Immuno-infiltration analysis
The expression data of patients with BLCA was
analyzed for immune infiltration, immune checkpoint and tumor
mutation load. R version 4.1.2 was used for analysis, with the R
packages reshape2 1.4.4 (https://github.com/hadley/reshape) and vioplot 0.5.1
(https://github.com/TomKellyGenetics/vioplot)
utilized.
Molecular docking and dynamics
simulation
In our preliminary screening study (unpublished
data), immunoprecipitation (IP) mass spectrometry using antibodies
against DHCR24 as the IP antibody and 293 cells was performed to
identify the DHCR24-interaction proteins. In total, 413 proteins
were identified as DHCR24-interaction proteins (Table SI). These data were combined with
the results of four publicly available protein interaction
databases Biogrid (https://thebiogrid.org/) (DHCR24 dataset), GeneMania
(http://genemania.org/) (DHCR24 dataset), Unihi
(http://193.136.227.168/UniHI/pages/unihiSearch.jsf)
(24-Dehydrocholesterol Reductase dataset) and Hitpredict
(http://www.hitpredict.org/) (dataset
Q15392) to form a DHCR24 candidate interaction proteome containing
492 protein molecules. Furthermore, the enrich Pathway function of
Clusterprofiler 3.21 (https://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
software of R 4.1.2 software was used to perform enrichment
analysis in terms of cellular pathways and to predict the important
cellular signaling pathways of DHCR24 and its interacting
proteins.
Subsequently, the interacting proteome was entered
into STRING 12.0 (https://string-db.org/) to form a protein-protein
interaction (PPI) network. The network map was then downloaded and
imported into Cytoscape3.9.1 (https://cytoscape.org/). DHCR24 with HRAS, a common
variant in cancer, was selected as a node, all proteins adjacent to
it were selected to form a new network map, and the network was
analyzed. The central node of the PPI network was determined.
Modeller 9.24 software (https://salilab.org/modeller/) was used to screen all
sequences with homology of >30% as templates for homology
modeling, and the obtained protein structures were optimized for
kinetic simulations using gromacs2019.5 (https://manual.gromacs.org/documentation/2019.6/download.html)
for 50,000 steps of energy minimization, constant temperature of
300 k, nd 1 nsec, with a Rasch plot of Modeller 9.24 (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) as
a standard for protein structure evaluation. Subsequently, using
the biomacromolecular docking platform ZDOCK2.3.2 (http://zdock.umassmed.edu/), the structure of DHCR24
homology modeled with the activated HRAS structure (PDB database,
https://www.rcsb.org/; 1HE8 B chain) was
submitted separately and docking calculations were performed. The
best-bound structural system of the aforementioned DHCR-HRAS
complex was determined as the initial conformation, and molecular
dynamics simulations were performed using the NAMD 2.13 (https://www.ks.uiuc.edu/Research/namd/2.13/notes.html)
program and Amber's ff14SB (Preliminary version; http://pubs.acs.org/doi/10.1021/acs.jctc.5b00255)
force field. A 10.0 Å TIP3P water box model was added around the
complex and the addition made the system electrically neutral. The
complex was slowly warmed by 30,000 steps of initial energy
minimization and 1,000 steps of 0–300 K using a constant
temperature and constant pressure system. Molecular dynamics
simulations of the complexes were performed for 70 nsecs to study
the interactions between DHCR24-HRAS complexes, and the results of
the molecular dynamics simulation calculations were analyzed using
Pymol2.6 software (https://pymol.org/).
Human protein atlas (HPA) database
analysis
In order to explore DHCR24 expression in patients
with BLCA, data was retrieved from the HPA database (https://www.proteinatlas.org/). The BLCA data and
normal bladder data in the DHCR24 immunohistochemistry dataset were
analyzed.
Cell culture
For in vitro cytological validation, the
human BLCA 5637 cell line was used. These cells can produce
products such as stem cell factor, interleukin-1, interleukin-6 and
granulocyte macrophage-colony stimulating factor. Moreover, 5637
cells were derived from the bladder epithelial cells of a
68-year-old male patient with BLCA which had adherent growth
characteristics. The cell line is widely used in the study of the
mechanism of BLCA development and drug treatment strategies
(18,19).
Human BLCA 5637 cells, purchased from Wuhan Punosai
Life Technology Co., Ltd., were seeded at 5×106
cells/well in six-well plates and cultured in 10% FBS/DMEM in a 5%
CO2 incubator at 37°C. Fetal calf serum and DMEM were
purchased from Hyclone, and the Penicillin-Streptomycin Solution
mixture was purchased from Donglin Changsheng Biotechnology Co.,
Ltd. The concentration of double antibody was 1%. When the cells
reached ~50% confluence, the old medium was discarded and the cells
were starved overnight. Subsequently, 5 ml DMEM was added to each
well, images of the cells were captured, and then drug treatment
was applied. To observe the number of adherent cells after 72 h,
the cells were counted in the same position of each group and each
well under the same field of view using the same microscope
magnification. U18666A, Filipin, EGF and paraformaldehyde were
purchased from Beijing Ding Guo Changsheng Biotechnology Co., Ltd.
Lonafarnib was purchased from Selleck Chemicals. The working
concentrations of EGF, U18666A and lonafarnib were 15 ng/ml, 2 µM
and 1.9 nM, respectively. The inverted and fluorescence microscopes
used were manufactured by Olympus Corporation. The number of
adherent cells per unit area was plotted using GraphPad Prism 9.3
(Dotmatics).
Cell viability assay
Cells were seeded in 96-well plates at a density of
1×105 cells/well and incubated for 24 h at 37°C. When
the cells reached ~50% confluence, the old medium was discarded and
the cells were starved overnight. Next, 0.5 mg/ml tetrazolium salt
(MTT) was added and the cells were incubated for 4 h at 37°C. The
working concentrations of EGF, U18666A and lonafarnib were 15
ng/ml, 2 µM and 1.9 nM, respectively. EGF can activate the
downstream signaling pathway to promote the proliferation of cancer
cells. U18666A is a DHCR24 inhibitor, and the cell viability and
proliferation changes after inhibition of DHCR24 can be explored.
Lonafarnib, an HARS inhibitor, can be used to investigate the
changes in cell viability and proliferation after inhibition of
HRAS activity. Several negative control groups were set up, namely
the untreated control group and the EGF, U18666A and lonafarnib
control groups. The EGF control group was cultured with 15 ng/ml
EGF for 15 min and then the medium was replaced. The U18666A
control group was treated with 2 µM U18666A, and the lonafarnib
control group was treated with 1.9 nM lonafarnib. The other two
experimental groups were treated with 15 ng/ml EGF for 15 min, then
the medium was replaced, and then 2 µM U18666A and 1.9 nM
lonafarnib were added, respectively. After drug addition, all cells
were treated at 37°C for 72 h. Following the incubation period, the
formazan crystals were dissolved using 100 µl of dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA). The absorbance of the samples was then
measured at 490 nm using a microplate reader (Spark®;
Tecan Group, Ltd.). The percentage of viable cells was calculated
and compared with that of the negative control.
Duolink
The cells were divided into two groups: One group
was cultured with medium containing 15 ng/ml EGF to activate HRAS,
whilst the other was cultured with the same amount of medium
without EGF as a control. After 15 min, the medium in the wells was
discarded, and the cells were eluted with 1 ml PBS per well. A
total of 1 ml 4% paraformaldehyde was added to the cells in each
well, and fixed at room temperature for 30 min after agitation at
room temperature. The paraformaldehyde was removed by suction, and
1 ml PBS was added to each well for shaking and cleaning for 3×10
min. Blocking was performed using 1 ml PBS containing a final
concentration of 1% BSA and 0.3% Triton-PBS, and the mixture was
shaken for 1 h at room temperature. Cells were eluted with PBS to
wash away residual blocking reagents. A sealing film of a suitable
size was cut, absorbent paper and tin foil was put under it, they
were placed in a Petri dish to form a wet box and then Round
Coverslip piece was transferred to the wet box. A drop of
Duolink® Blocking Solution (Sigma-Aldrich; Merck KGaA)
was dropped on each slide to ensure complete coverage of the slide,
and the slide was incubated at 37°C for 1 h. Duolink®
Antibody Diluent (Sigma-Aldrich; Merck KGaA) was then vortexed to
dilute the anti-HRAS [Santa Cruz Biotechnology (Shanghai) Co.,
Ltd.; cat. no. SC-35] and anti-DHCR24 (Wuhan Aibotaike
Biotechnology Co. Ltd.; cat. no. A5402) antibodies at a ratio of
1:250 (~0.2 µl of each antibody). The Duolink® Blocking
Solution was removed by suction, and the diluted antibodies were
evenly added to each slide. The slides were incubated at room
temperature for 1 h or 4°C overnight. PLUS and MINUS PLA probes
(Sigma-Aldrich; Merck KGaA) were then vortexed and added to the
Duolink® Antibody Diluent at a ratio of 1:5. The primary
antibodies were removed by aspiration, washed with PBS for 2×5 min,
and incubated with PLA solution for 1 h at 37°C. The 5×
Amplification buffer was then diluted into 1× Amplification buffer
with sterile water and mixed well. The PLA solution was
subsequently removed by aspiration and washed with PBS for 2×5 min.
During this period, polymerase was added to the 1× Amplification
buffer at a dilution of 1:80 to form an amplification reaction
solution and mixed. The amplification reaction solution was added
to the slide and incubated at 37°C for 100 min. The amplification
reaction solution was then removed by suction and washed with PBS
for 3×10 min. A drop of Duolink® In Situ Mounting
Medium with DAPI (Sigma-Aldrich; Merck KGaA) was then added onto
the slide and the slide was transferred from the wet box to the
slide. After 15 min, the cells were observed with a fluorescence
microscope at a minimum magnification of ×20. After the
observation, the carrier plate was placed in the refrigerator at
−20°C.
Filipin staining
5637 cells with good growth status were seeded into
six-well plates at 5×106 cells/well and cultured in a
constant temperature incubator at 37°C and 5% CO2. When
the cells reached ~50% confluency, the old medium was discarded and
the cells were starved overnight. Drug treatment was added as
aforementioned, and after 48 h of incubation, the old medium was
removed by aspiration and washed three times by addition of PBS.
After aspiration, the cells were fixed with 4% paraformaldehyde for
30 min at room temperature. The paraformaldehyde was then removed
by aspiration and the cells were washed three times with PBS,
stained with 1 ml PI added to each well, and incubated for 30 min
at room temperature. PI staining was removed by aspiration and the
cells were washed 3 times with PBS, treated with 1 ml glycine
solution (1.5 mg/ml) per well for 10 min at room temperature, and
washed 3 times with PBS at the end of treatment. PBS was removed by
aspiration and 1 ml filipin staining solution (5 mg/ml) was added
to each well at room temperature and they were left in the dark for
2 h. Filipin reagent was removed by suction and the cells were
eluted 3 times with PBS. A drop of anti-fluorescence quenching
solution was placed on the slide, a climbing slide was covered, the
slides were sealed and labeled, and the slides were observed using
fluorescence microscopy.
Statistical analysis
For bioinformatics analysis, the differential
expression analysis of DHCR24 in the normal and cancer groups was
performed using the unpaired t-test using the R language. The
paired t-test was used for the paired analysis of differential
expression of DHCR24 in cancer and adjacent tissues. The cell
experiments were repeated three times for each group. In the
filipin staining assays, differences between the two cell groups
(control and EGF groups) were compared using an unpaired t-test.
Differences between ≥3 groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. Data are presented as mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant different. Statistical analysis was
performed using GraphPad Prism 9.3 (Dotmatics).
Results
Pan-cancer analysis of DHCR24
expression
DHCR24 expression was first assessed across several
cancers in the TCGA pan-cancer dataset. The findings revealed that
DHCR24 exhibited differential expression across 12 cancer types
(Fig. 1). Specifically, DHCR24 was
significantly upregulated in seven cancers, including BLCA, BRCA,
cervical cancer, liver cancer, prostate cancer, endometrial cancer
and stomach cancer, whereas its expression was notably lower in
glioblastoma, kidney chromophobe carcinoma, kidney renal clear cell
carcinoma, lung squamous cell carcinoma (LUSC) and lung
adenocarcinoma (Fig. 2A). To
further assess these findings, the DHCR24 expression differences
between tumor tissues and adjacent normal tissues were analyzed for
these 12 cancers. The results demonstrated that, compared with in
normal tissues, DHCR24 was significantly upregulated in BLCA, BRCA,
liver cancer and prostate cancer tumor tissues, whilst it was
downregulated in kidney chromophobe carcinoma, kidney renal clear
cell carcinoma, lung adenocarcinoma and LUSC tumor tissues
(Fig. 2B). These findings suggest
that DHCR24 may serve distinct roles in different cancer types.
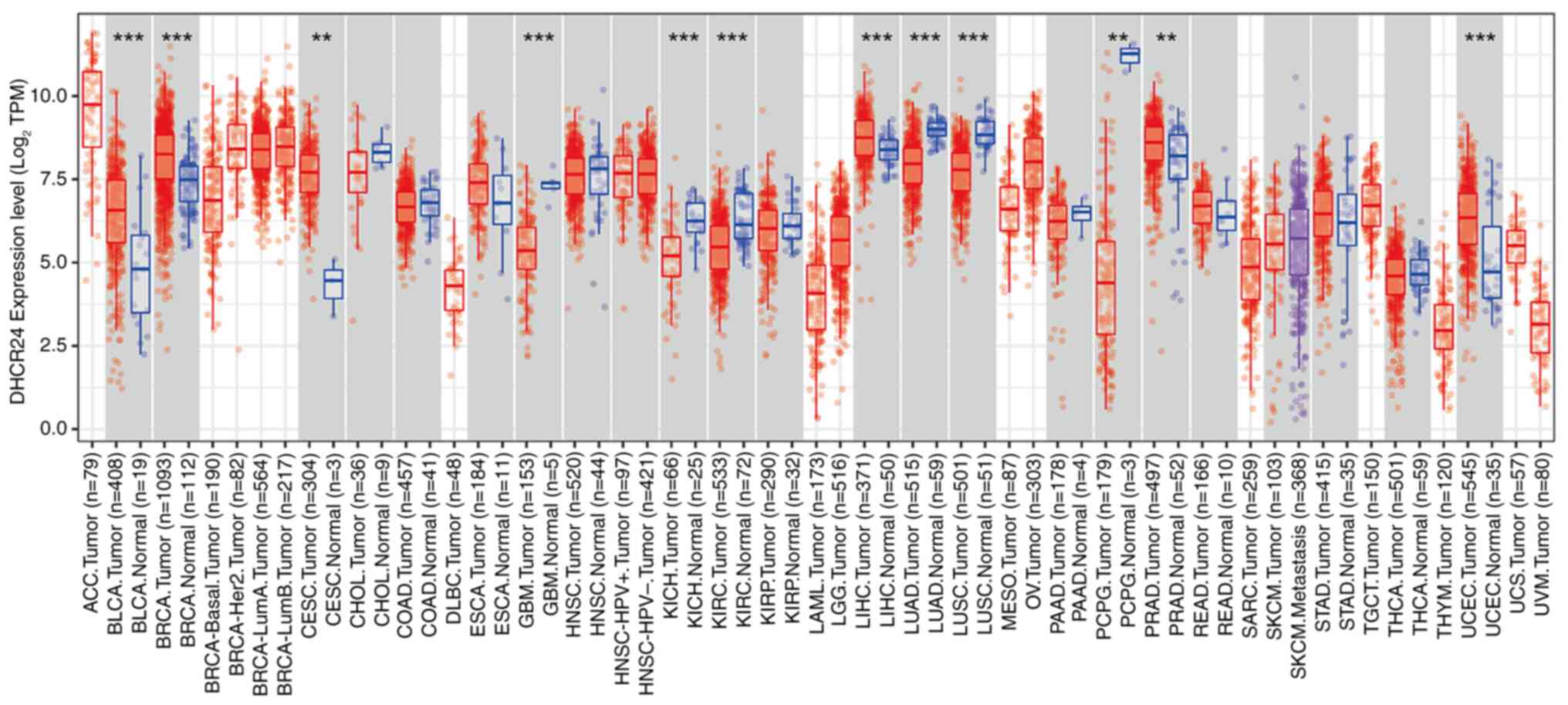 | Figure 1.Upregulated mRNA expression of DHCR24
in pan-cancer. The results obtained from Tumor Immune Estimation
Resource 2.0 were used for preliminary pan-cancer analysis of
cancer data in The Cancer Genome Atlas, and they revealed that
DHCR24 expression was significantly increased in 12 tumors compared
with that in normal tissue. The red and blue boxes represent tumor
tissues and normal tissues, respectively. **P<0.01;
***P<0.001. DHCR24, 3β-hydroxysteroid Δ24-reductase; TPM,
transcripts per million. BLCA, bladder cancer; BRCA, breast
invasive carcinoma; CESC, cervical squamous cell carcinoma and
endocervical adenocarcinoma; GBM, glioblastoma multiforme; KICH,
kidney chromophobe; KIRC, kidney renal clear cell carcinoma; LIHC,
liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC,
lung squamous cell carcinoma; PRAD, prostate adenocarcinoma; UCEC,
uterine corpus endometrial carcinoma; STAD, stomach
adenocarcinoma. |
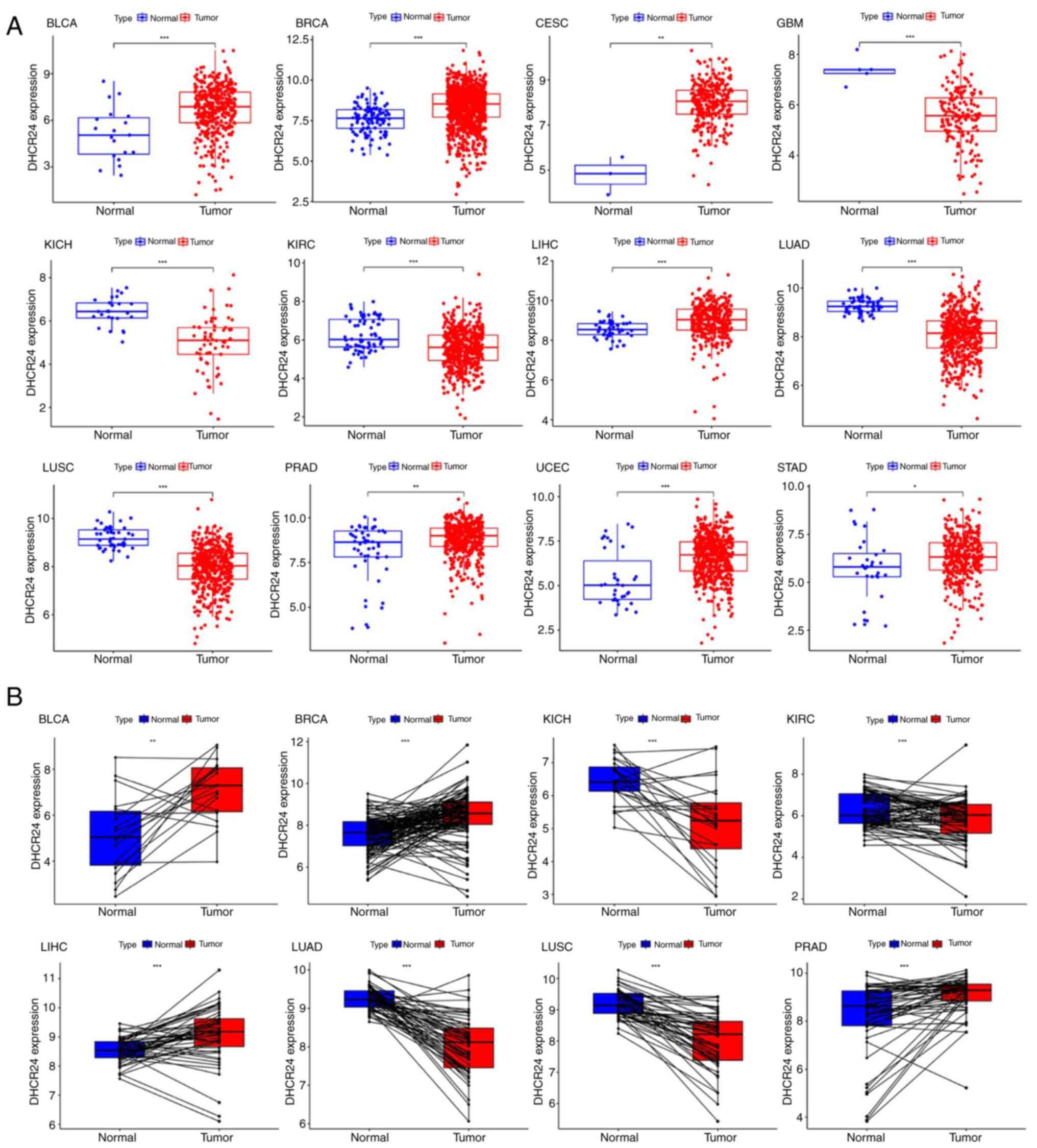 | Figure 2.Expression of DHCR24 in different
types of cancer. (A) Expression distribution of the DHCR24 gene in
tumor and normal tissues. The differences between the two groups
were compared. (B) Pan-cancer differential expression of DHCR24 in
paired tumor and adjacent normal tissues in the indicated tumor
types from The Cancer Genome Atlas database. *P<0.05,
**P<0.01; ***P<0.001. DHCR24, 3β-hydroxysteroid
Δ24-reductase; BLCA, bladder cancer; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma; GBM, glioblastoma multiforme; KICH, kidney
chromophobe; KIRC, kidney renal clear cell carcinoma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; PRAD, prostate adenocarcinoma; UCEC,
uterine corpus endometrial carcinoma; STAD, stomach
adenocarcinoma. |
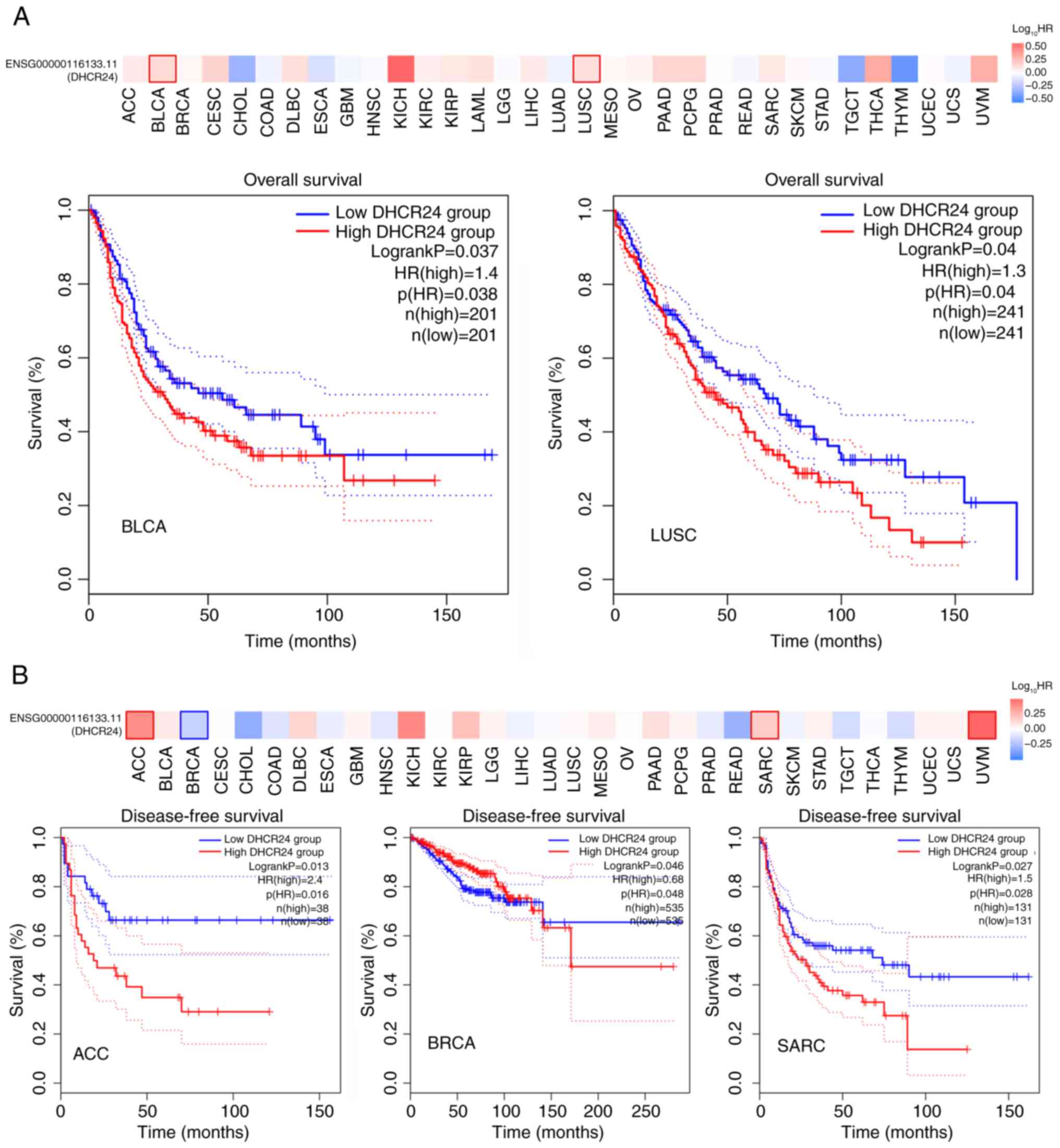
Prognostic and staging implications of
DHCR24
To evaluate the prognostic relevance of DHCR24 in
patients with cancer, a Kaplan-Meier OS analysis was performed,
which revealed that high DHCR24 expression was significantly
associated with a worse prognosis in BLCA compared with a low
expression of DHCR24 (Fig. 3A).
Furthermore, DFS analysis indicated that elevated DHCR24 expression
was significantly associated with a worse prognosis in BRCA
compared with a low expression of DHCR24 (Fig. 3B). Subsequently, DHCR24 expression
across different cancer stages was further assessed using the World
Health Organization staging system (20). The results demonstrated that DHCR24
expression increased with advancing cancer stages of BLCA
(P=0.0371; Fig. 4A), whereas the
opposite trend was observed in LUSC (P=0.0466; Fig. 4A); however, no significant
stage-related variations were noted in other cancers (Fig. 4A). Based on these findings, BLCA was
prioritized for further functional analysis. A one-way Cox
proportional hazards regression analysis was performed using TCGA
data, which demonstrated that DHCR24 acts as an independent risk
factor in BLCA (P=0.036; Fig. 4B).
Additionally, multivariate Cox regression analysis confirmed that
DHCR24 was an independent prognostic factor for BLCA (P=0.015;
Fig. 4B), suggesting that DHCR24
expression significantly influences the survival of patients with
BLCA. Moreover, ROC curve analysis further evaluated the prognostic
use of DHCR24. The risk scores effectively stratified patients with
BLCA based on prognosis at 1, 3 and 5 years (Fig. 4C). Notably, the predictive accuracy
for 1-year survival was relatively high, with an area under the
curve value of 0.575, whilst the predictive probability for
survival beyond 1 year reached 0.725.
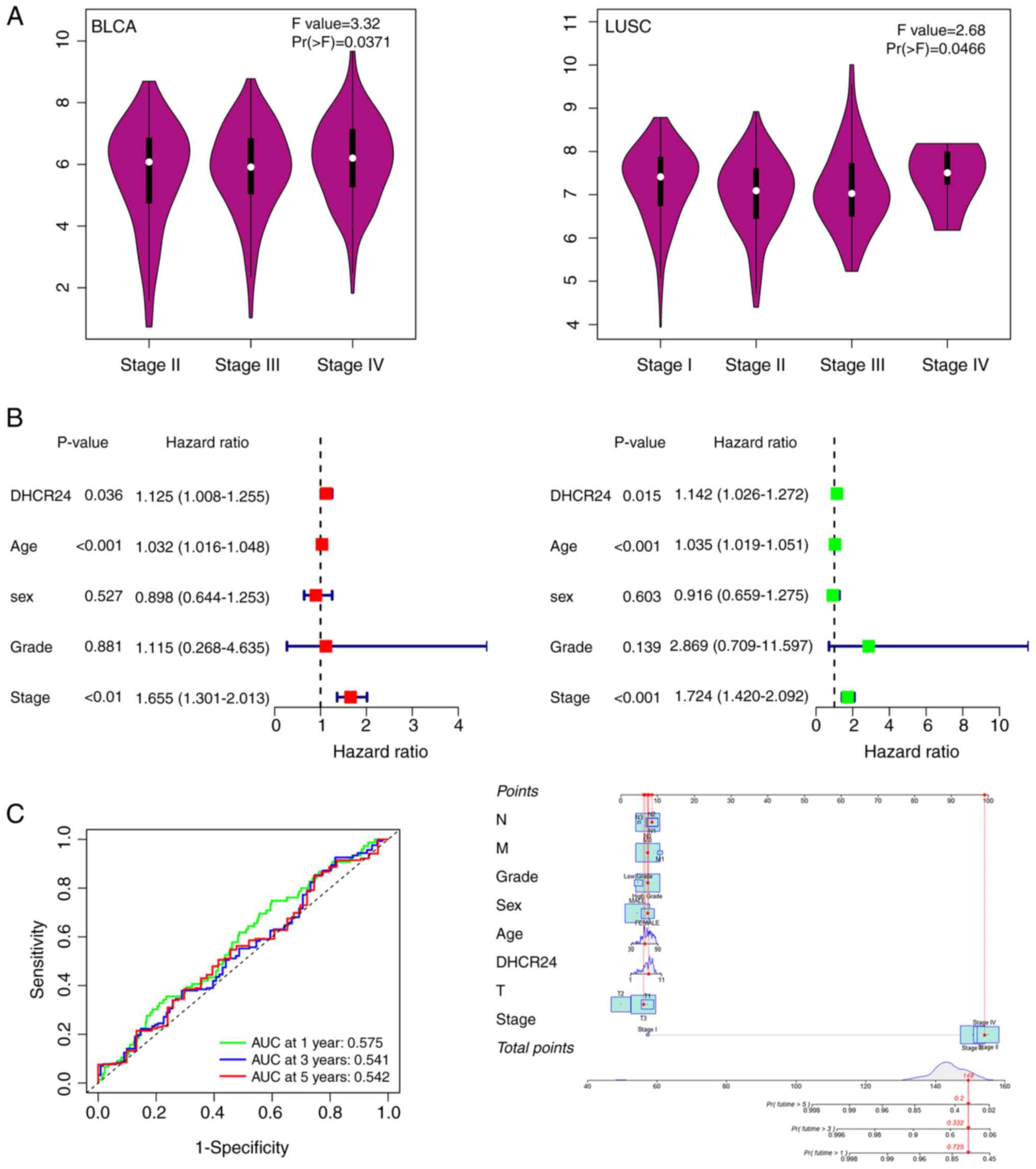 | Figure 4.DHCR24 is a factor for clinical stage
and the prognosis of cancer. (A) Based on The Cancer Genome Atlas
data, the association between expression levels of the DHCR24 gene
and the main pathological stages (stages I–IV) of BLCA and LUSC
were analyzed. Log2 (transcripts per million +1) was
applied for log-scale. (B) Univariate and multifactorial
proportional hazards model analyses. (C) Receiver operating
characteristic curves demonstrated efficacy in predicting risk
scores for the 1-, 3- and 5-year survival of patients, and the
nomogram revealed the probability of predicting the 1-, 3- and
5-year survival of patients. DHCR24, 3β-hydroxysteroid
Δ24-reductase; BLCA, bladder cancer; LUSC, lung squamous cell
carcinoma; AUC, area under the curve; N, lymph node stage; M,
metastasis stage; T, tumor stage. |
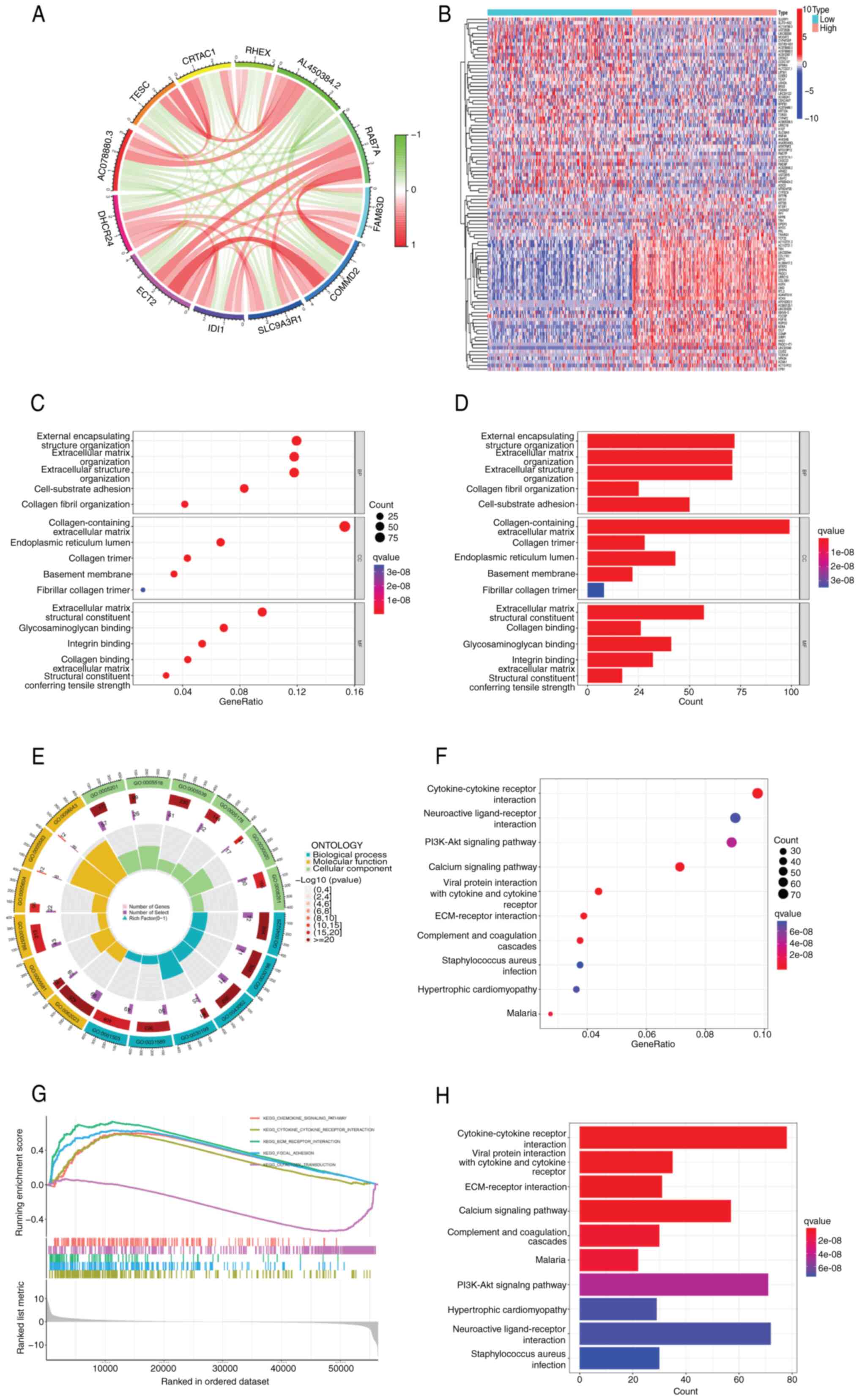
Biological characterization and
consensus clustering analysis of differential genes
Given the pivotal role of DHCR24 in BLCA, a
differential gene expression analysis was performed in BLCA using
the limma package. Consensus clustering analysis (Fig. 5A) identified 11 hub genes closely
associated with DHCR24, namely RAB7A, FAM83D, COMMD2, SLC9A3R1,
IDI1, ECT2, AC078880.3, TESC, CRTAC1, RHEX and AL450384.2. A
heatmap of differentially expressed genes in BLCA samples revealed
a total of 2,958 differentially expressed genes (Fig. 5B). Pathway and enrichment analyses
were performed using the clusterProfiler R package. GO enrichment
analysis (Fig. 5C-E) revealed that
these genes were primarily involved in extracellular matrix
organization, collagen fibril organization and other processes
critical to tumor microenvironment (TME) remodeling. They were
predominantly associated with cellular components such as the
collagen-containing extracellular matrix, collagen trimers and the
endoplasmic reticulum lumen. Additionally, the molecular functions
of these genes included extracellular matrix structural
constituents, collagen binding and glycosaminoglycan binding. KEGG
pathway enrichment analysis (Fig. 5F
and G) identified key pathways enriched for these genes,
including cytokine-cytokine receptor interactions, viral protein
interactions with cytokine receptors, extracellular matrix
(ECM)-receptor interactions, the calcium signaling pathway and the
PI3K-Akt signaling pathway. Furthermore, gene set enrichment
analysis revealed that DHCR24 expression was significantly
associated with the chemokine signaling pathway, cytokine-cytokine
receptor interactions and ECM-receptor interactions (Fig. 5H).
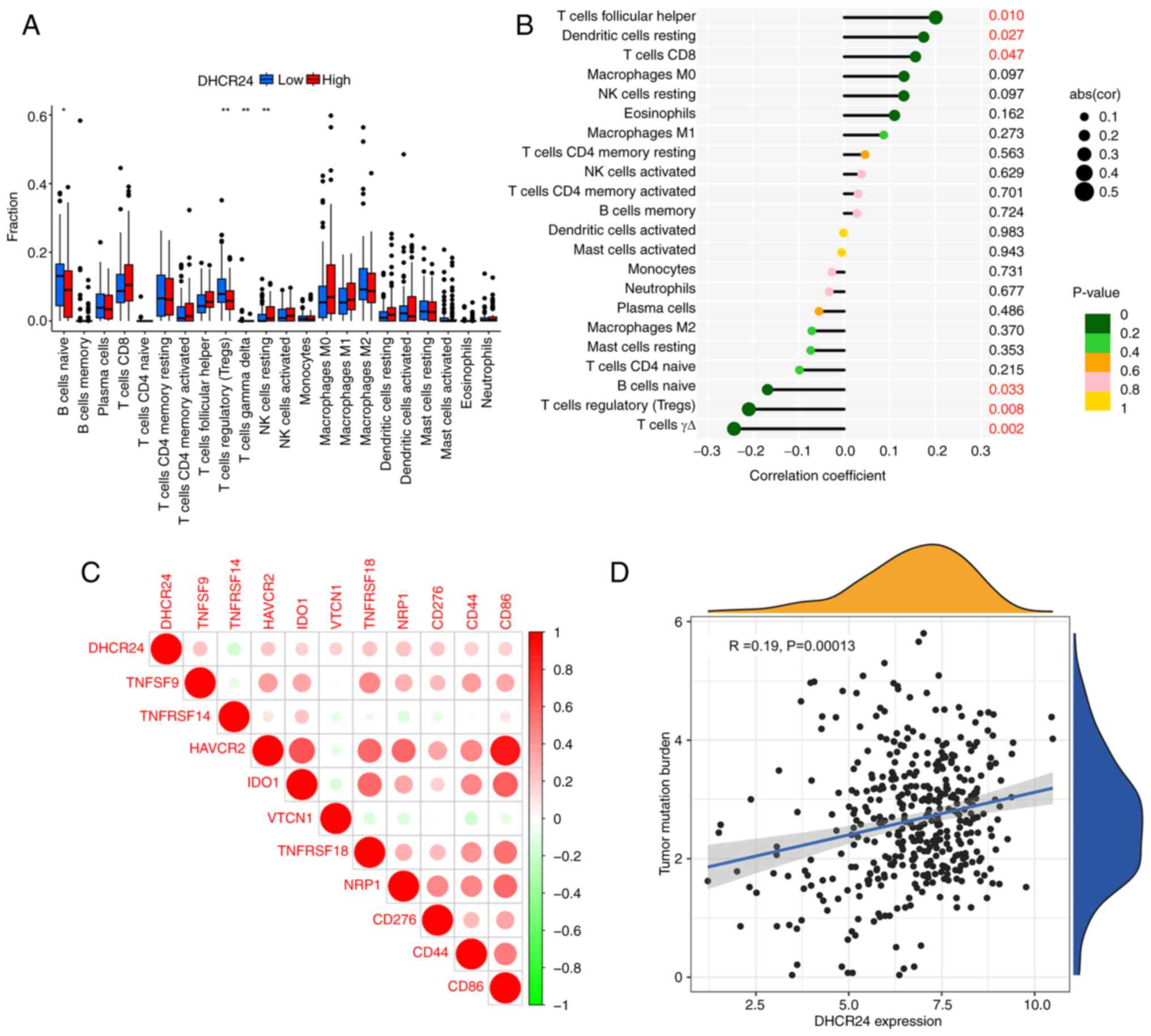
Analysis of DHCR24 expression and
immune cell infiltration
To assess the relationship between DHCR24 and tumor
immunity (Fig. 6A and B), the
correlation between DHCR24 expression and immune cell infiltration
levels in BLCA using the TCGA dataset was analyzed. The findings
indicated that DHCR24 expression in BLCA was positively correlated
with follicular helper T cells, dendritic cells and CD8+ T cells,
whereas it was negatively correlated with naïve B cells, regulatory
T cells and γΔ T cells. Immune surveillance serves a crucial role
in cancer prognosis, as tumors can evade the immune response by
exploiting immune checkpoints (21). The analysis revealed that DHCR24
expression was positively correlated with multiple immune
checkpoint markers, including tumor necrosis factor ligand
superfamily (TNFSF)9, hepatitis A virus cellular receptor 2,
indoleamine 2,3-dioxygenase 1, V-set domain containing T cell
activation inhibitor 1, TNFRSF18, neuropilin 1, CD276, CD44 and
CD86, whilst it was negatively correlated with TNFRSF14
(P<0.001; Fig. 6C). Furthermore,
DHCR24 expression showed a significant positive correlation with
tumor mutational burden (TMB) in BLCA (Fig. 6D), suggesting a potential role of
DHCR24 in the tumor immune microenvironment.
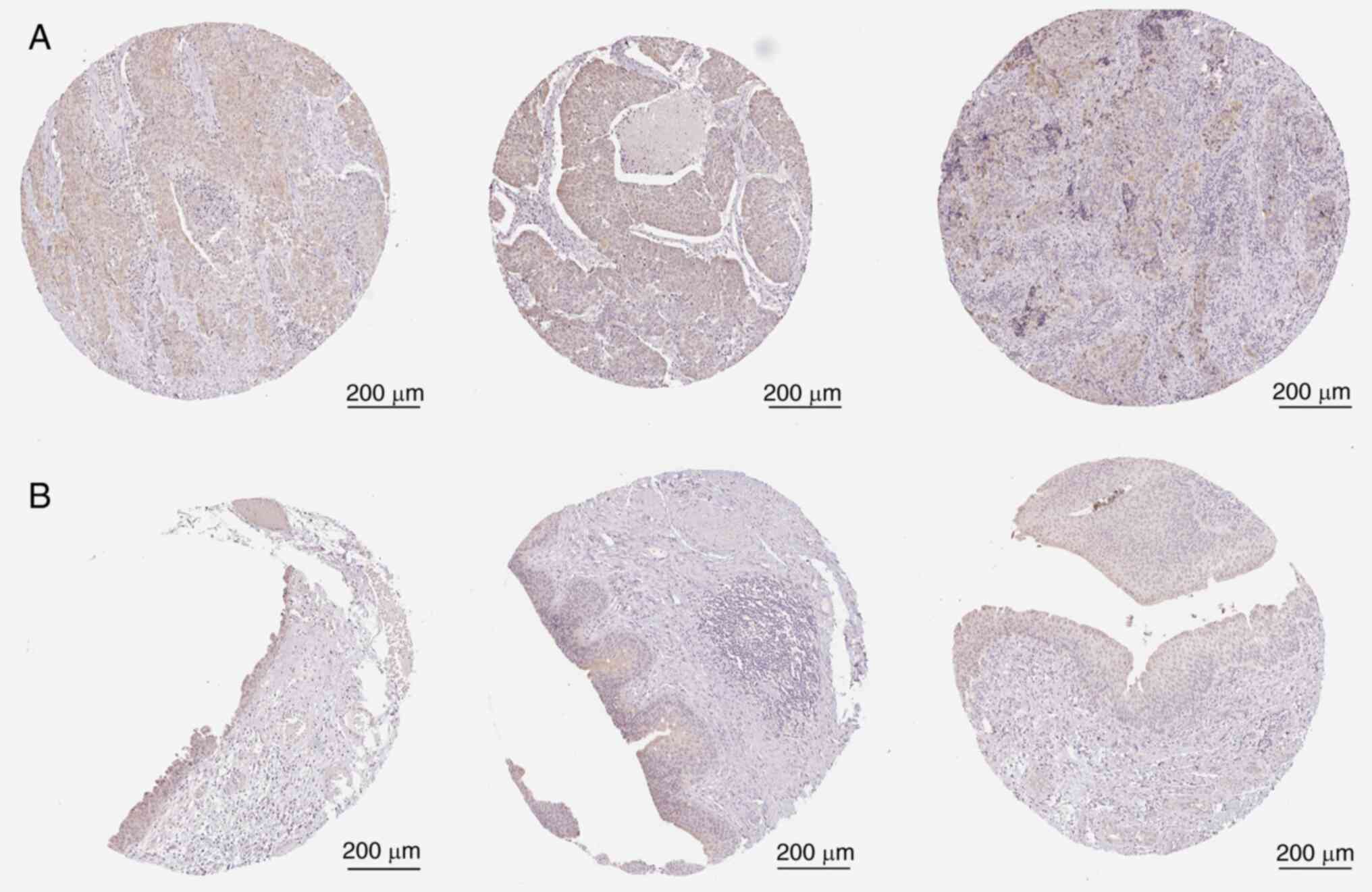
DHCR24 expression in BLCA in the HPA
database
To assess DHCR24 expression in BLCA,
immunohistochemical images from the HPA database were analyzed. The
results demonstrated that DHCR24 expression was markedly higher in
BLCA tissues than in normal tissues (Fig. 7).
Establishment and analysis of the
DHCR24 protein interaction database
Proteins rarely function in isolation; instead, they
interact with other proteins to exert their biological effects
(22). To elucidate the functional
role of DHCR24, a protein interaction analysis was performed to
identify its potential interacting partners. IP-mass spectrometry
using anti-DHCR24 antibodies and 293 cell lysates, combined with
four publicly available protein interaction datasets, identified
492 proteins interacting with DHCR24 (unpublished data). Pathway
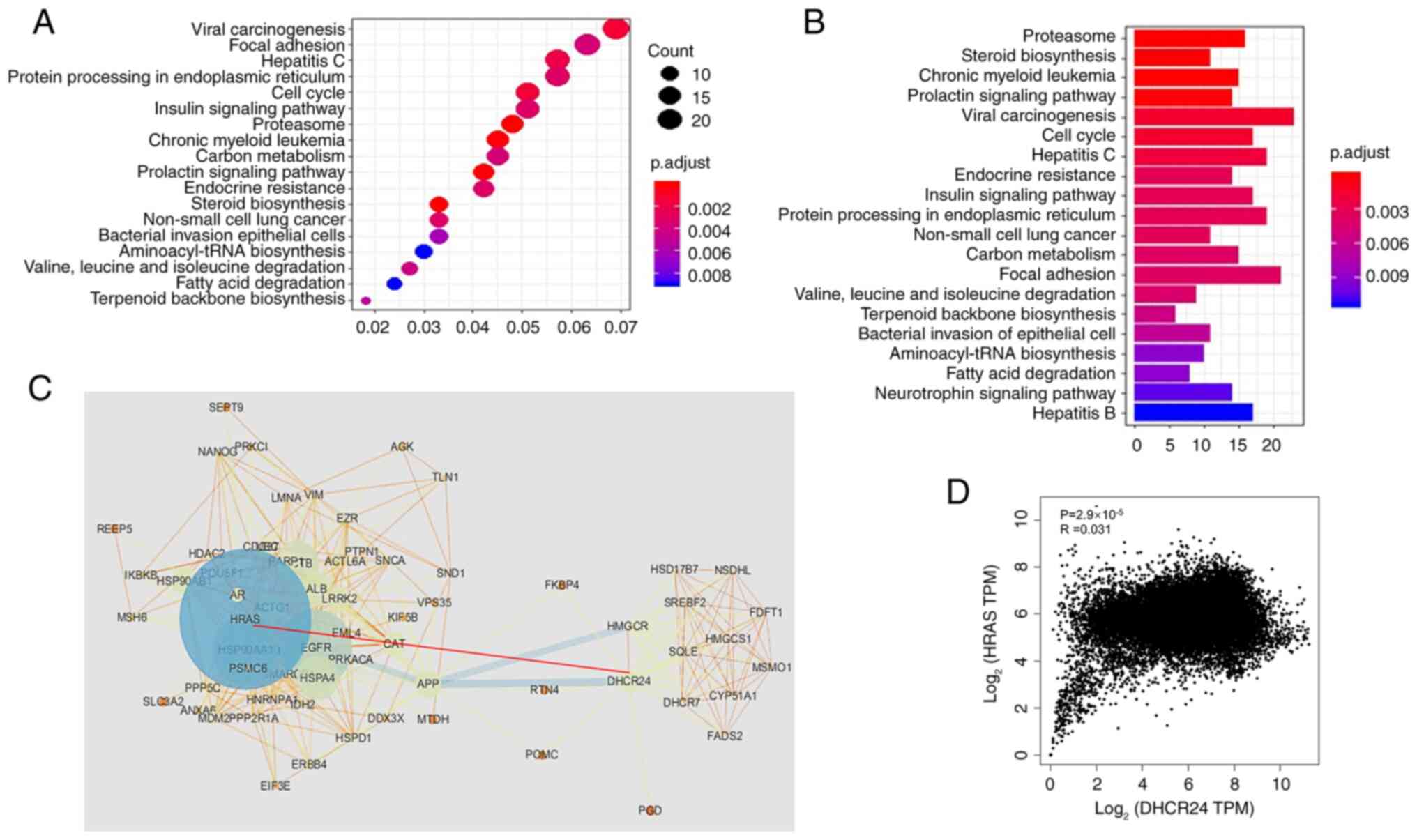
enrichment analysis of this DHCR24 interaction network revealed
significant enrichment in 20 signaling pathways, including the
proteasome pathway, sterol biosynthesis pathway, viral oncogenic
signaling pathway and endoplasmic reticulum protein processing
pathway (Fig. 8A and B). These
findings suggest that DHCR24 and its interacting proteins serve a
critical role in sterol metabolism and cancer progression. To
further assess the interaction network, a PPI network was
constructed using STRING (Fig. 8C),
with the dot size in the PPI network representing the contribution
of each protein to network stability. Notably, DHCR24 exhibited a
direct interaction with HRAS, as indicated by a red dotted line
between DHCR24 and HRAS. HRAS is a well-known oncogene that encodes
a small GTPase involved in cancer progression (23). To further evaluate the functional
significance of the DHCR24-HRAS interaction, DHCR24 and HRAS were
chosen for the main nodes and a subnetwork was constructed
consisting of all proteins directly interacting with them.
Correlation analysis in the GEPIA database further demonstrated a
positive correlation between DHCR24 and HRAS expression in cancer
samples (Fig. 8D), highlighting
their potential cooperative role in tumorigenesis.
Interaction between DHCR24 and
HRAS
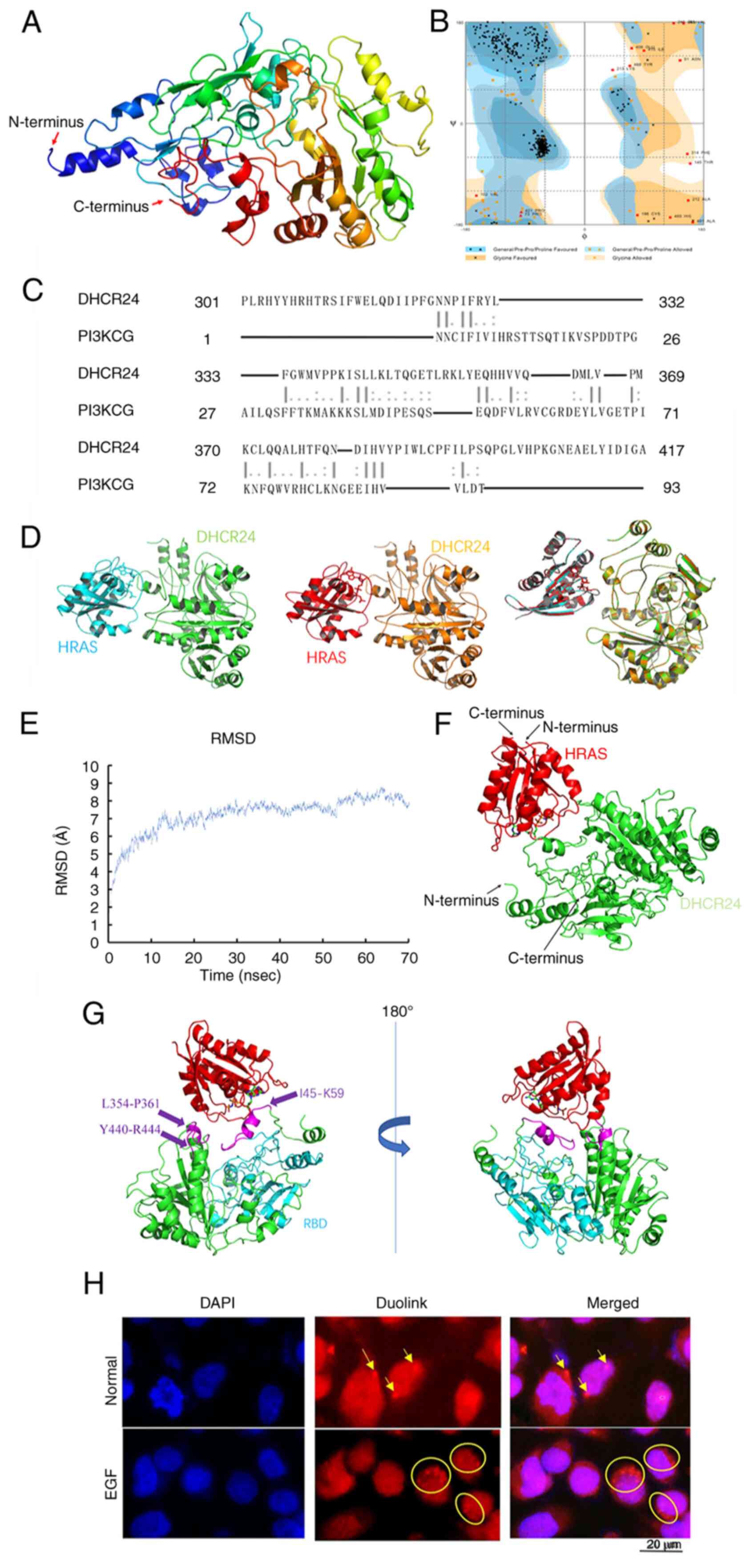
Using the Modeller sequence filtering function, all
templates with ≥30% sequence homology to DHCR24 were selected for
multitemplate modeling. The 3-dimensional (3D) model of DHCR24 was
subsequently constructed using the Modeller multitemplate modeling
function (Fig. 9A). Ramachandran
plot analysis revealed that 86.6% of the residues fell within the
most favorable region, 10.2% in the permissible region and only
3.3% in the outlier region. As an ideal 3D protein structure is
expected to have ≥95% of its residues in the favorable and
permissible regions (24), the
results indicate that the modeled DHCR24 structure was high quality
and well-suited for subsequent analyses (Fig. 9B).
HRAS interacts with the receptor-binding domain
(RBD) region of PI3K, and EGF-mediated activation of HRAS leads to
the activation of PI3K, thereby triggering downstream signaling
pathways (25). To assess the
molecular interaction between DHCR24 and HRAS, protein-protein
docking was performed using the ZDOCK online server (Fig. 9C). A total of two docking strategies
were used: Random docking and hotspot selection based on a DHCR24
homologous sequence similar to the RBD region of
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit γ.
Notably, the top-ranked docking models generated by both strategies
were nearly identical, as confirmed by Pymol-generated structural
overlays (Fig. 9D) (26). These results suggest a direct and
stable binding between DHCR24 and HRAS.
To further refine the binding mode between DHCR24
and HRAS, molecular dynamics simulations we performed using NAMD.
The top-ranked conformation from ZDOCK docking was selected as the
initial structure, and molecular dynamics simulations were
performed to recalculate and optimize the docking results,
providing a more accurate representation of the DHCR24-HRAS
interaction (27). The
root-mean-square deviation values for the DHCR24-HRAS binding
system demonstrated an initial sharp increase within 15 nsec,
followed by a gradual rise after 20 nsec and eventual stabilization
after 55 nsec (Fig. 9E). Based on
this, the stable complex conformation at 70 nsec was selected for
interaction analysis (Fig. 9F)
(28).
Using Pymol, the DHCR24-HRAS interaction interface
was identified, which involved three structural regions of DHCR24:
I45-K59, L354-P361 and Y440-R444. Notably, the L354-P361 region was
homologous to the RBD domain of PI3K, whilst the L45-K59 region was
located near the FAD-binding domain of DHCR24. Subsequently, the
interaction patterns of PI3K-HRAS (PDB: 1HE8) and DHCR24-HRAS were
further compared using Pymol (Fig.
9G). The PI3K-HRAS interface in 1HE8 consisted of two complete
α-helices and β-sheets, whereas the DHCR24-HRAS interface included
two α-helices and two irregular loop regions, with one of the loops
connecting to a β-sheet lamellar structure. These findings indicate
a structural resemblance between the DHCR24-HRAS and PI3K-HRAS
interaction models, suggesting that DHCR24 may functionally
interact with HRAS in a manner similar to PI3K. Collectively, the
aforementioned results provide strong evidence for a direct
interaction between DHCR24 and HRAS, and this interaction may
modulate DHCR24 activity via HRAS activation.
DHCR24 interacts with HRAS in
vivo
To assess the interaction between DHCR24 and HRAS at
the cellular level, 5637 cells, a human BLCA cell line with a
naturally mutated HRAS, were used and the Duolink technique was
applied for verification. The Duolink experiment results revealed
scattered red fluorescent dots outside the nucleus in untreated
5637 cells (Normal group), indicating a direct interaction between
DHCR24 and HRAS (Fig. 9H). Upon EGF
stimulation (EGF group), a marked increase in the number of red
fluorescent dots was observed compared with the Normal group,
strongly suggesting that EGF enhances the interaction between
DHCR24 and HRAS in 5637 cancer cells.
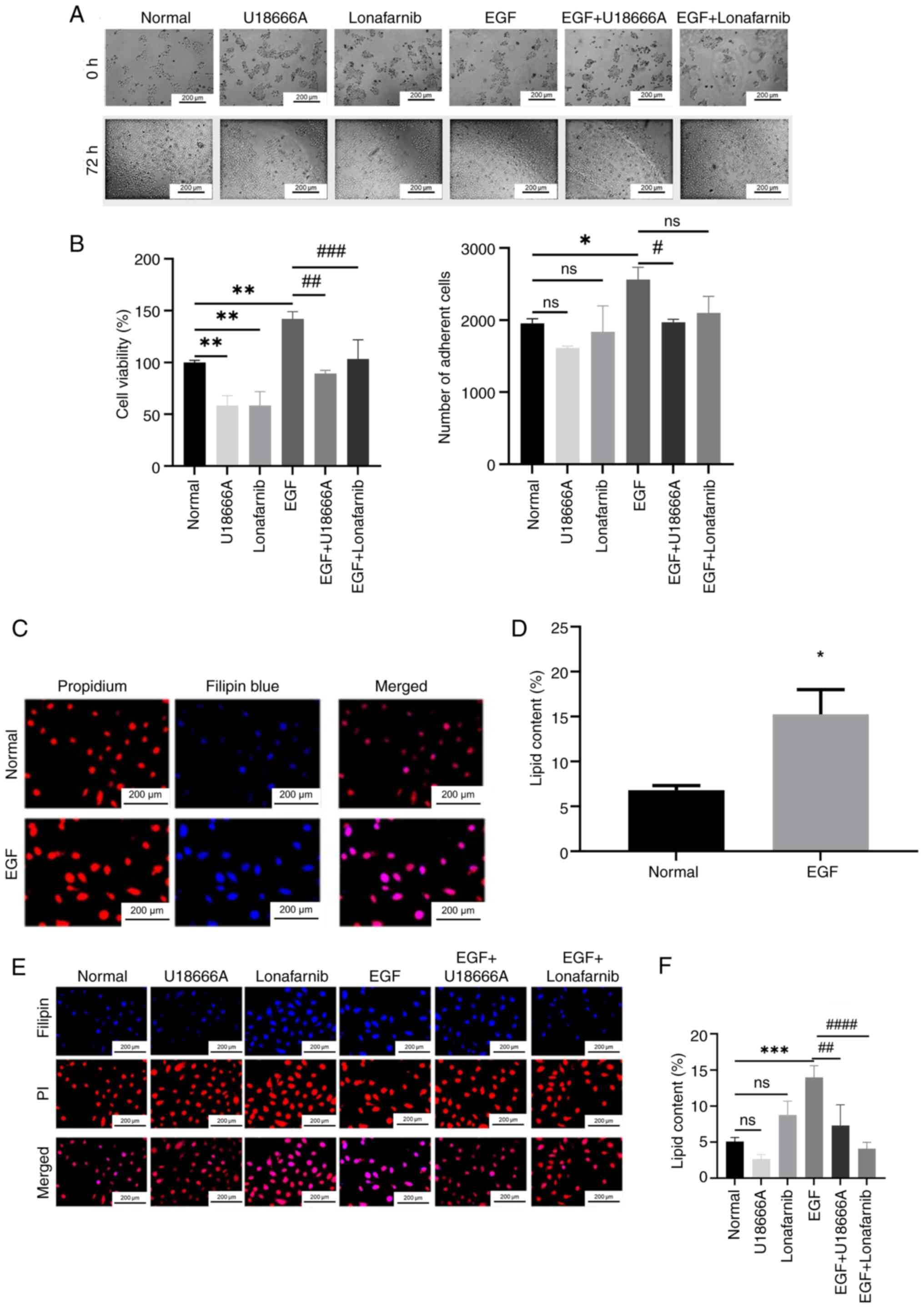
Role of DHCR24-HRAS interaction in
bladder carcinogenesis
To evaluate the role of the DHCR24-HRAS interaction
in bladder carcinogenesis, the present study assessed whether this
interaction influences cancer cell proliferation by catalyzing
cholesterol synthesis. 5637 cells were treated with EGF, lonafarnib
(an HRAS inhibitor) and U18666A (a DHCR24 inhibitor) in different
combinations and cell proliferation was assessed. In the absence of
EGF stimulation, similar numbers of adherent cells were observed
across all groups. However, in the U18666A-treated group, the
number of adherent cells was notably reduced compared with the
normal group, indicating that U18666A inhibited 5637 cell
proliferation (Fig. 10A).
Furthermore, Compared with that in the untreated control group, the
number of adherent cells in the EGF group was significantly
increased, demonstrating that EGF promotes 5637 cell proliferation.
Notably, the addition of U18666A to EGF-stimulated cells (EGF +
U18666A group) led to a marked reduction in adherent cells compared
with the EGF-only group, indicating that U18666A blocked
EGF-induced proliferation. Similarly, the EGF + lonafarnib group
also demonstrated a notable decrease in adherent cells compared
with the EGF group, suggesting that lonafarnib-mediated HRAS
inhibition suppressed EGF-induced cell proliferation. These
findings align with that of previous studies (29,30).
In addition, cell adhesion assessment has certain limitations, for
example, adherent cells do not directly represent the potential of
cell proliferation. In addition, only cells with both adherent and
proliferative activities can form cell clones, which can better
reflect cell life. So w the cell viability assay was performed.
Evaluated cell proliferation was further using the MTT assay, which
yielded results consistent with the aforementioned observations
(Fig. 10B).
To assess whether the EGF-driven proliferation of
5637 cells was associated with increased cholesterol synthesis,
fluorescence staining was performed using the cholesterol-specific
fluorescent dye, filipin. Fluorescence microscopy revealed that
blue fluorescence intensity corresponded with intracellular
cholesterol levels. Weak fluorescence was observed in untreated
5637 cells (Fig. 10C), whereas the
EGF group showed significantly enhanced fluorescence on the cell
membrane compared with the untreated control group, indicating
increased cholesterol content (Fig.
10D).
To determine whether EGF-induced cholesterol
synthesis is dependent on DHCR24 and HRAS, both untreated and
EGF-stimulated cells were treated with lonafarnib and U18666A and
the cholesterol content was assessed using filipin staining.
U18666A, but not lonafarib, significantly reduced cholesterol
synthesis in normal 5637 cells compared with the control (Fig. 10E), suggesting that cholesterol
synthesis in unstimulated 5637 cells requires DHCR24 activity but
is not primarily dependent on HRAS activation. However, in
EGF-treated cells, both U18666A and lonafarnib significantly
inhibited cholesterol synthesis compared with the EGF group
(Fig. 10F), indicating that
EGF-driven cholesterol synthesis relies on the interaction between
HRAS and DHCR24.
Discussion
Cancer remains a significant threat to human health
due to its high morbidity and mortality rates, which have been
rising in recent years (31).
Cancer treatments are broadly classified into chemotherapy,
radiotherapy and surgery; however, many patients lack access to
these treatments. For instance, patients with advanced metastases
rarely qualify for surgical resection, whilst certain individuals
are unable to tolerate the severe side effects of chemotherapy and
radiotherapy. Additionally, targeted therapy is only suitable for a
limited subset of patients. Certain genetically predisposed
cancers, such as CRC, pose particular challenges in achieving
optimal prognostic outcomes through tailored drug therapies
(32). Therefore, early detection
and timely intervention are critical for improving cancer prognosis
(33). Given the heterogeneity of
cancer, treatment strategies are often individualized, with
personalized therapy becoming increasingly prevalent (34). Pan-cancer analyses offer valuable
insights into the commonalities and differences among several
cancers, providing a foundation for personalized treatment
approaches (35). Moreover, this
type of analysis helps elucidate the relationship between genetic
mutations and cancer progression, and, as research advances, it may
serve a pivotal role in promoting early cancer diagnosis and
treatment (36,37).
DHCR24, the terminal enzyme in cholesterol
biosynthesis, is closely associated with several diseases,
particularly hyperlipidemia and hypercholesterolemia, which are
major risk factors for cardiovascular diseases such as
atherosclerosis, coronary artery disease and ischemic heart disease
(38). The liver, a key organ in
fat metabolism, has been reported to be markedly impacted by DHCR24
activity. Research indicates that suppression of DHCR24 expression
can prevent diet-induced hepatic steatosis and inflammation in an
Liver X receptor α-dependent manner (39). Furthermore, hypercholesterolemia has
been strongly associated with the development of steroid
hormone-regulated cancers, including breast and prostate cancer
(40,41). A previous study also suggested a
link between hypercholesterolemia and BLCA, although the underlying
mechanisms remain unclear (42).
Through a pan-cancer analysis of DHCR24, the present
study demonstrated aberrant DHCR24 expression across multiple
cancer types, and identifying it as an independent prognostic
factor for BLCA. This suggests that DHCR24 may serve as a biomarker
for early BLCA diagnosis and treatment. Enrichment analysis of
DHCR24-associated differentially expressed genes in BLCA also
revealed strong associations with cytokines and their receptors,
viral infections and the PI3K-Akt signaling pathway. Viral
infections are well-documented oncogenic factors; for example,
Epstein-Barr virus has been implicated in BRCA and gastric cancer
(43,44). The PI3K-Akt signaling pathway, a key
regulator of cell survival, is widely recognized as a major driver
of cancer progression and therapeutic resistance (45), Notably, numerous studies have
highlighted the notable role of PI3K-Akt pathway activation in the
development of urological malignancies (46,47).
TME, immune checkpoints and TMB are key areas of
focus in cancer research. Growing evidence indicates that
interactions between cancer cells and several components of the TME
contribute to immune evasion, ultimately promoting tumor
proliferation, recurrence and metastasis (48). Immune checkpoints and TMB partially
influence treatment strategies for patients with cancer (49). In the present study, in BLCA, DHCR24
expression was positively correlated with T-cell follicular helper
cells, dendritic cells and CD8+ T cells, whereas it was negatively
correlated with naïve B cells, regulatory T cells and γδ T cells.
This suggests that DHCR24 is also involved in the metastatic
progression of BLCA.
RAS proteins are the founding members of the RAS
superfamily of small GTPases and are central to a highly complex
signaling network that regulates key cellular processes, including
differentiation, survival and proliferation (50). These proteins serve a crucial role
in tumorigenesis, with point mutations leading to chronic RAS
activation in ~30% of human tumors (51). RAS proteins are typically activated
by guanine nucleotide exchange factors, such as Son of sevenless
homolog 1, and inactivated by GTPase-activating proteins (52). In cancer, RAS activation is enhanced
through multiple mechanisms, including amplification of signals
from oncogenic or wild-type RAS, increased upstream inputs such as
receptor tyrosine kinases or guanine nucleotide exchange factors
(51), or the loss of negative
regulators such as GTPase-activating proteins or Sprouty related
EVH1 domain/Sprouty-mediated feedback inhibition (53). The TCGA project identified RTK-RAS
signaling as the most frequently altered oncogenic pathway,
occurring in ~46% of cancer samples. RAS mutations are implicated
in 20–30% of human cancers, with KRAS mutations predominantly found
in pancreatic and CRCs, whereas NRAS mutations are more frequent in
melanoma, thyroid cancer and leukemia (54–57).
Although KRAS, NRAS and HRAS share similar functions, KRAS
mutations typically occur at codon 12, whilst HRAS and NRAS
mutations are more commonly observed at codon 61 (58). These mutations disrupt the
regulatory mechanisms of RAS signaling, leading to sustained
pro-proliferative signaling downstream of growth factor receptors
(58,59).
In a previous study, when DHCR24 was knocked down in
HepG2 cells cultured in the presence of fetal bovine serum, normal
cell proliferation was maintained. However, under fat-free serum
conditions, DHCR24 knockdown markedly reduced cell viability after
48 h, suggesting that desmosterol cannot fully compensate for
cholesterol, at least in terms of supporting cell proliferation
(60). By contrast, other studies
suggest that desmosterol can displace cholesterol in cellular
membranes (61). Although the
functional equivalence of desmosterol and cholesterol in the plasma
membrane remains debated, their effects appear to differ depending
on the tumor cell type. Our previous study using DHCR24 knockout
(DHCR24-/-) mouse embryonic fibroblasts demonstrated that while
desmosterol accumulated in these cells, its levels remained notably
lower than cholesterol levels in wild-type cells. This suggests
that the downregulation of DHCR24 exerts a broader inhibitory
effect on the cholesterol synthesis pathway (62). Thus, in certain tumor cells, reduced
DHCR24 expression not only leads to decreased cholesterol levels
but also results in a comparatively lower desmosterol concentration
than in cells with normal DHCR24 expression. Additionally, our
previous study demonstrated that DHCR24 functions as a hydrogen
peroxide scavenger, protecting cells from oxidative stress-induced
apoptosis (63). Moreover,
DHCR24-mediated sterol homeostasis is essential for mitochondrial
sheath formation during spermatogenesis, highlighting its critical
role in both the urinary and reproductive systems (64). These findings suggest that abnormal
DHCR24 expression in different cancer cells contributes to
tumorigenesis through diverse mechanisms beyond desmosterol
accumulation alone.
The androgen receptor-mediated PI3K/Akt signaling
pathway has been reported to regulate DHCR24 expression.
Additionally, DHCR24 has been implicated in the development of
human BLCA (65). This suggests
that DHCR24 overexpression is associated with BLCA pathogenesis in
males. Notably, studies have reported that a diet high in selenium
or isoflavones can markedly regulate DHCR24 enzyme activity
downstream of the androgen receptor, indicating that such dietary
habits may provide protective effects against the development of
hormone-related cancers (66,67).
Through molecular docking, kinetic simulations and
Duolink experiments, the present study demonstrated that DHCR24
interacts with HRAS and that HRAS activation may influence DHCR24
activity, thereby affecting its ability to synthesize cholesterol.
Furthermore, experiments using EGF, lonafarnib and U18666A (alone
or in combination) in BLCA 5637 cells revealed that the HRAS-DHCR24
interaction enhances cholesterol synthesis, ultimately promoting
cell proliferation in BLCA.
DHCR24 is an enzyme involved in the final step of
cholesterol synthesis and exhibits notable variation in cholesterol
content in BLCA cells (68);
however, a limitation of the present study is the use of a single
human BLCA cell line. In related BLCA research, other commonly used
cell lines, such as T24, RT4 and J82, are also frequently employed
(69). Further study of gene
function usually involves gene knockdown or deletion. The preset
study also has certain limitations in this respect. In the
follow-up study, a BLCA DHCR24 knockout cell line can be created to
further explore the relationship between DHCR24 and the occurrence
and development time of BLCA. Therefore, future research should
verify the findings of the present study across several BLCA cell
lines, which will be valuable for the classification and precision
treatment of BLCA. Moreover, it is currently unclear as to whether
there are differences in cholesterol content in other parts of the
body, and this should be addressed through subsequent animal
experiments in BLCA xenograft mice.
In summary, the results of the present study
demonstrated that DHCR24 is highly expressed in BLCA and serves as
an independent prognostic factor, with significant implications for
tumor progression and treatment strategies. Through pan-cancer
analysis, its association with the TME, TMB and immune cell
infiltration was established, suggesting its potential role in
tumorigenesis and metastasis. Further molecular docking, dynamic
simulations and cellular experiments revealed that DHCR24 interacts
with HRAS, enhancing cholesterol synthesis and promoting BLCA cell
proliferation. The effects of EGF, lonafarnib and U18666A on 5637
cells further demonstrated the role of DHCR24-HRAS interaction in
tumor growth. Given its involvement in key oncogenic pathways,
DHCR24 may serve as a biomarker for BLCA prognosis and a potential
therapeutic target. Future prospective studies and animal
experiments are warranted to explore the role of DHCR24 in immune
cell infiltration and its potential impact on immunotherapy
efficacy, paving the way for personalized treatment strategies
targeting DHCR24 in BLCA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by grants from the National Natural
Science Foundation of China (nos. 82070722, 82300865 and
82470798).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZW, XL and BG conceptualized the study, designed the
methodology and interpreted data. JM and ZL conducted cell
experiments. ZW and YZ performed data acquisition. WY and YZ
conducted the statistical analyses. YZ participated in the drafting
of the manuscript and critical revisions for important intellectual
content and images. BG and DS participated in the study design. XL
and BG confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
DHCR24
|
3β-hydroxysteroid Δ24-reductase
|
|
TIMER
|
Tumor Immune Estimation Resource
|
|
TPM
|
transcripts per million
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
BLCA
|
bladder cancer
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPI
|
protein-protein interaction
|
|
EGF
|
epidermal growth factor
|
|
BRCA
|
breast cancer
|
|
LUSC
|
lung squamous cell carcinoma
|
|
TME
|
tumor microenvironment
|
|
TCGA
|
The Cancer Genome Atlas
|
References
|
1
|
Haas MJ and Mooradian AD: Potential
therapeutic agents that target ATP binding cassette A1 (ABCA1) gene
expression. Drugs. 82:1055–1075. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wahida A, Buschhorn L, Fröhling S, Jost
PJ, Schneeweiss A, Lichter P and Kurzrock R: The coming decade in
precision oncology: Six riddles. Nat Rev Cancer. 23:43–54. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou C, Solomon B, Loong HH, Park K, Pérol
M, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A, et al:
First-line selpercatinib or chemotherapy and pembrolizumab in RET
Fusion-positive NSCLC. N Engl J Med. 389:1839–1850. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Last AR, Ference JD and Menzel ER:
Hyperlipidemia: Drugs for cardiovascular risk reduction in adults.
Am Fam Physician. 95:78–87. 2017.PubMed/NCBI
|
|
5
|
Nong S, Han X, Xiang Y, Qian Y, Wei Y,
Zhang T, Tian K, Shen K, Yang J and Ma X: Metabolic reprogramming
in cancer: Mechanisms and therapeutics. MedComm (2020). 4:e2182023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giacomini I, Gianfanti F, Desbats MA, Orso
G, Berretta M, Prayer-Galetti T, Ragazzi E and Cocetta V:
Cholesterol metabolic reprogramming in cancer and its
pharmacological modulation as therapeutic strategy. Front Oncol.
11:6829112021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel K and Kashfi K: Lipoproteins and
cancer: The role of HDL-C, LDL-C, and cholesterol-lowering drugs.
Biochem Pharmacol. 196:1146542022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelton K, Freeman M and Solomon K:
Cholesterol and prostate cancer. Curr Opin Pharmacol. 12:751–759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jun SY, Brown AJ, Chua NK, Yoon JY, Lee
JJ, Yang JO, Jang I, Jeon SJ, Choi TI, Kim CH and Kim NS: Reduction
of squalene epoxidase by cholesterol accumulation accelerates
colorectal cancer progression and metastasis. Gastroenterology.
160:1194–1207.e28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baek AE, Yu YA, He S, Wardell SE, Chang
CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, et al:
The cholesterol metabolite 27 hydroxycholesterol facilitates breast
cancer metastasis through its actions on immune cells. Nat Commun.
8:8642017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Chen J, Zheng H, Luo Y, An M, Lin
Y, Pang M, Li Y, Kong Y, He W, et al: SUMOylation-Driven mRNA
circularization enhances translation and promotes lymphatic
metastasis of bladder cancer. Cancer Res. 84:434–448. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luu W, Zerenturk EJ, Kristiana I, Bucknall
MP, Sharpe LJ and Brown AJ: Signaling regulates activity of DHCR24,
the final enzyme in cholesterol synthesis. J Lipid Res. 55:410–420.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Wang H, Zhang X, Huang X, Jiang S,
Li Y, Liu T, Lu X and Gao B: High fat diet induces brain injury and
neuronal apoptosis via down-regulating 3-β hydroxycholesterol 24
reductase (DHCR24). Cell Tissue Res. 393:471–487. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CH, Weng TH, Huang KY, Kao HJ, Liao
KW and Weng SL: Anticancer peptide Q7 suppresses the growth and
migration of human endometrial cancer by inhibiting DHCR24
expression and modulating the AKT-mediated pathway. Int J Med Sci.
19:2008–2021. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Guo L, Qiu X, Ren Y, Li F, Cui W and
Song S: Genkwadaphnin inhibits growth and invasion in
hepatocellular carcinoma by blocking DHCR24-mediated cholesterol
biosynthesis and lipid rafts formation. Br J Cancer. 123:1673–1685.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan W, Yong W, Zhu J and Shi D: DPP4
regulates DHCR24-mediated cholesterol biosynthesis to promote
methotrexate resistance in gestational trophoblastic neoplastic
cells. Front Oncol. 11:7040242021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan-Long S, Ming-Bo L, Hong-Ting W, Ye H
and Xuan H: GLTP is a potential prognostic biomarker and correlates
with immunotherapy efficacy in cervical cancer. Dis Markers.
2022:91093652022.PubMed/NCBI
|
|
18
|
Zeynab A, Nasrin Z and Roghayeh A:
Anticancer effects of cinnamaldehyde through inhibition of
ErbB2/HSF1/LDHA pathway in 5637 cell line of bladder cancer.
Anticancer Agents Med Chem. 22:1139–1148. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motie FM, Soltani Howyzeh M and
Ghanbariasad A: Synergic effects of DL-limonene, R-limonene, and
cisplatin on AKT, PI3K, and mTOR gene expression in MDA-MB-231 and
5637 cell lines. Int J Biol Macromol. 280:1362162024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pezeshki S, Hashemi P, Salimi A, Ebrahimi
S, Javanzad M and Monfaredan A: Evaluation of NUF2 and GMNN
expression in prostate cancer: Potential biomarkers for prostate
cancer screening. Rep Biochem Mol Biol. 10:224–232. 2021.PubMed/NCBI
|
|
21
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonzalez MW and Kann MG: Chapter 4:
Protein interactions and disease. PLoS Comput Biol. 8:e10028192012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Astrain G, Nikolova M and Smith MJ:
Functional diversity in the RAS subfamily of small GTPases. Biochem
Soc Trans. 50:921–933. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jumper J, Evans R, Pritzel A, Green T,
Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A,
Potapenko A, et al: Highly accurate protein structure prediction
with AlphaFold. Naturev. 596:583–589. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:522017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delano WL: The PyMol molecular graphics
system. Proteins Structure Function Bioinformatics. 30:442–454.
2002.
|
|
27
|
Phillips JC, Braun R, Wang W, Gumbart J,
Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L and Schulten K:
Scalable molecular dynamics with NAMD. J Comput Chem. 26:1781–802.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Lu Z, Li Y, Liu T, Zhao L, Gao T,
Lu X and Gao B: Virtual screening of Novel 24-dehydroxysterol
reductase (DHCR24) inhibitors and the biological evaluation of
irbesartan in Cholesterol-lowering effect. Molecules. 28:26432023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Li Y, Yang B, Wang H, Lu C, Chang
AK, Huang X, Zhang X, Lu Z, Lu X and Gao B: Suppression of neuronal
cholesterol biosynthesis impairs brain functions through
insulin-like growth factor I-Akt signaling. Int J Biol Sci.
17:3702–3716. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quan X, Chen X, Sun D, Xu B, Zhao L, Shi
X, Liu H, Gao B and Lu X: The mechanism of the effect of U18666a on
blocking the activity of 3β-hydroxysterol Δ-24-reductase (DHCR24):
Molecular dynamics simulation study and free energy analysis. J Mol
Model. 22:462016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Liu P, Zhou M, Yin L, Wang M, Liu
T, Jiang X and Gao H: Small-molecule drugs of colorectal cancer:
Current status and future directions. Biochim Biophys Acta Mol
Basis Dis. 1870:1668802024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crosby D, Bhatia S, Brindle KM, Coussens
LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS and
Kuhn P: Early detection of cancer. Science. 375:eaay90402022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Xie J, Yang Z, Yu CKW, Hu Y and
Qin J: Tumour heterogeneity and personalized treatment screening
based on single-cell transcriptomics. Comput Struct Biotechnol J.
27:307–320. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Li X, Huang H, Tao L, Zhang C, Xie Y
and Jiang Y: SERCA3Role of in the prognosis and immune function in
Pan-cancer. J Oncol. 2022:93598792022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song J, Yang R, Wei R, Du Y, He P and Liu
X: Pan-cancer analysis reveals RIPK2 predicts prognosis and
promotes immune therapy resistance via triggering cytotoxic T
lymphocytes dysfunction. Mol Med. 28:472022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fang Z, Li P, Li H, Chong W, Li L, Shang L
and Li F: New insights into PTBP3 in human cancers: Immune cell
infiltration, TMB, MSI, PDCD1 and m6A markers. Front Pharmacol.
13:8113382022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rossini E, Biscetti F, Rando MM, Nardella
E, Cecchini AL, Nicolazzi MA, Covino M, Gasbarrini A, Massetti M
and Flex A: Statins in high cardiovascular risk patients: Do
comorbidities and characteristics matter? Int J Mol Sci.
23:93262022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou E, Ge X, Nakashima H, Li R, van der
Zande HJP, Liu C, Li Z, Müller C, Bracher F, Mohammed Y, et al:
Inhibition of DHCR24 activates LXRα to ameliorate hepatic steatosis
and inflammation. EMBO Mol Med. 15:e168452023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bravi F, Scotti L, Bosetti C, Talamini R,
Negri E, Montella M, Franceschi S and La Vecchia C: Self-reported
history of hypercholesterolaemia and gallstones and the risk of
prostate cancer. Ann Oncol. 17:1014–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murtola TJ, Kasurinen TVJ, Talala K, Taari
K, Tammela TLJ and Auvinen A: Serum cholesterol and prostate cancer
risk in the Finnish randomized study of screening for prostate
cancer. Prostate Cancer Prostatic Dis. 22:66–76. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang L, Sun J, Li M, Long Y, Zhang D, Guo
H, Huang R and Yan J: Oxidized Low-density lipoprotein links
hypercholesterolemia and bladder cancer aggressiveness by promoting
cancer stemness. Cancer Res. 81:5720–5732. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Glaser S, Hsu J and Gulley M: Epstein-Barr
virus and breast cancer: State of the evidence for viral
carcinogenesis. Cancer Epidemiol Biomarkers Prev. 13:688–697. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liang Q, Yao X, Tang S, Zhang J, Yau TO,
Li X, Tang CM, Kang W, Lung RW, Li JW, et al: Integrative
identification of Epstein-Barr virus-associated mutations and
epigenetic alterations in gastric cancer. Gastroenterology.
147:350–1362.e4. 2014. View Article : Google Scholar
|
|
45
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rezaei S, Nikpanjeh N, Rezaee A, Gholami
S, Hashemipour R, Biavarz N, Yousefi F, Tashakori A, Salmani F,
Rajabi R, et al: PI3K/Akt signaling in urological cancers:
Tumorigenesis function, therapeutic potential, and therapy response
regulation. Eur J Pharmacol. 955:1759092023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Houédé N and Pourquier P: Targeting the
genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use
in the treatment of bladder cancers. Pharmacol Ther. 145:1–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sordo-Bahamonde C, Lorenzo-Herrero S,
Granda-Díaz R, Martínez-Pérez A, Aguilar-García C, Rodrigo JP,
García-Pedrero JM and Gonzalez S: Beyond the anti-PD-1/PD-L1 era:
Promising role of the BTLA/HVEM axis as a future target for cancer
immunotherapy. Mol Cancer. 22:1422023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cox A and Der C: Ras history: The saga
continues. Small GTPases. 1:2–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coley AB, Ward A, Keeton AB, Chen X,
Maxuitenko Y, Prakash A, Li F, Foote JB, Buchsbaum DJ and Piazza
GA: Pan-RAS inhibitors: Hitting multiple RAS isozymes with one
stone. Adv Cancer Res. 153:131–168. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McCormick F: A brief history of RAS and
the RAS initiative. Adv Cancer Res. 153:1–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li D, Jackson RA, Yusoff P and Guy GR:
Direct association of Sprouty-related protein with an EVH1 domain
(SPRED) 1 or SPRED2 with DYRK1A modifies substrate/kinase
interactions. J Biol Chem. 285:35374–35385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Prior I, Hood F and Hartley J: The
frequency of ras mutations in cancer. Cancer Res. 80:2969–2974.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zehir A, Benayed R, Shah RH, Syed A,
Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et
al: Mutational landscape of metastatic cancer revealed from
prospective clinical sequencing of 10,000 patients. Nat Med.
23:703–713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kodaz H, Kostek O, Hacioglu MB, Erdogan B,
Kodaz CE, Hacibekiroglu I, Turkmen T, Uzunoglu S and Cicin I:
Frequency of RAS mutations (KRAS, NRAS, HRAS) in human solid
cancer. EJMO. 1:1–7. 2017.
|
|
59
|
Prior I, Lewis P and Mattos C: A
comprehensive survey of Ras mutations in cancer. Cancer Res.
72:2457–2467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Skubic C, Trček H, Nassib P, Kreft T,
Walakira A, Pohar K, Petek S, Režen T, Ihan A and Rozman D:
Knockouts of CYP51A1, DHCR24, or SC5D from cholesterol synthesis
reveal pathways modulated by sterol intermediates. iScience.
27:1106512024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Daniel H, Holger AS, Klaus A, Andreas H
and Peter M: Desmosterol may replace cholesterol in lipid
membranes. Biophys J. 88:1838–1844. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lu X, Kambe F, Cao X, Yoshida T, Ohmori S,
Murakami K, Kaji T, Ishii T, Zadworny D and Seo H: DHCR24-knockout
embryonic fibroblasts are susceptible to serum withdrawal-induced
apoptosis because of dysfunction of caveolae and insulin-Akt-Bad
signaling. Endocrinology. 147:3123–3132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu X, Kambe F, Cao X, Kozaki Y, Kaji T,
Ishii T and Seo H: 3beta-Hydroxysteroid-delta24 reductase is a
hydrogen peroxide scavenger, protecting cells from oxidative
stress-induced apoptosis. Endocrinology. 149:3267–3273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Relovska S, Wang H, Zhang X,
Fernández-Tussy P, Jeong KJ, Choi J, Suárez Y, McDonald JG,
Fernández-Hernando C and Chung JJ: DHCR24-mediated sterol
homeostasis during spermatogenesis is required for sperm
mitochondrial sheath formation and impacts male fertility over
time. bioRxiv. Feb 11–2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hengbing Z, Junfeng W, Jianfeng Z, Min Y
and Zhen H: Testosterone up-regulates seladin-1 expression by iAR
and PI3-K/Akt signaling pathway in C6 cells. Neurosci Lett.
514:122–126. 2012. View Article : Google Scholar
|
|
66
|
Legg RL, Tolman JR, Lovinger CT, Lephart
ED, Setchell KD and Christensen MJ: Diets high in selenium and
isoflavones decrease androgen-regulated gene expression in healthy
rat dorsolateral prostate. Reprod Biol Endocrinol. 6:572008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nakken HL, Lephart ED, Hopkins TJ, Shaw B,
Urie PM and Christensen MJ: Prenatal exposure to soy and selenium
reduces prostate cancer risk factors in tramp mice more than
exposure beginning at six weeks. Prostate. 76:588–596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wei H, Li Z, Qian K, Du W, Ju L, Shan D,
Yu M, Fang Y, Zhang Y, Xiao Y, et al: Unveiling the association
between HMG-CoA reductase inhibitors and bladder cancer: A
comprehensive analysis using mendelian randomization, animal
models, and transcriptomics. Pharmacogenomics J. 24:242024.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang X, Wang L, Lin P, Ning Y, Lin Y, Xie
Y, Zhao C, Mu L and Xu C: Discovery of artesunate (ARS) PROTACs as
GPX4 protein degraders for the treatment of bladder cancer. Eur J
Med Chem. 293:1177102025. View Article : Google Scholar : PubMed/NCBI
|