Introduction
Thyroid cancer (TC) is the most common type of
malignant tumor in the endocrine system, which accounts for ~90% of
all endocrine malignancies (1).
Among the TC subtypes, anaplastic thyroid cancer (ATC) is extremely
rare and represents only 1.7% of all TC cases (2). However, ATC is the most lethal TC
subtype, with a high mortality rate (20–40 %) and poor prognosis
(3). The average survival time is
~6 months and the 1-year overall survival (OS) rate is only 20%
(4), largely due to the high
invasiveness and resistance to treatment (5). Clinically, ATC often presents with a
rapidly enlarging neck mass, frequently accompanied by dysphagia,
hoarseness, dyspnea and occasionally neck pain (6). Metastasis is observed in 50% of
patients, with the lungs, brain and bones as the most common sites
of distant spread (7).
The core pathogenesis of TC involves genetic and
epigenetic alterations, which include gene mutations,
amplifications, copy-number gains, aberrant methylation, gene
translocations, decreased expression of the Na/I symporter and
non-coding RNA imbalances (8). Key
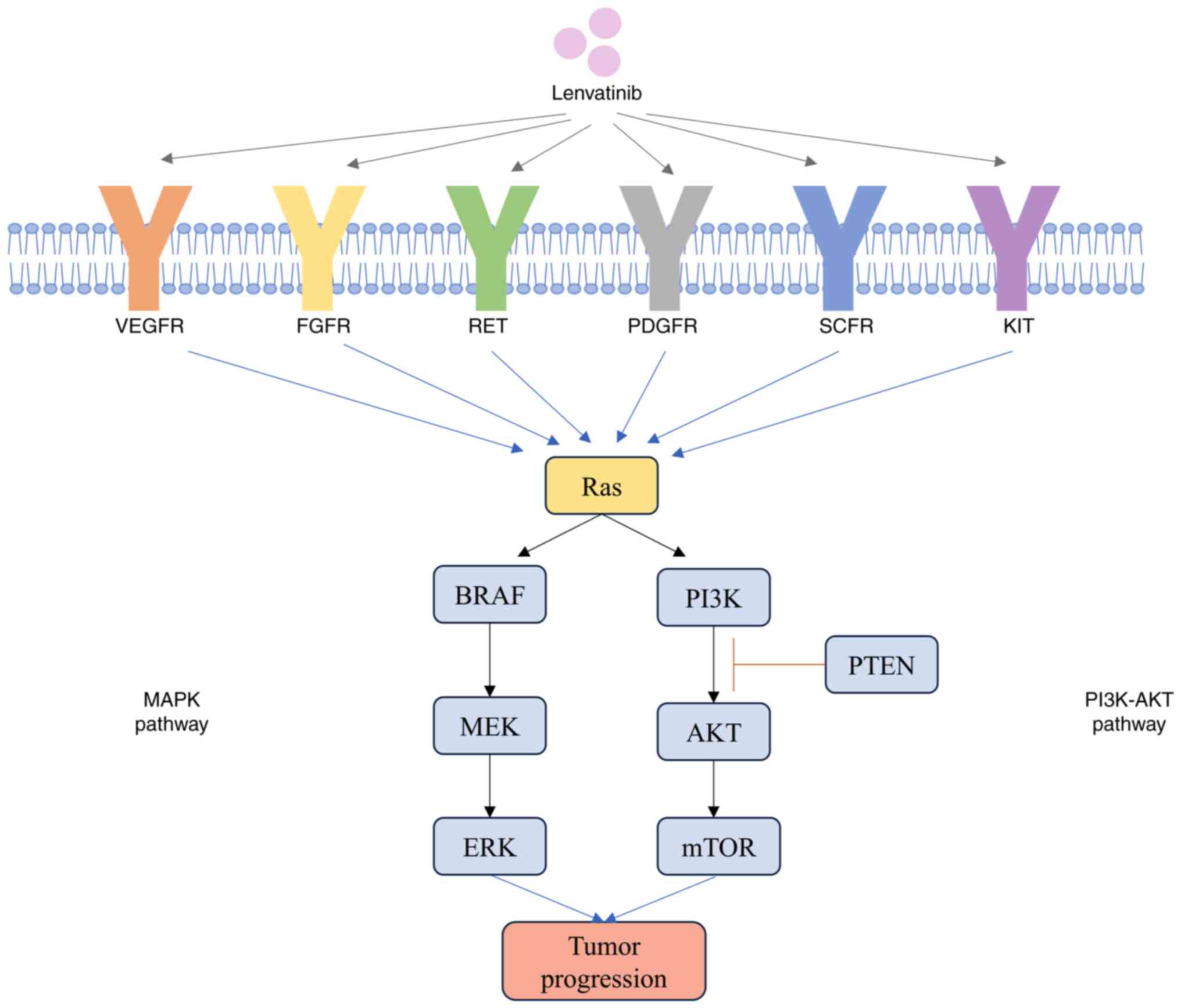
molecular pathways implicated in TC are the MAPK and PI3K-AKT
pathways (Fig. 1). Mutations
commonly involve oncogenes such as BRAF (~60%), Ras (~13%) and
rearrangements of anaplastic lymphoma kinase, rearranged during
transfection (RET) and neurotrophic tyrosine receptor kinase genes
(~5%), as well as loss-of-function mutations in tumor suppressor
genes, such as peroxisome proliferator-activated receptor γ, PTEN
and TP53 (~10%) (9,10).
Notably, ATC displays distinct genetic profiles
compared with differentiated TC (DTC). The prevalence of telomerase
reverse transcriptase (TERT) promoter and TP53 mutations in ATC are
markedly higher compared with mutations in BRAF and Ras, which are
less frequent in ATC. TERT promoter mutations are common in both
poorly DTC and ATC, but TP53 mutations are more common in ATC and
may serve a key role in tumor aggressiveness. Mutations in PTEN,
PIK3CA and immune-modulatory genes [for example, programmed death
ligand (PD-L)-1; PD-L2 and Janus kinase 2] are also relatively
common in ATC (9–11). The higher overall tumor mutation
burden in ATC contributes to the invasiveness and resistance to
conventional therapies, including chemotherapy, radiotherapy and
single targeted therapy, which makes ATC treatment highly
challenging.
Current treatments for ATC include surgery,
chemotherapy, radiotherapy, immunotherapy and targeted therapy. For
cases without distant metastasis, complete surgical resection is
the primary treatment and has demonstrated efficacy in early-stage
tumors (12). As with the most
common type of TC-papillary thyroid carcinoma, early surgical
treatment without lymph node dissection still results in an
extremely low recurrence rate within 8 years (13,14).
However, ~80% of ATC cases were diagnosed with invasion of
surrounding tissues, lymph node involvement or distant metastases,
where surgery alone has a poor prognosis (4). In such instances, adjuvant therapies
such as chemotherapy or radiotherapy are typically required.
Chemotherapy often includes doxorubicin, cisplatin and paclitaxel
(or docetaxel), administered either alone or in combination
(doxorubicin + docetaxel or cisplatin + paclitaxel) (15). While the American Thyroid
Association recognizes the survival benefits of chemotherapy in
ATC, the survival rate for late-stage ATC remains dismal, as the
median survival was only 2.7–3 months (4). Additionally, the notable off-target
toxicities of chemotherapy drugs frequently lead to dose reductions
or treatment discontinuation, which severely affect the quality of
life in patients (16). Common
adverse effects include cardiotoxicity from doxorubicin (17), ototoxicity, neurotoxicity and
nephrotoxicity from cisplatin (17), peripheral neuropathy,
gastrointestinal disturbances and hematologic toxicities from
paclitaxel, with elderly patients being particularly vulnerable
compared with younger patients (18). ATC is also generally unresponsive to
radioactive iodine (RAI) therapy. Previous studies have reported
that BRAF and TERT promoter mutations, which are prevalent in ATC
(9,19), were associated with the loss of RAI
avidity and impaired expression of thyroid-specific genes (20). These findings highlight the need for
alternative therapeutic approaches.
Targeted therapy has become a promising area of
research when conventional therapeutic approaches fail. Lenvatinib
(E7080), a tyrosine kinase inhibitor (TKI) approved by the US Food
and Drug Administration (FDA) and European Medicines Agency for
progressive, RAI-refractory DTC, has demonstrated efficacy in the
treatment of ATC (21,22). However, lenvatinib monotherapy has
notable limitations, which include a high incidence of adverse
events (AE) (23).
In the present review, the efficacy and safety of
lenvatinib monotherapy and combination therapies for ATC were
analyzed. The present review aimed to provide insights into the
feasibility of various therapeutic combinations to improve survival
outcomes and quality of life for patients with ATC.
Database search strategy
The following search words were used for data mining
in PubMed (https://pubmed.ncbi.nlm.nih.gov/.) and Web of Science
(https://www.webofscience.com/)
databases: {‘lenvatinib’ or all its synonyms
[4-(3-chloro-4-((cyclopropylaminocarbonyl)amino)phenoxy)-7-methoxy-6-quinolinecarboxamide,
E7080, Lenvima] in the Mesh database (Title/Abstract)} and
[‘anaplastic thyroid cancer’ or all its synonyms (anaplastic
thyroid carcinoma, anaplastic thyroid carcinomas, anaplastic
thyroid cancers) in the Mesh database (Title/Abstract)].
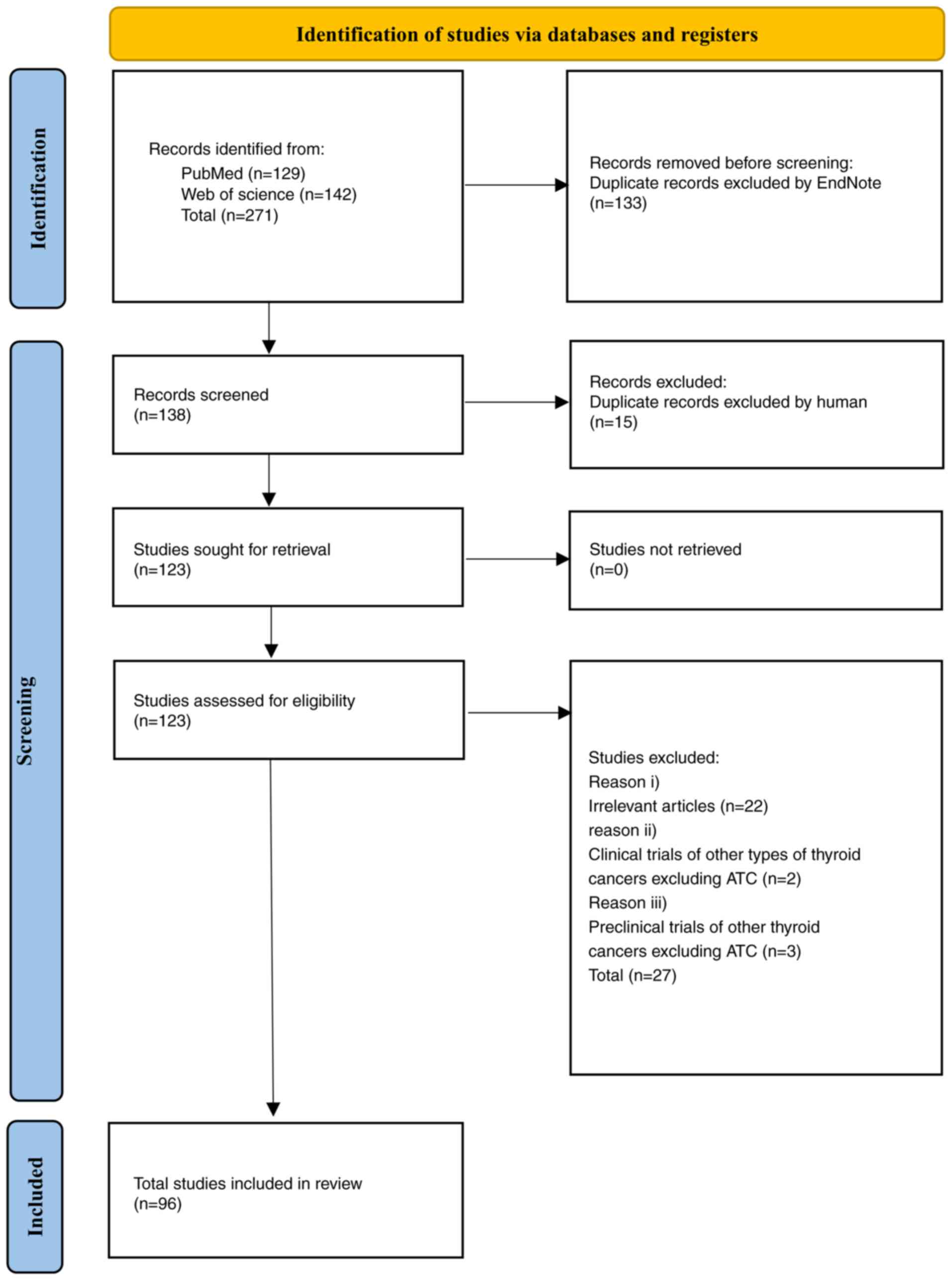
Publications dated from 1st January 2024 to 7th February 2024 were
imported into EndNote X9. After automatic elimination of duplicate
documents, a total of 138 papers were obtained.
Inclusion criteria were as follows: i) Clinical
trials of lenvatinib on ATC; ii) preclinical trials of lenvatinib
on ATC; iii) clinical trial of lenvatinib combined with other
drugs; and iv) preclinical trials of lenvatinib combined with other
drugs on ATC. Exclusion criteria were as follows: i) Repeated
literature and research; ii) irrelevant articles; iii) clinical
trials of other TC types excluding ATC; and iv) preclinical trials
of other types of TC excluding ATC.
Based on the aforementioned criteria, 42 studies
were rejected (Fig. 2). The
remaining 96 studies were examined and relevant materials were
analyzed to extract the information and data required for the
present review. After integrated analysis, the clinical efficacy
and safety of lenvatinib and the combination with chemotherapy,
radiotherapy, targeted therapy, immunotherapy and histone
deacetylase inhibitors (HDACIs) on ATC were summarized. For the
combination drugs that have not been clinically studied, the
pre-clinical experiments were analyzed to assess their
potential.
Lenvatinib
Lenvatinib is an oral, multi-target TKI that
suppresses several key signaling pathways involved in tumor growth
and angiogenesis. Lenvatinib targets include vascular endothelial
growth factor receptor (VEGFR)1, VEGFR2 and VEGFR3,
platelet-derived growth factor receptor (PDGFR)α, fibroblast growth
factor receptor (FGFR)1, FGFR2, FGFR3 and FGFR4, mast/stem cell
growth factor receptor, RET and the tumor angiogenesis-related
proto-oncogene, receptor tyrosine kinase (c-KIT) signaling network,
among others (24).
VEGF is a key cytokine in tumor neovascularization,
which promotes tumor growth and cell proliferation. Upregulation of
VEGFR was associated with increased tumor invasiveness and reduced
recurrence-free survival. Among VEGFRs, VEGFR-2 served a pivotal
role in tumor angiogenesis, while VEGFR-3 was key for lymph
angiogenesis (27,28). By inhibition of the tyrosine kinase
domain of VEGFR, lenvatinib disrupted intracellular signaling,
which thereby suppressed tumor growth and migration (25–27).
PDGFR complements VEGFR in the mediation of
angiogenesis. The PDGFRα signaling pathway has been identified as
essential for cell migration and lenvatinib targets the PDGFRα
pathway to impede tumor progression (28).
The FGFR family is upregulated in TC and regulates
processes such as tumor cell proliferation, differentiation and
survival (29). Lenvatinib exhibits
notable potency against FGFR-1 by inhibition of the downstream
effector, fibroblast growth factor receptor substrate 2
phosphorylation, which sets lenvatinib apart from other
anti-angiogenic TKIs (imatinib, sorafenib), this ability may help
overcome resistance to other angiogenesis inhibitors (30). FGFR-2 is the only FGFR detected in
normal thyroid tissues and FGFR-2 expression is reduced in TC
tissues (29). FGFR-3 was more
highly upregulated in less aggressive TC types, whereas FGFR-4 was
highly upregulated in more aggressive primary thyroid tumors,
including ATC and PDTC, FGFR-4 was not upregulated in
well-differentiated, less aggressive TC types (31). Thus, the FGFR family may be able to
serve as a prognostic factor for patients with TC (32).
RET gene fusions are frequently observed in TC
(33). Lenvatinib demonstrated
antitumor activity in RET gene fusion-driven tumor models by
blocking oncogenic signaling (34).
The c-KIT receptor is suggested to serve a role in thyroid
epithelial cell proliferation, but this function may be lost during
malignant transformation. Lenvatinib inhibited tumor progression by
targeting this pathway (Fig. 1) as
well (34).
Lenvatinib has demonstrated efficacy in the
treatment of various solid tumors, including liver cancer, renal
cell carcinoma and adenoid cystic carcinoma. The role of lenvatinib
in TC, particularly in ATC, involves disrupting tumor angiogenesis
and growth through the aforementioned mechanisms. The present
review focused on the development of lenvatinib and the
effectiveness in managing ATC (35).
Lenvatinib monotherapy in ATC
Preclinical studies
Tohyama et al (30) evaluated the antiproliferative
effects of lenvatinib by comparing the half-maximal inhibitory
concentration (IC50) values in TC cell lines and
Nthy-ori 3-1 cells (human thyroid follicular epithelial cells).
Among 11 TC cell lines, only RO82-W-1 and TT cells demonstrated
notable antiproliferative activity in vitro. However, in
five ATC xenograft models, lenvatinib markedly reduced tumor micro
vessel density, which suggested that the antitumor effects of
lenvatinib in TC were primarily driven by antivascular
activity.
Ferrari et al (36) investigated the effects of lenvatinib
on primary ATC cells, 8305C cells and an ATC cell line (AF).
Dose-dependent pro-apoptotic, anti-proliferative and inhibitory
effects on migration and invasion were observed in primary ATC
cells. Similar effects were seen in 8305C and AF cells, which
attributed to the inhibition of EGFR, AKT and ERK1/2
phosphorylation. Lenvatinib also downregulated cyclin D1, a key
regulator of cell cycle progression in ATC, which thereby
suppressed tumor cell proliferation.
In vivo, lenvatinib reduced tumor growth,
VEGF-A expression and blood vessel density in AF xenograft mice
without affecting body weight. Additionally, while both lenvatinib
and sorafenib inhibited subcutaneous ATC tumor growth, lenvatinib
uniquely crossed the blood-brain barrier, which enabled
intracranial tumor inhibition (37).
Clinical studies
Preclinical findings have been corroborated by
clinical studies, which demonstrated the efficacy of lenvatinib in
patients with ATC. In total, two Phase II trials (28,29)
that included 54 and 17 patients with ATC, respectively, evaluated
lenvatinib at an initial dose of 24 mg/day (adjusted based on
patient tolerance). In the first trial, 20 patients failed to meet
the minimum overall response rate (ORR) threshold of 15%, which led
to early termination of treatment for those participants. Among the
remaining 34 patients, 1 patient (2.9%) achieved partial response
(PR), 17 patients (50%) had stable disease (SD) and the disease
control rate (DCR) reached 90%. Median OS and progression-free
survival (PFS) were 3.2 and 2.6 months, respectively. In the second
trial, 4 patients (24%) achieved PR, 12 patients (71%) had SD, with
a DCR of 94.1%. Median OS was 10.6 months and median PFS was 7.4
months.
Differences in clinical efficacy between the two
Phase II clinical trials could be attributed to patient
demographics. The first trial primarily included White and Black
patients (88%), while the second study enrolled Japanese patients.
Additionally, 9% of participants in the first trial received
treatment with monoclonal antibodies or protein kinase inhibitors
prior to lenvatinib, which potentially influenced patient
outcomes.
Retrospective studies have further supported the
benefits of lenvatinib in prolonging survival for patients with ATC
(Table I). Murayama et al
(38) reported that
lenvatinib-induced pulmonary cavitation, observed in 46.2% of
patients diagnosed with ATC with lung metastases, was associated
with markedly longer OS (186 vs. 89 days for patients without
cavitation). Another previous study (39) reported that 7 out of 10 patients
with TC, who developed lung cavitation during treatment with
lenvatinib or sorafenib were in the lenvatinib group, which
suggested the potential efficacy of lenvatinib in the management of
ATC with lung metastases.
 | Table I.Summary of clinical trials on the
efficacy of lenvatinib in the treatment of patients with ATC. |
Table I.
Summary of clinical trials on the
efficacy of lenvatinib in the treatment of patients with ATC.
| First author,
year | Study type | Initial dose,
mg/day | Patients, n | Group | Median survival,
(days/months) | Responses, n
(%) | DCRa, n (%) | Observations | (Refs.) |
|---|
| Murayama et
al, 2022 | Retrospective | 24 | 26 | Total (all
patients) | OS, 128 d; PFS,
NA | CR, 0 (0); PR, 6
(23.1); SD, 17 (65.4); PD, 2 (7.7); NE, 1 (3.8) | 23 (88.5) | Nearly half of the
patients developed LC, which demonstrated improved clinical
outcomes compared with patients without LC. | (38) |
|
|
|
| 12 | LC (+) | OS, 186 d; PFS,
NA | CR, 0 (0); PR, 2
(16.7); SD, 8 (66.7); PD, 2, (16.7); NE, 0 (0) | 10 (83.3) |
|
|
|
|
|
| 14 | LC (−) | OS, 89 d; PFS,
NA | CR, 0 (0); PR, 4
(28.6); SD, 9 (64.3), PD, 0 (0); NE, 1 (7.1) | 13 (92.9) |
|
|
| Wirth et al,
2021 | Phase II
prospective | 24 | 34 | IAS | OS, 2.9 m; PFS, 2.6
m | CR, 0 (0); PR, 1
(5); SD, 9 (45); PD, 5 (25); NE, 5 (25) | 10 (29.4) | Lenvatinib
monotherapy might not have been an effective treatment for
ATC. | (45) |
|
|
|
| 20 | FAS | OS, 3.2 m; PFS, 2.6
m | CR, 0, (0); PR, 1
(2.9); SD, 17 (50); PD, 9 (26.5);
NE, 7 (20.6) | 18 (90) |
|
|
| Iwasaki et
al, 2021 | Retrospective | 24 | 32 |
| Total, 3.2 m | CR, 6 (18.8); PR, 8
(25); SD, 6 (18.8); PD, 12 (37.5);
NE, 0 (0) | 20 (62.5) | Dose reduction or
discontinuation of lenvatinib monotherapy was frequently required
due to SAEs. | (23) |
| Yamazaki et
al, 2020 | Phase II
prospective | 10-24 | 12 |
| OS, NA; PFS, 5
m | CR, 0 (0); PR, 4
(33.3); SD, 3 (25); PD, 0 (0); NE,
5 (41.7) | 7 (58.3) | FGFR4 may have
served as a prognostic predictor for lenvatinib treatment
response. | (86) |
| Takahashi et
al, 2020 | Retrospective | 24 | 124 |
| OS, 101 d; PFS,
NA | CR, 3 (2.9); PR, 43
(41); SD, 34 (32.4); PD, 25
(23.8); NE, 0 (0) | 80 (64.5) | The safety profile
of lenvatinib in ATC was confirmed to be favorable. | (47) |
| Kim et al,
2021 | Retrospective | 20-23 | 14 |
| OS, 6.7 m; PFS, 5.7
m | CR, 0 (0); PR, 4
(29); SD, 9 (64); PD, 1 (7); NE, 0 (0) | 13 (92.9) | The therapeutic
benefits of lenvatinib remained modest in advanced ATC cases. | (49) |
| Fukuda et
al, 2020 | Retrospective | 24 | 13 | Total | OS, 10.2 m; PFS,
3.8 m | CR, 0 (0); PR, 3
(23); SD, 6 (46.2); PD, 4 (30.8);
NE, 0 (0) | 9 (69.2) | NLR may have served
as a potential prognostic biomarker for lenvatinib treatment
response. | (41) |
|
|
|
| 9 | NLR <8 | OS, 10.2 m; PFS,
NA | CR, 0 (0); PR, 3
(33.3); SD, 5 (55.6); PD, 1 (11.1); NE, 0 (0) | 8 (88.9) |
|
|
|
|
|
| 4 | NLR ≥8 | Total OS, 3.8
m | CR, 0 (0); PR, 0
(0); SD, 1 (25); PD, 3 (75); NE, 0 (0) | 1 (25) |
|
|
| Iwasaki et
al, 2020 | Phase II
prospective | 10-24 | 16 |
| OS, 4.2 m; PFS,
NA | CR, 0 (0); PR, 7
(43.75); SD, 5 (31.3); PD, 4 (25);
NE, 0 (0) | 12 (75) | Judicious dose
reduction of lenvatinib represented a necessary therapeutic
strategy. | (87) |
| Iwasaki et
al, 2018 | Retrospective | 20-24 | 23 |
| OS, 166 d; PFS,
NA | CR, 0 (0); PR, 4
(17.4); SD, 6 (26.1); PD, 7 (30.4); NE, 6 (26.1) | 10 (43.5) | Lenvatinib
exhibited clinical activity in patients with chemotherapy
refractory ATC. | (91) |
| Tahara et
al, 2017 | Phase II
prospective | 24 | 17 |
| OS, 10.6 m; PFS 7.4
m, | CR, 0 (0); PR, 4
(24); SD, 12 (71); PD, 1 (6); NE, 0 (0) | 16 (94.1) | Lenvatinib
demonstrated clinical benefits with manageable toxicity. | (44) |
| Koyama et
al, 2018 | Retrospective | 24 | 5 |
| Total OS, 165
d | CR, 0 (0); PR, 3
(60); SD, 2 (40); PD, 0 (0); NE, 0 (0) | 5 (100) | Lenvatinib improved
survival in patients with unresectable ATC. | (92) |
| Iyer et al,
2018 | Retrospective | 24 | 8 |
| OS, 3.9 m; PFS, 2.6
m | CR, 0 (0); PR, 3
(37.5); SD, 0 (0); PD, 5 (62.5); NE, 0 (0) | 3 (37.5) | Lenvatinib
demonstrated therapeutic efficacy in patients with ATC who were
ineligible for clinical trials. | (93) |
The neutrophil-lymphocyte ratio (NLR) of patients
with ATC was higher compared with that of radioiodine-refractory
(RR)-DTC patients and NLR was also a diagnostic marker for
distinguishing ATC from RR-DTC (40). In addition, NLR has also emerged as
a prognostic marker for lenvatinib-treated patients with ATC.
Fukuda et al (41)
demonstrated that patients with low NLR had higher ORR (33.3%),
longer median PFS (4.0 months) and longer median OS (10.2 months)
compared with patients with high NLR. The study by Fukuda et
al (41) was a single-center
and small-sample retrospective study. The clinical stages of the
experimental individuals were different and patients had undergone
different degrees of radiotherapy, chemotherapy and surgical
treatment before inclusion in the trial, which had an impact on the
basic level of NLR. Another previous study (42) ruled out inflammatory interference
and the statistical results indicated that NLR did not affect the
therapeutic effect of lenvatinib. Nonetheless, monitoring dynamic
changes in NLR before and after lenvatinib treatment could provide
some prognostic insight for patients with ATC. Another previous
study (43) on TKI treatment in ATC
reported a notable increase in NLR levels at three key points:
Before TKI initiation, after TKI initiation and at the time of
death. However, current studies are influenced by numerous
confounding factors such as prior treatments, recurrence or
metastasis, infections, glucocorticoid use and physiological
stress. Therefore, NLR requires validation through large-scale,
multi-center prospective trials with stricter patient inclusion
criteria.
Safety
Previous studies on lenvatinib in ATC reported at
least 1 AE per patient, wherein hypertension, reduced appetite,
fatigue and proteinuria were the most common AEs (23,27,44,45).
These AEs were linked to the inhibition of VEGFR by lenvatinib
(46). While the incidence of AEs
is high, severe AEs (SAEs) associated with lenvatinib are rare and
treatment discontinuation due to AEs is uncommon.
In a previous study conducted in Japan (47), of 124 patients with ATC (mean age,
73 years), 76.6% experienced grade ≥3 AEs, primarily hypertension
(46.8%) and thrombocytopenia (12.9%). The high rate of severe AEs
may be attributed to the advanced age of participants and the use
of a 24 mg/day dose, which may be excessive for Asian populations
(48).
Another study conducted in Korea on 14 patients with
ATC, who were administered an initial dose of 20 mg/day reported
100% incidence of AEs, with 50% classified as grade ≥3 (49). At a maintenance dose of 13 mg/day,
DCR reached 93% and median OS was 6.73 months. These findings
suggested that lower starting doses may improve tolerability and
efficacy in Asian populations despite differences in participant
numbers between the studies.
Lenvatinib combination therapy in ATC
Preclinical studies
Lenvatinib and other targeted inhibitors
i) MEK inhibitors. Enomoto et al (50) investigated the combination of
lenvatinib with MEK inhibitors (U0126 and selumetinib) in ATC.
Lenvatinib inhibited AKT signaling but not MAPK signaling, which
prompted the use of MEK inhibitors to complement its effects. In
vitro assays demonstrated that a combination of 5 µM lenvatinib
and 5 µM U0126 demonstrated synergistic antiproliferative effects
in ATC cell lines (8505C and TCO1). The antiproliferative rate
increased significantly compared with lenvatinib alone (8505C, 32
to 66%; TCO1, 58 to 72%), The present review concluded that
lenvatinib suppressed AKT phosphorylation, while U0126 suppressed
ERK phosphorylation and cell cycle protein D1 expression. In
vivo assays demonstrated that mice treated with lenvatinib (30
mg/kg/day) and selumetinib (30 mg/kg/day) experienced significantly
larger tumor volume reduction compared with tumor volumes in
monotherapy groups. Tumor cell apoptosis increased via caspase-3
activation, while toxicity levels remained manageable.
The aforementioned results highlight the potential
of lenvatinib and MEK inhibitors as a synergistic therapy for ATC,
which targets both AKT and MAPK signaling pathways effectively.
ii) BRAF inhibitors. In a previous study, lenvatinib
was combined with vemurafenib, a BRAF inhibitor, to treat
BRAF-mutated (8505C) and non-BRAF-mutated (HTh7) ATC cell lines
(51).
The results demonstrated that under low
concentrations (0.25 µM) of vemurafenib, the proliferation of BRAF
mutated 8505C cells was significantly inhibited, whereas the HTh7
cell line exhibited resistance to vemurafenib by 4 µM. Lenvatinib
inhibited 8505C cell proliferation in a dose-dependent manner,
whereas HTh7 cells did not exhibit an antiproliferative pattern
compared with that of 8505C until lenvatinib reached 12. 5 µM and
cell viability of HTh7 and 8505C did not differ statistically until
25 µM. HTh7 cells demonstrated only additive cytotoxic effects when
combined treatment with lenvatinib and vemurafenib. Vemurafenib
effectively inhibited the proliferation of 8505C cells but
vemurafenib was less effective in HTh7 cells. The combination of
lenvatinib and vemurafenib demonstrated cooperative cytotoxic
effects in BRAF-mutated cells, which induced apoptosis through
poly(ADP-ribose) polymerase cleavage.
In addition, combined BRAF and MEK inhibitors
increased sodium/iodide symporter expression and RAI uptake in
BRAF-mutated TC types (52,53), which offered a potential strategy
for BRAF-mutated ATC. Further clinical trials are required to
establish safety and optimal dosing for BRAF and MEK inhibitors
combination therapy.
Lenvatinib and chemotherapeutic agents
i) Lenvatinib and doxorubicin. Doxorubicin, an
approved chemotherapy for ATC, cannot improve the OS rate of
advanced ATC and has strong cardiotoxicity as monotherapy. A
preclinical study (54) combining
lenvatinib (1 µM) and doxorubicin (10 nM) demonstrated synergistic
effects in ATC cell lines (8305C, C643 and 8505C), which inhibited
cell proliferation, migration, invasion and colony formation. In
vivo assays demonstrated that the combination therapy group
exhibited lighter tumor weights and lower Ki-67 levels compared
with both the lenvatinib monotherapy and doxorubicin monotherapy
groups. The mechanism may be to inhibit ERK phosphorylation and
induce cell cycle arrest. The MAPK pathway has a notable effect on
DNA repair in reaction to DNA damage and lenvatinib can impair DNA
repair capacity by suppressing receptor tyrosine kinases and the
downstream.
The lenvatinib and doxorubicin combination therapy
may further enhance the damage of doxorubicin to DNA and thereby
exert a synergistic anticancer effect (55,56)
without added toxicity, which highlights the clinical potential of
lenvatinib and doxorubicin.
ii) Lenvatinib and paclitaxel. The combination of
lenvatinib and paclitaxel demonstrated similar cooperative effects
to lenvatinib and doxorubicin, which includes inhibition of tumor
cell growth, colony formation, increase of the proportion of
G2/M phase and induction of apoptosis in ATC cells. The
effects were dose-dependent, which supports the potential for
combination therapy (5).
iii) Lenvatinib and vinorelbine. Vinorelbine is a
microtubule-targeting drug, which induces tumor cell death via
apoptosis (57). The drug has also
been demonstrated to block angiogenesis (58). In vitro assays demonstrated
that lenvatinib in combination with vinorelbine has synergistic
anti-proliferative activity, whereas sorafenib in combination with
vinorelbine did not exhibit synergistic effects (59).
As an efflux pump, the ATP-binding cassette
subfamily (ABC) transporter can excrete TKIs, which may increase
tumor resistance to lenvatinib and vinorelbine (60). Lenvatinib reduced ABCB1 expression,
which increased intracellular drug concentrations and overcame
resistance (59). Wide permeation
of tumor associated macrophages (TAMs) was observed in 95% of
patients with ATC (61). The
colony-stimulating factor-1 receptor (CSF-1R) gene, as a
TAMs-related gene, was associated with low survival and was
markedly upregulated in ATC (59).
CSF-1 can also induce the generation of VEGF (62). However, both lenvatinib monotherapy
and the combination with vinorelbine can inhibit the expression of
CSF-1 mRNA, wherein lenvatinib combined with vinorelbine is more
effective (63). This combination
regimen might serve as an option to mitigate TKI resistance in
patients with ATC. In vivo findings: Combination therapy
reduced tumor volume, mitotic activity and Ki-67 levels, while
increasing apoptosis markers such as caspase-3 (59). Lenvatinib combined with vinorelbine
may mitigate resistance to TKIs in patients with ATC.
Lenvatinib and immunotherapy
Immunotherapy, particularly anti-programmed death-1
(PD-1)/PD-L1 agents, is a major research focus in ATC. ATC markedly
upregulates PD-L1 compared with DTC (64). Gunda et al (3) reported that lenvatinib combined with
anti-PD-1/PD-L1 therapy reduced tumor size and improved survival.
ATC cells treated with lenvatinib alone did not affect PD-L1
expression but increased TAMs, CD8+ T cells, regulatory
T cells and polymorphonuclear myeloid derived suppressor cells
(PMN-MDSCs). The lenvatinib treatment group also demonstrated an
increase in granulocyte-CSF levels in tumor lysis fluid, which may
drive the proliferation of MDSCs. T-cell defection and natural
killer cell toxicity were linked to the increase in these cells,
which had a negative effect on the efficacy of lenvatinib (65–67).
The effect of lenvatinib to increase PMN-MDSCs was also
demonstrated in patients with ATC and only the lenvatinib combined
with anti-PD-1 group exhibited reduced PMN-MDSCs (3). Combining lenvatinib with therapies
targeting PMN-MDSCs or TAMs, such as CSF-1R blockade, may enhance
therapeutic efficacy and overcome immune evasion in ATC.
Lenvatinib and HDACIs
Histone deacetylase (HNHA) is a HDACI that removes
acetyl groups from histone lysine residues. This process is called
deacetylation, which suppresses cellular transcription and stalls
tumor growth, polarization and apoptosis. In addition, it can
sensitize tumor cells to radiation, which increases the uptake of
RAI and the accumulation of RAI in tumors (68). A previous study (69) reported that the combination of HNHA
and lenvatinib has a strong anti-proliferative effect, which
increased apoptosis in GSA2 (first patient-derived ATC cells) and
GSA1 (second patient-derived ATC cells) cell lines by increasing
p53 and p21 levels, reduced cyclin D1 and CDK4 and induced cell
cycle arrest. In the mouse xenograft model, both the monotherapy
group and the HNHA combined with sorafenib group did not
significantly suppress the development of GSA1 and GSA2 cell
xenografts. However, HNHA combined with lenvatinib could
significantly inhibit tumor growth.
Previous studies have suggested that drug resistance
in poorly differentiated cancer stem cells is associated with
epithelial mesenchymal transition (EMT) and the FGFR signaling
pathway is involved in this process (70,71). A
previous study reported that the expression of the FGFR signaling
pathway and EMT markers in ATC cell lines is higher compared with
that in DTC (69). Therefore, when
combined lenvatinib with HNHA in GSA2 and GSA1 cell lines,
lenvatinib combined with HNHA group had a stronger inhibitory
effect on the FGFR signaling pathway compared with other groups. In
addition, the EMT marker, β-catenin, is important in EMT nuclear
positioning induction in late-stage TC cells. Other studies have
demonstrated that downregulating zinc finger E-box binding homeobox
1 (ZEB1) expression can restore drug sensitivity (72,73),
which blocks β-catenin and downregulation of ZEB1, which may
potentially be novel methods for the treatment of ATC resistance in
the future. These findings suggest that combining lenvatinib with
HDACIs could potentially be a promising strategy to overcome drug
resistance in ATC.
Clinical studies
Although research is limited, clinical trials that
investigated lenvatinib in combination with other agents for ATC
have demonstrated promising efficacy.
Pembrolizumab, a monoclonal antibody targeting the
PD-1 receptor, was approved by the US FDA for the treatment of
several types of cancer, including non-small cell lung cancer,
biliary tract cancer and ATC (64,74).
ATC tumors exhibit diffuse expression of PD-L1, which makes this
combination a rational therapeutic approach (31). Lenvatinib in combination with
pembrolizumab has been evaluated in patients with ATC in two
studies, with an initial lenvatinib dose of 24 mg/day and
pembrolizumab at 200 mg every 3 weeks (31,64).
The first study (64) initially used with lenvatinib
monotherapy and later introduced pembrolizumab upon ATC
progression. Among 5 patients, 3 patients (60%) achieved PR, 1
patient (20%) achieved SD and the DCR was 83%. The median OS was
8.25 months. While the lack of a control group for continuous
lenvatinib monotherapy limits direct comparisons, the median
survival time for lenvatinib monotherapy before pembrolizumab
addition was 10.4 months, which suggested an improvement in
survival with combination therapy.
In the second study (31), 6 patients were treated with the
combination therapy from the outset. Complete response (CR) was
achieved in 4 patients (66.7%), 1 patient (16.7%) achieved SD and
the DCR was 66%. The median OS was 17.3 months and the median PFS
was 16.8 months. Treatment was well-tolerated, with a maximum
treatment duration of 40 months and 3 patients (50%) were still
receiving therapy at the time of data cut-off.
Another previous study reported that pembrolizumab
is only partially effective in patients with prior resistance to
TKIs, which resulted in a PFS of just 2.96 months (69). These findings underscore the
importance of timing in adding pembrolizumab to TKI therapy.
Previous studies have suggested that early addition of
pembrolizumab may provide greater benefit compared with sequential
therapy, though the optimal timing requires further investigation
(64,75).
Another previous study (76) evaluated a combination regimen
involving paclitaxel, intensity-modulated radiotherapy and
lenvatinib. Patients began with one cycle of paclitaxel and IMRT.
After 6 weeks, imaging determined the suitability for surgery.
Patients, who were ineligible for surgery continued chemotherapy
and radiotherapy, with lenvatinib (10 mg/day) added upon disease
progression. Among 18 patients, the median OS was 230 days, with
6-month and 1-year survival rates of 61.1 and 22.2%, respectively.
Notably, 3 patients (16.7%) survived beyond 1 year. While this
study demonstrated a survival benefit, the optimal timing for the
introduction of lenvatinib during radiotherapy remained unclear due
to the absence of a control group initiating paclitaxel,
radiotherapy and lenvatinib simultaneously. Furthermore, the
toxicity profile of the three-drug combination requires additional
investigation through larger sample sizes and more comprehensive
clinical studies.
Lenvatinib combined with pembrolizumab or
traditional chemoradiotherapy has demonstrated positive results for
patients with ATC, particularly in improving survival outcomes
(64,75,76).
However, the timing of lenvatinib initiation and the role of
lenvatinib in combination regimens require further clinical
exploration. The aforementioned studies lay a foundation for future
research aimed at the optimization of lenvatinib-based therapies
for ATC.
Discussion
Clinical studies have demonstrated that lenvatinib
monotherapy offers notable efficacy in the treatment of ATC, with
PR rates ranging from 2.9 to 60%, DCR ranging from 25 to 100% and
median OS ranging from 2.9 to 10.6 months. The difference in the
PR, DCR and median OS rates may stem from the predominance of
retrospective studies, which carry a high risk of selection bias,
such as differences in enrollment criteria, sample sizes, prior
treatments and demographic characteristics. For example: i) Age,
poorer prognosis in trials with predominantly elderly participants
(47); ii) sex; and iii) ethnicity,
the initial recommended dose of lenvatinib (based on a phase III
trial for RR-DTC) may not be optimal for Asian populations
(77). Second, the external
validity of retrospective studies is also questionable, as
participants are often recruited from large single-center hospitals
with more complex conditions. To address the aforementioned issues,
it is essential to establish a standardized inclusion framework,
recruit volunteers across multiple centers, dynamically adjust
exclusion criteria and explore the optimal monotherapy dose under
varying conditions. Third, the lack of predictive markers for ATC
prognosis and lenvatinib efficacy. Previous studies have identified
potential prognostic indicators, such as acute symptoms, white
blood cell count, tumor diameter (≥5 cm), distant metastases, NLR,
age >70 years and extra-thyroidal infiltration (T4b), which were
associated with poor prognosis (78–80).
Furthermore, a low NLR was associated with improved outcomes for
lenvatinib-treated patients. Another potential predictive factor
includes the presence of hand-foot syndrome, which was associated
with improved a 24-month OS rate (81), and lower FGFR4 expression, which has
been linked to better lenvatinib response (82). However, the lack of standardized
detection methods, incomplete data collection and common selection
biases require key interpretation of these results. Future research
may employ more rigorous trial designs to validate these prognostic
factors, which potentially leverage existing data to screen
candidate biomarkers, establish uniform detection and enrollment
criteria and conduct multi-center prospective validation
trials.
Combination therapies including lenvatinib have
demonstrated increased efficacy. For instance, in two studies
investigating lenvatinib combined with pembrolizumab (64,75),
the first study reported a DCR of 80% and a median OS of 18.65
months, while the second reported a DCR of 66% and a median OS of
17.3 months-substantial improvements compared with lenvatinib
monotherapy. Additionally, a previous study that examined
lenvatinib combined with paclitaxel and radiotherapy observed a
median OS of 230 days, although the efficacy was lower compared
with that of pembrolizumab combinations. Notably, conventional
chemotherapeutic agents combined with lenvatinib yielded less
favorable results (76), likely due
to the late introduction of targeted therapies. Nevertheless, the
combination of lenvatinib and pembrolizumab demonstrated robust
potential. A recent consensus supported the effectiveness of
lenvatinib and pembrolizumab combination therapy in patients with
ATC with BRAF mutations (83).
According to the Thyroid Neck Morbidity Complexity (TNMC) Scoring
System (84), current guidelines
(4,85) recommend surgery as the first-line
treatment for patients with stage IVB BRAFv-ATC (TNMC, 0) with
resectable primary tumors, followed by adjuvant radiotherapy and
chemotherapy. The consensus recommended first-line use of BRAF/MEK
inhibitors [dabrafenib-trametinib (DT)] for patients with stage IVB
BRAFv-ATC or patients with unresectable or advanced disease (TNMC
≥1), followed by surgery. The consensus also advised combining the
regimen with pembrolizumab from the outset or adding pembrolizumab
upon disease progression. If surgery is not feasible after 6 months
of neoadjuvant treatment, radiotherapy and chemotherapy should be
considered. Similar to lenvatinib, combination therapy with
pembrolizumab demonstrates survival benefits in patients with ATC.
However, a previous study (69)
indicated that prior resistance to TKI inhibitors notably limits
the efficacy of pembrolizumab, while the consensus suggested that
the limited effectiveness of adding pembrolizumab midway was due to
the delayed onset of action. Additionally, combining pembrolizumab
with immune checkpoint inhibitors increased treatment toxicity
(64). Therefore, clinical
decisions should be individualized based on the condition of the
patient. For patients with stage IVC BRAFv-ATC, both the guidelines
and consensus recommended routine DT plus pembrolizumab (DTP), with
reassessment after 2–3 months. If surgery is not possible or
recurrence risk is high, DTP should be continued. If surgery is
resectable, DTP should continue postoperatively and discontinuation
of pembrolizumab after 1 year may offer enhanced survival
benefits.
Targeted therapy combined with immunotherapy is a
promising direction for the future treatment of ATC. However,
several clinical studies (44,45,86,87) on
combination therapies are single-arm phase II trials with low
levels of evidence, which potentially overestimate treatment
efficacy. Future studies should incorporate control groups for
causal inference, avoid selecting only patients with favorable
baseline characteristics and extend follow-up duration to improve
long-term safety data collection.
ATC remains one of the most challenging malignancies
in TC, with limited benefits from traditional treatments (16,17,86–93).
Targeted therapies such as lenvatinib represent a vital avenue for
improving patient outcomes (Table
I) (23,38,41,44,45,47,49,86,87,91–93).
The present review highlighted the efficacy and safety of
lenvatinib as both a monotherapy and in combination regimens. There
are still several directions worth exploring in the future.
Notably, lenvatinib has exhibited superiority to sorafenib in
preclinical models of ATC brain metastases and has provided notable
survival benefits in patients with ATC lung metastases (37,39).
Combination regimens have also proven effective in mitigating
toxicity, with specific combinations that demonstrate sensitivity
in certain ATC subgroups. For example, lenvatinib combined with the
BRAF inhibitor vemurafenib or HDACIs increased RAI sensitivity and
lenvatinib combined with vinorelbine or HDACIs reduced drug
resistance (52,68). However, relevant studies remain
scarce, with most evidence derived solely from animal models and
lacking clinical validation. Future research is warranted to verify
the aforementioned hypotheses and provide improved therapeutic
options for specific ATC subgroups.
The findings from the aforementioned studies
highlight promising directions for future clinical trials, aimed at
optimizing lenvatinib combinations to enhance DCRs, minimize toxic
side effects and improve survival outcomes for patients with ATC.
The present review serves as a foundation for future research and
clinical trial design, with the ultimate goal of developing
individualized therapies to meet the diverse needs of patients with
ATC.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Ningbo Public Welfare
Program (grant no. 2023S139).
Availability of data and materials
Not applicable.
Authors' contributions
LX conceptualized the present review. YW, YX, and KY
conducted the literature search, preliminary screening, and data
collection. LX and QL performed the data analysis and prepared the
original draft. WZ and XW reviewed and edited the manuscript. XW
acquired funding and supervised the present review. All authors
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fugazzola L, Elisei R, Fuhrer D, Jarzab B,
Leboulleux S, Newbold K and Smit J: 2019 European thyroid
association guidelines for the treatment and follow-up of advanced
radioiodine-refractory thyroid cancer. Eur Thyroid J. 8:227–245.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunda V, Gigliotti B, Ashry T, Ndishabandi
D, McCarthy M, Zhou Z, Amin S, Lee KE, Stork T, Wirth L, et al:
Anti-PD-1/PD-L1 therapy augments lenvatinib's efficacy by favorably
altering the immune microenvironment of murine anaplastic thyroid
cancer. Int J Cancer. 144:2266–2278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American thyroid association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duan H, Li Y, Hu P, Gao J, Ying J, Xu W,
Zhao D, Wang Z, Ye J, Lizaso A, et al: Mutational profiling of
poorly differentiated and anaplastic thyroid carcinoma by the use
of targeted next-generation sequencing. Histopathology. 75:890–899.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari SM, Elia G, Ragusa F, Ruffilli I,
La Motta C, Paparo SR, Patrizio A, Vita R, Benvenga S, Materazzi G,
et al: Novel treatments for anaplastic thyroid carcinoma. Gland
Surg. 9 (Suppl 1):S28–S42. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ragazzi M, Ciarrocchi A, Sancisi V,
Gandolfi G, Bisagni A and Piana S: Update on anaplastic thyroid
carcinoma: morphological, molecular, and genetic features of the
most aggressive thyroid cancer. Int J Endocrinol. 2014:7908342014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landa I, Ibrahimpasic T, Boucai L, Sinha
R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP,
Xu B, et al: Genomic and transcriptomic hallmarks of poorly
differentiated and anaplastic thyroid cancers. J Clin Invest.
126:1052–1066. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romei C, Tacito A, Molinaro E, Piaggi P,
Cappagli V, Pieruzzi L, Matrone A, Viola D, Agate L, Torregrossa L,
et al: Clinical, pathological and genetic features of anaplastic
and poorly differentiated thyroid cancer: A single institute
experience. Oncol Lett. 15:9174–9182. 2018.PubMed/NCBI
|
|
12
|
Sacks D, Baxter B, Campbell BCV, Carpenter
JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA,
et al: Multisociety consensus quality improvement revised consensus
statement for endovascular therapy of acute ischemic stroke: From
the American association of neurological surgeons (AANS), American
society of neuroradiology (ASNR), cardiovascular and interventional
radiology society of Europe (CIRSE), Canadian Interventional
Radiology Association (CIRA), Congress of Neurological Surgeons
(CNS), European society of minimally invasive neurological therapy
(ESMINT), European society of neuroradiology (ESNR), European
stroke organization (ESO), Society for Cardiovascular Angiography
and Interventions (SCAI), Society of Interventional Radiology
(SIR), Society of NeuroInterventional Surgery (SNIS), and World
stroke organization (WSO). J Vasc Interv Radiol. 29:441–453. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conzo G, Docimo G, Mauriello C,
Gambardella C, Esposito D, Cavallo F, Tartaglia E, Napolitano S and
Santini L: The current status of lymph node dissection in the
treatment of papillary thyroid cancer. A literature review. Clin
Ter. 164:e343–e346. 2013.PubMed/NCBI
|
|
14
|
Conzo G, Mauriello C, Docimo G,
Gambardella C, Thomas G, Cavallo F, Tartaglia E, Napolitano S,
Varriale R, Rossetti G, et al: Clinicopathological pattern of lymph
node recurrence of papillary thyroid cancer. Implications for
surgery. Int J Surg. 12 (Suppl 1):S194–S197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laetitia G, Sven S and Fabrice J:
Combinatorial therapies in thyroid cancer: An overview of
preclinical and clinical progresses. Cells. 9:8302020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American Thyroid association guidelines
for management of patients with anaplastic thyroid cancer: American
thyroid association anaplastic thyroid cancer guidelines task force
by bible. Thyroid. 31:337–386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trendowski MR, El Charif O, Dinh PC Jr,
Travis LB and Dolan ME: Genetic and modifiable risk factors
contributing to cisplatin-induced toxicities. Clin Cancer Res.
25:1147–1155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frederiks CN, Lam SW, Guchelaar HJ and
Boven E: Genetic polymorphisms and paclitaxel- or docetaxel-induced
toxicities: A systematic review. Cancer Treat Rev. 41:935–950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tirrò E, Martorana F, Romano C, Vitale SR,
Motta G, Di Gregorio S, Massimino M, Pennisi MS, Stella S, Puma A,
et al: Molecular alterations in thyroid cancer: From bench to
clinical practice. Genes (Basel). 10:7092019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Liu R, Shen X, Zhu G, Li B and Xing
M: The genetic duet of BRAF V600E and TERT promoter mutations
robustly predicts loss of radioiodine avidity in recurrent
papillary thyroid cancer. J Nucl Med. 61:177–182. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussein Z, Mizuo H, Hayato S, Namiki M and
Shumaker R: Clinical pharmacokinetic and pharmacodynamic profile of
lenvatinib, an orally active, small-molecule, multitargeted
tyrosine kinase inhibitor. Eur J Drug Metab Pharmacokinet.
42:903–914. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nair A, Lemery SJ, Yang J, Marathe A, Zhao
L, Zhao H, Jiang X, He K, Ladouceur G, Mitra AK, et al: FDA
approval summary: lenvatinib for progressive,
radio-iodine-refractory differentiated thyroid cancer. Clin Cancer
Res. 21:5205–5208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwasaki H, Toda S, Murayama D, Kato S and
Matsui A: Relationship between adverse events associated with
lenvatinib treatment for thyroid cancer and patient prognosis. Mol
Clin Oncol. 14:282021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cabanillas ME and Habra MA: Lenvatinib:
Role in thyroid cancer and other solid tumors. Cancer Treat Rev.
42:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng H, Dvorak HF and Mukhopadhyay D:
Vascular permeability factor (VPF)/vascular endothelial growth
factor (VEGF) peceptor-1 down-modulates VPF/VEGF
receptor-2-mediated endothelial cell proliferation, but not
migration, through phosphatidylinositol 3-kinase-dependent
pathways. J Biol Chem. 276:26969–26979. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnhart BJ and Cox SH: DNA replication of
induced prophage in Haemophilus influenzae. J Virol. 12:165–176.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wedge SR, Ogilvie DJ, Dukes M, Kendrew J,
Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove
HL, et al: ZD6474 inhibits vascular endothelial growth factor
signaling, angiogenesis, and tumor growth following oral
administration. Cancer Res. 62:4645–4655. 2002.PubMed/NCBI
|
|
28
|
Glen H, Mason S, Patel H, Macleod K and
Brunton VG: E7080, a multi-targeted tyrosine kinase inhibitor
suppresses tumor cell migration and invasion. BMC Cancer.
11:3092011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wesche J, Haglund K and Haugsten EM:
Fibroblast growth factors and their receptors in cancer. Biochem J.
437:199–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adam P, Kircher S, Sbiera I, Koehler VF,
Berg E, Knösel T, Sandner B, Fenske WK, Bläker H, Smaxwil C, et al:
FGF-Receptors and PD-L1 in Anaplastic and Poorly Differentiated
Thyroid Cancer: Evaluation of the Preclinical Rationale. Front
Endocrinol (Lausanne). 12:7121072021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamoto K, Kodama K, Takase K, Sugi NH,
Yamamoto Y, Iwata M and Tsuruoka A: Antitumor activities of the
targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against
RET gene fusion-driven tumor models. Cancer Lett. 340:97–103. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santoro M, Melillo RM and Fusco A: RET/PTC
activation in papillary thyroid carcinoma: European Journal of
Endocrinology Prize Lecture. Eur J Endocrinol. 155:645–653. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mazeh H, Mizrahi I, Halle D, Ilyayev N,
Stojadinovic A, Trink B, Mitrani-Rosenbaum S, Roistacher M, Ariel
I, Eid A, et al: Development of a microRNA-based molecular assay
for the detection of papillary thyroid carcinoma in aspiration
biopsy samples. Thyroid. 21:111–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hao Z and Wang P: Lenvatinib in management
of solid tumors. Oncologist. 25:e302–e310. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrari SM, Bocci G, Di Desidero T, Elia
G, Ruffilli I, Ragusa F, Orlandi P, Paparo SR, Patrizio A, Piaggi
S, et al: Lenvatinib exhibits antineoplastic activity in anaplastic
thyroid cancer in vitro and in vivo. Oncol Rep. 39:2225–2234.
2018.PubMed/NCBI
|
|
37
|
Wang R, Yamada T, Arai S, Fukuda K,
Taniguchi H, Tanimoto A, Nishiyama A, Takeuchi S, Yamashita K,
Ohtsubo K, et al: Distribution and activity of lenvatinib in brain
tumor models of human anaplastic thyroid cancer cells in severe
combined immune deficient mice. Mol Cancer Ther. 18:947–956. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murayama D, Yamamoto Y, Matsui A, Yasukawa
M, Okamoto S, Toda S and Iwasaki H: Lung cavitation in patients
with anaplastic thyroid cancer treated with lenvatinib. Gland Surg.
11:963–969. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Datar S, Cabanillas M, Dadu R, Ost D and
Grosu HB: Pulmonary cavitation in patients with thyroid cancer
receiving antiangiogenic agents. BMC Cancer. 20:11812020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cho JS, Park MH, Ryu YJ and Yoon JH: The
neutrophil to lymphocyte ratio can discriminate anaplastic thyroid
cancer against poorly or well differentiated cancer. Ann Surg Treat
Res. 88:187–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukuda N, Toda K, Fujiwara YU, Wang X,
Ohmoto A, Urasaki T, Hayashi N, Sato Y, Nakano K, Yunokawa M, et
al: Neutrophil-to-lymphocyte ratio as a prognostic marker for
anaplastic thyroid cancer treated with lenvatinib. In Vivo.
34:2859–2864. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamazaki H, Iwasaki H, Suganuma N, Toda S,
Masudo K, Nakayama H, Rino Y and Masuda M: Inflammatory biomarkers
and dynamics of neutrophil-to-lymphocyte ratio in lenvatinib
treatment for anaplastic thyroid carcinoma. Gland Surg. 10:852–860.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tomoda C, Sugino K, Kitagawa W, Nagahama M
and Ito K: The time series behavior of neutrophil-to-lymphocyte
ratio in thyroid cancer patients on tyrosine kinase inhibitor
therapy. ORL J Otorhinolaryngol Relat Spec. 83:347–353. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for anaplastic thyroid cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wirth LJ, Brose MS, Sherman EJ, Licitra L,
Schlumberger M, Sherman SI, Bible KC, Robinson B, Rodien P, Godbert
Y, et al: Open-label, single-arm, multicenter, phase II trial of
lenvatinib for the treatment of patients with anaplastic thyroid
cancer. J Clin Oncol. 39:2359–2366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; a review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takahashi S, Tahara M, Ito K, Tori M,
Kiyota N, Yoshida K, Sakata Y and Yoshida A: Safety and
effectiveness of lenvatinib in 594 patients with unresectable
thyroid cancer in an all-case post-marketing observational study in
Japan. Adv Ther. 37:3850–3862. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamada K, Yamamoto N, Yamada Y, Nokihara
H, Fujiwara Y, Hirata T, Koizumi F, Nishio K, Koyama N and Tamura
T: Phase I dose-escalation study and biomarker analysis of E7080 in
patients with advanced solid tumors. Clin Cancer Res. 17:2528–2537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim M, Ahn J, Song DE, Yoon JH, Kang HC,
Lim DJ, Kim WG, Kim TY, Kim WB, Shong YK, et al: Real-world
experience of lenvatinib in patients with advanced anaplastic
thyroid cancer. Endocrine. 71:427–433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Enomoto K, Hirayama S, Kumashiro N, Jing
X, Kimura T, Tamagawa S, Matsuzaki I, Murata SI and Hotomi M:
Synergistic effects of lenvatinib (E7080) and MEK inhibitors
against anaplastic thyroid cancer in preclinical models. Cancers
(Basel). 13:8622021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong CM, Oh JM, Gangadaran P, Rajendran RL
and Ahn BC: treatment effect of combining lenvatinib and
vemurafenib for BRAF mutated anaplastic thyroid cancer. Int J
Thyroidol. 14:127–134. 2021. View Article : Google Scholar
|
|
52
|
Zhang H and Chen D: Synergistic inhibition
of MEK/ERK and BRAF V600E with PD98059 and PLX4032 induces
sodium/iodide symporter (NIS) expression and radioiodine uptake in
BRAF mutated papillary thyroid cancer cells. Thyroid Res.
11:132018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song H, Zhang J, Ning L, Zhang H, Chen D,
Jiao X and Zhang K: The MEK1/2 Inhibitor AZD6244 Sensitizes
BRAF-mutant thyroid cancer to vemurafenib. Med Sci Monit.
24:3002–3010. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Su X, Liu J, Zhang H, Gu Q, Zhou X, Ji M
and Yao D: Lenvatinib promotes the antitumor effect of doxorubicin
in anaplastic thyroid cancer. Onco Targets Ther. 13:11183–11192.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ulm R, Revenkova E, di Sansebastiano GP,
Bechtold N and Paszkowski J: Mitogen-activated protein kinase
phosphatase is required for genotoxic stress relief in Arabidopsis.
Genes Dev. 15:699–709. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wood CD, Thornton TM, Sabio G, Davis RA
and Rincon M: Nuclear localization of p38 MAPK in response to DNA
damage. Int J Biol Sci. 5:428–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Martino E, Casamassima G, Castiglione S,
Cellupica E, Pantalone S, Papagni F, Rui M, Siciliano AM and
Collina S: Vinca alkaloids and analogues as anti-cancer agents:
Looking back, peering ahead. Bioorg Med Chem Lett. 28:2816–2826.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shaked Y, Emmenegger U, Man S, Cervi D,
Bertolini F, Ben-David Y and Kerbel RS: Optimal biologic dose of
metronomic chemotherapy regimens is associated with maximum
antiangiogenic activity. Blood. 106:3058–3061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Di Desidero T, Orlandi P, Gentile D,
Banchi M, Alì G, Kusmic C, Armanetti P, Cayme GJ, Menichetti L,
Fontanini G, et al: Pharmacological effects of vinorelbine in
combination with lenvatinib in anaplastic thyroid cancer. Pharmacol
Res. 158:1049202020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu S and Fu L: Tyrosine kinase inhibitors
enhanced the efficacy of conventional chemotherapeutic agent in
multidrug resistant cancer cells. Mol Cancer. 17:252018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ryder M, Ghossein RA, Ricarte-Filho JC,
Knauf JA and Fagin JA: Increased density of tumor-associated
macrophages is associated with decreased survival in advanced
thyroid cancer. Endocr Relat Cancer. 15:1069–1074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eubank TD, Galloway M, Montague CM,
Waldman WJ and Marsh CB: M-CSF induces vascular endothelial growth
factor production and angiogenic activity from human monocytes. J
Immunol. 171:2637–2643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Abbasifarid E, Sajjadi-Jazi SM, Beheshtian
M, Samimi H, Larijani B and Haghpanah V: The role of ATP-binding
cassette transporters in the chemoresistance of anaplastic thyroid
cancer: A systematic review. Endocrinology. 160:2015–2023. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Iyer PC, Dadu R, Gule-Monroe M, Busaidy
NL, Ferrarotto R, Habra MA, Zafereo M, Williams MD, Gunn GB, Grosu
H, et al: Salvage pembrolizumab added to kinase inhibitor therapy
for the treatment of anaplastic thyroid carcinoma. J Immunother
Cancer. 6:682018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
French JD, Bible K, Spitzweg C, Haugen BR
and Ryder M: Leveraging the immune system to treat advanced thyroid
cancers. Lancet Diabetes Endocrinol. 5:469–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Suzuki S, Shibata M, Gonda K, Kanke Y,
Ashizawa M, Ujiie D, Suzushino S, Nakano K, Fukushima T, Sakurai K,
et al: Immunosuppression involving increased myeloid-derived
suppressor cell levels, systemic inflammation and hypoalbuminemia
are present in patients with anaplastic thyroid cancer. Mol Clin
Oncol. 1:959–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Galdiero MR, Varricchi G and Marone G: The
immune network in thyroid cancer. Oncoimmunology. 5:e11685562016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Woyach JA, Kloos RT, Ringel MD, Arbogast
D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M and Shah
MH: Lack of therapeutic effect of the histone deacetylase inhibitor
vorinostat in patients with metastatic radioiodine-refractory
thyroid carcinoma. J Clin Endocrinol Metab. 94:164–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee YS, Kim SM, Kim BW, Chang HJ, Kim SY,
Park CS, Park KC and Chang HS: Anti-cancer effects of HNHA and
lenvatinib by the suppression of EMT-mediated drug resistance in
cancer stem cells. Neoplasia. 20:197–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Brown WS, Akhand SS and Wendt MK: FGFR
signaling maintains a drug persistent cell population following
epithelial-mesenchymal transition. Oncotarget. 7:83424–83436. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Meidhof S, Brabletz S, Lehmann W, Preca
BT, Mock K, Ruh M, Schüler J, Berthold M, Weber A, Burk U, et al:
ZEB1-associated drug resistance in cancer cells is reversed by the
class I HDAC inhibitor mocetinostat. EMBO Mol Med. 7:831–847. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou G, Zhang F, Guo Y, Huang J, Xie Y,
Yue S, Chen M, Jiang H and Li M: miR-200c enhances sensitivity of
drug-resistant non-small cell lung cancer to gefitinib by
suppression of PI3K/Akt signaling pathway and inhibites cell
migration via targeting ZEB1. Biomed Pharmacother. 85:113–119.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kwok G, Yau TC, Chiu JW, Tse E and Kwong
YL: Pembrolizumab (Keytruda). Hum Vaccin Immunother. 12:2777–2789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dierks C, Seufert J, Aumann K, Ruf J,
Klein C, Kiefer S, Rassner M, Boerries M, Zielke A, la Rosee P, et
al: Combination of lenvatinib and pembrolizumab is an effective
treatment option for anaplastic and poorly differentiated thyroid
carcinoma. Thyroid. 31:1076–1085. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim SY, Kim SM, Kim JW, Lee IJ, Jeon TJ,
Chang H, Kim BW, Lee YS, Chang HS and Park CS: Survival with
lenvatinib for the treatment of progressive anaplastic thyroid
cancer: A single-center, retrospective analysis. Front Endocrinol
(Lausanne). 11:5992020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Are C and Shaha AR: Anaplastic thyroid
carcinoma: Biology, pathogenesis, prognostic factors, and treatment
approaches. Ann Surg Oncol. 13:453–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sugitani I, Miyauchi A, Sugino K, Okamoto
T, Yoshida A and Suzuki S: Prognostic factors and treatment
outcomes for anaplastic thyroid carcinoma: ATC research consortium
of Japan cohort study of 677 patients. World J Surg. 36:1247–1254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu L, Wang E, Li L, Chen D, Peng K, Wang
M and Han G: As clinical markers, hand-foot-skin reaction and
diarrhea can predict better outcomes for hepatocellular carcinoma
patients receiving transarterial chemoembolization plus sorafenib.
Can J Gastroenterol Hepatol. 2019:25763492019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Robinson B, Schlumberger M, Wirth LJ,
Dutcus CE, Song J, Taylor MH, Kim SB, Krzyzanowska MK, Capdevila J,
Sherman SI and Tahara M: Characterization of tumor size changes
over time from the phase 3 study of lenvatinib in thyroid cancer. J
Clin Endocrinol Metab. 101:4103–4109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hamidi S, Dadu R, Zafereo ME, Ferrarotto
R, Wang JR, Maniakas A, Gunn GB, Lee A, Spiotto MT, Iyer PC, et al:
Initial management of BRAF V600E-variant anaplastic thyroid cancer:
The FAST multidisciplinary group consensus statement. JAMA Oncol.
10:1264–1271. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhao X, Wang JR, Dadu R, Busaidy NL, Xu L,
Learned KO, Chasen NN, Vu T, Maniakas A, Eguia AA, et al: Surgery
after BRAF-directed therapy is associated with improved survival in
BRAF(V600E) mutant anaplastic thyroid cancer: A single-center
retrospective cohort study. Thyroid. 33:484–491. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cabanillas ME, Williams MD, Gunn GB,
Weitzman SP, Burke L, Busaidy NL, Ying AK, Yiin YH, William WN, Lu
C and Lai SY: Facilitating anaplastic thyroid cancer specialized
treatment: A model for improving access to multidisciplinary care
for patients with anaplastic thyroid cancer. Head Neck.
39:1291–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yamazaki H, Yokose T, Hayashi H, Iwasaki
H, Osanai S, Suganuma N, Nakayama H, Masudo K, Rino Y and Masuda M:
Expression of fibroblast growth factor receptor 4 and clinical
response to lenvatinib in patients with anaplastic thyroid
carcinoma: A pilot study. Eur J Clin Pharmacol. 76:703–709. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Iwasaki H, Toda S, Suganuma N, Murayama D,
Nakayama H and Masudo K: Lenvatinib vs. palliative therapy for
stage IVC anaplastic thyroid cancer. Mol Clin Oncol. 12:138–143.
2020.PubMed/NCBI
|
|
88
|
Yang J and Barletta JA: Anaplastic thyroid
carcinoma. Semin Diagn Pathol. 37:248–256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hu S, Helman SN, Hanly E and Likhterov I:
The role of surgery in anaplastic thyroid cancer: A systematic
review. Am J Otolaryngol. 38:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI
|
|
92
|
Koyama S, Miyake N, Fujiwara K, Morisaki
T, Fukuhara T, Kitano H and Takeuchi H: Lenvatinib for anaplastic
thyroid cancer and lenvatinib-induced thyroid dysfunction. Eur
Thyroid J. 7:139–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Iyer PC, Dadu R, Ferrarotto R, Busaidy NL,
Habra MA, Zafereo M, Gross N, Hess KR, Gule-Monroe M, Williams MD
and Cabanillas ME: Real-world experience with targeted therapy for
the treatment of anaplastic thyroid carcinoma. Thyroid. 28:79–87.
2018. View Article : Google Scholar : PubMed/NCBI
|