Introduction
Wilms tumor (WT), also known as nephroblastoma, is
one of the most common malignant renal tumors in children, with an
incidence rate of ~7.1 cases per 100,000 children in the US
(1). In Asian populations, the
incidence is slightly lower, with China reporting an incidence rate
of approximately 2.5 cases per million children (2), accounting for >90% of all pediatric
renal malignancies (3). It may
affect a single kidney or occur bilaterally (4). Based on histological classification,
WT is divided into favorable histology (FH) WT, which comprises
~90% of cases, and anaplastic WT. The typical age of onset is
between 2–3 years, although it can occur in infants and in children
aged >10 years old (5). With
advances in imaging technologies and the adoption of
multidisciplinary treatment strategies, the survival rate of WT has
markedly improved, with a 5-year survival rate of >90% (6). However, a subset of patients still
experiences recurrence or metastasis after radical surgery, leading
to a poor prognosis and compromised long-term survival and quality
of life (7–9). Therefore, identifying prognostic
factors and establishing effective prediction models is essential
to improving treatment outcomes and long-term prognosis in
pediatric WT.
Over the past decades, research on WT has focused
primarily on genetics, molecular biology and clinical management.
Studies have reported that WT is closely associated with aberrant
expression of genes such as WT1, catenin β1 and WNT signaling
components (10,11). In addition, clinical stage, tumor
size, histological subtype and preoperative chemotherapy have been
identified as important prognostic factors (12–15).
Although numerous studies have explored the impact of these
factors, the findings remain inconsistent due to differences in
study populations, sample sizes and methodologies (16,17).
Therefore, further research is needed to clarify the prognostic
significance of these variables across diverse populations and to
develop multifactorial prognostic models that can guide clinical
decision-making more accurately and comprehensively.
The present study retrospectively analyzed the
clinical data of 180 pediatric patients with WT who underwent
radical nephrectomy at the Department of Surgical Oncology, Baoding
Branch of Beijing Children's Hospital (Baoding, China). The study
aimed to identify prognostic factors and to construct a predictive
model based on these variables, with the ultimate goal of providing
clinicians with valuable evidence to support more precise treatment
strategies and improve survival outcomes and quality of life for
children with WT.
Materials and methods
Study population
Clinical data were retrospectively collected from
pediatric patients with WT who underwent radical nephrectomy at
Baoding Branch of Beijing Children's Hospital between January 2015
and January 2019. Data sources included electronic medical records
and handwritten treatment documentation. To ensure data integrity
and consistency, all handwritten treatment records were
independently reviewed and cross-checked against corresponding
entries in the electronic medical record system by two trained
clinical researchers. Discrepancies, if identified, were resolved
through consensus review and, when necessary, verification with the
original source documents or consultation with treating physicians.
For cases with missing or incomplete data, predefined exclusion
criteria were applied to maintain the accuracy and completeness of
the dataset; patients with notable missing data were excluded from
the final analysis. The inclusion criteria were as follows: i) Age
of <18 years; ii) histopathology-confirmed diagnosis of WT; iii)
radical nephrectomy performed; and iv) complete clinical data
available. The exclusion criteria included the following: i) No
pathological diagnosis or unclear pathology; ii) presence of a
second primary malignancy or hematologic disease; iii) notable
comorbid injury to other organs; and iv) incomplete medical
records.
The present study was approved by the Ethics
Committee of the Baoding Branch of Beijing Children's Hospital
(approval no. H-BDETKJ-SOP006-03-A/2; project no. 2341ZF378).
Informed consent was obtained from the guardians of all patients
prior to treatment.
Postoperative adjuvant therapy
Diagnosis, staging and treatment for all patients
were performed in accordance with the guidelines of the Children's
Oncology Group (COG)-Renal Tumor Committee and national protocols
developed by the Chinese Children Cancer Group (CCCG), including
CCCG-WT-2003 (18), CCCG-WT-2009
(19) and CCCG-WT-2015 (20). Stages I–II were defined as early
stage and stages III–IV as advanced stage. All patients received
standard postoperative therapy. The early-stage group was treated
with the vincristine + actinomycin D (EE4A) regimen. Vincristine
was administered via intravenous bolus on day 1 only, at a dosage
of 0.025 mg/kg for patients aged <1 year, 0.05 mg/kg for those
aged 1–3 years, and 1.5 mg/m2 (maximum 2.0 mg) for those
aged >3 years. Actinomycin D was administered via intravenous
infusion on day 1 only, at a dosage of 0.023 mg/kg for patients
aged <1 year and 0.045 mg/kg (maximum 2.3 mg) for those aged
>1 year. The total treatment duration was 19 weeks. The
advanced-stage group received the vincristine + actinomycin D +
doxorubicin (DD4A) regimen for 25 weeks, combined with radiotherapy
(180 cGy/day; 5 days/week). Vincristine was administered via
intravenous bolus on day 1 only, at a dosage of 0.025 mg/kg for
patients aged <1 year, 0.05 mg/kg for those aged 1–3 years and
1.5 mg/m2 (maximum 2.0 mg) for those aged >3 years.
Actinomycin D was administered via intravenous infusion on day 1
only, at a dosage of 0.023 mg/kg for patients aged <1 year and
0.045 mg/kg (maximum 2.3 mg) for those aged >1 year. Doxorubicin
was administered via intravenous infusion on day 1 only, at a
dosage of 1 mg/kg for patients aged ≤1 year and 30 mg/m2
for those aged >1 year.
Follow-up and outcome evaluation
Patients who completed WT treatment were followed up
every 3 months during the first and second postoperative years,
every 4 months in the third year, every 6 months in the fourth
year, and once in the fifth year. Follow-up frequency was further
individualized based on clinical stage at diagnosis. Specifically,
patients with advanced-stage disease (stage III–IV) underwent more
intensive monitoring during the first 2 postoperative years,
including clinical evaluations and imaging every 2 months during
the first year, and every 3 months during the second year. This
stage-adapted surveillance strategy was intended to facilitate
early detection of recurrence or metastasis. Follow-up examinations
included abdominal ultrasonography of the kidney, liver, spleen and
gallbladder, and chest X-rays (anteroposterior and lateral views).
Follow-up beyond the fifth year was not mandatory. Progression-free
survival (PFS) was defined as the interval from the date of radical
nephrectomy to the date of tumor recurrence or last follow-up.
Overall survival (OS) was defined as the time from surgery to death
from any cause or last follow-up. The median follow-up duration was
67 months (range, 29–101 months), with the cutoff date of follow-up
in July 2024.
Statistical analysis
All data were analyzed using SPSS version 27.0 (IBM
Corp.). Categorical variables were compared using the χ2
test or Fisher's exact text, depending on the expected count in the
contingency table. PFS was used as the primary survival endpoint in
the present study, as it captures the time from treatment to
disease progression, recurrence or death. Moreover, whilst OS is an
important outcome, the small number of deaths (n=16) in the present
study limited the ability to performed an extensive OS analysis.
Therefore, PFS was used as the main survival measure. Survival
analyses were performed using the Kaplan-Meier method, and
differences between groups were assessed using the log-rank test.
Multivariate analysis was performed using the Cox proportional
hazards model. Receiver operating characteristic (ROC) curves were
generated, and the area under the curve (AUC) was calculated to
evaluate model performance. All tests were two-sided, and P<0.05
was considered to indicate a statistically significant difference.
Variables with P<0.05 in the univariate analysis were
prioritized for multivariate modeling; however, tumor thrombus was
intentionally retained due to its clinical relevance despite its
nonsignificant multivariable P-value.
Results
Baseline clinical characteristics
A total of 180 pediatric patients with WT were
included in the present study, comprising 114 male (63.3%) and 66
female (36.7%) patients. A total of 74 patients (41.1%) were aged
≤2 years and 106 (58.9%) were aged >2 years. The median age of
the patients was 3.3 years, with an age range of 0.5–8.5 years.
Clinical manifestations included abdominal mass in 107 (59.4%)
cases, hematuria in 34 (18.9%) cases and other symptoms in 39
(21.7%) cases. Clinical staging revealed 62 (34.4%) patients in
stage I, 53 (29.4%) patients in stage II, 61 (33.9%) patients in
stage III and 4 (2.2%) patients in stage IV. Histologically, 158
patients (87.8%) had FH, whilst 22 patients (12.2%) had unfavorable
histology (UFH). Additional clinical details are presented in
Table I.
 | Table I.Univariate analysis of prognostic
factors in patients with pediatric Wilms tumor. |
Table I.
Univariate analysis of prognostic
factors in patients with pediatric Wilms tumor.
| Variable | Cases, n (%) | Recurrence cases, n
(%) | Total recurrence
rate, % | HR (95% CI) | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
| ≤2 | 74 (41.1) | 10 (40.0) | 13.5 | 1.000 |
|
|
|
>2 | 106 (58.9) | 15 (60.0) | 14.2 | 1.253
(0.527–3.657) | 0.190 | 0.663 |
| Sex |
|
|
|
|
|
|
|
Male | 114 (63.3) | 15 (60.0) | 13.2 | 1.000 |
|
|
|
Female | 66 (36.7) | 10 (40.0) | 15.2 | 1.051
(0.489–3.527) | 0.008 | 0.929 |
| Clinical
presentation |
|
|
|
|
|
|
|
Abdominal mass | 107 (59.4) | 15 (60.0) | 14.0 | 1.000 |
|
|
|
Hematuria | 34 (18.9) | 8 (32.0) | 23.5 | 1.124
(0.639–4.021) |
|
|
|
Other | 39 (21.7) | 2 (8.0) | 5.1 | 1.405
(0.695–3.963) | - | 0.104a |
| Hypertension |
|
|
|
|
|
|
| No | 136 (75.6) | 20 (80.0) | 14.7 | 1.000 |
|
|
|
Yes | 44 (24.4) | 5 (20.0) | 11.4 | 1.090
(0.578–2.957) | 0.012 | 0.912 |
| Tumor
laterality |
|
|
|
|
|
|
|
Left | 86 (47.8) | 13 (52.0) | 15.1 | 1.000 |
|
|
|
Right | 94 (52.2) | 12 (48.0) | 12.8 | 0.954
(0.698–4.538) | 0.292 | 0.589 |
| Clinical stage |
|
|
|
|
|
|
|
I–II | 115 (63.9) | 5 (20.0) | 4.4 | 1.000 |
|
|
|
III–IV | 65 (36.1) | 20 (80.0) | 30.8 | 4.981
(2.515–9.634) | 20.852 | <0.001 |
| Tumor diameter |
|
|
|
|
|
|
| ≤5
cm | 72 (40.0) | 9 (36.0) | 12.5 | 1.000 |
|
|
| >5
cm | 108 (60.0) | 16 (64.0) | 14.8 | 1.263
(0.602–3.451) | 0.378 | 0.539 |
| Tumor volume |
|
|
|
|
|
|
| ≤1,000
ml | 121 (67.2) | 16 (64.0) | 13.2 | 1.000 |
|
|
|
>1,000 ml | 59 (32.8) | 9 (36.0) | 37.3 | 1.327
(0.581–3.036) | 0.508 | 0.476 |
| Tumor rupture |
|
|
|
|
|
|
| No | 166 (92.2) | 22 (88.0) | 1.8 | 1.000 |
|
|
|
Yes | 14 (7.8) | 3 (12.0) | 7.1 | 1.871
(0.723–5.063) | - | 0.443a |
| Lymph node
metastasis |
|
|
|
|
|
|
| No | 172 (95.6) | 24 (96.0) | 14.0 | 1.000 |
|
|
|
Yes | 8 (4.4) | 1 (4.0) | 12.5 | 1.682
(0.762–6.309) | - | 0.032a |
| Tumor thrombus |
|
|
|
|
|
|
| No | 173 (96.1) | 21 (84.0) | 12.1 | 1.000 |
|
|
|
Yes | 7 (3.9) | 4 (16.0) | 57.1 | 5.332
(2.354–9.658) | - | 0.032a |
| Histological
type |
|
|
|
|
|
|
| FH | 158 (87.8) | 14 (56.0) | 8.9 | 1.000 |
|
|
|
UFH | 22 (12.2) | 11 (44.0) | 50.0 | 5.658
(3.064–10.325) | 34.221 | <0.001 |
| Number of lymph
nodes dissected |
|
|
|
|
|
|
|
<7 | 13 (7.2) | 2 (8.0) | 15.4 | 1.000 |
|
|
| ≥7 | 167 (92.8) | 23 (92.0) | 13.8 | 1.136
(0.581–4.964) | - | 0.795a |
| Postoperative
chemotherapy |
|
|
|
|
|
|
| No | 4 (2.2) | 1 (4.0) | 25.0 | 1.000 |
|
|
|
Yes | 176 (97.8) | 24 (96.0) | 13.6 | 0.857
(0.519–3.725) | - | 0.482a |
| Postoperative
radiotherapy |
|
|
|
|
|
|
| No | 114 (63.3) | 15 (60.0) | 13.2 | 1.000 |
|
|
|
Yes | 66 (36.7) | 10 (40.0) | 15.2 | 0.986
(0.693–3.928) | 0.334 | 0.564 |
Univariate and multivariate analysis
of prognostic factors
To identify prognostic factors following radical
surgery, univariate analysis was performed which revealed that
clinical stage, lymph node metastasis, histological subtype and the
presence of tumor thrombus were significantly associated with
prognosis (P<0.05; Table I).
Furthermore, multivariate Cox regression analysis demonstrated that
advanced clinical stage [stage III–IV; hazard ratio (HR), 4.151;
95% confidence interval (CI), 1.440–11.922; P=0.009] and UFH (HR,
3.842; 95% CI, 1.592–9.283; P=0.002) were independent risk factors
for adverse outcomes (Table II).
Notably, tumor thrombus demonstrated the highest univariate HR of
5.332 (P<0.001) among all variables, although its effect size
decreased after adjustment for stage and histology in the
multivariate analysis (P>0.05).
 | Table II.Multivariate cox regression analysis
of prognostic factors in pediatric Wilms tumor. |
Table II.
Multivariate cox regression analysis
of prognostic factors in pediatric Wilms tumor.
| Variable | HR | 95% CI | P-value |
|---|
| Clinical stage
(III–IV vs. I–II | 4.151 | 1.440–11.922 | 0.009 |
| Histological type
(UFH vs. FH) | 3.842 | 1.592–9.283 | 0.002 |
| Tumor thrombus | 2.284 | 0.731–7.186 | 0.156 |
| Lymph node
metastasis | 1.568 | 0.624.681 | 0.339 |
Prognostic outcomes
By the end of follow-up, 25 patients (13.9%)
experienced recurrence and 16 patients (8.9%) had died. The 5-year
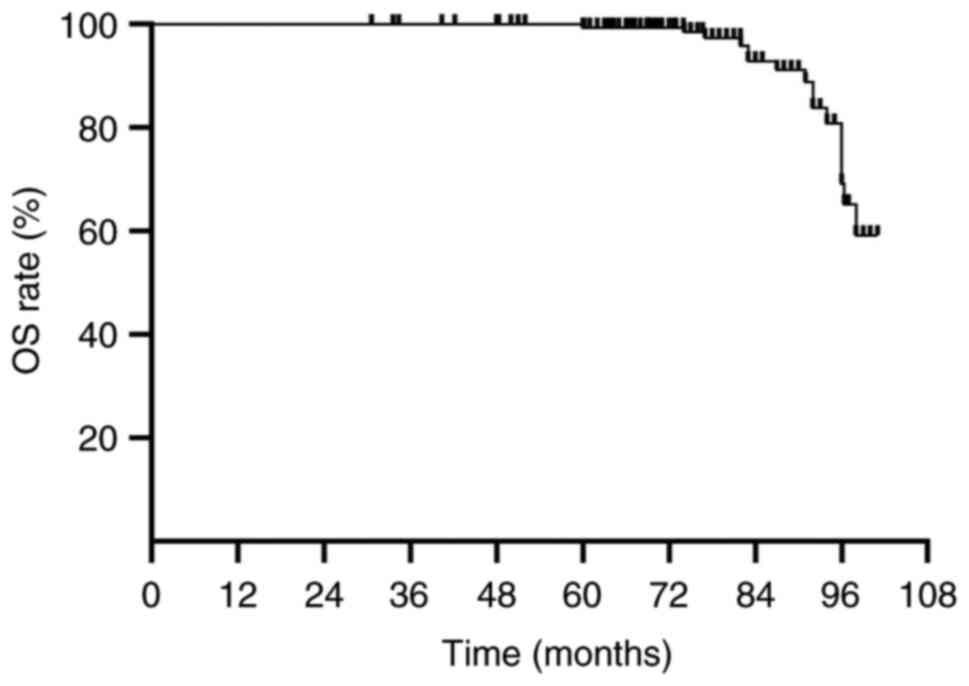
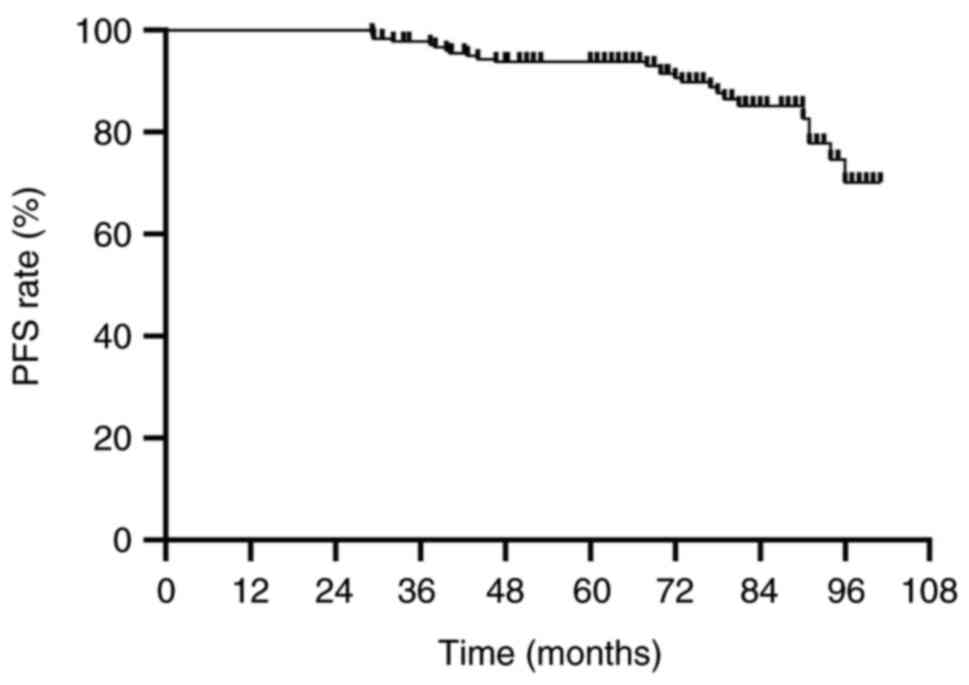
OS rate was 99.6% and the 5-year PFS rate was 94.3%. The median OS
was 76.9 months (range, 30–101 months) and the median PFS was 75.5
months (range, 29–101 months) (Figs.
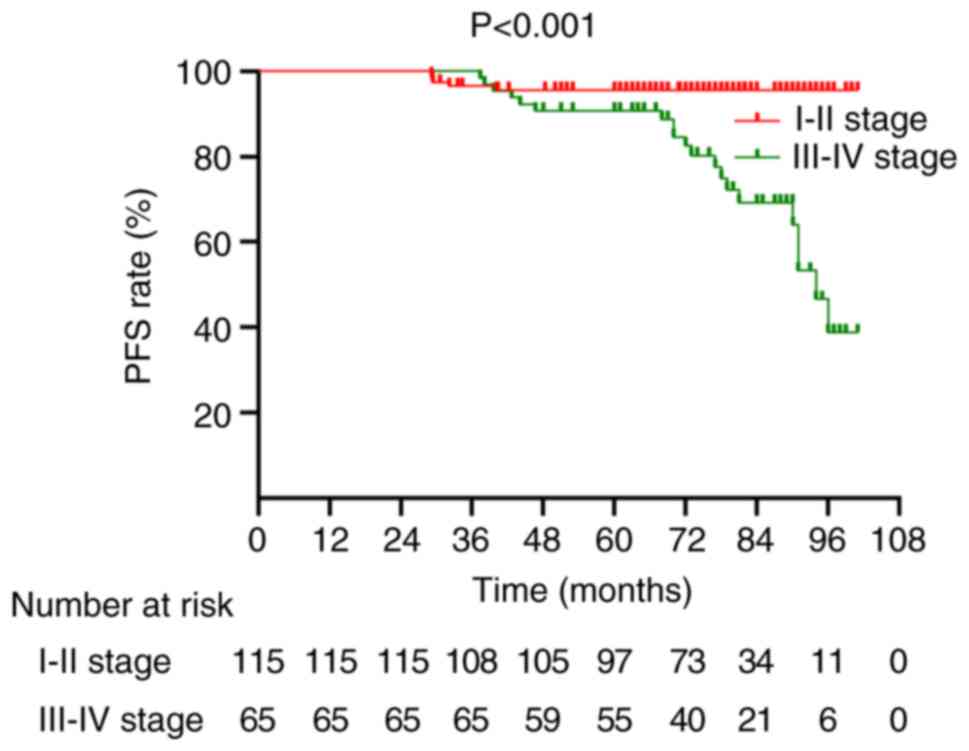
1 and 2). Further stratified
analysis revealed that patients with stage I–II WT had
significantly longer PFS than those with stage III–IV WT
(P<0.001; Fig. 3). Additionally,
despite more frequent follow-up in patients with advanced-stage
disease (stage III–IV), these patients still experienced
significantly lower PFS compared to those with early-stage disease
(P<0.001). This suggests that while increased surveillance may
facilitate earlier detection, it does not fully mitigate the impact
of more aggressive disease biology in advanced-stage Wilms tumor.
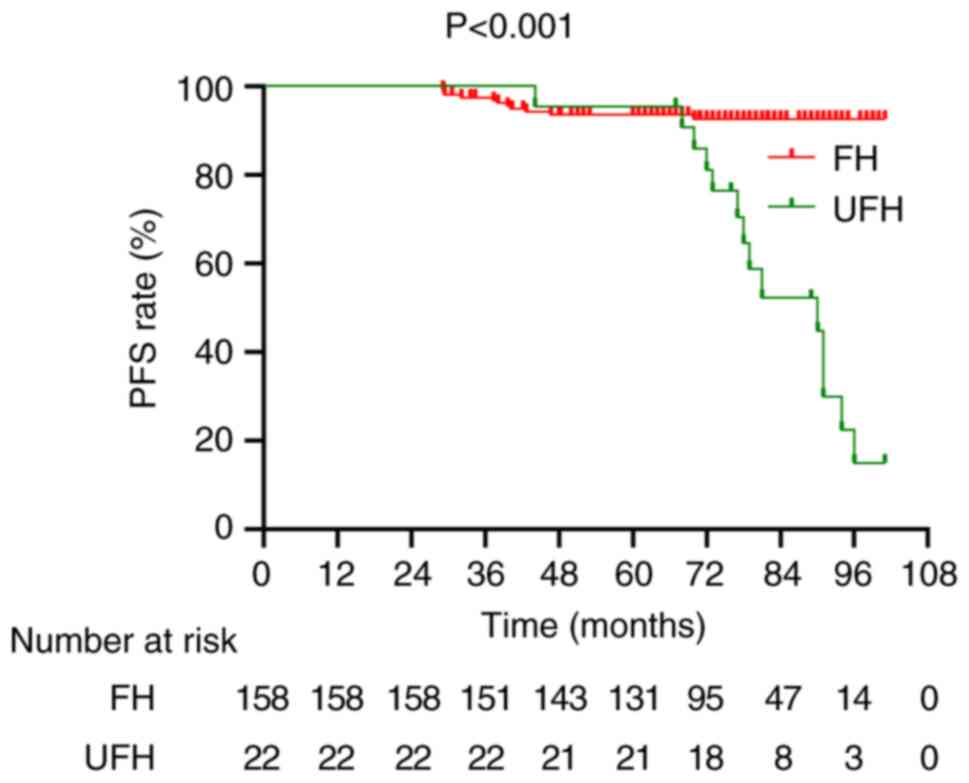
Similarly, patients with FH demonstrated significantly longer PFS
compared with those with UFH (P<0.001; Fig. 4).
Development of the predictive
model
A prognostic model was developed based on clinical
stage and histological subtype. The discriminative performance of
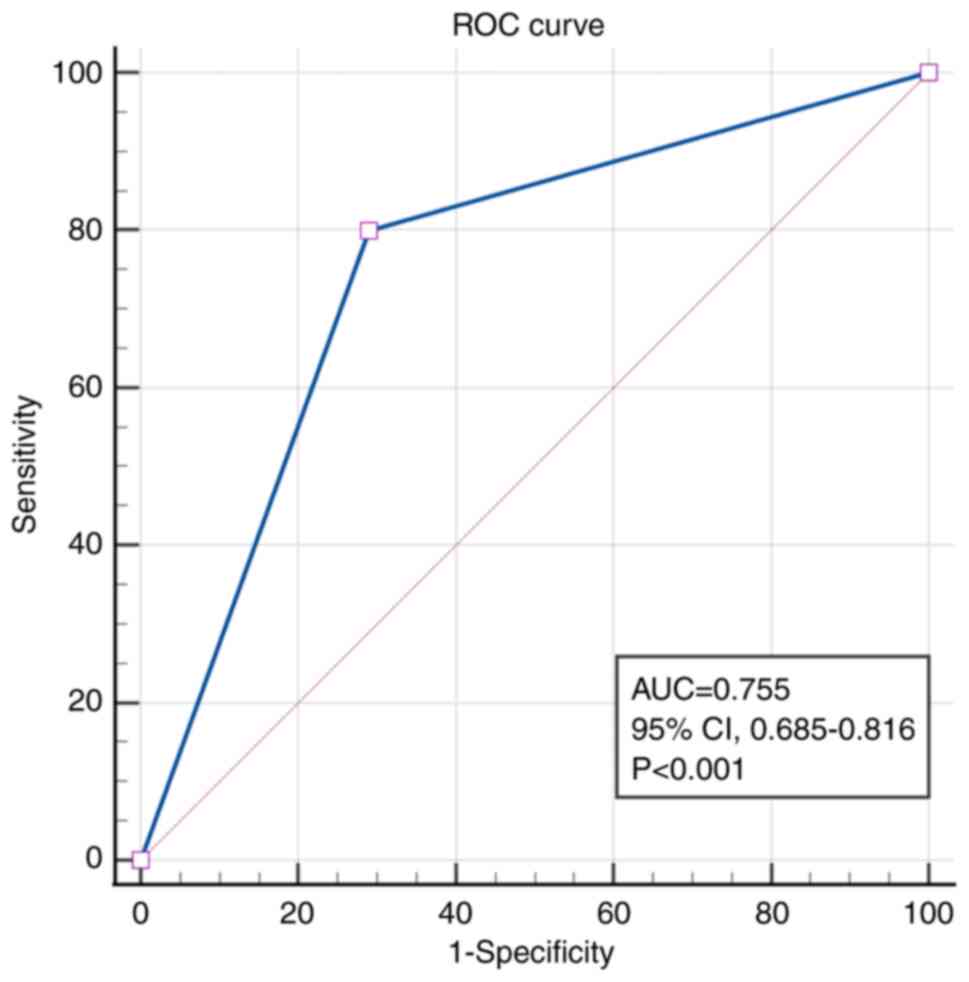
the model was evaluated using ROC analysis, which yielded an AUC of
0.755 (95% CI, 0.685–0.816; P<0.001), indicating good predictive
accuracy (Fig. 5).
Discussion
WT is a common malignant renal tumor in children.
With the refinement of risk-adapted diagnostic and treatment
protocols developed by the COG and the International Society of
Paediatric Oncology (SIOP) (3,21),
long-term survival outcomes for WT have steadily improved, with
current 5-year OS rates reaching ~90% (6,22).
Nevertheless, ~15% of patients with FHWT still experience
recurrence or metastasis, leading to disease progression (13,23).
Accordingly, the present study aimed to analyze the clinical
characteristics and prognostic factors in patients with WT treated
at the Baoding Branch of Beijing Children's Hospital.
The present study primarily focused on PFS due to
its ability to comprehensively reflect disease progression,
recurrence, and death. The 5-year PFS rate was 94.3%, with a median
PFS of 75.5 months. Continued follow-up and expanded sample sizes
will be necessary to investigate factors influencing long-term
survival.
The selection of clinical stage and histological
subtype as core predictors was driven by three main criteria: i)
Statistical strength: Both factors demonstrated the highest HRs in
univariate analysis (stage III–IV HR, 4.981 and UFH HR, 5.658; both
P<0.001) and retained independence in multivariate modeling.
Other variables, such as tumor thrombus, became non-significant
when adjusted (adjusted P=0.154); ii) clinical feasibility: These
parameters are routinely confirmed within 48 h post-nephrectomy,
enabling rapid risk stratification according to COG/SIOP guidelines
(24); and iii) biological
relevance: Advanced stage reflects metastatic potential, whilst
anaplasia is associated with tumor protein P53-mediated treatment
resistance, which has been validated in prior studies (25–27).
Additionally, other clinical factors, such as tumor size and lymph
node involvement, were considered in the multivariate analysis.
Lymph node involvement showed statistical significance in the
univariate analysis and was therefore included in the multivariate
model. Whilst excluding these factors may limit the sensitivity of
the model in certain patient subgroups, focusing on clinically
relevant and easily obtainable variables allows for more practical
application in routine clinical settings.
In the present study, univariate factors
significantly associated with PFS included clinical stage,
histological subtype and tumor thrombus (P<0.05). Multivariate
Cox regression confirmed that stage III–IV disease (HR, 4.151; 95%
CI, 1.440–11.922; P=0.009) and UFH (HR, 3.842; 95% CI, 1.592–9.283;
P=0.002) were independent risk factors for PFS. However, as PFS was
the primary endpoint, competing risks, such as death from causes
other than WT or comorbid conditions, were did not explicitly
accounted for, which may affect survival outcomes. Whilst the small
number of deaths (n=16) limited the ability to perform detailed
competing risk analysis, it is acknowledged that such factors
should be considered in future studies. A more comprehensive
survival analysis, incorporating competing risks, would provide a
clearer picture of the influence of several factors on patient
survival and help refine prognostic models.
Tumor stage has been consistently identified as a
major prognostic determinant in WT. As all cases in the present
study underwent upfront surgery, clinical staging was defined
according to COG guidelines, where stage I–II is considered early
stage and stage III–IV as advanced stage. A national registry by
the Japan Wilms Tumor Study Group reported relapse-free survival
(RFS) rates of >90% for stages I–III but markedly worse outcomes
for stage IV (RFS, 66.2%) (28).
Similarly, combined analyses of the SIOP 93-01 and 2001 studies
reported that patients with stage III WT had a higher risk of
recurrence than those with stage I (29). In general, patients with
advanced-stage disease (III–IV) present with more extensive tumor
burden and consequently worse prognosis than those diagnosed at
earlier stages (30). Therefore,
early detection and accurate staging are critical to improving
outcomes in pediatric WT. Even in patients who undergo radical
nephrectomy, those with advanced-stage disease should receive
timely postoperative adjuvant therapy and close follow-up to
prevent recurrence. Whilst all patients in the present study
received standard postoperative therapy, treatment regimens varied
slightly based on individual patient characteristics, such as
disease stage, histological subtype and tumor size. Specifically,
patients with advanced-stage disease (stage III–IV) received the
more intensive DD4A regimen combined with radiotherapy, whereas
those with early-stage disease (stage I–II) were treated with the
EE4A regimen. These regimens differ in both the number of drugs and
the inclusion of radiotherapy, which was administered to
advanced-stage patients to address the higher risk of recurrence.
However, the potential impact of these differences on survival
outcomes was not fully analyzed in the present study. In future
studies, it would be valuable to explore how the interaction
between different treatment modalities (chemotherapy compared with
radiotherapy) and clinical variables, such as tumor size, stage or
histological subtype, influences survival outcomes. This could help
identify which patient subgroups benefit most from specific
treatment strategies and guide personalized treatment approaches.
Further analyses may also provide insight into whether certain
treatment regimens could be more effective for specific
histological types or tumor sizes, potentially improving patient
outcomes.
Histological subtype was also demonstrated to be a
crucial prognostic factor in the present study. Previous studies
have reported that patients with FHWT have notably improved
survival outcomes compared with those with UFH (31–33).
In the present study, patients with UFH had notably higher
recurrence and mortality rates. These tumors are typically more
aggressive and prone to relapse, potentially due to underlying
immunological and biochemical alterations such as oxidative and
glycoxidative stress responses (34).
In addition to histological factors, molecular
markers such as WT1 mutations and loss of heterozygosity at 1p/16q,
have emerged as notable prognostic indicators in WT. Although these
molecular data were not included in the present study due to data
limitations, future studies should explore their potential role in
refining prognostic models. The incorporation of genetic and
molecular data could enhance prognostic accuracy, provide a more
comprehensive understanding of tumor biology, and offer insights
into personalized treatment strategies. For example, WT1 mutations,
which are frequently associated with UFH and poor prognosis
(35), could serve as a powerful
predictor for treatment response and long-term outcomes. Therefore,
pathological assessment serves a pivotal role in risk
stratification and treatment planning.
Furthermore, individualized therapeutic approaches
based on histological subtype may improve patient outcomes. Whilst
the COG and SIOP risk stratification systems for WT are
well-established and widely used, the present study provided
important new insights that may enhance current risk stratification
and inform treatment strategies. Specifically, the following three
clinically actionable findings of the present study from a cohort
treated at a single center in China suggest the need for further
refinement of existing protocols. i) Higher incidence of stage III
disease: A total of 33.9% of patients presented with stage III
disease, compared with 28% reported in the SIOP 2001 trials
(36). This finding suggests
potential regional differences in disease aggressiveness, which may
influence the choice of treatment regimens and surveillance
strategies in different populations. ii) Tumor thrombus as a
prognostic factor: Although tumor thrombus was present in only 3.9%
of cases, it demonstrated a strong univariate association with
recurrence (57.1 vs. 12.1%; P<0.001). This highlights that the
presence of tumor thrombus, although rare, should be considered a
notable prognostic factor, particularly in cases of recurrence
risk. Whilst its prognostic significance was reduced after
adjusting for stage and histology, it still warrants consideration
in clinical decision-making. iii) Interaction between stage III–IV
and tumor thrombus: The data also suggested that the interaction
between stage III–IV disease and the presence of tumor thrombus
accounts for ~75% of thrombus-associated recurrences. This finding
indicates a synergistic risk escalation, suggesting that a more
personalized approach may be needed for patients with these
combined factors. These findings underscore the need for a more
nuanced approach to risk stratification for WT, one that considers
both regional differences in disease presentation and additional
prognostic factors not fully captured in existing models. Although
COG and SIOP provide robust guidelines, the findings from the
present study indicate that further refinements may be needed to
improve the accuracy of recurrence prediction and optimize
treatment strategies, particularly in populations with unique
disease patterns.
Based on clinical stage and histology, the present
study developed a predictive model for postoperative prognosis in
children with WT. The model demonstrated good discriminatory power,
with an area under the ROC curve of 0.755 (95% CI, 0.685–0.816;
P<0.001), supporting its clinical utility in forecasting
recurrence risk and guiding personalized management strategies.
From a clinical perspective, the predictive model based on clinical
stage and histological subtype offers a practical tool for early
postoperative risk stratification. Both variables are readily
available within 48–72 h following radical nephrectomy, allowing
clinicians to implement early decisions regarding surveillance
intensity and adjuvant treatment. For example, patients identified
as high-risk by the model (namely, stage III–IV and/or UFH) may
benefit from intensified imaging surveillance during the first 2
postoperative years, earlier initiation or escalation of
chemotherapy, or closer multidisciplinary follow-up. Notably, this
model complements, rather than replaces, existing risk
stratification systems such as those established by the COG. Whilst
COG guidelines provide protocol-driven treatment based on operative
and pathological findings, the model in the present study serves to
refine prognostic predictions post-surgery, particularly in
settings where clinical discretion is allowed (such as borderline
stage II/III cases or histologically ambiguous presentations).
Thus, integration of this model into routine clinical practice
could enhance individualized care by aligning early prognostic
assessment with standardized treatment protocols.
Further refinement of the model could include
additional clinical, molecular and genetic factors such as WT1
mutations, loss of heterozygosity at 1p/16q or other emerging
biomarkers. Incorporating these factors could potentially improve
the accuracy of the model and broaden its applicability,
particularly in genetically heterogeneous populations. Furthermore,
external validation was not performed using independent cohorts,
which is a crucial step to assess the robustness and
generalizability of the model in diverse clinical settings. Future
studies should aim to perform internal validation (such as
bootstrapping or k-fold cross-validation) as well as external
validation to refine and validate the predictive power of the model
in different clinical environments.
Moreover, the present study has several limitations.
First, it was a single-center retrospective analysis with a
relatively limited sample size, which may reduce the statistical
power and generalizability of the findings. An important
consideration for future studies is the potential impact of
postoperative complications, such as renal insufficiency,
infections or other treatment-related side effects. These
complications may negatively affect prognosis and overall survival
outcomes, particularly in children with WT who may experience
long-term treatment-related toxicities. Whilst these factors were
not specifically addressed in the current study due to data
limitations, future research should aim to evaluate the role of
postoperative complications in survival outcomes. Including such
factors in prognostic models could provide a more comprehensive
assessment of patient risk and guide individualized treatment
approaches. Whilst this approach ensures consistency in data
collection, the results may not fully reflect the broader pediatric
WT population, particularly with respect to regional or demographic
differences. For instance, variations in treatment protocols,
patient characteristics and disease prevalence across different
regions or institutions could influence outcomes. Therefore, the
external validity of these findings could be limited. To confirm
and validate the results of the present, future multicenter
studies, incorporating diverse patient populations from several
geographic regions, would be valuable. A larger and more
heterogeneous cohort would enhance the generalizability of the
model and improve its applicability to different clinical settings.
Second, the prognostic value of tumor thrombus requires cautious
interpretation due to small event numbers (n=7), although its
univariate significance aligns with emerging evidence from AREN03B2
trial sub-studies (37). Third, an
important aspect to consider in future studies is the use of
biomarkers in postoperative follow-up. Biomarkers such as
circulating tumor DNA, WT1 mutations, 1q gain and microRNAs have
shown promise as early indicators of recurrence or metastasis
(38,39). For example, 1q gain is associated
with more aggressive disease and worse prognosis in patients with
WT, making it an important biomarker for risk stratification
(40). Similarly, microRNA
expression changes can help predict disease progression and
outcomes (41,42). Additionally, prohibitin levels have
been reported to be associated with relapse in WT, highlighting its
potential as a non-invasive biomarker for monitoring recurrence
(43). Incorporating these
biomarkers into routine postoperative surveillance could improve
the accuracy of recurrence prediction and allow for more
personalized treatment strategies. Future research should focus on
validating the reliability and feasibility of these biomarkers in
clinical practice. Additionally, certain clinical variables, such
as tumor rupture timing and long-term treatment-related
complications (for example, cardiac or renal dysfunction), were not
fully captured. The reliance on a single data source may also
introduce selection and information biases, which are inherent in
retrospective studies and single-center data collection. Another
limitation is the potential for follow-up loss, as not all patients
could be consistently monitored for the entire follow-up period.
Additionally, variability in protocol adherence could affect the
consistency of treatment and outcomes. Furthermore, the study did
not incorporate genetic or molecular biomarkers, such as WT1,
1p/16q LOH and 1q gain, which have been identified as important
prognostic indicators in WT. The absence of these markers in the
current study was due to data limitations, including the
unavailability of genetic profiling for certain patients. Future
research should aim to include these molecular markers to improve
the prognostic accuracy of the model and improve the stratification
of patient risk. Finally, internal cross-validation (such as
bootstrapping or k-fold cross-validation) was not performed and
external validation cohorts were not used in the development of the
predictive model. This limitation may affect the robustness and
generalizability of the model. Future studies should incorporate
internal validation techniques and use independent cohorts to
enhance the accuracy and applicability of the model.
In conclusion, the present study primarily focused
on PFS as the key prognostic indicator, as it provides a useful
reflection of the risk of recurrence, progression and death in
patients with WT. Nevertheless, the findings of the present study
provide meaningful guidance for clinicians and offer a foundation
for future research. Future studies should incorporate additional
clinical and genetic factors to refine the predictive model
further. For instance, integrating molecular markers such as WT1
mutations, 1p/16q loss of heterozygosity or other genetic
alterations could markedly improve the accuracy of the model.
Furthermore, additional cohort studies, especially multicenter or
international studies, would help validate the findings of the
study and assess the robustness of the model across diverse
populations. Incorporating data from different clinical settings
and populations may enhance the generalizability of the model,
ensuring its applicability in routine clinical practice.
Furthermore, another important aspect that should be considered in
future studies is the assessment of long-term quality of life in
pediatric patients with WT. Whilst the present study focused on PFS
and OS, the impact of postoperative treatments, recurrence and
treatment-related side effects on the physical function, mental
health and social adaptation of patients is also crucial.
Incorporating quality of life assessments into future research
would provide a more comprehensive understanding of the treatment
impacts on the overall well-being of patients and help guide
personalized and holistic care. Through early diagnosis, accurate
staging, individualized treatment and comprehensive perioperative
management, the prognosis of children with WT can be substantially
improved. Moreover, the development of a multifactorial prognostic
model may facilitate more precise and evidence-based clinical
decision-making, ultimately enhancing survival and quality-of-life
in this patient population.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Baoding Municipal Science
and Technology Bureau (grant no. 2341ZF378).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HT and DM conceived and designed the study. HT
collected clinical data, performed the statistical analysis and
drafted the manuscript. BH, KS, HL, JW and JS contributed to data
interpretation and literature review. DM critically revised the
manuscript for important intellectual content and supervised the
overall project. HT and DM confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of the Declaration of Helsinki and was approved by
the Ethics Committee of the Baoding Branch of Beijing Children's
Hospital, Baoding Children's Hospital (approval no.
H-BDETKJ-SOP006-03-A/2). Written informed consent was obtained from
the legal guardians of all participating patients prior to
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
WT
|
Wilms tumor
|
|
FH
|
favorable histology
|
|
UFH
|
unfavorable histology
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
RFS
|
relapse-free survival
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
|
COG
|
Children's Oncology Group
|
|
SIOP
|
International Society of Paediatric
Oncology
|
|
CCCG
|
Chinese Children Cancer Group
|
|
EE4A
|
vincristine + actinomycin D
regimen
|
|
DD4A
|
vincristine + actinomycin D +
doxorubicin regimen
|
References
|
1
|
Doganis D, Panagopoulou P, Tragiannidis A,
Vichos T, Moschovi M, Polychronopoulou S, Rigatou E,
Papakonstantinou E, Stiakaki E, Dana H, et al: Survival and
mortality rates of Wilms tumour in Southern and Eastern European
countries: Socioeconomic differentials compared with the United
States of America. Eur J Cancer. 101:38–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan X, Wang J, Tang J, Tian X, Jin L, Li
M, Zhang Z and He D: A nomogram for predicting Cancer-specific
survival in children with wilms tumor: A study based on SEER
database and external validation in China. Front Public Health.
10:8298402022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vujanić GM, Gessler M, Ooms AHAG, Collini
P, Coulomb-l'Hermine A, D'Hooghe E, de Krijger RR, Perotti D,
Pritchard-Jones K, Vokuhl C, et al: The UMBRELLA SIOP-RTSG 2016
Wilms tumour pathology and molecular biology protocol. Nat Rev
Urol. 15:693–701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spreafico F, Fernandez CV, Brok J, Nakata
K, Vujanic G, Geller JI, Gessler M, Maschietto M, Behjati S,
Polanco A, et al: Wilms tumour. Nat Rev Dis Primers. 1:752021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain J, Sutton KS and Hong AL: Progress
update in pediatric renal tumors. Curr Oncol Rep. 23:332021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahoush G and Saeedi E: Outcome of
Children with Wilms'tumor indeveloping countries. Med Life.
13:484–489. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan CC, To KF, Yuen HL, Shing Chiang AK,
Ling SC, Li CH, Cheuk DK, Li CK and Shing MM: A 20-year prospective
study of Wilms tumor and other kidney tumors: A report from Hong
Kong pediatric hematology and oncology study group. J Pediatr
Hematol Oncol. 36:445–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Millar AJW, Cox S and Davidson A:
Management of bilateral Wilms tumours. Pediatr Surg Int.
33:737–745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dome JS, Graf N, Geller JI, Fernandez CV,
Mullen EA, Spreafico F, Van den Heuvel-Eibrink M and
Pritchard-Jones K: Advances in wilmstumor treatment and biology:
Progress Through In-ternational Collaboration. J Clin Oncol.
27:2999–3007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JE, Noh OK, Lee Y, Choi HS, Han JW,
Hahn SM, Lyu CJ, Lee JW, Yoo KH, Koo HH, et al: Loss of
Heterozygosity at chromosome 16q is a negative prognostic factor in
Korean pediatric patients with favorable histology Wilms tumor: A
report of the Korean pediatric hematology oncology group (K-PHOG).
Cancer Res Treat. 52:438–445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dix DB, Fernandez CV, Chi YY, Mullen EA,
Geller JI, Gratias EJ, Khanna G, Kalapurakal JA, Perlman EJ, Seibel
NL, et al: AREN0532 and AREN0533 study committees. Augmentation of
therapy for combined loss of heterozygosity 1p and 16q in favorable
histology wilms tumor: A Children's oncology group AREN0532 and
AREN0533 study report. J Clin Oncol. 30:2769–2777. 2019. View Article : Google Scholar
|
|
12
|
Li K, Zhang K, Yuan H and Fan C:
Prognostic role of primary tumor size in Wilms tumor. Oncol Lett.
27:1642024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez CV, Mullen EA, Chi YY, Ehrlich
PF, Perlman EJ, Kalapurakal JA, Khanna G, Paulino AC, Hamilton TE,
Gow KW, et al: Outcome and prognostic factors in stage III
Favorable-histology wilms tumor: A report from the Children's
oncology group study AREN0532. J Clin Oncol. 36:254–261. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dome JS, Mullen EA, Dix DB, Gratias EJ,
Ehrlich PF, Daw NC, Geller JI, Chintagumpala M, Khanna G,
Kalapurakal JA, et al: Impact of the first generation of Children's
oncology group clinical trials on clinical practice for wilms
tumor. J Natl Compr Canc Netw. 19:978–985. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nelson MV, van den Heuvel-Eibrink MM, Graf
N and Dome JS: New approaches to risk stratification for Wilms
tumor. Curr Opin Pediatr. 33:40–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haruta M, Arai Y, Okita H, Tanaka Y,
Takimoto T, Sugino RP, Yamada Y, Kamijo T, Oue T, Fukuzawa M, et
al: Combined genetic and chromosomal characterization of wilms
tumors identifies chromosome 12 Gain as a potential new marker
predicting a favorable outcome. Neoplasia. 21:117–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao W, Weng S, Li K, Shen J, Dong R and
Dong K: Bilateral Wilms tumor: 10-year experience from a single
center in China. Transl Cancer Res. 13:879–887. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian XM, Ma W, Shi QL, Lu P, Liu X, Lin T,
He D and Wei G: Clinical analysis of 43 cases of Wilms tumor
treated with the CCCG-WT-2016 regimen. J Clin Pediatrics.
12:915–920. 2020.
|
|
19
|
Urology Group, Pediatric Surgery Society,
Chinese Medical Association, . Pediatric nephroblastoma diagnosis
and treatment expert consensus. Chin J Pediatr Surge. 7:585–590.
2020.
|
|
20
|
de Faria LL, Ponich Clementino C, Véras
FASE, Khalil DDC, Otto DY, Oranges Filho M, Suzuki L and Bedoya MA:
Staging and restaging pediatric abdominal and pelvic tumors: A
practical guide. Radiographics. 44:e2301752024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geller JI, Hong AL, Vallance KL, Evageliou
N, Aldrink JH, Cost NG, Treece AL, Renfro LA and Mullen EA; COG
Renal Tumor Committee, : Children's oncology Group's 2023 blueprint
for research: Renal tumors. Pediatr Blood Cancer. 70 (Suppl
6):e305862023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dome JS, Graf N, Geller JI, Fernandez CV,
Mullen EA, Spreafico F, Van den Heuvel-Eibrink M and
Pritchard-Jones K: Advances in wilms tumor treatment and biology:
Progress through international collaboration. J Clin Oncol.
33:2999–3007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong KF, Reulen RC, Winter DL, Guha J,
Fidler MM, Kelly J, Lancashire ER, Pritchard-Jones K, Jenkinson HC,
Sugden E, et al: Risk of adverse healthand social outcomes up to 50
years after Wilms tumor: The British childhood cancer survivor
study. J Clin Oncol. 34:1772–1779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brok J, Mavinkurve-Groothuis AMC, Drost J,
Perotti D, Geller JI, Walz AL, Geoerger B, Pasqualini C, Verschuur
A, Polanco A, et al: Unmet needs for relapsed or refractory Wilms
tumour: Mapping the molecular features, exploring organoids and
designing early phase trials-A collaborative SIOP-RTSG, COG and
ITCC session at the first SIOPE meeting. Eur J Cancer. 144:113–122.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo X, Deng C, Liu F, Liu X, Lin T, He D
and Wei G: HnRNPL promotes Wilms tumor progression by regulating
the p53 and Bcl2 pathways. Onco Targets Ther. 12:4269–4279. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phelps HM, Al-Jadiry MF, Corbitt NM,
Pierce JM, Li B, Wei Q, Flores RR, Correa H, Uccini S, Frangoul H,
et al: Molecular and epidemiologic characterization of Wilms tumor
from Baghdad, Iraq. World J Pediatr. 14:585–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Lou S, Huang X, Mo Y, Wang Z, Zhu
J, Tian X, Shi J, Zhou H, He J and Ruan J: The association of
miR34b/c and TP53 gene polymorphisms with Wilms tumor risk in
Chinese children. Biosci Rep. 40:BSR201942022020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koshinaga T, Takimoto T, Oue T, Okita H,
Tanaka Y, Nozaki M, Tsuchiya K, Inoue E, Haruta M, Kaneko Y and
Fukuzawa M: Outcome of renal tumors registered in Japan Wilms Tumor
Study-2 (JWiTS-2): A report from the Japan Children's Cancer Group
(JCCG). Pediatr Blood Cancer. 65:e270562018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hol JA, Lopez-Yurda MI, Van Tinteren H,
Van Grotel M, Godzinski J, Vujanic G, Oldenburger F, De Camargo B,
Ramírez-Villar GL, Bergeron C, et al: Prognostic significance of
age in 5631 patients with Wilms tumour prospectively registered in
International Society of Paediatric Oncology (SIOP) 93-01 and 2001.
PLoS One. 14:e02213732019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chagtai T, Zill C, Dainese L, Wegert J,
Savola S, Popov S, Mifsud W, Vujanić G, Sebire N, Le Bouc Y, et al:
Gain of 1q as a prognostic biomarker in wilms tumors (WTs) treated
with preoperative chemotherapy in the international society of
paediatric oncology (SIOP) WT 2001 Trial: A SIOP renal tumours
biology consortium study. J Clin Oncol. 34:3195–3203. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ehrlich PF, Anderson JR, Ritchey ML, Dome
JS, Green DM, Grundy PE, Perlman EJ, Kalapurakal JA, Breslow NE and
Shamberger RC: Clinicopathologic findings predictive of relapse in
children with stage III favorable-histology Wilms tumor. J Clin
Oncol. 31:1196–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oue T, Koshinaga T, Takimoto T, Okita H,
Tanaka Y, Nozaki M, Haruta M, Kaneko Y and Fukuzawa M; Renal Tumor
Committee of the Japanese Children's Cancer Group, : Renal tumor
committee of the Japanese Children's cancer group. Anaplastic
histology Wilms' tumors registered to the Japan Wilms' Tumor study
group are less aggressive than that in the National Wilms' tumor
study 5. Pediatr Surg Int. 32:851–855. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Groenendijk A, Spreafico F, de Krijger RR,
Drost J, Brok J, Perotti D, van Tinteren H, Venkatramani R,
Godziński J, Rübe C, et al: Prognostic factors for wilms tumor
recurrence: A review of the literature. Cancers (Basel).
13:31422021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Islam S, Moinuddin Mir AR, Raghav A, Habib
S, Alam K and Ali A: Glycation, oxidation and glycoxidation of IgG:
A biophysical, biochemical, immunological and hematological study.
J Biomol Struct Dyn. 36:2637–2653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gadd S, Huff V, Walz AL, Ooms AHAG,
Armstrong AE, Gerhard DS, Smith MA, Guidry Auvil JM, Meerzaman D,
Chen QR, et al: A Children's oncology group and TARGET initiative
exploring the genetic landscape of Wilms tumor. Nat Genet.
49:1487–1494. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hol JA, Lopez-Yurda MI, Van Tinteren H,
Van Grotel M, Godzinski J, Vujanic G, Oldenburger F, De Camargo B,
Ramírez-Villar GL, Bergeron C, et al: Prognostic significance of
age in 5631 patients with Wilms tumour prospectively registered in
SIOP 93-01 and 2001. PLoS One. 14:e02213732019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dome JS, Perlman EJ and Graf N: Risk
stratification for wilms tumor: Current approach and future
directions. Am Soc Clin Oncol Educ Book. 215–223. 2014.doi:
10.14694/EdBook_AM.2014.34.215. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perotti D, Williams RD, Wegert J,
Brzezinski J, Maschietto M, Ciceri S, Gisselsson D, Gadd S, Walz
AL, Furtwaengler R, et al: Hallmark discoveries in the biology of
Wilms tumour. Nat Rev Urol. 21:158–180. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Madanat-Harjuoja LM, Renfro LA, Klega K,
Tornwall B, Thorner AR, Nag A, Dix D, Dome JS, Diller LR, Fernandez
CV, et al: Circulating tumor DNA as a biomarker in patients with
stage iii and iv Wilms tumor: Analysis from a Children's oncology
group trial, AREN0533. J Clin Oncol. 40:3047–3056. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gratias EJ, Dome JS, Jennings LJ, Chi YY,
Tian J, Anderson J, Grundy P, Mullen EA, Geller JI, Fernandez CV
and Perlman EJ: Association of chromosome 1q gain with inferior
survival in Favorable-histology wilms tumor: A report from the
Children's oncology group. J Clin Oncol. 34:3189–3194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Q, Chen J, Wang C, Chen X, Liu J,
Zhou L and Liu Y: MicroRNA-466o-3p mediates β-catenin-induced
podocyte injury by targeting Wilms tumor 1. FASEB J.
34:14424–14439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mohamed FS, Jalal D, Fadel YM, El-Mashtoly
SF, Khaled WZ, Sayed AA and Ghazy MA: Characterization and
comparative profiling of piRNAs in serum biopsies of pediatric
Wilms tumor patients. Cancer Cell Int. 25:1632025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ortiz MV, Ahmed S, Burns M, Henssen AG,
Hollmann TJ, MacArthur I, Gunasekera S, Gaewsky L, Bradwin G, Ryan
J, et al: Prohibitin is a prognostic marker and therapeutic target
to block chemotherapy resistance in Wilms' tumor. JCI Insight.
4:e1270982019. View Article : Google Scholar : PubMed/NCBI
|