Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal
antibodies used for cancer treatment that augment host immune
responses toward tumor cells by blocking signaling pathways
responsible for T cell inhibition [such as cytotoxic
T-lymphocyte-associated protein 4 (CTLA4), programmed cell death
protein 1 (PD-1) and programmed death-ligand 1 (PD-L1)]. Despite
their notable oncological efficacy, ICIs can potentially elicit
undesired immune responses, such as dermatitis, pneumonitis, joint
and muscle pain, colitis, hypothyroidism or other endocrinopathies
(1,2). In a systematic review of neurological
adverse events associated with ICIs, Cuzzubbo et al reported
that the incidence of severe neurological immune-related adverse
events (irAEs) was <1%. Although rare, they have the potential
to cause severe disability or life-threatening consequences
(3). Thus, early identification and
effective management of these irAEs is imperative; albeit, further
research is warranted to establish optimal clinical guidelines.
Longitudinally extensive transverse myelitis (LETM) is one of the
most fatal and rapidly progressive neurological syndromes, most
commonly associated with neuromyelitis optica spectrum disorder
(NMOSD). Other causes include spinal cord infarction,
parainfectious myelopathy, or, as in the present case, a
complication of treatment with ICIs (4). The symptoms usually include motor
weakness, para- or tetraparesis, sensory deficits, bowel/bladder
disturbances, and in severe cases, respiratory failure. Radiologic
imaging, usually MRI of the spinal cord, can reveal a variety of
lesions, although the extent of abnormalities may not always be
associated with clinical findings (5). Risk factors for LETM vary, depending
on the etiology and underlying disease. The present paper reports a
comprehensive case elucidating the efficacious treatment and
eventual resolution of LETM as an irAE.
Case report
The oncological history of the patient in the
present report, a 26-year-old woman, began in December 2020 with a
sudden onset of hematuria. The patient admitted themselves to the
Emergency Department, Kazimierz Hołoga Hospital (Nowy Tomyśl,
Poland). CT imaging revealed a 95×76 mm nodular mass in the right
kidney (data not shown), which led to the performance of a
right-sided nephrectomy, without any post-operative complications.
Pathological and immunohistochemical examination, performed in
accordance with widely recognized standards for diagnosing renal
cell carcinoma (RCC) variants (6),
revealed a rare subtype of RCC-a TFE3-rearranged (t)RCC (data not
shown). The patient started receiving 400 mg intravenous (i.v.)
pembrolizumab every 6 weeks and 20 mg lenvatinib orally in May 2021
as the first-line therapy. A total of 2 months later, the patient
underwent a simultaneous integrated boost-intensity-modulated
radiotherapy aimed at multiple metastatic sites in 10 courses of
treatment for 2 weeks: A total dose of 30 Gy for the thoracic spine
at the Th8-Th10 level and a total dose of 48.5 Gy for several
locations, such as the liver and lungs. Other interventions
included a right-sided adrenalectomy and oral L-thyroxine
supplementation titrated according to fluctuating TSH levels. The
diagnosis and treatment timeline of the patient is presented in
Table I.
 | Table I.Timeline of diagnosis and treatment
details of the patient in the present study. |
Table I.
Timeline of diagnosis and treatment
details of the patient in the present study.
| Timeline | Diagnosis and
treatment |
|---|
| December 2020 | Diagnosis of
transcription factor E3-rearranged renal cell carcinoma |
| May 2021 | Implementation of
pembrolizumab and lenvatinib therapy |
| July 2021 | Simultaneous
integrated boost-intensity-modulated radiotherapy for Th8-Th10,
total dose of 30 Gy |
| March 2022 | Onset of symptoms
Gradual loss of muscular strength of the lower extremities and
urinary and fecal incontinence |
|
| Paraparesis and
urinary retention-admission to the Department of Genitourinary
Oncology, Maria Skłodowska-Curie National Research Institute of
Oncology (Warsaw, Poland) |
| April 2022 | MRI revealed a
diffused spinal cord lesion at the Th9-Th10 level, with a possible
autoimmune or inflammatory origin |
|
| Diagnosis of
longitudinally extensive transverse myelitis and implementation of
methylprednisolone and intravenous immunoglobulin |
|
| Patient was gradually
regaining muscular strength, with control of urination and
superficial feel Discharge from hospital, pembrolizumab
discontinued and lenvatinib upheld |
The patient was then admitted to the Department of
Genitourinary Oncology of the Maria Skłodowska-Curie National
Research Institute of Oncology (Warsaw, Poland) in March 2022 due
to progressing hyposthenia of the lower extremities and urinary
retention. The onset of symptoms occurred 3 days before the patient
reported to the hospital. Initially, the patient experienced muscle
weakness in the right lower limb, and then developed weakness in
the other leg, leading to disability and the need for a wheelchair.
Apart from the weakness of the lower limbs, there was a concurrent
report of pain localized to the left gluteal region. The patient
also experienced urinary and fecal incontinence 1 day prior to
admission. Upon arrival at the Emergency Department, the
performance status of the patient was assessed and classified as
Eastern Cooperative Oncology Group (ECOG) 4 (7). A total of 2 mg/kg i.v.
methylprednisolone was administered on the day of admission.
Moreover, until an infectious background could be ruled out, the
patient was administered 3×1,000 mg i.v. meropenem. MRI of the
spinal cord and a CT scan of the thorax, abdomen and small pelvis
were performed. MRI revealed a new nodular lesion in the piriformis
muscle area, alongside an irregular enhancement of the contrast at
the level of Th9-Th10 of the spinal cord, indicative of an
autoimmune or inflammatory origin. Additionally, oedema of the
spinal cord ranging from the Th2 to the conus medullaris was
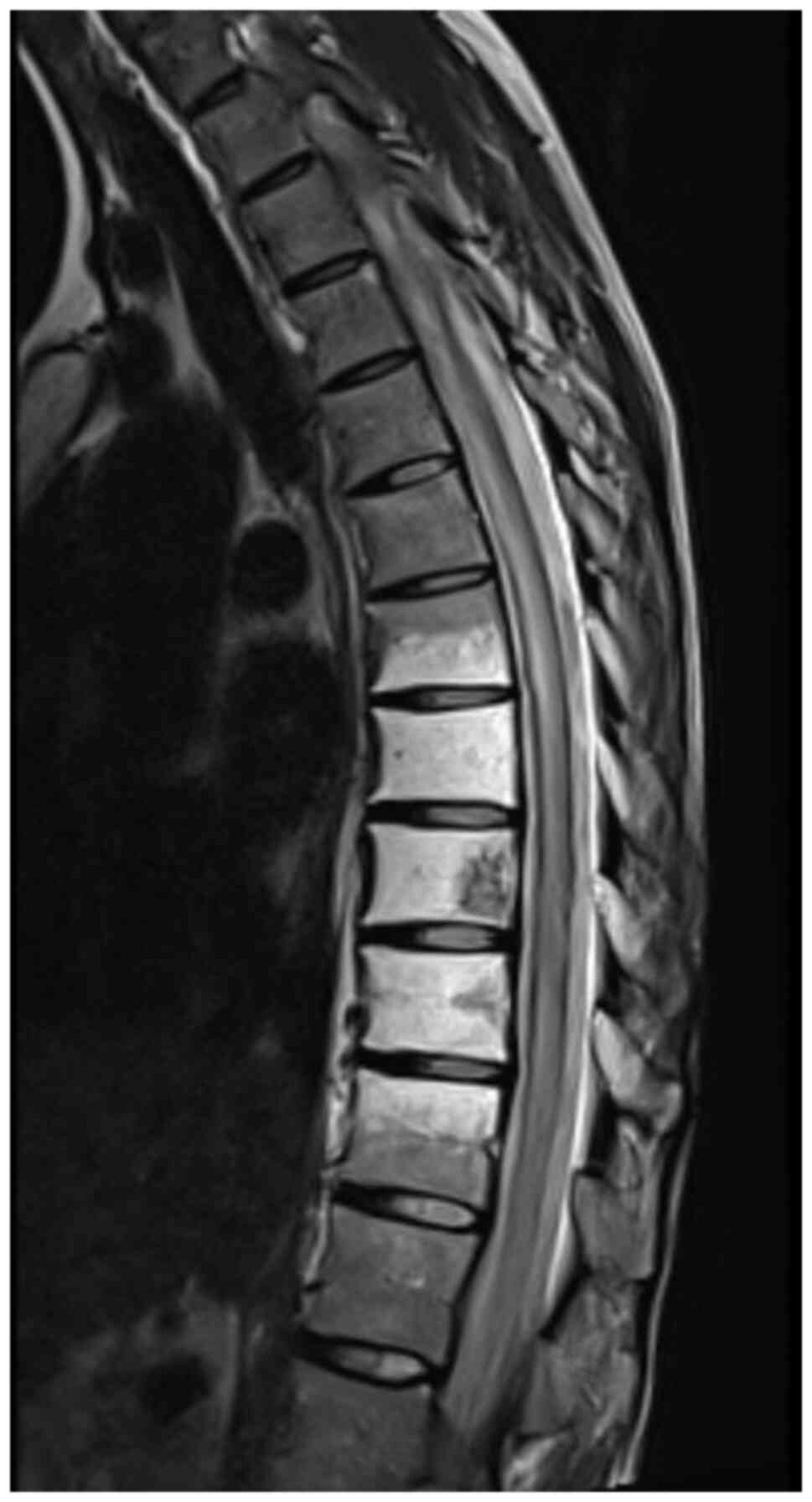
revealed (Fig. 1). CT imaging
demonstrated extensive nodular infiltration propagating through the
obturator foramen into the soft tissues of the left buttock,
possibly involving the gluteal muscles (data not shown).
Subsequently, an ultrasound-guided biopsy of the lesion was
performed, revealing oedema, mixed inflammatory infiltrates and
fibrinous exudate (data not shown). To exclude infectious
transverse myelitis, the more common cause of myelitis (namely,
viral: herpes zoster and Cytomegalovirus; bacterial: Lyme disease
and tuberculosis; and fungal) (8),
a comprehensive assessment was performed, including a complete
blood count with inflammatory markers, as well as cerebrospinal
fluid (CSF) analysis and flow cytometry which revealed a lack of
neoplastic cells and pleocytosis (data not shown), indicating an
underlying inflammatory process (9,10). As
none of these investigations indicated an infectious etiology,
meropenem was discontinued. Based on the radiological findings and
the neurological examination, a diagnosis of LETM was established
in April 2022. Thus, the patient began receiving 1,000 mg i.v.
methylprednisolone daily for 3 days and 2 mg/kg intravenous
immunoglobulin for 4 days. Over the course of 11 days, the patient
gradually regained muscular strength and superficial sensation
(exteroceptive sensation). The ability to perform certain daily
tasks, as well as the control of urination, was also regained, and
the performance score of the patient improved from ECOG 4 to 3.
Therefore, the urinary catheter was removed. Furthermore, the
patient regained the ability to walk using a walker. Pembrolizumab
was discontinued, due to G4 toxicity, with lenvatinib upheld. A
total of 11 days after the patient was diagnosed with LETM, they
were discharged from the hospital. Due to the long half-time life
of ICIs, it was crucial to provide the patient with long-term
treatment with oral prednisone, with tapered dosage, to decrease
the possibility of reoccurrence.
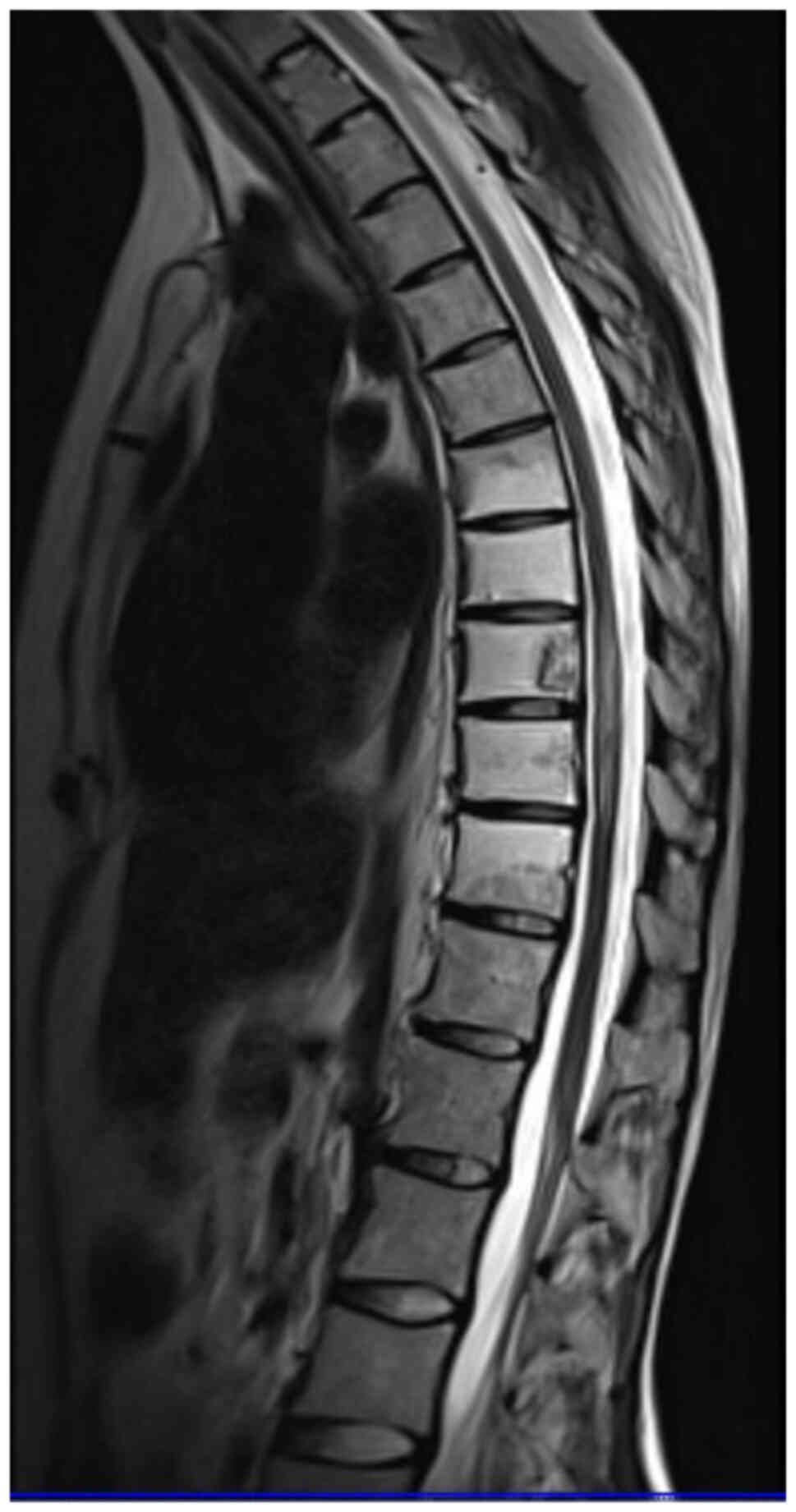
MRI after 5 months was performed as a follow-up. The
oedema of the spinal cord had markedly decreased, and the contrast
enhancement of an area with an elevated signal intensity at the
level of Th9-Th10 was more pronounced than in the previous scan
(Fig. 2). At follow-up, the patient
was still walking with the aid of a walker and had not experienced
any other irAEs.
Discussion
tRCC is a rare RCC subtype harboring TFE3
translocations. It is typically diagnosed in younger patients than
clear cell RCC, and when diagnosed, it is often already at a
metastatic stage due to its typically aggressive clinical behavior
(11). Most patients are usually at
stage III or IV of the disease upon diagnosis. Certain studies
(12,13) estimate that tRCC accounts for ~5% of
all RCCs in adults and between 23–50% of all RCCs in children.
However, these numbers are likely to be higher in reality. To
identify tRCC, fluorescence in situ hybridization is
required. Immunohistochemical staining targeted at TFE3 can be
insufficient due to possible false-positive results. tRCC also has
many overlapping pathological characteristics with other types of
RCC (14). Therefore, it is usually
diagnosed at an advanced stage, and it may remain undiagnosed in
many patients. It is presumed that a history of chemotherapy could
be a risk factor for developing tRCC; however, there are no other
established risk factors (15).
Surgical treatment is usually performed in localized
tRCC as long as there is a possibility for a radical procedure.
ICIs are also one of the possible treatment options in the advanced
stage of the disease. ICIs have been gaining popularity in recent
years when it comes to their efficacy in several cancer types.
However, due to their mechanism of action, irAEs such as dermatitis
and colitis can arise during the therapy. Moreover, irAEs of any
kind can affect ≤40% of patients during ICI treatment (16). One of the possible systems that can
be affected is the central nervous system, although this occurs
relatively infrequently compared with other irAEs. Furthermore,
LETM is a rare but potentially life-threatening condition that can
present with pain, sensory deficits, motor deficits, or bladder and
rectal sphincter dysfunction (17).
Apart from MRI, it is vital to exclude infectious causes, perform
CSF analysis, and assess for paraneoplastic antibodies.
NMOSD is one of the most common causes of LETM and
should be taken into consideration in differential diagnosis
(4). To confirm the diagnosis of
NMOSD, testing for the presence of NMOSD-specific antibodies such
as anti-aquaporin 4 and anti-myelin oligodendrocyte should be
performed (18). However, the Maria
Skłodowska-Curie National Research Institute of Oncology could not
assess these parameters and the nearest neurological clinic could
not provide them quickly enough, as it would significantly postpone
the treatment. Therefore, in the present case, the therapy was
implemented without assessing the presence of the antibodies.
There are reports of LETM as an adverse effect of
both radiotherapy near the spinal cord and ICI treatment (19,20).
In cases such as in the present report, where both risk factors
appear, it was challenging to establish the main trigger of LETM.
Nonetheless in this particular case, it seemed that this adverse
effect occurred as a complication of immunotherapy with
pembrolizumab, rather than the radiation therapy itself. The
patient in the present case had also been receiving lenvatinib (a
vascular endothelial growth factor receptor tyrosine kinase
inhibitor) together with pembrolizumab. However, it is highly
unlikely that lenvatinib caused LETM in the patient, as this drug
is not associated with causing myelitis.
irAEs can occur at any time during immunotherapy
treatment (21). The time interval
for developing myelitis from the initiation of ICI differs
throughout the literature, ranging from several days to >40
weeks (15). The patient in the
present report developed LETM 9 months after pembrolizumab was
initiated, and 3 weeks after its last infusion. The changes in the
spinal cord were described as inflammatory or of an autoimmune
origin, hinting at the underlying cause, whilst CSF analysis
suggested an inflammatory process: Pleocytosis and borderline
protein level. Moreover, the biopsy of the piriformis muscle
revealed an inflammatory infiltrate and fibrous exudation. Such
co-occurrence of auto-aggressive incidents implies that
pembrolizumab was the trigger for LETM.
The National Comprehensive Cancer Network (NCCN)
guidelines for the management of immunotherapy-related toxicities
state that patients with suspicion of LETM should be treated with
methylprednisolone boluses with dosing of 1 g per day for 3–5 days
and strongly suggest considering intravenous immunoglobulin or
plasmapheresis (22). Additionally,
the NCCN recommends discontinuing treatment with ICIs. Therefore,
upon admission to Department of Genitourinary Oncology at Maria
Skłodowska-Curie National Research Institute of Oncology,
pembrolizumab was discontinued and lenvatinib was upheld. Apart
from the aforementioned recommended treatment, there are reports of
treatment of LETMs with cyclophosphamide or infliximab, a
monoclonal antibody targeting tumor necrosis factor, with outcomes
varying depending on the condition of the patient (23,24).
The spinal cord has a high sensitivity to radiation
and is susceptible to damage by irradiation. However, such effects
are dose-related and a safe dose for spinal radiotherapy has been
established. The probability of myelitis is 0.03 and 0.2% for 45
and 50 Gy, respectively (25),
whilst the median tolerance dose for myelopathy has been estimated
at 69.4 Gy (26). The patient in
the present report received a total of 30 Gy in 10 fractions at the
region of Th8-Th10. This dose is well below the safe dose for the
spine, therefore it seems unlikely that radiation could be the
cause of LETM. Moreover, the occurrence of lesions revealed during
MRI performed on the day of the admission (Th9-Th10) corresponded
to the irradiated location (Th8-Th10). However, these lesions were
already visible in previous MRI scans, a few months before the LETM
developed. Furthermore, the radiologist evaluating the MRI scan
assessed these changes as mostly of an autoimmune or inflammatory
origin.
The patient in the present report received
radiotherapy in July 2021, whilst the neurological symptoms began
in March 2022, so there were almost nine months in between. Such a
delayed onset of LETM is possible, as radiation myelopathy has two
different manifestations: i) Early delayed or transient myelopathy,
which occurs usually between 6 weeks to 6 months after irradiation
and presents with Lhermitte's sign. This is usually benign and
self-limiting; and ii) Delayed or progressive myelopathy, which is
a more malignant manifestation, that develops usually >6 months
after radiotherapy (most commonly between 9–15 months after) and
can consist of a wide range of clinical manifestations, such as
minor motor and sensory deficits, Brown-Séquard syndrome,
transverse myelopathy, and bladder and bowel sphincters
dysfunctions (27). The precise
mechanism by which radiation causes myelitis is still unknown, but
oligodendrocytes and endothelial cells of the blood vessels of the
spinal cord have been reported to be especially susceptible to
radiation. Both tissues, however, differ in the usual latency in
which the potential damage manifests itself. Endothelial cells are
more sensitive to radiation and the damaging effects have a longer
latency period in contrast to the glial cells, which are less
sensitive and have a shorter latency of the onset of the symptoms.
Therefore, the early transient form of myelopathy is associated
more with the damage of the neuroglia, whilst more persistent and
delayed damage is linked to the damage of the small vessels and
small areas of ischemia within the spinal cord (28). Radio-sensitizing chemotherapeutics
are a risk factor for developing radiation myelopathy. Khan et
al (28) reported that the
incidence of radiation myelitis was higher in patients with
previous or concurrent chemotherapy treatment. This finding is also
consistent with reports by Seddon et al (29), Rubin (30) and Chao et al (31).
A previous study suggested that apart from having a
collaborative effect on cancer treatment, combining radiotherapy
and immunotherapy also has an effect on the incidence of adverse
effects (32). Radiotherapy
increases the immunogenic death of tumor and host cells, and it is
possible that this mechanism underlies an ‘abscopal effect’ of
radiotherapy, in which non-irradiated metastases regress after
irradiation of another neoplastic lesions. Radiotherapy stimulates
the release of tumor cell antigens, antigens of non-malignant,
healthy tissues, as well as damage-associated molecular patterns
which activate antigen-presenting cells (APCs), and thus results in
increased antigen presentation to T-cells (33). APCs that have taken up the antigens
of tumor cells migrate to the draining lymph nodes where priming
and activation of naive T-cells occurs. Radiotherapy increases the
repertoire of circulating T-cell receptors of the circulating T
lymphocytes and induces the release of inflammatory chemokines and
cytokines, which can in turn recruit and promote migration of the
immune cells to the tumor microenvironment, promoting antitumor
responses. However, it also promotes auto-aggressive events.
Additionally, anti-PD-L1 antibodies decrease T-cell exhaustion.
Their synergistic mechanism of action in terms of immunologic
response might also affect the spectrum and severity of
treatment-related toxicities (34).
In conclusion, the initial diagnosis of LETM is
often difficult. The differential diagnosis should include NMOSD,
infectious causes, paraneoplastic myelitis, multiple sclerosis or
other autoimmunological conditions (35). The patient in the present report was
diagnosed with LETM and treated adequately rather quickly: Symptoms
began in March and the treatment was implemented in April. The
patient eventually regained the ability to walk and their quality
of life improved significantly. However, permanent neurological
deficits are a common complication of LETM, and death is also a
possible outcome. To the best of our knowledge, the present case
report is the first to describe LETM that occurred during the
treatment of tRCC. As ICIs gain more indications for oncological
treatment, it is important to emphasize that such therapy can
result in serious or even life-threatening adverse effects, which
should be diagnosed and treated as quickly as possible.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MP and BK wrote and revised the manuscript. JK, TD,
AH, PD and JJ initiated and designed the project. MP, BK, JK, TD,
PD, JJ and AH collected and organized all data. All authors
reviewed and edited the manuscript, and read and approved the final
version of the manuscript. MP and JK confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Franzin R, Netti GS, Spadaccino F, Porta
C, Gesualdo L, Stallone G, Castellano G and Ranieri E: The use of
immune checkpoint inhibitors in oncology and the occurrence of AKI:
Where do we stand? Front Immunol. 11:5742712020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuzzubbo S, Javeri F, Tissier M, Roumi A,
Barlog C, Doridam J, Lebbe C, Belin C, Ursu R and Carpentier AF:
Neurological adverse events associated with immune checkpoint
inhibitors: Review of the literature. Eur J Cancer. 73:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tobin WO, Weinshenker BG and Lucchinetti
CF: Longitudinally extensive transverse myelitis. Curr Opin Neurol.
27:279–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitley J, Leite M, George J and Palace J:
The differential diagnosis of longitudinally extensive transverse
myelitis. Mult Scler. 18:271–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CR, Suh J, Jang D, Jin BY, Cho J, Lee
M, Sim H, Kang M, Lee J, Park JH, et al: Comprehensive molecular
characterization of TFE3-rearranged renal cell carcinoma. Exp Mol
Med. 56:1807–1815. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–656. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gritsch D and Valencia-Sanchez C:
Drug-related immune-mediated myelopathies. Front Neurol.
13:10032702022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLean BN, Luxton RW and Thompson EJ: A
study of immunoglobulin G in the cerebrospinal fluid of 1007
patients with suspected neurological disease using isoelectric
focusing and the Log IgG-Index. A comparison and diagnostic
applications. Brain. 113:1269–1289. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tumani H, Petereit HF, Gerritzen A, Gross
CC, Huss A, Isenmann S, Jesse S, Khalil M, Lewczuk P, Lewerenz J,
et al: S1 guidelines ‘lumbar puncture and cerebrospinal fluid
analysis’ (abridged and translated version). Neurol Res Pract.
2:82020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simonaggio A, Ambrosetti D, Verkarre V,
Auvray M, Oudard S and Vano YA: MiTF/TFE translocation renal cell
carcinomas: From clinical entities to molecular insights. Int J Mol
Sci. 23:76492022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross H and Argani P: Xp11 translocation
renal cell carcinoma. Pathology. 42:369–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magers MJ, Udager AM and Mehra R: MiT
family translocation-associated renal cell carcinoma: A
contemporary update with emphasis on morphologic, immunophenotypic,
and molecular mimics. Arch Pathol Lab Med. 139:1224–1233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manucha V, Sessums MT, Lewin J and Akhtar
I: Cyto-histological correlation of Xp11.2 translocation/TFE3 gene
fusion associated renal cell carcinoma: Report of a case with
review of literature. Diagn Cytopathol. 46:267–270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Argani P, Laé M, Ballard ET, Amin M,
Manivel C, Hutchinson B, Reuter VE and Ladanyi M: Translocation
carcinomas of the kidney after chemotherapy in childhood. J Clin
Oncol. 24:1529–1534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antonia S, Goldberg SB, Balmanoukian A,
Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel
JJ and Rizvi NA: Safety and antitumour activity of durvalumab plus
tremelimumab in non-small cell lung cancer: A multicentre, phase 1b
study. Lancet Oncol. 17:299–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moodie T, Alshaqi O and Alchaki A:
Longitudinal extensive transverse myelitis after chemoradiation
therapy with durvalumab, a rare complication: Case report. BMC
Neurol. 22:1072022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carnero Contentti E and Correale J:
Neuromyelitis optica spectrum disorders: From pathophysiology to
therapeutic strategies. J Neuroinflammation. 18:2082021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chatterton S, Xi S, Jia JX, Krause M, Long
GV, Atkinson V, Menzies AM, Fernando SL, Boyle T, Kwok S, et al:
Case series: Immune checkpoint inhibitor-induced transverse
myelitis. Front Neurol. 14:11303132023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Picca A, Berzero G, Bihan K, Jachiet V,
Januel E, Coustans M, Cauquil C, Perrin J, Berlanga P, Kramkimel N,
et al: Longitudinally extensive myelitis associated with immune
checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm.
8:e9672021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoest JM: Clinical features, predictive
correlates, and pathophysiology of immune-related adverse events in
immune checkpoint inhibitor treatments in cancer: A short review.
Immunotargets Ther. 6:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang VA, Simpson DR, Daniels GA and
Piccioni DE: Infliximab for treatment-refractory transverse
myelitis following immune therapy and radiation. J Immunother
Cancer. 6:1532018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garcia CA, El-Ali A, Rath TJ, Contis LC,
Gorantla V, Drappatz J and Davar D: Neurologic immune-related
adverse events associated with adjuvant ipilimumab: Report of two
cases. J Immunother Cancer. 6:832018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halperin EC, Brady LW, Perez CA and Wazer
DE: Perez & Brady's principles and practice of radiation
oncology. 6th. Lippincott Williams & Wilkins; Philadephia:
2013
|
|
26
|
Schultheiss TE: The radiation
dose-response of the human spinal cord. Int J Radiat Oncol Biol
Phys. 71:1455–1459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okada S and Okeda R: Pathology of
radiation myelopathy. Neuropathology. 21:247–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khan M, Ambady P, Kimbrough D, Shoemaker
T, Terezakis S, Blakeley J, Newsome SD and Izbudak I:
Radiation-induced myelitis: Initial and follow-up MRI and clinical
features in patients at a single tertiary care institution during
20 years. AJNR Am J Neuroradiol. 39:1576–1581. 2018.PubMed/NCBI
|
|
29
|
Seddon B, Cassoni A, Galloway M, Rees J
and Whelan J: Fatal radiation myelopathy after high-dose busulfan
and melphalan chemotherapy and radiotherapy for Ewing's sarcoma: A
review of the literature and implications for practice. Clin Oncol
(R Coll Radiol). 17:385–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubin P: The Franz Buschke lecture: Late
effects of chemotherapy and radiation therapy: A new hypothesis.
Int J Radiat Oncol Biol Phys. 10:5–34. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chao MW, Wirth A, Ryan G, MacManus M and
Liew K: Radiation myelopathy following transplantation and
radiotherapy for non-Hodgkin's lymphoma. Int J Radiat Oncol Biol
Phys. 41:1057–1061. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sridharan V, Margalit DN, Lynch SA,
Severgnini M, Hodi FS, Haddad RI, Tishler RB and Schoenfeld JD:
Effects of definitive chemoradiation on circulating immunologic
angiogenic cytokines in head and neck cancer patients. J Immunother
Cancer. 4:322016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ludgate CM: Optimizing cancer treatments
to induce an acute immune response: Radiation Abscopal effects,
PAMPs, and DAMPs. Clin Cancer Res. 18:4522–4525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hwang WL, Pike LR, Royce TJ, Mahal BA and
Loeffler JS: Safety of combining radiotherapy with
immune-checkpoint inhibition. Nat Rev Clin Oncol. 15:477–494. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nightingale H, Witherick J and Wilkins A:
Diagnosis of longitudinally extensive transverse myelitis. BMJ Case
Rep. 2011:bcr10201034442011. View Article : Google Scholar : PubMed/NCBI
|