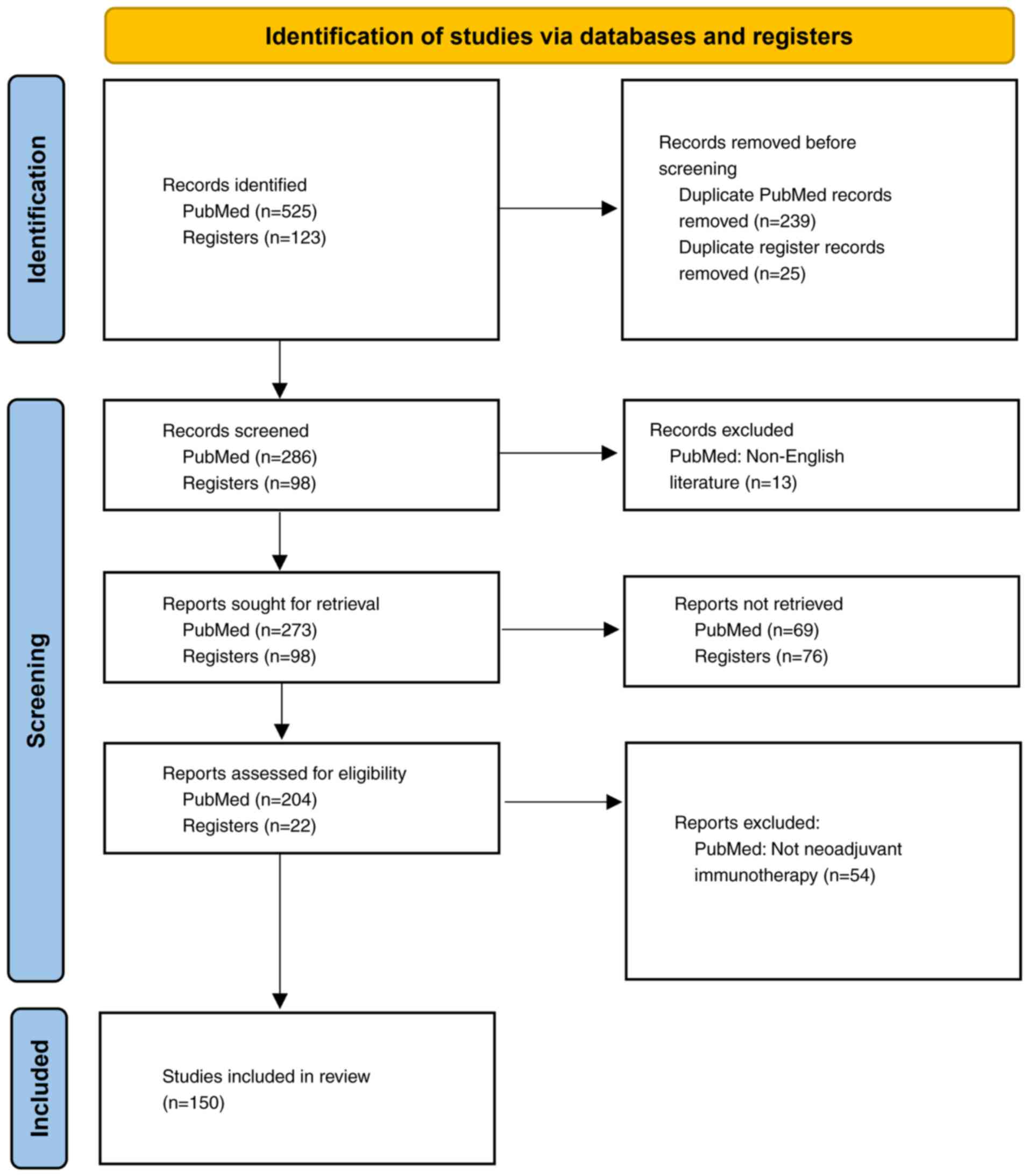
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Reveron-Thornton RF, Teng MLP, Lee EY,
Tran A, Vajanaphanich S, Tan EX, Nerurkar SN, Ng RX, Teh R,
Tripathy DP, et al: Global and regional long-term survival
following resection for HCC in the recent decade: A meta-analysis
of 110 studies. Hepatol Commun. 6:1813–1826. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banales JM, Marin JJG, Lamarca A,
Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen
JB, Braconi C, et al: Cholangiocarcinoma 2020: The next horizon in
mechanisms and management. Nat Rev Gastroenterol Hepatol.
17:557–588. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng H, Chen W, Zheng R, Zhang S, Ji JS,
Zou X, Xia C, Sun K, Yang Z, Li H, et al: Changing cancer survival
in China during 2003-15: A pooled analysis of 17 population-based
cancer registries. Lancet Glob Health. 6:e555–e567. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen N, Cai Y, Li F, Ye H, Tang W, Song P
and Cheng N: The clinical management of hepatocellular carcinoma
worldwide: A concise review and comparison of current guidelines:
2022 update. Biosci Trends. 16:20–30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glantzounis GK, Karampa A, Peristeri DV,
Pappas-Gogos G, Tepelenis K, Tzimas P and Cyrochristos DJ: Recent
advances in the surgical management of hepatocellular carcinoma.
Ann Gastroenterol. 34:453–465. 2021.PubMed/NCBI
|
|
7
|
Ryon EL, Kronenfeld JP, Lee RM, Yopp A,
Wang A, Lee AY, Luu S, Hsu C, Silberfein E, Russell MC, et al:
Surgical management of hepatocellular carcinoma patients with
portal vein thrombosis: The United States safety net and academic
center collaborative analysis. J Surg Oncol. 123:407–415. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu P, Liao W, Zhang WG, Chen L, Shu C,
Zhang ZW, Huang ZY, Chen YF, Lau WY, Zhang BX and Chen XP: A
prospective study using propensity score matching to compare
Long-term survival outcomes after robotic-assisted, laparoscopic,
or open liver resection for patients with BCLC stage 0-A
hepatocellular carcinoma. Ann Surg. 277:e103–e111. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhir M, Melin AA, Douaiher J, Lin C, Zhen
WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK and Are C: A
review and update of treatment options and controversies in the
management of hepatocellular carcinoma. Ann Surg. 263:1112–1125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabrizian P, Jibara G, Shrager B, Schwartz
M and Roayaie S: Recurrence of hepatocellular cancer after
resection: Patterns, treatments, and prognosis. Ann Surg.
261:947–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erstad DJ and Tanabe KK: Prognostic and
therapeutic implications of microvascular invasion in
hepatocellular carcinoma. Ann Surg Oncol. 26:1474–1493. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roayaie S, Obeidat K, Sposito C, Mariani
L, Bhoori S, Pellegrinelli A, Labow D, Llovet JM, Schwartz M and
Mazzaferro V: Resection of hepatocellular cancer </=2 cm:
Results from two Western centers. Hepatology. 57:1426–1435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng YX, Wang T, Deng YZ, Yang P, Li JJ,
Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, et al: Sorafenib suppresses
postsurgical recurrence and metastasis of hepatocellular carcinoma
in an orthotopic mouse model. Hepatology. 53:483–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang SN, Chuang SC and Lee KT: Efficacy of
sorafenib as adjuvant therapy to prevent early recurrence of
hepatocellular carcinoma after curative surgery: A pilot study.
Hepatol Res. 44:523–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang W, Zhao G, Wei K, Zhang Q, Ma W,
Song T, Wu Q, Zhang T, Kong D and Li Q: Adjuvant sorafenib reduced
mortality and prolonged overall survival and post-recurrence
survival in hepatocellular carcinoma patients after curative
resection: A single-center experience. Biosci Trends. 8:333–338.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You A, Cao M, Guo Z, Zuo B, Gao J, Zhou H,
Li H, Cui Y, Fang F, Zhang W, et al: Metformin sensitizes sorafenib
to inhibit postoperative recurrence and metastasis of
hepatocellular carcinoma in orthotopic mouse models. J Hematol
Oncol. 9:202016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruix J, Takayama T, Mazzaferro V, Chau
GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al: Adjuvant
sorafenib for hepatocellular carcinoma after resection or ablation
(STORM): A phase 3, randomised, double-blind, placebo-controlled
trial. Lancet Oncol. 16:1344–1354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yau T, Kang YK, Kim TY, El-Khoueiry AB,
Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al:
Efficacy and safety of nivolumab plus ipilimumab in patients with
advanced hepatocellular carcinoma previously treated with
sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol.
6:e2045642020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lygidakis NJ, Ziras N and Parissis J:
Resection versus resection combined with adjuvant pre- and
post-operative chemotherapy-immunotherapy for metastatic colorectal
liver cancer. A new look at an old problem. Hepatogastroenterology.
42:155–161. 1995.PubMed/NCBI
|
|
22
|
Lygidakis NJ, Stringaris K, Kokinis K,
Lyberopoulos K and Raptis S: Locoregional chemotherapy versus
locoregional combined immuno-chemotherapy for patients with
advanced metastatic liver disease of colorectal origin: A
prospective randomized study. Hepatogastroenterology. 43:212–220.
1996.PubMed/NCBI
|
|
23
|
Lygidakis NJ, Sgourakis G, Vlachos L,
Raptis S, Safioleas M, Boura P, Kountouras J and Alamani M:
Metastatic liver disease of colorectal origin: the value of
locoregional immunochemotherapy combined with systemic chemotherapy
following liver resection. Results of a prospective randomized
study. Hepatogastroenterology. 48:1685–1691. 2001.PubMed/NCBI
|
|
24
|
Gardini A, Ercolani G, Riccobon A,
Ravaioli M, Ridolfi L, Flamini E, Ridolfi R, Grazi GL, Cavallari A
and Amadori D: Adjuvant, adoptive immunotherapy with tumor
infiltrating lymphocytes plus interleukin-2 after radical hepatic
resection for colorectal liver metastases: 5-year analysis. J Surg
Oncol. 87:46–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiang J, Chen PC, Pham J, Nguyen CQ, Kaur
K, Raman SS and Jewett A: Characterizing hepatocellular carcinoma
stem markers and their corresponding susceptibility to NK-cell
based immunotherapy. Front Immunol. 14:12846692023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Zhang H, Sun B and Karin M: The
immunobiology of hepatocellular carcinoma in humans and mice: Basic
concepts and therapeutic implications. J Hepatol. 72:167–182. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Tong S, Hu B, Wan T, Tang H, Zhao
F, Jiao T, Li J, Zhang Z, Cai J, et al: Lenvatinib plus anti-PD-1
antibodies as conversion therapy for patients with unresectable
intermediate-advanced hepatocellular carcinoma: A single-arm, phase
II trial. J Immunother Cancer. 11:e0073662023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen DS and Hurwitz H: Combinations of
bevacizumab with cancer immunotherapy. Cancer J. 24:193–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Finn RS, Ryoo B, Merle P, Kudo M,
Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et
al: Pembrolizumab as second-line therapy in patients with advanced
hepatocellular carcinoma in KEYNOTE-240: A randomized,
double-blind, phase III trial. J Clin Oncol. 38:193–202. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao M, Chen S, Li C, Du Y and Li P:
Neoadjuvant immune checkpoint inhibitors for resectable
hepatocellular carcinoma: A systematic review and meta-analysis.
Cancers (Basel). 15:6002023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelley RK, Abou-Alfa G, Bendell JC, Kim
TY, Borad MJ, Yong WP, Morse M, Kang YK, Rebelatto M, Makowsky M,
et al: Phase I/II study of durvalumab and tremelimumab in patients
with unresectable hepatocellular carcinoma (HCC): Phase I safety
and efficacy analyses. J Clin Oncol. 35:40732017. View Article : Google Scholar
|
|
33
|
Stein S, Pishvaian M, Lee MS, Lee KH,
Hernandez S, Kwan A, Liu B, Grossman W, Iizuka K and Ryoo BY:
Safety and clinical activity of 1L atezolizumab + bevacizumab in a
phase Ib study in hepatocellular carcinoma (HCC). J Clin Oncol.
36:40742018. View Article : Google Scholar
|
|
34
|
Sangro B, Gomez-Martin C, de la Mata M,
Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E,
Alfaro C, Sarobe P, et al: A clinical trial of CTLA-4 blockade with
tremelimumab in patients with hepatocellular carcinoma and chronic
hepatitis C. J Hepatol. 59:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaseb AO, Hasanov E, Cao HST, Xiao L,
Vauthey JN, Lee SS, Yavuz BG, Mohamed YI, Qayyum A, et al:
Perioperative nivolumab monotherapy versus nivolumab plus
ipilimumab in resectable hepatocellular carcinoma: a randomised,
open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 7:208–218.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WQ, Zhang Q, Tan L, Guan ZF, Tian F,
Tang HT, He K and Chen WQ: Postoperative adjuvant immunotherapy for
high-risk hepatocellular carcinoma patients. Front Oncol.
13:12899162023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen W, Hu S, Liu Z, Sun Y, Wu J, Shen S
and Peng Z: Adjuvant anti-PD-1 antibody for hepatocellular
carcinoma with high recurrence risks after hepatectomy. Hepatol
Int. 17:406–416. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M,
Lee HC, Yopp AC, Zhou J, Wang L, Wen X, et al: Atezolizumab plus
bevacizumab versus active surveillance in patients with resected or
ablated high-risk hepatocellular carcinoma (IMbrave050): A
randomised, open-label, multicentre, phase 3 trial. Lancet.
402:1835–1847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu X, Wang MD, Xu JH, Fan ZQ, Diao YK,
Chen Z, Jia HD, Liu FB, Zeng YY, Wang XM, et al: Adjuvant
immunotherapy improves recurrence-free and overall survival
following surgical resection for intermediate/advanced
hepatocellular carcinoma a multicenter propensity matching
analysis. Front Immunol. 14:13222332024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Wu PS, Liang XM, Chen K, Zhang GL,
Su QB, Huo RR, Xie RW, Huang S, Ma L and Zhong JH: Adjuvant immune
checkpoint inhibitors associated with higher recurrence-free
survival in postoperative hepatocellular carcinoma (PREVENT): A
prospective, multicentric cohort study. J Gastroenterol.
58:1043–1054. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang K, Xiang YJ, Yu HM, Cheng YQ, Liu ZH,
Qin YY, Shi J, Guo WX, Lu CD, Zheng YX, et al: Adjuvant sintilimab
in resected high-risk hepatocellular carcinoma: A randomized,
controlled, phase 2 trial. Nat Med. 30:708–715. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zimmer L, Livingstone E, Hassel JC, Fluck
M, Eigentler T, Loquai C, Haferkamp S, Gutzmer R, Meier F, Mohr P,
et al: Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy
versus placebo in patients with resected stage IV melanoma with no
evidence of disease (IMMUNED): A randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet. 395:1558–1568. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wakelee HA, Altorki N, Zhou C, Csőszi T,
Vynnychenko IO, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: IMpower010: Primary results of a phase III
global study of atezolizumab versus best supportive care after
adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung
cancer (NSCLC). J Clin Oncol. 39 (15_suppl):S85002021. View Article : Google Scholar
|
|
44
|
Tang F, Tie Y, Tu C and Wei X: Surgical
trauma-induced immunosuppression in cancer: Recent advances and the
potential therapies. Clin Transl Med. 10:199–223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jia R, Zhou M, Tuttle CSL and Maier AB:
Immune capacity determines outcome following surgery or trauma: A
systematic review and meta-analysis. Eur J Trauma Emerg Surg.
46:979–991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bakos O, Lawson C, Rouleau S and Tai LH:
Combining surgery and immunotherapy: Turning an immunosuppressive
effect into a therapeutic opportunity. J Immunother Cancer.
6:862018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng X, Zhang H, Hamad A, Huang H and
Tsung A: Surgery-mediated tumor-promoting effects on the immune
microenvironment. Semin Cancer Biol. 86:408–419. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blank CU, Rozeman EA, Fanchi LF, Sikorska
K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M,
Philips D, Broeks A, et al: Neoadjuvant versus adjuvant ipilimumab
plus nivolumab in macroscopic stage III melanoma. Nat Med.
24:1655–1661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garris CS, Arlauckas SP, Kohler RH, Trefny
MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M,
Gungabeesoon J, et al: Successful Anti-PD-1 cancer immunotherapy
requires T cell-dendritic cell crosstalk involving the cytokines
IFN-gamma and IL-12. Immunity. 49:1148–1161. e72018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu J, Blake SJ, Yong MC, Harjunpää H,
Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ and
Teng MW: Improved efficacy of neoadjuvant compared to adjuvant
immunotherapy to eradicate metastatic disease. Cancer Discov.
6:1382–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xia Y, Tang W, Qian X, Li X, Cheng F, Wang
K, Zhang F, Zhang C, Li D, Song J, et al: Efficacy and safety of
camrelizumab plus apatinib during the perioperative period in
resectable hepatocellular carcinoma: A single-arm, open label,
phase II clinical trial. J Immunother Cancer. 10:e0046562022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qiao ZY, Zhang ZJ, Lv ZC, Tong H, Xi ZF,
Wu HX, Chen XS, Xia L, Feng H, Zhang JJ and Xia Q: Neoadjuvant
programmed cell death 1 (PD-1) inhibitor treatment in patients with
hepatocellular carcinoma before liver transplant: A cohort study
and literature review. Front Immunol. 12:6534372021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Marron TU, Fiel MI, Hamon P, Fiaschi N,
Kim E, Ward SC, Zhao Z, Kim J, Kennedy P, Gunasekaran G, et al:
Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: A
single-arm, open-label, phase 2 trial. Lancet Gastroenterol
Hepatol. 7:219–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pinato DJ, Cortellini A, Sukumaran A, Cole
T, Pai M, Habib N, Spalding D, Sodergren MH, Martinez M, Dhillon T,
et al: PRIME-HCC: Phase Ib study of neoadjuvant ipilimumab and
nivolumab prior to liver resection for hepatocellular carcinoma.
BMC Cancer. 21:3012021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 29:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Topalian SL, Bhatia S, Amin A, Kudchadkar
RR, Sharfman WH, Lebbé C, Delord JP, Dunn LA, Shinohara MM,
Kulikauskas R, et al: Neoadjuvant nivolumab for patients with
resectable merkel cell carcinoma in the checkmate 358 trial. J Clin
Oncol. 38:2476–2487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Forde PM, Anagnostou V, Sun Z, Dahlberg
SE, Kindler HL, Niknafs N, Purcell T, Santana-Davila R, Dudek AZ,
Borghaei H, et al: Durvalumab with platinum-pemetrexed for
unresectable pleural mesothelioma: Survival, genomic and
immunologic analyses from the phase 2 PrE0505 trial. Nat Med.
27:1910–1920. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schmid P, Cortes JA, Dent R, Pusztai L,
McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, et al:
VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant
pembrolizumab+ chemotherapy vs. placebo+chemotherapy, followed by
adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann Oncol.
32:1198–1200. 2021. View Article : Google Scholar
|
|
60
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick S, Brahmer J, Swanson SJ,
et al: Abstract CT003: Nivolumab (NIVO)+ platinum-doublet
chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for
resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the
phase 3 CheckMate 816 trial. Cancer Res. 81 (13_Suppl):CT0032021.
View Article : Google Scholar
|
|
61
|
Ho WJ, Zhu Q, Durham J, Popovic A, Xavier
S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, et al:
Neoadjuvant cabozantinib and nivolumab converts locally advanced
HCC into resectable disease with enhanced antitumor immunity. Nat
Cancer. 2:891–903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huang AC, Orlowski RJ, Xu X, Mick R,
George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, et
al: A single dose of neoadjuvant PD-1 blockade predicts clinical
outcomes in resectable melanoma. Nat Med. 25:454–461. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Uppaluri R, Campbell KM, Egloff AM,
Zolkind P, Skidmore ZL, Nussenbaum B, Paniello RC, Rich JT, Jackson
R, Pipkorn P, et al: Neoadjuvant and adjuvant pembrolizumab in
resectable locally advanced, human papillomavirus-unrelated head
and neck cancer: A multicenter, phase II Trial. Clin Cancer Res.
26:5140–5152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gatteschi L, Iannopollo M and Gonfiotti A:
Neoadjuvant immunotherapy in resectable non-small cell lung cancer.
A narrative review. Life (Basel). 11:10362021.PubMed/NCBI
|
|
66
|
Hou S, Pan Z, Hao X, Hang Q and Ding Y:
Recent progress in the neoadjuvant treatment strategy for locally
advanced esophageal cancer. Cancers (Basel). 13:51622021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY
and Chen YF: Long-term outcome of resection of large hepatocellular
carcinoma. Br J Surg. 93:600–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sotiropoulos GC, Molmenti E, Lösch C,
Beckebaum S, Broelsch CE and Lang H: Meta-analysis of tumor
recurrence after liver transplantation for hepatocellular carcinoma
based on 1,198 cases. Eur J Med Res. 12:527–534. 2007.PubMed/NCBI
|
|
69
|
Rich NE, Parikh ND and Singal AG:
Hepatocellular carcinoma and liver transplantation: Changing
patterns and practices. Curr Treat Options Gastroenterol.
15:296–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kayashima H, Toshima T, Okano S, Taketomi
A, Harada N, Yamashita Y, Tomita Y, Shirabe K and Maehara Y:
Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic
cells is an effective strategy to control recurrence of murine
hepatocellular carcinoma in immunosuppressed mice. J Immunol.
185:698–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schwacha-Eipper B, Minciuna I, Banz V and
Dufour JF: Immunotherapy as a downstaging therapy for liver
transplantation. Hepatology. 72:1488–1490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Abdelrahim M, Esmail A, Umoru G, Westhart
K, Abudayyeh A, Saharia A and Ghobrial RM: Immunotherapy as a
neoadjuvant therapy for a patient with hepatocellular carcinoma in
the pretransplant setting: A case report. Curr Oncol. 29:4267–4273.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Finn RS, Ryoo B, Merle P, Kudo M,
Bouattour M, Lim HY, Breder VV, Edeline J, Chao Y, Ogasawara S, et
al: Results of KEYNOTE-240: Phase 3 study of pembrolizumab (pembro)
vs best supportive care (BSC) for second lien therapy in advanced
hepatocellular carcinoma (HCC). J Clin Oncol. 37:40042019.
View Article : Google Scholar
|
|
74
|
Gassmann D, Weiler S, Mertens JC, Reiner
CS, Vrugt B, Nägeli M, Mangana J, Müllhaupt B, Jenni F and
Misselwitz B: Liver allograft failure after nivolumab treatment-a
case report with systematic literature research. Transplant Direct.
4:e3762018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ho CM, Chen HL, Hu RH and Lee PH:
Harnessing immunotherapy for liver recipients with hepatocellular
carcinoma: A review from a transplant oncology perspective. Ther
Adv Med Oncol. 11:17588359198434632019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tabrizian P, Holzner M, Ajmera V, Kim AK,
Zhou K, Schnickel GT, Torosian K, Hoteit M, Marino R, Li M, et al:
Intention-to-treat outcomes of patients with hepatocellular
carcinoma receiving immunotherapy before liver transplant: The
multicenter VITALITY study. J Hepatol. 82:512–522. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Abdelrahim M, Esmail A, Divatia MK, Xu J,
Kodali S, Victor DW, Brombosz E, Connor AA, Saharia A, Elaileh A,
et al: Utilization of immunotherapy as a neoadjuvant therapy for
liver transplant recipients with hepatocellular carcinoma. J Clin
Med. 13:30682024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mazzaferro V, Citterio D, Bhoori S,
Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M,
Romagnoli R, Antonelli B, et al: Liver transplantation in
hepatocellular carcinoma after tumour downstaging (XXL): A
randomised, controlled, phase 2b/3 trial. Lancet Oncol. 21:947–956.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lu X, Zhu Q, Cai J, Yang Z, Gu G, Pang L,
Su M, Zhang F, Lin H, Wu W, et al: Pretransplant immunotherapy
increases acute rejection yet improves survival outcome of HCC
patients with MVI post-liver transplantation. Cancer Immunol
Immunother. 74:182024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu Y, Yan Y, Liu D, Tang J, Zhang H, Liu
X, Wu Y and Cui X: Risk of transplant rejection associated with
ICIs prior to liver transplantation in HCC: A multicenter
retrospective study. Int Immunopharmacol. 143:1134002024.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Rezaee-Zavareh MS, Yeo YH, Wang T, Guo Z,
Tabrizian P, Ward SC, Barakat F, Hassanein TI, Dave S, Ajmera V, et
al: Impact of pre-transplant immune checkpoint inhibitor use on
post-transplant outcomes in HCC: A systematic review and individual
patient data meta-analysis. J Hepatol. 82:107–119. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tabrizian P, Marino R, Bhoori S,
Zeitlhoefler M, Mehta N, Banz V, Gruttadauria S, Iavarone M,
Mazzarelli C, Simonotti N, et al: Neoadjuvant atezolizumab plus
bevacizumab prior liver transplantation for hepatocellular
carcinoma. JHEP Rep. 7:1012462025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kuo FC, Chen CY, Lin NC, Liu C, Hsia CY
and Loong CC: Optimizing the safe washout period for liver
transplantation following immune checkpoint inhibitors with
atezolizumab, nivolumab, or pembrolizumab. Transplant Proc.
55:878–883. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kumar V, Shinagare AB, Rennke HG, Ghai S,
Lorch JH, Ott PA and Rahma OE: The safety and efficacy of
checkpoint inhibitors in transplant recipients: A case series and
systematic review of literature. Oncologist. 25:505–514. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Toso C, Meeberg G, Hernandez-Alejandro R,
Dufour JF, Marotta P, Majno P and Kneteman N: Total tumor volume
and alpha-fetoprotein for selection of transplant candidates with
hepatocellular carcinoma: A prospective validation. Hepatology.
62:158–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Laschtowitz A, Roderburg C, Tacke F and
Mohr R: Preoperative immunotherapy in hepatocellular carcinoma:
Current state of the art. J Hepatocell Carcinoma. 10:181–191. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Singal AG, Yarchoan M, Yopp A, Sapisochin
G, Pinato DJ and Pillai A: Neoadjuvant and adjuvant systemic
therapy in HCC: Current status and the future. Hepatol Commun.
8:e04302024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Elias D, Farace F, Triebel F, Hattchouel
JM, Pignon JP, Lecesne A, Rougier P, Lasser P, Duvillard P and
Escudier B: Phase I–II randomized study on prehepatectomy
recombinant interleukin-2 immunotherapy in patients with metastatic
carcinoma of the colon and rectum. J Am Coll Surg. 181:303–310.
1995.PubMed/NCBI
|
|
89
|
Nakajima M, Hazama S, Tokumitsu Y, Shindo
Y, Matsui H, Matsukuma S, Nakagami Y, Tamada K, Udaka K, Sakamoto
M, et al: Phase I study of a novel therapeutic vaccine as
perioperative treatment for patients with surgically resectable
hepatocellular carcinoma: The YCP02 trial. Hepatol Res. 53:649–660.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kaseb AO, Vence L, Blando J, Yadav SS,
Ikoma N, Pestana RC, Vauthey JN, Allison JP and Sharma P:
Immunologic correlates of pathologic complete response to
preoperative immunotherapy in hepatocellular carcinoma. Cancer
Immunol Res. 7:1390–1395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Seckler F, Doussot A, Colpart P, Turco C,
Calame P, Aubin F, Algros MP, Borg C, Nardin C and Heyd B:
Preoperative immunotherapy for resectable hepatocellular carcinoma:
Toward a paradigm shift? J Hepatol. 73:1588–1590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liao H, Zhang Z, Chen J, Liao M, Xu L, Wu
Z, Yuan K, Song B and Zeng Y: Preoperative radiomic approach to
evaluate tumor-infiltrating CD8(+) T cells in hepatocellular
carcinoma patients using contrast-enhanced computed tomography. Ann
Surg Oncol. 26:4537–4547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tian C, Yu Y, Wang Y, Yang L, Tang Y, Yu
C, Feng G, Zheng D and Wang X: Neoadjuvant immune checkpoint
inhibitors in hepatocellular carcinoma: A meta-analysis and
systematic review. Front Immunol. 15:13528732024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
D'Alessio A, Stefanini B, Blanter J,
Adegbite B, Crowley F, Yip V, Slater S, Fulgenzi CAM, Celsa C,
Manfredi GF, et al: Pathological response following neoadjuvant
immune checkpoint inhibitors in patients with hepatocellular
carcinoma: A cross-trial, patient-level analysis. Lancet Oncol.
25:1465–1475. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen X, Zhang Y, Zhang N, Ge Y and Jia W:
Lenvatinib combined nivolumab injection followed by extended right
hepatectomy is a feasible treatment for patients with massive
hepatocellular carcinoma: A case report. Onco Targets Ther.
12:7355–7359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ho WJ, Sharma G, Zhu Q, Stein-O'Brien G,
Durham J, Anders R, Popovic A, Mo G, Kamel I, Weiss M, et al:
Integrated immunological analysis of a successful conversion of
locally advanced hepatocellular carcinoma to resectability with
neoadjuvant therapy. J Immunother Cancer. 8:e0009322020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nakazawa M, Fang M, Vong T, Zorzi J,
Griffith P, Anders RA, Oshima K, Kim AK, Laurin J, Lafaro KJ, et
al: Impact of neoadjuvant immunotherapy on recurrence-free survival
in patients with high-risk localized HCC. Cancer Res Commun.
4:2123–2132. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Han YH, Bo JQ and Liu LX: Neoadjuvant
immunotherapy for resectable hepatocellular carcinoma: A systematic
review and meta-analysis. Eur Rev Med Pharmacol Sci. 27:7134–7147.
2023.PubMed/NCBI
|
|
99
|
European Association for the Study of the
Liver, . EASL clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud
A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al:
Evaluation of immune-related response criteria and RECIST v1.1 in
patients with advanced melanoma treated with pembrolizumab. J Clin
Oncol. 34:1510–1517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lencioni R, Montal R, Torres F, Park JW,
Decaens T, Raoul JL, Kudo M, Chang C, Ríos J, Boige V, et al:
Objective response by mRECIST as a predictor and potential
surrogate end-point of overall survival in advanced HCC. J Hepatol.
66:1166–1172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vernuccio F, Godfrey D, Meyer M,
Williamson HV, Salama JK, Niedzwiecki D, Stephens SJ, Ronald J,
Palta M and Marin D: Local tumor control and patient outcome using
stereotactic body radiation therapy for hepatocellular carcinoma:
iRECIST as a potential substitute for traditional criteria. AJR Am
J Roentgenol. 213:1232–1239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cheung TT, Ho DWH, Lyu SX, Zhang Q, Tsui
YM, Yu TC, Sze KM, Lee JM, Lau VW, Chu EY, et al: Multimodal
integrative genomics and pathology analyses in neoadjuvant
nivolumab treatment for intermediate and locally advanced
hepatocellular carcinoma. Liver Cancer. 13:70–88. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gani RA, Teressa M, Budiman RA, Kalista KF
and Lesmana CRA: Meta analysis of radiofrequency ablation versus
surgical resection in small and large nodule of hepatocellular
carcinoma. HPB (Oxford). 26:1216–1228. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Han JW and Yoon SK: Immune responses
following locoregional treatment for hepatocellular carcinoma:
Possible roles of adjuvant immunotherapy. Pharmaceutics.
13:13872021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xi M, Yang Z, Hu L, Fu Y, Hu D, Zhou Z,
Liu M, Zhao J, Shen J, Li Q, et al: Radiofrequency ablation versus
stereotactic body radiotherapy for recurrent small hepatocellular
carcinoma: A randomized, open-label, controlled trial. J Clin
Oncol. 43:1073–1082. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Campelo SN, Huang PH, Buie CR and Davalos
RV: Recent advancements in electroporation technologies: From bench
to clinic. Annu Rev Biomed Eng. 25:77–100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kumar P, Nagarajan A and Uchil PD:
Electroporation. Cold Spring Harb Protoc.
2019.10.1101/pdb.top096271. 2019. View Article : Google Scholar
|
|
110
|
Tasu JP, Tougeron D and Rols MP:
Irreversible electroporation and electrochemotherapy in oncology:
State of the art. Diagn Interv Imaging. 103:499–509. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bernelin-Cottet C, Urien C, McCaffrey J,
Collins D, Donadei A, McDaid D, Jakob V, Barnier-Quer C, Collin N,
Bouguyon E, et al: Electroporation of a nanoparticle-associated DNA
vaccine induces higher inflammation and immunity compared to its
delivery with microneedle patches in pigs. J Control Release.
308:14–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rizzo A and Brandi G: Neoadjuvant therapy
for cholangiocarcinoma: A comprehensive literature review. Cancer
Treat Res Commun. 27:1003542021.PubMed/NCBI
|
|
113
|
Zhu SG, Li HB, Dai TX, Li H and Wang GY:
Successful treatment of stage IIIB intrahepatic cholangiocarcinoma
using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: A
case report. World J Clin Cases. 10:9743–9749. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Abudalou M, Vega EA, Kondratiev S, Conrad
C and Kozyreva O: Complete pathological response to neoadjuvant
chemoimmunotherapy in a patient with metastatic intrahepatic
cholangiocarcinoma with high tumor mutational burden. Cureus.
13:e201872021.PubMed/NCBI
|
|
115
|
Oh DY, Lee KH, Lee DW, Yoon J, Kim TY,
Bang JH, Nam AR, Oh KS, Kim JM, Lee Y, et al: Gemcitabine and
cisplatin plus durvalumab with or without tremelimumab in
chemotherapy-naive patients with advanced biliary tract cancer: An
open-label, single-centre, phase 2 study. Lancet Gastroenterol
Hepatol. 7:522–532. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou J, Sun H, Wang Z, Cong W, Zeng M,
Zhou W, Bie P, Liu L, Wen T, Kuang M, et al: Guidelines for the
diagnosis and treatment of hepatocellular carcinoma (2019 Edition).
Liver Cancer. 9:682–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Marie PK, Haymaker C, Parra ER, Kim YU,
Lazcano R, Gite S, Lorenzini D, Wistuba II, Tidwell RSS, Song X, et
al: Pilot clinical trial of perioperative durvalumab and
tremelimumab in the treatment of resectable colorectal cancer liver
metastases. Clin Cancer Res. 27:3039–3049. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang BC, Kuang BH, Lin GH and Fu C:
Durvalumab and pembrolizumab in advanced biliary tract cancer: A
reconstructed patient-level mimic head-to-head comparative
analysis. Front Immunol. 15:14974152024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Piha-Paul SA, Oh DY, Ueno M, Malka D,
Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, et
al: Efficacy and safety of pembrolizumab for the treatment of
advanced biliary cancer: Results from the KEYNOTE-158 and
KEYNOTE-028 studies. Int J Cancer. 147:2190–2198. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Guidelines Working Committee of Chinese
Society of Clinical Oncology, . Guideline of Chinese Society of
Clinical Oncology (CSCO) Biliary Tract Cancer. People's Medical
Publishing House. (Beijing, China). 2023.(In Chinese).
|
|
121
|
Shi GM, Huang XY, Wu D, Sun HC, Liang F,
Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, et al: Toripalimab combined
with lenvatinib and GEMOX is a promising regimen as first-line
treatment for advanced intrahepatic cholangiocarcinoma: A
single-center, single-arm, phase 2 study. Signal Transduct Target
Ther. 8:1062023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen X, Wu X, Wu H, Gu Y, Shao Y, Shao Q,
Zhu F, Li X, Qian X, Hu J, et al: Camrelizumab plus gemcitabine and
oxaliplatin (GEMOX) in patients with advanced biliary tract cancer:
A single-arm, open-label, phase II trial. J Immunother Cancer.
8:e0012402020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen X, Qin S, Gu S, Ren Z, Chen Z, Xiong
J, Liu Y, Meng Z, Zhang X, Wang L, et al: Camrelizumab plus
oxaliplatin-based chemotherapy as first-line therapy for advanced
biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer.
149:1944–1954. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Oh DY, He AR, Qin S, Chen LT, Okusaka T,
Vogel A, Kim JW, Suksombooncharoen T, Lee MA, Kitano M, et al:
Durvalumab plus Gemcitabine and cisplatin in advanced biliary tract
cancer. NEJM Evid. 1:EVIDoa22000152022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pinter M, Scheiner B and Pinato DJ: Immune
checkpoint inhibitors in hepatocellular carcinoma: Emerging
challenges in clinical practice. Lancet Gastroenterol Hepatol.
8:760–770. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Mayoral M, Castañer E, Gallardo X, Andreu
M, Dalmau E and Garcia Y: Tumor pseudoprogression during nivolumab
immunotherapy for lung cancer. Radiología (Engl Ed). 61:498–505.
2019.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Billan S, Kaidar-Person O and Gil Z:
Treatment after progression in the era of immunotherapy. Lancet
Oncol. 21:e463–e476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Peng X, Gong C, Zhang W and Zhou A:
Advanced development of biomarkers for immunotherapy in
hepatocellular carcinoma. Front Oncol. 12:10910882023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Vignali DAA, Collison LW and Workman CJ:
How regulatory T cells work. Nat Rev Immunol. 8:523–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wen J, Zhang X, Wong CC, Zhang Y, Pan Y,
Zhou Y, Cheung AH, Liu Y, Ji F, Kang X, et al: Targeting squalene
epoxidase restores anti-PD-1 efficacy in metabolic
dysfunction-associated steatohepatitis-induced hepatocellular
carcinoma. Gut. 73:2023–2036. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Goh CC, Roggerson KM, Lee HC, Golden-Mason
L, Rosen HR and Hahn YS: Hepatitis C virus-induced myeloid-derived
suppressor cells suppress NK cell IFN-γ production by altering
cellular metabolism via arginase-1. J Immunol. 196:2283–2292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hoechst B, Ormandy LA, Ballmaier M, Lehner
F, Krüger C, Manns MP, Greten TF and Korangy F: A new population of
myeloid-derived suppressor cells in hepatocellular carcinoma
patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology.
135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cheng JN, Yuan YX, Zhu B and Jia Q:
Myeloid-derived suppressor cells: A Multifaceted accomplice in
tumor progression. Front Cell Dev Biol. 9:7408272021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hudspeth K, Donadon M, Cimino M, Pontarini
E, Tentorio P, Preti M, Hong M, Bertoletti A, Bicciato S,
Invernizzi P, et al: Human liver-resident CD56bright/CD16neg NK
cells are retained within hepatic sinusoids via the engagement of
CCR5 and CXCR6 pathways. J Autoimmun. 66:40–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sanjabi S, Oh SA and Li MO: Regulation of
the immune response by TGF-β: From conception to autoimmunity and
infection. Cold Spring Harb Perspect Biol. 9:a0222362017.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhao J, Zhang Y, Qin S, Zou B and Wang Y:
Hepatitis B virus reactivation in cancer patients undergoing immune
checkpoint inhibitors therapy: A systematic review. J Cancer.
13:3539–3553. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Burns EA, Muhsen I, Anand K, Xu J, Umoru
G, Arain AN and Abdelrahim M: Hepatitis B virus reactivation in
cancer patients treated with immune checkpoint inhibitors. J
Immunother. 44:132–139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Xia Z, Zhang J, Chen W, Zhou H, Du D, Zhu
K, Chen H, Meng J and Yang J: Hepatitis B reactivation in cancer
patients receiving immune checkpoint inhibitors: A systematic
review and meta-analysis. Infect Dis Poverty. 12:872023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lee PC, Chao Y, Chen MH, Lan KH, Lee IC,
Hou MC and Huang YH: Risk of HBV reactivation in patients with
immune checkpoint inhibitor-treated unresectable hepatocellular
carcinoma. J Immunother Cancer. 8:e0010722020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yibirin M, Mustafayev K, Hosry J, Pundhir
P, Klingen J, Guevara EY, Granwehr BP, Kaseb A, Naing A, Patel S,
et al: Immune checkpoint inhibitors suppress hepatitis C virus
replication in infected patients with solid tumors. Am J
Gastroenterol. 118:1609–1617. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Pinto E, Meneghel P, Farinati F, Russo FP,
Pelizzaro F and Gambato M: Efficacy of immunotherapy in
hepatocellular carcinoma: Does liver disease etiology have a role?
Dig Liver Dis. 56:579–588. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Guo Z, Liu Y, Ling Q, Xu L, Wang T, Zhu J,
Lin Y, Lu X, Qu W, Zhang F, et al: Pretransplant use of immune
checkpoint inhibitors for hepatocellular carcinoma: A multicenter,
retrospective cohort study. Am J Transplant. 24:1837–1856. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Sedano R, Cabrera D, Jiménez A, Ma C,
Jairath V, Arrese M and Arab JP: Immunotherapy for cancer: Common
gastrointestinal, liver, and pancreatic side effects and their
management. Am J Gastroenterol. 117:1917–1932. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
De Martin E, Michot JM, Papouin B,
Champiat S, Mateus C, Lambotte O, Roche B, Antonini TM, Coilly A,
Laghouati S, et al: Characterization of liver injury induced by
cancer immunotherapy using immune checkpoint inhibitors. J Hepatol.
68:1181–1190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu Z, Zhu Y, Xie H and Zou Z:
Immune-mediated hepatitis induced by immune checkpoint inhibitors:
Current updates and future perspectives. Front Pharmacol.
13:10774682023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zen Y and Yeh MM: Checkpoint
inhibitor-induced liver injury: A novel form of liver disease
emerging in the era of cancer immunotherapy. Semin Diagn Pathol.
36:434–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhang N, Li Z, Liu Y, Shi X, Shi D, Li Y,
Si X, Xun Z, Shao J, Zhao H and Wang H: Management and treatment of
severe immune-related hepatotoxicity based on clinical and
pathological characteristics. Hepatol Int. 18:1770–1780. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Chaft JE, Oezkan F, Kris MG, Bunn PA,
Wistuba II, Kwiatkowski DJ, Owen DH, Tang Y, Johnson BE, Lee JM, et
al: Neoadjuvant atezolizumab for resectable non-small cell lung
cancer: An open-label, single-arm phase II trial. Nat Med.
28:2155–2161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Qin R, Jin T and Xu F: Biomarkers
predicting the efficacy of immune checkpoint inhibitors in
hepatocellular carcinoma. Front Immunol. 14:13260972023. View Article : Google Scholar : PubMed/NCBI
|