Introduction
According to GLOBOCAN 2022 statistics, lung cancer
has the highest incidence and mortality rate in both men and women
(1). Non-small cell lung cancer
(NSCLC) is the most common type of lung cancer, accounting for
80–85% of cases (2). In recent
years, the advent of targeted therapies for NSCLC has significantly
improved the survival of patients, with 5-year survival rates for
patients with EGFR mutations reaching 30–40% and for ALK-positive
patients achieving 50–60%, compared with 5-year survival rates of
only 5–10% in the pre-targeted therapy era (3–5).
Epidermal growth factor receptor (EGFR) has the highest proportion,
occurring in 45% of Asian patients and 20% of Caucasian patients
with adenocarcinoma histology (6).
In these patients, EGFR-tyrosine kinase inhibitors (TKIs) are
indicated as the first-line treatment. Classical activating
mutations (exon 19 deletions and the L858R point mutation) account
for most EGFR mutations and are strong predictors for a good
response to EGFR-TKIs (7). By
contrast, 10–20% of patients with NSCLC harbor uncommon or rare
EGFR mutations, including G719X (Exon 18), L861Q (Exon21) and S768I
(Exon 20), which have lower response rates (8). Anaplastic lymphoma kinase (ALK)
rearrangement is less common than EGFR mutation and is found in ~5%
of patients with NSCLC (9).
ALK-TKIs are the optimal first-line treatment for individuals with
ALK rearrangement. The simultaneous or sequential appearance of
both mutations (EGFR and ALK) in a patient is rare, as it is
considered mutually exclusive. This raises questions regarding the
appropriate time and specimens for investigation, as well as
optimal treatment options for these patients. In the present
report, the case of a patient with simultaneous EGFR and ALK
mutations is detailed.
Case report
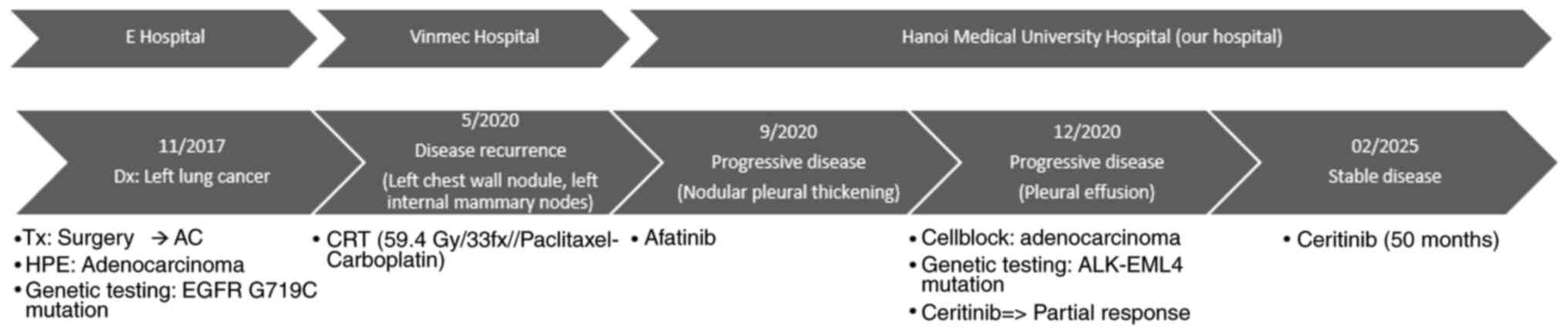
Patient
A 58-year-old asymptomatic female patient, with no
past medical history, was admitted with a left upper lung cancer
with the pathology of adenocarcinoma on a routine health check in
November 2017 at E Hospital (Hanoi, Vietnam). The patient underwent
laparoscopic surgery to remove the left upper lobe of the lung,
lymph node dissection and then 6 cycles of adjuvant chemotherapy
[paclitaxel 175 mg/m2 and carboplatin area under the
curve (AUC) 5 every 21 days]. PCR combined with molecular probe
hybridization of the tumor specimens was performed to detect gene
mutations, revealing a G719C mutation on exon 18. The patient was
then periodically monitored every 3 months.
In May 2020, the disease recurred at the left chest
wall nodule and left internal mammary nodes. The patient's clinical
examination was normal. The patient received chemoradiotherapy (33
fractions at 1.8 Gy) concurrent with paclitaxel 50 mg/m2
and carboplatin AUC 2 (repeated weekly for 6 weeks), followed by
consolidation chemotherapy of paclitaxel 200 mg/m2 and
carboplatin AUC 6 (repeated every 3 weeks for 2 cycles) at Vinmec
Hospital (Hanoi, Vietnam).
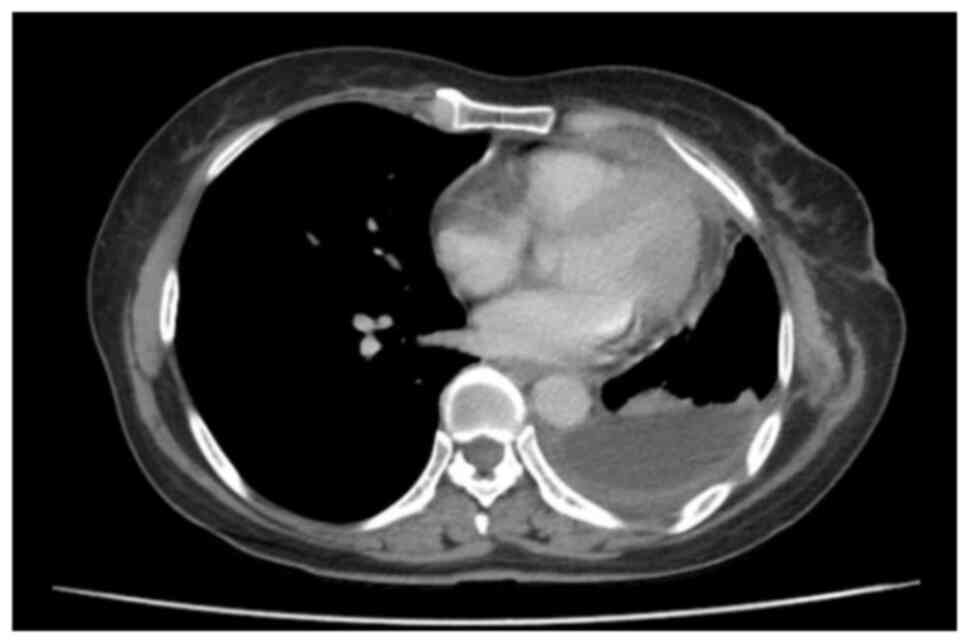
In September 2020, the patient was transferred to
Hanoi Medical University Hospital (Hanoi, Vietnam), and a chest CT
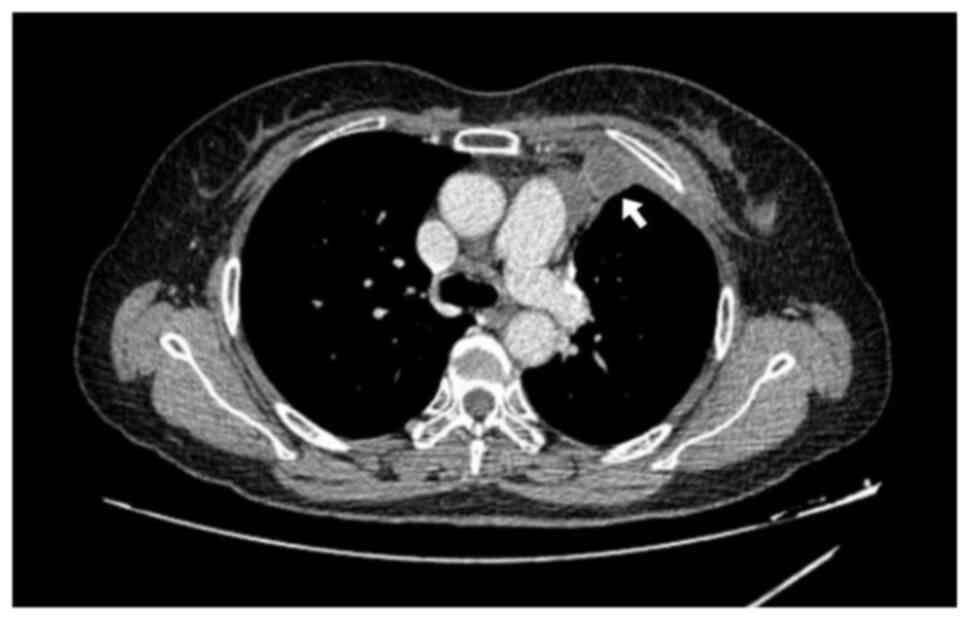
scan performed 2 months after chemoradiation showed an upper
lobectomy, pleural thickening with enhanced nodule measuring 18×40
mm with no abnormal mediastinal lymph nodes and no bilateral
pleural effusion (Fig. 1). The
patient was further treated with a second-generation EGFR-TKI drug,
afatinib (40 mg once a day, 1 h before food or 3 h after food).
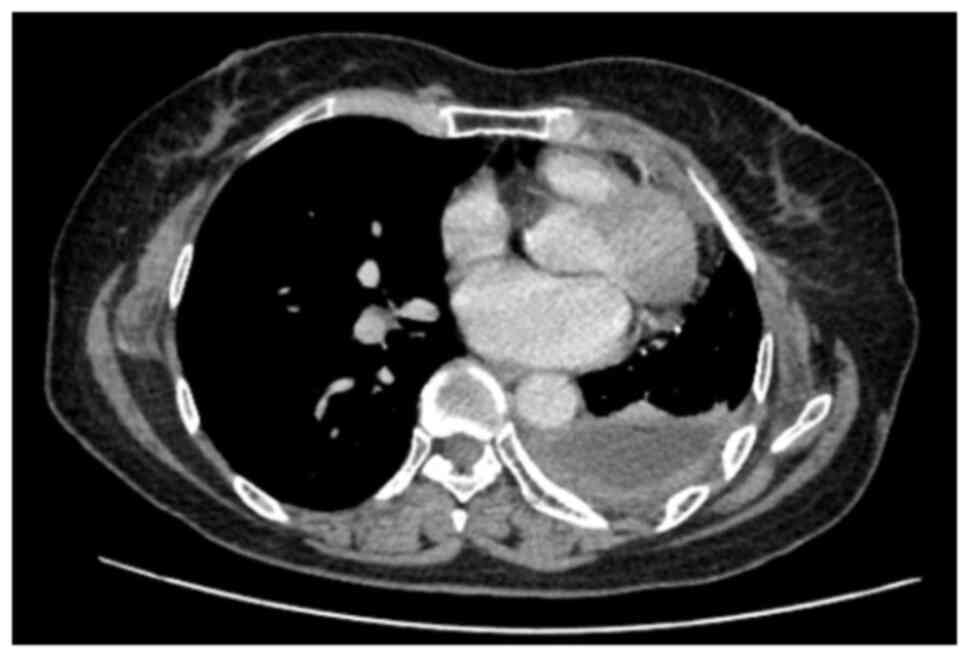
After 3 months of treatment, in December 2020, the patient
developed rapidly increasing dyspnea and the disease progressed
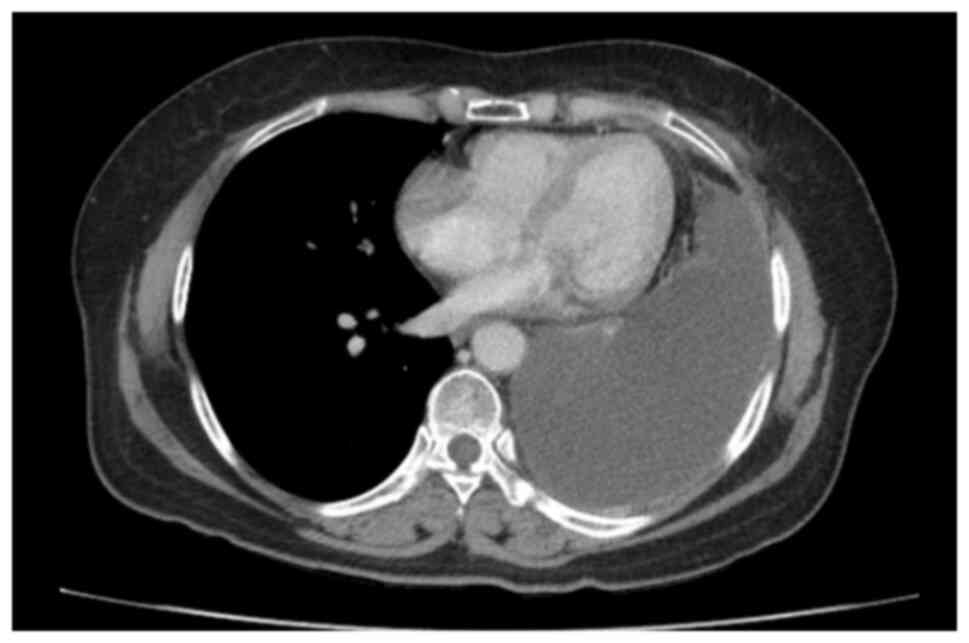
with a large amount of pleural effusion. A CT scan image showed
irregular left pleural thickening, a large left pleural effusion of
75 mm causing passive atelectasis, a left chest wall enhancing
nodule of 15×13 mm, pericardial fluid of 16 mm and no unusual
mediastinal lymph nodes (Fig. 2).
The patient then underwent a pleural puncture to reduce the
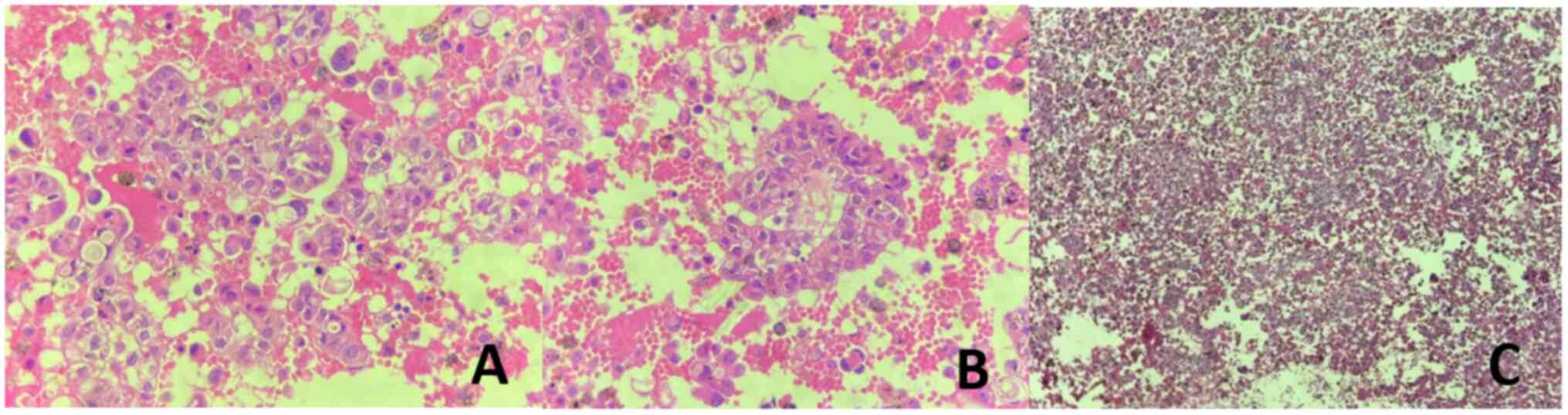
difficulty breathing and to collect specimens for testing. The cell
block from the left pleural effusion, stained with hematoxylin and
eosin (H&E), revealed cells arranged in clusters resembling
glandular structures. These cells displayed large, basophilic
nuclei and a high nucleus-to-cytoplasm ratio, raising a strong
suspicion of adenocarcinoma (Fig.
3). However, other differential diagnoses, including reactive
mesothelial cells and mesothelioma, needed to be excluded. To
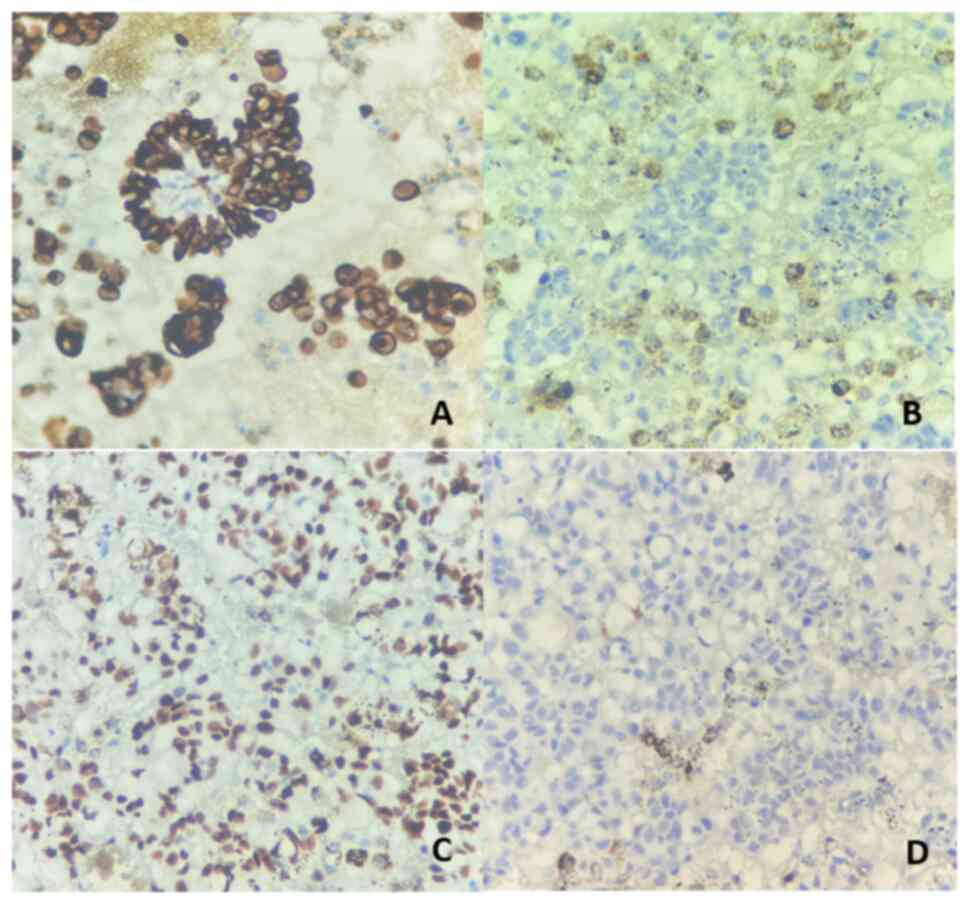
clarify the diagnosis, additional immunohistochemical staining was
performed. The tumor cells demonstrated positivity for epithelial
markers such as CK7 and lung adenocarcinoma markers such as
transcription termination factor 1 (TTF-1), while being negative
for mesothelial markers such as calretinin. These findings
confirmed the diagnosis of metastatic lung adenocarcinoma with
associated pleural effusion (Fig.
4).
Gene mutation testing on pleural fluid specimens
using the new-generation gene sequencing method (test performed by
the Medical Genetics Institute using the Miseq system; Illumina,
Inc.) detected the ALK-EMAP like 4 (EML4) fusion mutation. The
patient was then switched to the second-generation ALK-TKI drug,
ceritinib (450 mg, once a day on an empty stomach, at least 2 h
before or after food), and re-evaluated every 3 months Fig. 5 shows the CT scan obtained after the
first 3 months of ceritinib treatment, indicating a significant
reduction in pleural effusion. During the first month of taking
ceritinib, the patient had diarrhea, but it was mild and
intervention was not indicated. After that, the patient tolerated
the drug well, with no further side effects noted. Since then, the
disease has responded well to treatment and has become stable.
Fig. 6 displays the most recent
follow-up CT scan after 50 months of receiving ceritinib, showing
that the amount of pleural effusion has remained nearly unchanged
with no evidence of disease progression. The diagnostic, treatment
and response of the patient are summarized in Fig. 7.
Histopathological and
immunohistochemical analysis protocol
The specimen consisted of 200 ml of pleural fluid
(sent to the pathology laboratory) and was stored at 4°C. The
specimen was allowed to sediment naturally for 10 h, after which
the supernatant was discarded and the sediment was collected. The
sediment was transferred to test tubes (15×45 mm), then centrifuged
for 10 min at 447 × g and room temperature. The supernatant was
removed and the sediment was fixed in 10% neutral buffered formalin
(NBF) at room temperature for 5 h, with a volume ratio of NBF to
sediment of 10:1. Next, the sample was again centrifuged for 10 min
at 447 × g and room temperature and then incubated at 55°C for 1 h.
After fixation, the specimen formed a firm pellet that was easily
retrievable, which was wrapped in non-adhesive paper and placed in
a cassette for routine histopathological processing and embedding
within paraffin. Then, 3–5 µm-thick sections were cut from the
paraffin block using a microtome and stained with H&E at room
temperature for 30–45 min. Immunohistochemical staining of CK7,
CK20, TTF-1 and calretinin (Table
I) was also performed using the fully automated DAKO Omnis
immunostaining system from Agilent Technologies, following the
manufacturer's protocol, with both positive and negative controls
included. The H&E and immunohistochemically stained slides were
examined under a light optical microscope.
 | Table I.Primary antibodies used in
immunohistochemistry. |
Table I.
Primary antibodies used in
immunohistochemistry.
| Primary antibody | Clone | Supplier | Cat. no. | Dilution |
|---|
| CK7 (monoclonal) | OV-TL12/30 | Dako (Agilent
Technologies, Inc.) | M7018 | 1:250 |
| CK20
(monoclonal) |
Ks20.8 | Dako (Agilent
Technologies, Inc.) | M7019 | 1:250 |
| TTF-1
(monoclonal) | 8G7G3/1 | Dako (Agilent
Technologies, Inc.) | M3575 | 1:100 |
| Calretinin
(monoclonal) | DAK-Calret 1 | Dako (Agilent
Technologies, Inc.) | M7245 | 1:100 |
Discussion
In the present study, the patient was initially
diagnosed with local regional lung cancer and underwent surgery
followed by adjuvant chemotherapy. Subsequent analysis of the
post-operative tumor specimen revealed an EGFR gene mutation,
specifically G719C mutation, which is relatively rare compared with
the more common EGFR mutations such as exon 19 deletions and L858R.
The G719C mutation, along with other exon 18 mutations (such as
G719S and G719A), accounts for 3–5% of all EGFR mutations in NSCLC
(10). With first-generation TKIs,
patients with G719C and similar exon 18 mutations exhibit response
rates of 30–50%, with progression-free survival (PFS) times shorter
than those typically observed in patients with exon 19 deletions or
L858R mutations. Afatinib has shown improved efficacy for uncommon
mutations, including G719C. Clinical data suggest that afatinib
provides higher response rates and a longer PFS time for G719
mutations compared with first-generation TKIs (11–13).
Although primarily used for T790M resistance mutations, some
studies suggest that osimertinib may also have activity against
G719C. However, afatinib tends to be the preferred option in
clinical practice for this mutation, owing to more robust data on
uncommon mutations (13,14). In the present study, upon disease
recurrence, the response to EGFR-TKI was notably poor, marked by
the development of pleural effusion within the first 3 months. This
prompted the conduction of a genetic mutation test on pleural fluid
specimens. Notably, EGFR gene mutations were not detected; instead,
ALK mutations were identified. Based on these findings, the patient
was treated with anti-ALK drugs and achieved long-term disease
stability.
This change in gene mutation status at different
times could be explained by the influence of chemicals on genetic
mutation. In patients with advanced disease stages, it is crucial
to prioritize EGFR mutation detection before initiating systemic
therapy. However, the efficacy of TKI therapy as a second-line
treatment appears to be inferior to that as a first-line treatment,
suggesting a potential change in gene mutation status due to the
influence of chemicals. A study by Bai et al (15) showed that chemicals (such as
platinum-based chemotherapy) can initially significantly reduce the
frequency of EGFR mutations in tumor tissue and plasma. Among 264
patients with advanced NSCLC, plasma EGFR mutations were found in
34.5% of samples collected before 2 chemotherapy cycles, but only
in 23.1% of the post-chemotherapy plasma samples. Honda et
al (16) also reported the case
of a Japanese woman with the disappearance of an activated EGFR
mutation in malignant pleural effusion after treatment with
chemotherapy and TKIs. Therefore, chemotherapy and TKI treatment
may have influences on gene mutation status, and thus, EGFR
mutation status collected from the initial specimens might be
inadequate for predicting the efficacy of TKI treatment in
subsequent lines.
After failure with first- or second-generation
EGFR-TKIs, clinicians typically utilize liquid biopsies to detect
drug-resistant mutations, such as T790M, or identify other gene
mutations with lower frequencies. Liquid biopsy, primarily through
the analysis of circulating tumor DNA, offers a non-invasive
approach to monitor tumor dynamics and emerging resistance
mutations. For example, Iwama et al (17) demonstrated that an increase in
EGFR-activating mutation alleles in plasma during treatment was
correlated with disease progression, underscoring the value of
liquid biopsy in predicting EGFR-TKI efficacy and assessing clonal
evolution. Beyond detecting EGFR mutations in lung cancer, liquid
biopsy has expanded to identify various other genetic alterations,
including mutations in KRAS and BRAF as well as ALK rearrangements
(18). Qvick et al (19) demonstrated that liquid biopsy could
detect mutations in KRAS and BRAF, which were not identified in
tumor tissue samples, highlighting its potential to uncover
additional actionable mutations. In summary, liquid biopsy is a
powerful tool for identifying a broad range of genetic mutations in
lung cancer, such as KRAS, BRAF and ALK rearrangements, while also
providing insights into epigenetic changes and copy number
alterations (20,21). This broad utility enhances its role
in personalized treatment strategies and monitoring therapeutic
resistance.
In the early years following their discovery, the
two main genetic alterations, EGFR mutations and ALK
rearrangements, were previously believed to be mutually exclusive
(13–15). A study indicated that ALK
rearrangements were more frequently found in patients with poorly
differentiated adenocarcinoma, while EGFR mutations were more
typically found in well-differentiated cancer (22). Similarly, the coexistence of KRAS
mutations with either of these alterations was considered nearly
impossible (23,24). As a result, the initial algorithms
for biomolecular characterization of non-squamous NSCLC recommended
testing samples for KRAS mutations first, followed by EGFR testing
only if KRAS was wild-type (24).
ALK testing was reserved for cases where no alterations were
detected in the prior tests. However, further studies have shown
that the coexistence of EML4-ALK rearrangements and EGFR mutations,
though uncommon, is possible, as is the presence of KRAS and other
mutations (22,25–28).
Therefore, the previously established diagnostic algorithm can no
longer be the gold standard. Recent studies and reports have shown
that the simultaneous appearance of both EGFR and ALK is uncommon
(0.1–1.6%) (27,28). Intratumor heterogeneity, defined as
the presence of sub-clonal diversities of cells within a lung
tumor, may explain the occurrence of multiple genetic alterations
concurrently. Nonetheless, the clinical and pathological
characteristics of these patients have not been fully described,
and the optimal treatment approach for this patient group is
unclear. Hu et al (28)
reported a clinical case with concomitant EGFR mutation and ALK
rearrangement, progression on osimertinib and partial response to
alectinib. Recently, there have been studies reporting similar
cases in which 107 patients harboring both EGFR mutation and ALK
rearrangement have been documented, revealing variable responses to
treatment (28,29). The summary from these reports
indicated a reduced overall response rate (ORR) to EGFR-TKIs,
whereas patients receiving ALK-TKIs demonstrated an improved ORR.
Due to the variability in response, and since there are no clinical
guidelines for choosing the optimal targeted therapies in this
specific group of patients, further research is required to gain an
improved understanding and explore potential combination or
sequential therapy strategies. Although there is no consensus in
the literature, ALK-TKIs appear to be marginally more effective
than EGFR-TKIs in patients with both EGFR and ALK alterations.
Disease control and response has been reported as the best outcome
in 69.8 and 43.4% of cases treated with EGFR-TKIs, compared with
79.5 and 51.3% of cases treated with ALK-TKIs, respectively
(30,31). However, due to the limited number of
evaluable patients, definitive conclusions cannot be drawn.
Following a literature review of 100 cases, ALK-TKIs may be
considered the preferred first-line treatment, provided no other
data are available to guide the therapeutic decision (32). A potential future approach could
involve investigating the safety and efficacy of dual inhibition of
both ALK and EGFR, as these alterations may coexist in some
patients. Designing clinical trials specifically targeting this
patient subset would be a valuable step toward addressing several
unanswered questions. However, due to the rarity of this condition,
recruiting a sufficient number of patients would likely be a
notable challenge. Assuming that there is consistency in mutation
status across specimens (primary tumor and malignant pleural
fluid), the transformation of EGFR gene mutations from positive to
negative with the appearance of new ALK rearrangement is extremely
rare. To the best of our knowledge, no other similar clinical cases
have been reported. This raises questions about the appropriate
time and specimens to survey genetic mutations.
Tumor heterogeneity serves a notable role in the
diversity of tumor mutation status. There have been different
levels of heterogeneity in cancer: Interpatient, intratumor and
intertumor (28). Generally,
patients with NSCLC harboring targetable driver mutations respond
well to specific inhibitors. However, some patients show poor or
mixed responses to targeted therapy, which may be explained by
intertumor molecular heterogeneity (28,32).
In the literature, several lung cancer studies have reported
differences in EGFR and ALK mutational status between primary and
metastatic sites (22–25). Several different models have been
proposed to explain this difference in genetic profile. A classic
model states that primary tumor cells have a low metastatic
potential, so the accumulation of enough genetic mutations will
promote metastasis (32). Besides,
there can be inconsistencies between genetic testing methods, as in
the present case, two gene mutation tests were performed using two
different methods (32,33).
In conclusion, the simultaneous or sequential
appearance of EGFR/ALK mutation in lung cancer is very rare. This
can be explained by tumor heterogeneity and the effects of
chemotherapy. The approach and treatment need to be individualized
and remain a clinical challenge. According to reports, EGFR
mutations, ALK translocations and various other biomolecular
alterations can emerge during disease progression as mechanisms of
acquired resistance following treatment with EGFR and ALK-TKIs
(33,34). Therefore, whenever technically
feasible, it is essential to perform re-biopsies in all patients
with disease progression to identify any new alterations and tailor
subsequent therapies based on the updated biomolecular profile.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The next-generation
sequencing data generated in the present study are not publicly
available due to ethical and confidentiality restrictions but may
be requested from the corresponding author.
Authors' contributions
HVN is the clinical oncologist who treated the
patient and revised the manuscript. CHD is the assistant doctor who
wrote the manuscript and made contributions to the conception of
the study. BTT and HLT assisted in the patient treatment, collected
clinical information and assisted with the drafting of the
manuscript. HVN and CHD confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of this
study was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou YJ, Zheng W, Zeng QH, Ye Y, Wang C,
Fang C, Liu CJ, Niu L and Wu LM: Targeted exome sequencing
identifies mutational landscape in a cohort of 1500 Chinese
patients with non-small cell lung carcinoma (NSCLC). Hum Genomics.
15:212021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camidge DR, Dziadziuszko R, Peters S, Mok
T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis
F, et al: Updated efficacy and safety data and impact of the
EML4-ALK fusion variant on the efficacy of alectinib in untreated
ALK-positive advanced non-small cell lung cancer in the global
phase III ALEX study. J Thorac Oncol. 14:1233–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR,
Threapleton D, Yang ZY, Mao C and Tang JL: The prevalence of EGFR
mutation in patients with non-small cell lung cancer: A systematic
review and meta-analysis. Oncotarget. 7:78985–78993. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grigoriu B, Berghmans T and Meert AP:
Management of EGFR mutated nonsmall cell lung carcinoma patients.
Eur Respir J. 45:1132–1141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harrison PT, Vyse S and Huang PH: Rare
epidermal growth factor receptor (EGFR) mutations in non-small cell
lung cancer. Semin Cancer Biol. 61:167–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jahanzeb M, Lin HM, Pan X, Yin Y, Wu Y,
Nordstrom B and Socinski MA: Real-world treatment patterns and
progression-free survival associated with anaplastic lymphoma
kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small
cell lung cancer. Oncologist. 25:867–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H, Yang G, Li W, Li J, Hao X, Xing P,
Yang Y and Wang Y: EGFR exon 18 mutations in advanced non-small
cell lung cancer: A real-world study on diverse treatment patterns
and clinical outcomes. Front Oncol. 11:7134832021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T,
Zhan P and Song Y: Treatment of uncommon EGFR mutations in
non-small cell lung cancer: new evidence and treatment. Transl Lung
Cancer Res. 8:302–316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heigener DF, Schumann C, Sebastian M,
Sadjadian P, Stehle I, Märten A, Lüers A, Griesinger F and
Scheffler M; Afatinib Compassionate Use Consortium (ACUC), :
Afatinib in non-small cell lung cancer harboring uncommon EGFR
mutations pretreated with reversible EGFR inhibitors. Oncologist.
20:1167–1174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sehgal K, Rangachari D, VanderLaan PA,
Kobayashi SS and Costa DB: Clinical benefit of tyrosine kinase
inhibitors in advanced lung cancer with EGFR-G719A and other
uncommon EGFR mutations. Oncologist. 26:281–287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russo A, Franchina T, Ricciardi G,
Battaglia A, Picciotto M and Adamo V: Heterogeneous responses to
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
(TKIs) in patients with uncommon EGFR mutations: New Insights and
future perspectives in this complex clinical scenario. Int J Mol
Sci. 20:14312019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai H, Wang Z, Chen K, Zhao J, Lee JJ,
Wang S, Zhou Q, Zhuo M, Mao L, An T, et al: Influence of
chemotherapy on EGFR mutation status among patients with
non-small-cell lung cancer. J Clin Oncol. 30:3077–3083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honda Y, Takigawa N, Fushimi S, Ochi N,
Kubo T, Ozaki S, Tanimoto M and Kiura K: Disappearance of an
activated EGFR mutation after treatment with EGFR tyrosine kinase
inhibitors. Lung Cancer. 78:121–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwama E, Sakai K, Azuma K, Harada T,
Harada D, Nosaki K, Hotta K, Ohyanagi F, Kurata T, Fukuhara T, et
al: Monitoring of somatic mutations in circulating cell-free DNA by
digital PCR and next-generation sequencing during afatinib
treatment in patients with lung adenocarcinoma positive for EGFR
activating mutations. Ann Oncol. 28:136–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagasaka M, Uddin MH, Al-Hallak MN, Rahman
S, Balasubramanian S, Sukari A and Azmi AS: Liquid biopsy for
therapy monitoring in early-stage non-small cell lung cancer. Mol
Cancer. 20:822021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qvick A, Stenmark B, Carlsson J, Isaksson
J, Karlsson C and Helenius G: Liquid biopsy as an option for
predictive testing and prognosis in patients with lung cancer. Mol
Med. 27:682021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rolfo C, Mack PC, Scagliotti GV, Baas P,
Barlesi F, Bivona TG, Herbst RS, Mok TS, Peled N, Pirker R, et al:
Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A
statement paper from the IASLC. J Thorac Oncol. 13:1248–1268. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ansari J, Yun JW, Kompelli AR, Moufarrej
YE, Alexander JS, Herrera GA and Shackelford RE: The liquid biopsy
in lung cancer. Genes Cancer. 7:355–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gainor JF, Varghese AM, Ou SH, Kabraji S,
Awad MM, Katayama R, Pawlak A, Mino-Kenudson M, Yeap BY, Riely GJ,
et al: ALK rearrangements are mutually exclusive with mutations in
EGFR or KRAS: An analysis of 1,683 patients with non-small cell
lung cancer. Clin Cancer Res. 19:4273–4281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horn L and Pao W: EML4-ALK: Honing in on a
new target in non-small-cell lung cancer. J Clin Oncol.
27:4232–4235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu L, Lei J, Wang QZ, Li J and Wu L:
Clinical characteristics of patients with non-small cell lung
cancers harboring anaplastic lymphoma kinase rearrangements and
primary lung adenocarcinoma harboring epidermal growth factor
receptor mutations. Genet Mol Res. 14:12973–12983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas D, Maloney ME and Raval G:
Concomitant EGFR mutations and ALK rearrangements in lung
adenocarcinoma treated with osimertinib. Cureus.
15:e481222023.PubMed/NCBI
|
|
28
|
Hu H, Tan S, Xie M, Guo P, Yu Q, Xiao J,
Zhao K, Liao Q and Wang Y: Case report: Concomitant EGFR mutation
and ALK rearrangement in non-small cell lung cancer. Front
Pharmacol. 14:11679592023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santelmo C, Ravaioli A, Barzotti E, Papi
M, Poggi B, Drudi F, Mangianti M, Salvi M and Crinò L: Coexistence
of EGFR mutation and ALK translocation in NSCLC: Literature review
and case report of response to gefitinib. Lung Cancer. 81:294–296.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zito Marino F, Ronchi A, Accardo M and
Franco R: Concomitant ALK/KRAS and ALK/EGFR mutations in non small
cell lung cancer: Different profile of response to target
therapies. Transl Cancer Res. 6 (Suppl 3):S457–S460. 2017.
View Article : Google Scholar
|
|
31
|
Zhao N, Zheng SY, Yang JJ, Zhang XC, Xie
Z, Xie B, Su J, Chen ZH, Chen SL, Zhang N, et al: Lung
adenocarcinoma harboring concomitant EGFR mutation and EML4-ALK
fusion that benefits from three kinds of tyrosine kinase
inhibitors: A case report and literature review. Clin Lung Cancer.
16:e5–e9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lo Russo G, Imbimbo M, Corrao G, Proto C,
Signorelli D, Vitali M, Ganzinelli M, Botta L, Zilembo N, de Braud
F and Garassino MC: Concomitant EML4-ALK rearrangement and EGFR
mutation in non-small cell lung cancer patients: A literature
review of 100 cases. Oncotarget. 8:59889–59900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zito Marino F, Bianco R, Accardo M, Ronchi
A, Cozzolino I, Morgillo F, Rossi G and Franco R: Molecular
heterogeneity in lung cancer: From mechanisms of origin to clinical
implications. Int J Med Sci. 16:981–989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen ZY, Zhong WZ, Zhang XC, Su J, Yang
XN, Chen ZH, Yang JJ, Zhou Q, Yan HH, An SJ, et al: EGFR mutation
heterogeneity and the mixed response to EGFR tyrosine kinase
inhibitors of lung adenocarcinomas. Oncologist. 17:978–985. 2012.
View Article : Google Scholar : PubMed/NCBI
|