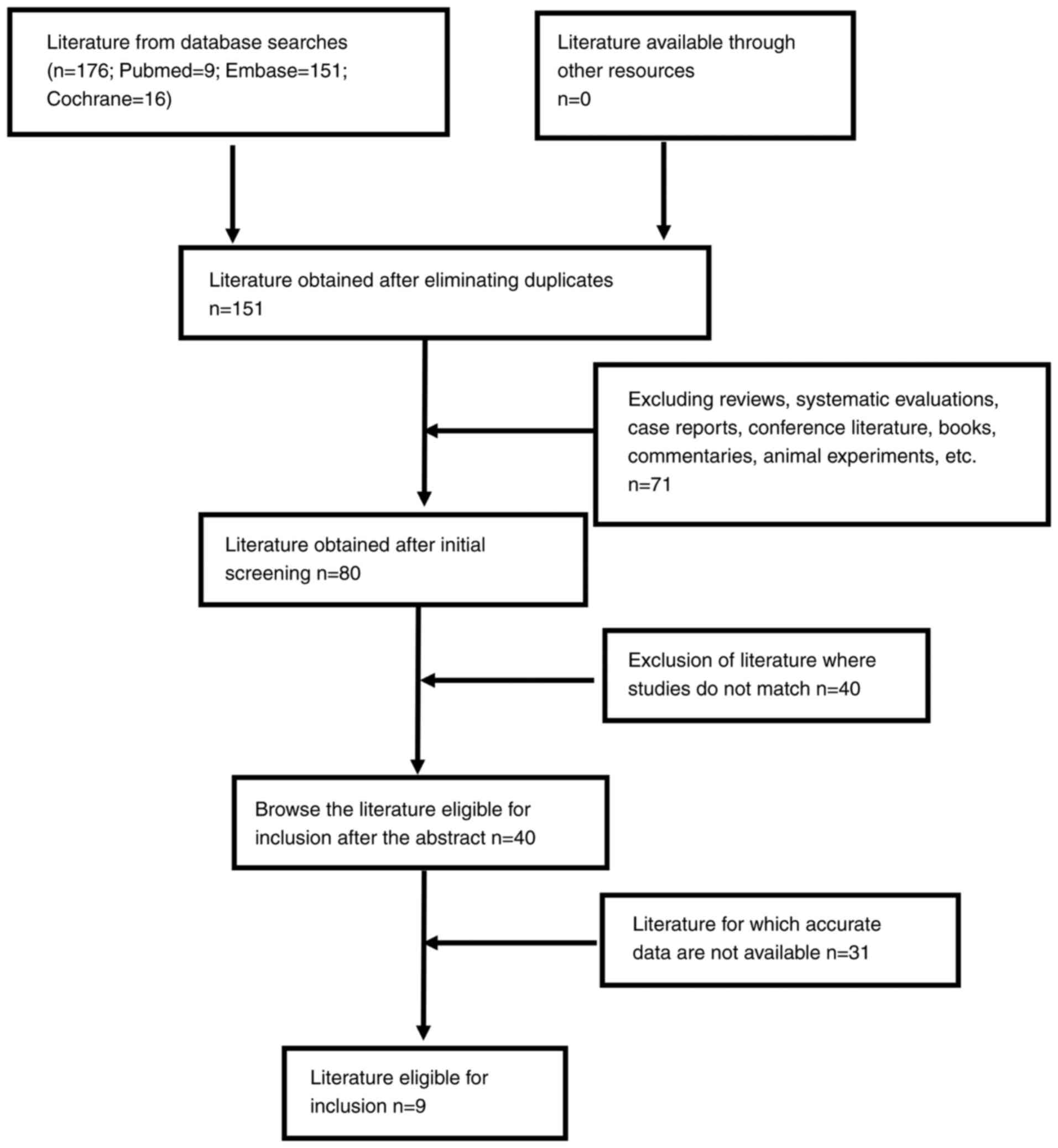
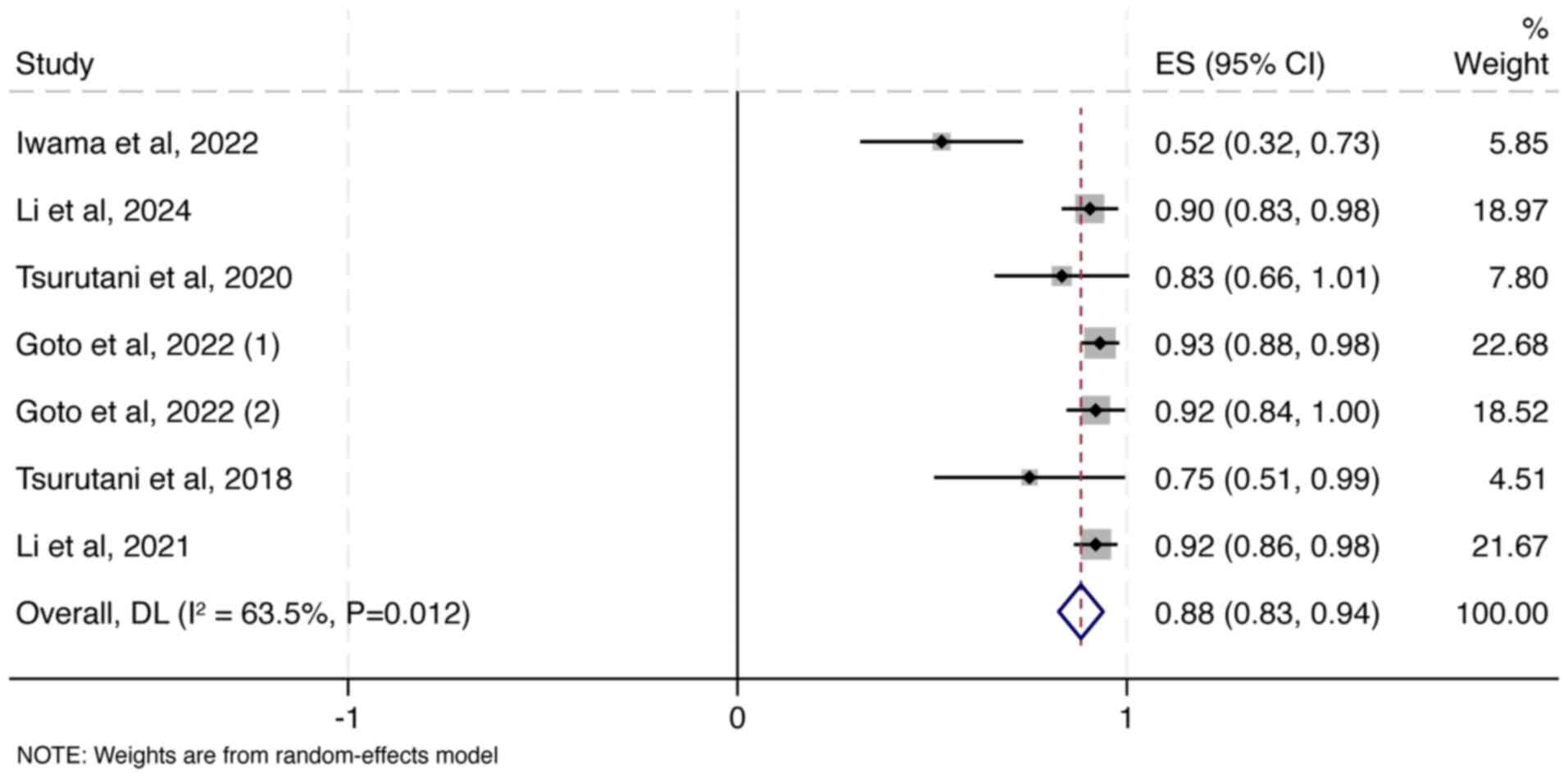
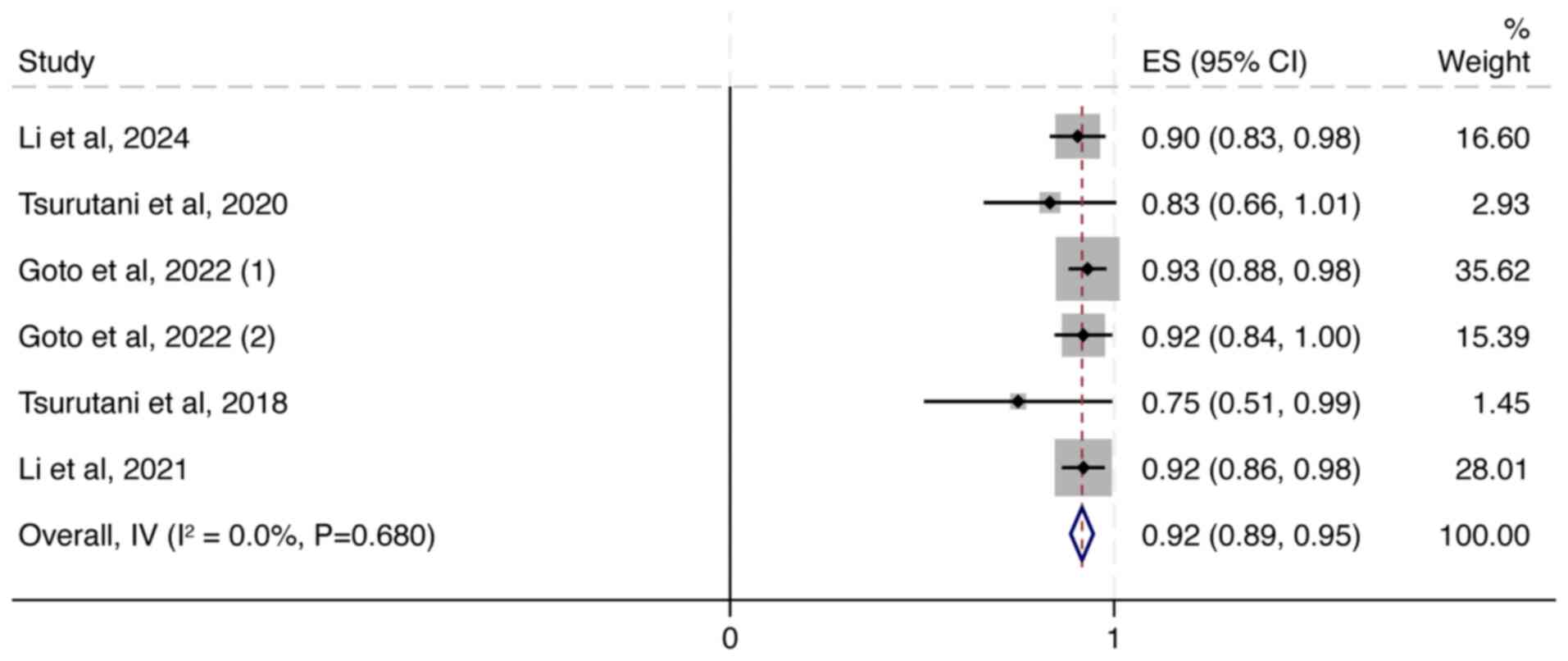
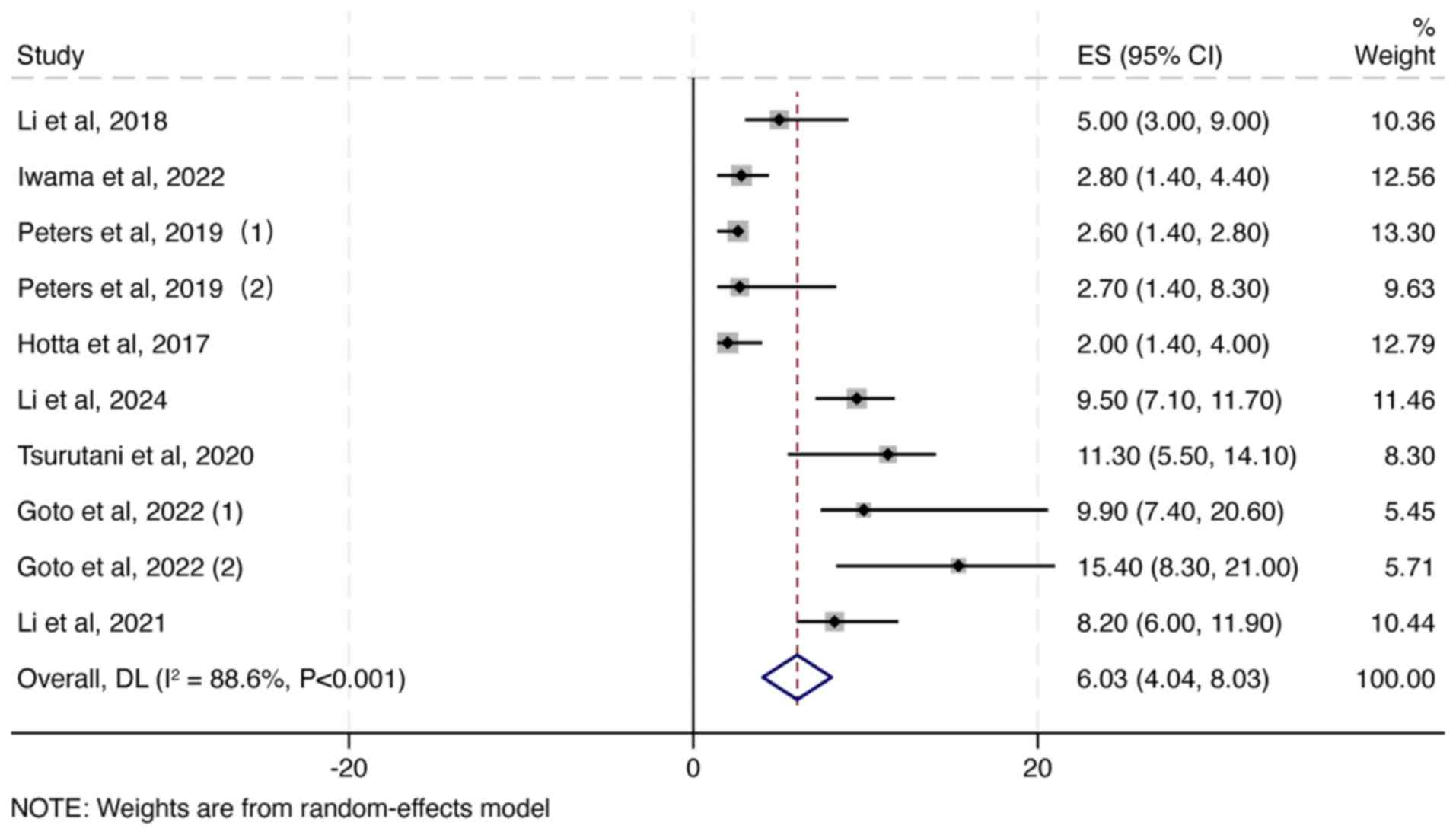
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Zhou L, Lu Y, Liu W, Wang S, Wang L, Zheng
P, Zi G, Liu H, Liu W and Wei S: Drug conjugates for the treatment
of lung cancer: From drug discovery to clinical practice. Exp
Hematol Oncol. 13:262024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104.
2020.PubMed/NCBI
|
|
4
|
Kroeze SGC, Pavic M, Stellamans K, Lievens
Y, Becherini C, Scorsetti M, Alongi F, Ricardi U, Jereczek-Fossa
BA, Westhoff P, et al: Metastases-directed stereotactic body
radiotherapy in combination with targeted therapy or immunotherapy:
Systematic review and consensus recommendations by the EORTC-ESTRO
OligoCare consortium. Lancet Oncol. 24:e121–e132. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi K, Wang G, Pei J, Zhang J, Wang J,
Ouyang L, Wang Y and Li W: Emerging strategies to overcome
resistance to third-generation EGFR inhibitors. J Hematol Oncol.
15:942022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conilh L, Sadilkova L, Viricel W and
Dumontet C: Payload diversification: A key step in the development
of antibody-drug conjugates. J Hematol Oncol. 16:32023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phillips GD, Li G, Dugge DL, Crocker LM,
Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, et
al: Targeting HER2-positive breast cancer with trastuzumab-DM1, an
antibody-cytotoxic drug conjugate. Cancer Res. 68:9280–9290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keam SJ: Trastuzumab deruxtecan: First
approval. Drugs. 80:501–508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YH, Lian W, Zhao X, Qi W, Xu J, Xiao
L, Qing Y, Xue T and Wang J: A first in-human study of A166 in
patients with locally advanced/metastatic solid tumors which are
HER2-positive or HER2-amplified who did not respond or stopped
responding to approved therapies. J Clin Oncol. 38:10492020.
View Article : Google Scholar
|
|
11
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (MINORS): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JPT, Thomas J, Chandler J,
Cumpston M, Li T, Page MJ and Welch VA: Cochrane handbook for
systematic reviews of interventions version 6.3 (updated February
2022). Cochrane. 2022.Available from:. https://training.cochrane.org/handbook
|
|
13
|
Goto K, Goto Y, Kubo T, Ninomiya K, Kim
SW, Planchard D, Ahn MJ, Smit EF, de Langen AJ, Pérol M, et al:
Trastuzumab deruxtecan in patients with HER2-mutant metastatic
non-small-cell lung cancer: Primary results from the randomized,
phase II DESTINY-Lung02 trial. J Clin Oncol. 41:4852–4863. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li BT, Shen R, Buonocore D, Olah ZT, Ni A,
Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T, et al:
Ado-Trastuzumab emtansine for patients with HER2-mutant lung
cancers: Results from a phase II basket trial. J Clin Oncol.
36:2532–2537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwama E, Zenke Y, Sugawara S, Daga H,
Morise M, Yanagitani N, Sakamoto T, Murakami H, Kishimoto J,
Matsumoto S, et al: Trastuzumab emtansine for patients with
non-small cell lung cancer positive for human epidermal growth
factor receptor 2 exon-20 insertion mutations. Eur J Cancer.
162:99–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peters S, Stahel R, Bubendorf L, Bonomi P,
Villegas A, Kowalski DM, Baik CS, Isla D, Carpeno JC, Garrido P, et
al: Trastuzumab emtansine (T-DM1) in patients with previously
treated HER2-overexpressing metastatic non-small cell lung cancer:
Efficacy, safety, and biomarkers. Clin Cancer Res. 25:64–72. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hotta K, Aoe K, Kozuki T, Ohashi K,
Ninomiya K, Ichihara E, Kubo T, Ninomiya T, Chikamori K, Harada D,
et al: A phase II study of trastuzumab emtansine in HER2-positive
non-small cell lung cancer. J Thorac Oncol. 13:273–279. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Song Z, Hong W, Yang N, Wang Y, Jian
H, Liang Z, Hu S, Peng M, Yu Y, et al: SHR-A1811 (antibody-drug
conjugate) in advanced HER2-mutant non-small cell lung cancer: A
multicenter, open-label, phase 1/2 study. Signal Transduct Target
Ther. 9:1822024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsurutani J, Iwata H, Krop I, Jänne PA,
Doi T, Takahashi S, Park H, Redfern C, Tamura K, Wise-Draper TM, et
al: Targeting her2 with trastuzumab deruxtecan: A dose-expansion,
phase i study in multiple advanced solid tumors. Cancer Discov.
10:688–701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsurutani J, Park H, Doi T, Modi S,
Takahashi S, Nakagawa K, Krop I, Waqar S, Yoh K, Li B, et al:
Updated results of phase 1 study of DS-8201a in HER2-expressing or
-mutated advanced non-small-cell lung cancer. J Thorac Oncol.
13:S3242018. View Article : Google Scholar
|
|
21
|
Li BT, Smit EF, Goto Y, Nakagawa K,
Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E,
et al: Trastuzumab deruxtecan in HER2-mutant non-small-cell lung
cancer. N Engl J Med. 386:241–251. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abuhelwa Z, Alloghbi A and Nagasaka M: A
comprehensive review on antibody-drug conjugates (ADCs) in the
treatment landscape of non-small cell lung cancer (NSCLC). Cancer
Treat Rev. 106:1024232022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He J, Yu SF, Yee S, Kaur S and Xu K:
Characterization of in vivo biotransformations for trastuzumab
emtansine by high-resolution accurate-mass mass spectrometry. MAbs.
10:960–967. 2018.PubMed/NCBI
|
|
24
|
Ogitani Y, Hagihara K, Oitate M, Naito H
and Agatsuma T: Bystander killing effect of DS-8201a, a novel
anti-human epidermal growth factor receptor 2 antibody-drug
conjugate, in tumors with human epidermal growth factor receptor 2
heterogeneity. Cancer Sci. 107:1039–1046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu HX, Zhuo KQ and Wang K: Efficacy of
targeted therapy in patients with HER2-positive non-small cell lung
cancer: A systematic review and meta-analysis. Br J Clin Pharmacol.
88:2019–2034. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwata TN, Ishii C, Ishida S, Ogitani Y,
Wada T and Agatsuma T: A HER2-targeting antibody-drug conjugate,
trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a
mouse model. Mol Cancer Ther. 17:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Liu K, Wang K and Zhu H:
Treatment-related adverse events of antibody-drug conjugates in
clinical trials: A systematic review and meta-analysis. Cancer.
129:283–295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colombo R and Rich JR: The therapeutic
window of antibody drug conjugates: A dogma in need of revision.
Cancer Cell. 40:1255–1263. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masters JC, Nickens DJ, Xuan D, Shazer RL
and Amantea M: Clinical toxicity of antibody drug conjugates: A
meta-analysis of payloads. Invest New Drugs. 36:121–135. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stepan LP, Trueblood ES, Hale K, Babcook
J, Borges L and Sutherland CL: Expression of Trop2 cell surface
glycoprotein in normal and tumor tissues: Potential implications as
a cancer therapeutic target. J Histochem Cytochem. 59:701–710.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahalingaiah PK, Ciurlionis R, Durbin KR,
Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ
and Van Vleet TR: Potential mechanisms of target-independent uptake
and toxicity of antibody-drug conjugates. Pharmacol Ther.
200:110–125. 2019. View Article : Google Scholar : PubMed/NCBI
|