Introduction
KORTUC (Kochi Oxidol-Radiation Therapy for
Unresectable Carcinomas) was proposed and developed by Kochi
University in 2006 and is currently the most widely used
radiosensitizer in clinical practice in Japan. Ogawa et al
(1) developed a novel treatment
method (KORTUC II), in which hydrogen peroxide is locally injected
into tumors as a new type of radiosensitizer, and confirmed its
safety and efficacy in patients with locally advanced cancers
(2,3). Hydrogen peroxide
(H2O2) is the only agent known to inactivate
antioxidant enzymes and simultaneously generate oxygen when
injected into tumor tissues (1–3).
KORTUC I involves the application of a sensitizer to
the tumor surface, whereas KORTUC II is an advanced formulation
that allows direct intratumoral injection. The solution consists of
0.5% H2O2 and 0.83% sodium hyaluronate (HA).
H2O2 is the active component of the
radiosensitizer, and HA functions to delay its decomposition,
thereby maintaining an elevated oxygen concentration within the
tumor for a sustained period. A characteristic feature of KORTUC II
is the injection of these two components at a specific ratio.
Since May 2010, Osaka Medical and Pharmaceutical
University has clinically implemented this treatment and has
treated over 400 patients with combined KORTUC I and II. The
effectiveness of KORTUC has previously been reported in cases of
recurrent and locally advanced breast cancer. Currently, a Phase II
trial targeting locally advanced and recurrent breast cancer is
underway in the United Kingdom (NCT03946202), and various clinical
studies targeting different solid tumors are in progress in
Japan.
Although standard treatment is generally recommended
for primary breast cancer, some patients refuse surgery or drug
therapy due to cosmetic concerns, comorbidities, or personal
preferences, and alternative approaches are limited for these
individuals. In this report, we present a case series of
breast-conserving therapy using KORTUC (KORTUC-BCT) for patients
with primary breast cancer stages 0-IIIC who declined standard
treatment.
Materials and methods
Patient selection
KORTUC therapy was administered to patients with
newly diagnosed primary breast cancer who had no distant metastases
and had refused standard treatments such as surgery. All patients
provided written informed consent after receiving a detailed
explanation of the procedure. Hormonal therapy or systemic
chemotherapy was administered in some cases, based on the clinical
judgment of the attending breast surgeon and patient
preference.
The present KORTUC II clinical study was approved by
the Ethics Committee of Osaka Medical and Pharmaceutical University
[Trial no. 1973, May 10, 2010; UMIN Clinical Trials Registry,
UMIN000003734, June 10, 2010].
Method for dosing the KORTUC
sensitizer
The sensitizer consisted of 0.83% HA and 0.5%
H2O2 (H2O2, also known
as ‘oxydol’ in Japan). It was prepared aseptically before each use
by adding 2.5 ml of HA (Adant Dispo) and 1 ml of 1% xylocaine to
0.5 ml of oxydol, mixing them into a total volume of 4 ml in a
single vial. Our standard dosing protocol prescribed one vial for
tumors <3 cm in diameter, two vials for tumors 3-<5 cm, and
≥3 vials for tumors ≥5 cm in diameter, with a maximum dose of five
vials for giant tumors. However, the optimal dose remains unclear.
The sensitizer was administered intratumorally under direct vision
or with ultrasound or computed tomography (CT) guidance twice
weekly immediately before each radiotherapy session. Under
ultrasound guidance, when the sensitizer is injected into a tumor,
oxygen is generated in the form of microbubbles, and the tumor is
immediately recognized as a high-echo area.
The sensitizer is injected to distribute oxygen
throughout the tumor. Usually, sensitizer injections are
administered after the patient has received approximately 20 Gy at
the beginning of the radiotherapy course. This was to prevent
increased intratumor pressure from the injections, causing viable
tumor cells to infiltrate the nearby lymphatic and blood vessels
(4). To prevent dissemination along
the injection route, punctures were made on the skin surface in the
irradiation field whenever possible. When multiple lesions were
present within the irradiation field, the sensitizer was injected
into both the primary and accessible subcutaneous lesions under
ultrasound guidance. The injection was administered through the
skin within the irradiated field whenever possible, to avoid
seeding along the needle tract. In cases of lymph node metastases,
sensitizer injections were administered only when the nodes were
sufficiently large to be safely punctured, considering the
associated risks.
Radiotherapy
Radiotherapy was administered according to standard
hypofractionation protocols. For stage 0-IIA, all patients received
whole-breast irradiation with tangential fields at a dose of 44 Gy
in 16 fractions, followed by a tumor bed boost of 9 Gy in three
fractions. For patients with stage IIB-IIIC disease, the
supraclavicular and internal mammary lymph node areas were included
depending on the case. The total dose ranged from 53 to 56 Gy in
19–20 fractions (tangential field, 44 Gy/16 fractions; boost, 9
Gy/3 fractions in 18 patients, and 12 Gy/4 fractions in one case).
X-rays were used for tangential irradiation, and either X-rays or
electron beams were used for boost irradiation.
Items examined
The treatment efficacy was comprehensively evaluated
using computed findings from CT, magnetic resonance imaging,
positron emission tomography/CT, breast ultrasonography, and
mammography. The local control duration was defined as the time
from the end of radiotherapy to the point at which tumor regrowth
was confirmed by imaging within the irradiated fields. Local
control and overall survival (OS) rates were estimated using the
Kaplan-Meier method.
Statistical analysis
The survival time was calculated from the day after
treatment completion. Continuous variables are expressed as mean ±
standard deviation, and categorical variables are presented as
numbers (percentages). Survival analyses were conducted using the
Kaplan-Meier method, and comparisons were made using the Wilcoxon
rank-sum test. Differences between groups, including comparisons by
radiation dose, were analyzed using the Wilcoxon rank-sum test.
Statistical analyses were performed using the EZR software (version
1.54) and Microsoft Excel 2016. Statistical significance was set at
P<0.05.
Results
Between February 2013 and April 2022, 50 patients
underwent KORTUC breast-conserving therapy (KORTUC-BCT) and were
followed for at least 1 year. Patient characteristics are
summarized in Table I. All patients
were female, with a mean age of 57 years (range: 38–83 years). The
staging distribution was as follows: stage 0 (n=2); stage I (n=12);
stage IIA (n=17); stage IIB (n=5); stage IIIA (n=5); stage IIIB
(n=4); and stage IIIC (n=5). The molecular subtypes were Luminal A
(n=30), Luminal B (n=8), Luminal-HER2 (n=6), HER2 (n=3), triple
negative (n=1), and unknown (n=2).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Total |
|---|
| Patients, n
(range) | 50 |
| Median
age, years | 57 (29–83) |
| Median
follow-up period, months | 27 (12–95) |
| Clinical stage, n
(%) |
|
| 0 | 2 (4.0) |
| I | 12 (24.0) |
|
IIA | 17 (34.0) |
|
IIB | 5 (10.0) |
|
IIIA | 5 (10.0) |
|
IIIB | 4 (8.0) |
|
IIIC | 5 (10.0) |
| Chemotherapy, n
(%) |
|
|
Yes | 7 (14.0) |
| No | 43 (86.0) |
| Hormone therapy, n
(%) |
|
|
Yes | 29 (58.0) |
| No | 21 (42.0) |
| Molecular subtype,
n (%) |
|
| Luminal
A or B | 38 (76.0) |
| Luminal
HER2 | 6 (12.0) |
|
HER2 | 3 (6.0) |
| Triple
negative | 1 (2.0) |
|
Unknown | 2 (4.0) |
The median radiation dose was 53 Gy delivered in 19
fractions. The number of sensitizer injections ranged from four to
six, with a median of five injections. Among the 50 patients, 29
received hormone therapy and seven received chemotherapy. Notably,
17 patients (34.0%) refused hormone therapy, and among 34 patients
with tumors classified as T2 or higher, 28 (82.3%) refused
chemotherapy. The median follow-up period was 27 months (range,
12–95 months). All patients initially achieved a clinical complete
response (cCR) with a median time to cCR of 12 months (range: 5–33
months). Recurrence occurred in 11 patients: five experienced local
recurrence, and eight experienced distant recurrence (two had both)
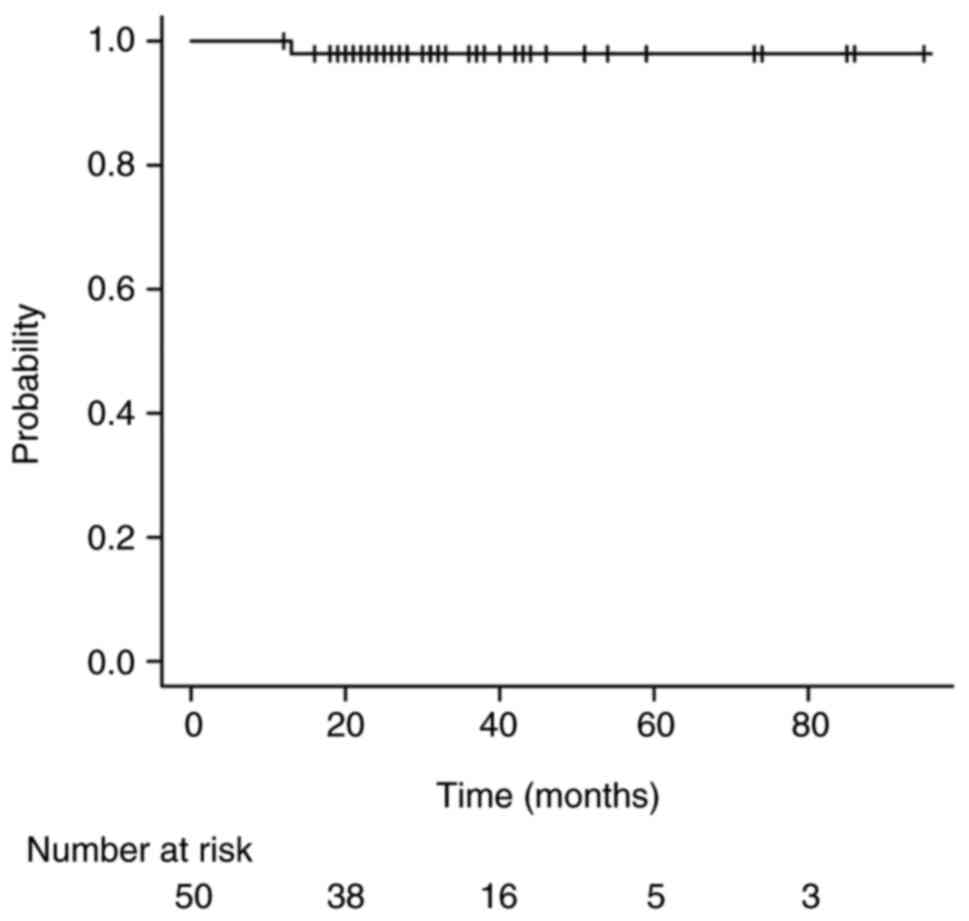
(Table II). The 3-year OS rate for
all patients was 98.0% (95% CI: 86.4–99.7), with no
disease-specific deaths during the observation period. One patient
died of an unrelated disease (bladder cancer) (Fig. 1).
 | Table II.recurrence pattern. |
Table II.
recurrence pattern.
| Clinical stage | Site of
recurrence | Time to recurrence,
months |
|---|
| I | Local | 47 |
| IIA | Liver | 13 |
| IIB | Local, ovary, bone,
gluteal muscle | 18 |
| IIB | Local | 28 |
| IIIA | Local | 20 |
| IIIA | Local, liver, lung,
bone | 6 |
| IIIB | Contralateral lymph
node | 12 |
| IIIC | Bone | 30 |
| IIIC | Brain | 22 |
| IIIC | Bone | 9 |
| IIIC | Liver | 11 |
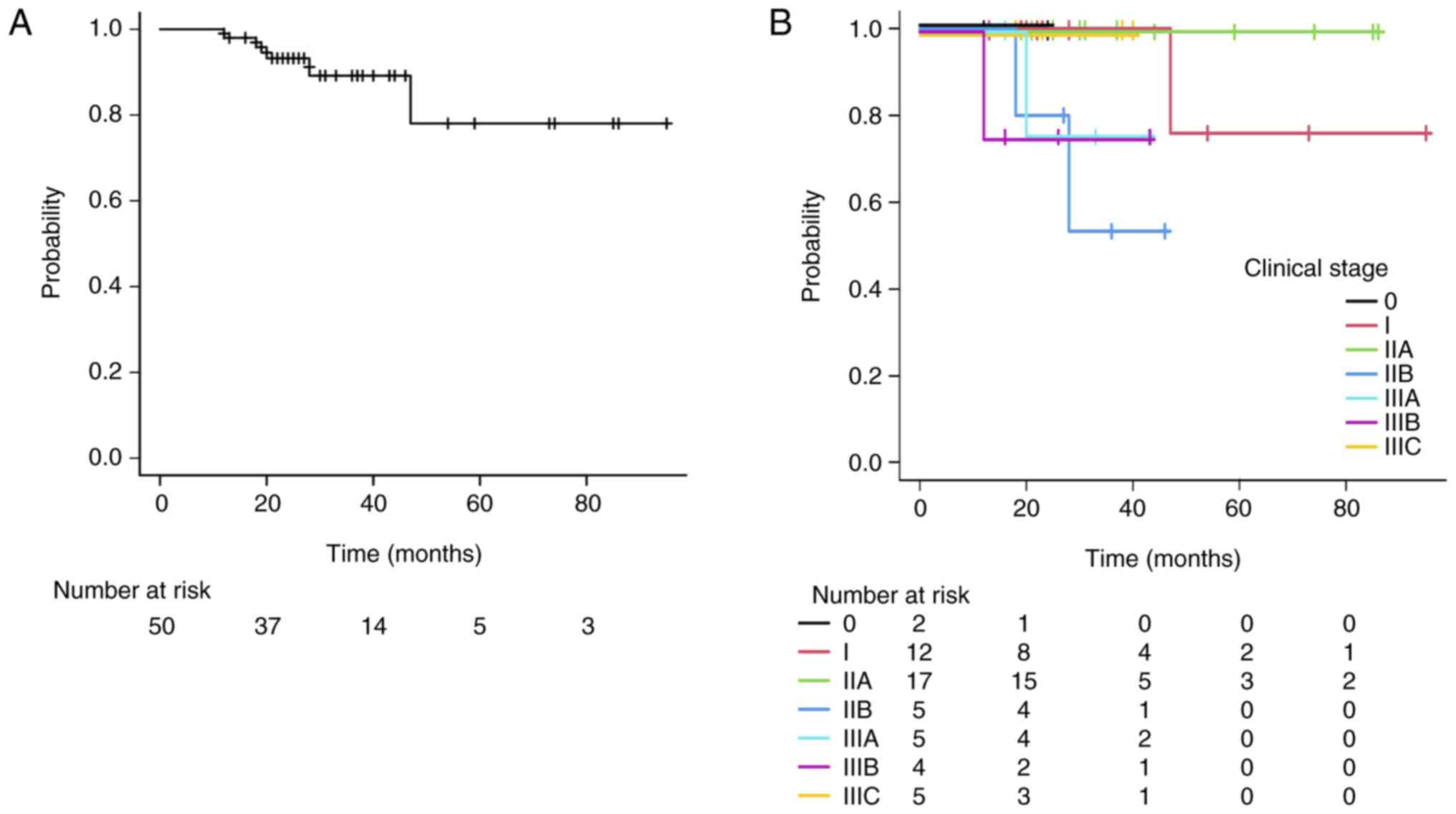
The overall 3-year local control rate was 89.3% (95%
CI: 72.5–96.0). When analyzed by clinical stage, the 3-year local
control rates were 100% for 0-I and IIA, 53.3% for IIB, 75.0% for
IIIA, 75.0% for IIIB, and 100% for IIIC (Fig. 2A and B). The 3-year disease-free
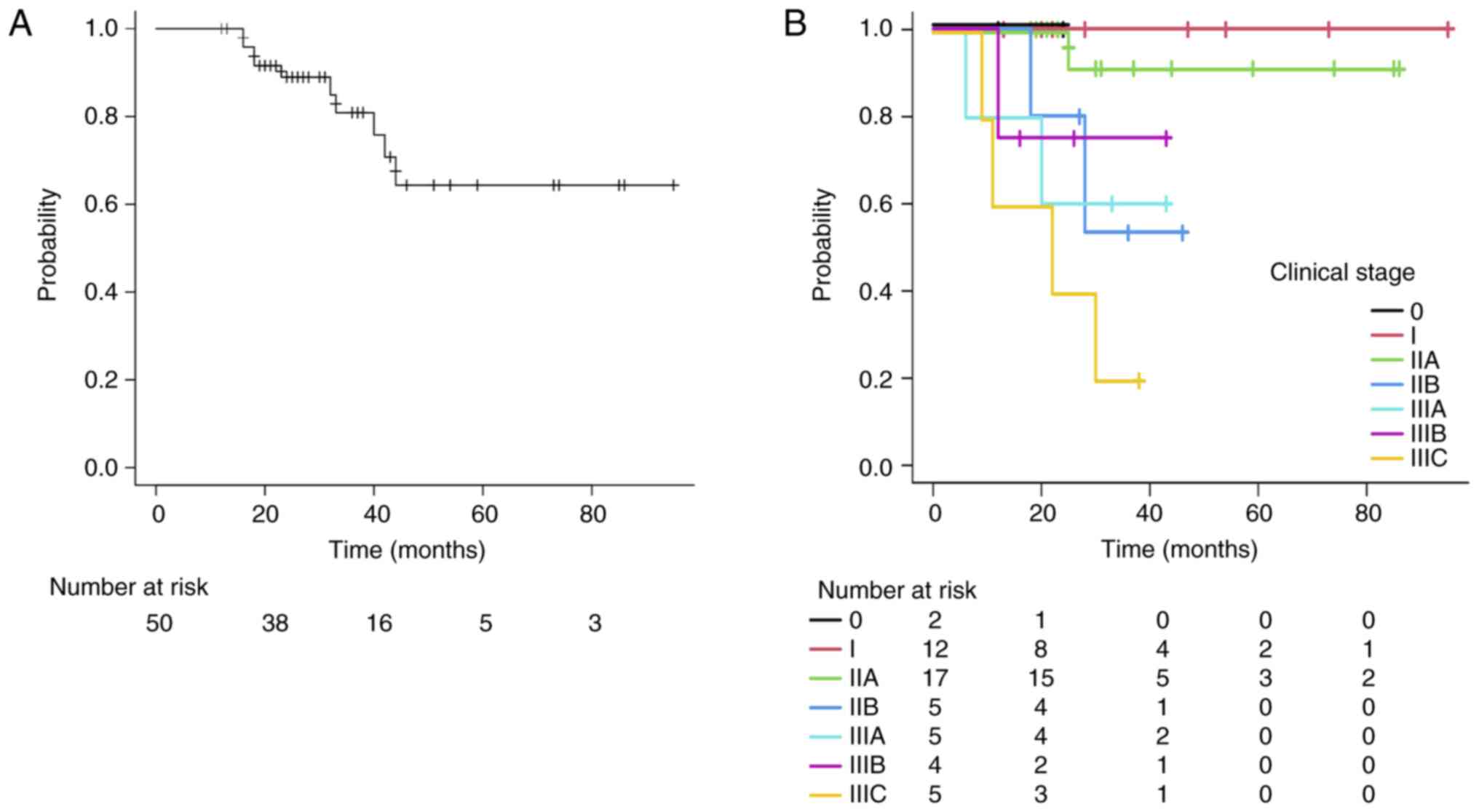
survival (DFS) rate for all patients was 80.9% (95% CI: 62.6–90.8).
Stage-specific analysis revealed 3-year DFS rates of 100% for 0-I,
91.7% for IIA, 53.3% for IIB, 60.0% for IIIA, 75.0% for IIIB, and
20.0% for IIIC (Fig. 3A and B).
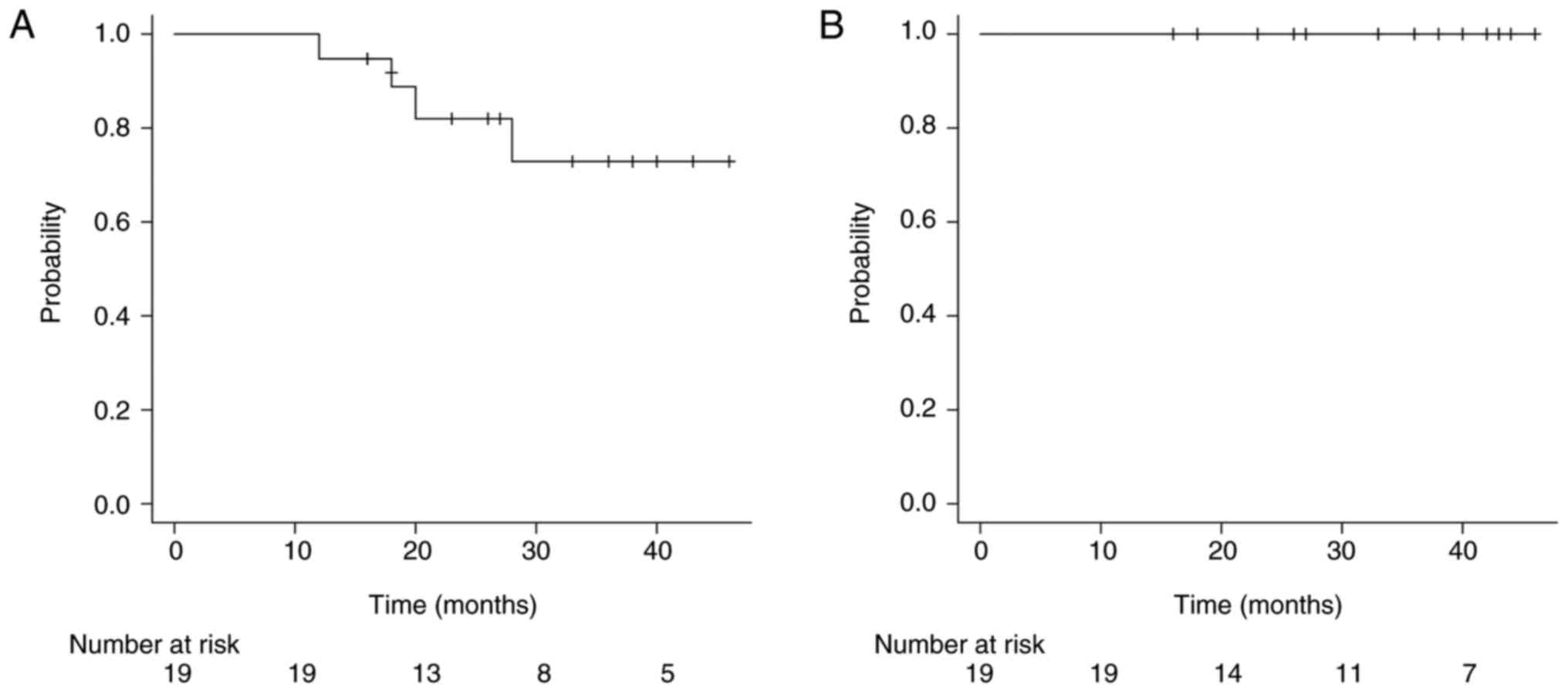
Among the node-positive patients, the 3-year local control rate for
primary tumors was 72.9% (95% CI: 41.4–89.3), (Fig. 4A), and the 3-year control rate for
metastatic lymph nodes was 100% (Fig.
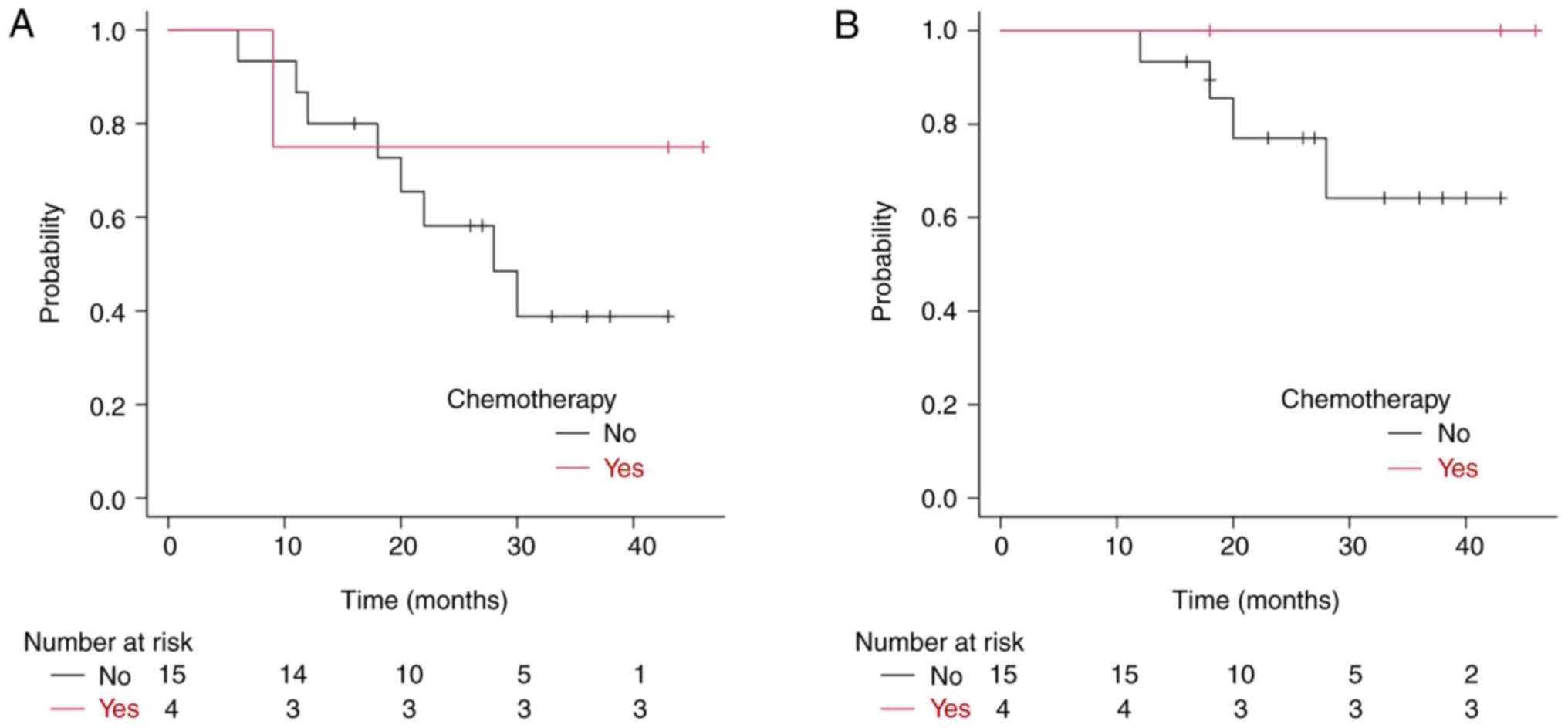
4B). In patients with stage IIB-IIIC disease and lymph node
metastases, comparison between those with and without chemotherapy
showed 3-year DFS and local control rates of 38.8% (95% CI:
13.3–64.2) and 64.2% (95% CI: 28.8–85.7), respectively, in the
non-chemotherapy group and 75.0% (95% CI: 12.8–96.1) and 100%,
respectively, in the chemotherapy group. Although the differences
were not statistically significant (P=0.347 and P=0.238), this
trend favored chemotherapy (Fig. 5A and
B). No Grade 3 or higher acute adverse events were observed.
The only reported acute reaction was mild pain during the
sensitizer injection. No significant cosmetic deterioration was
observed following treatment.
Discussion
KORTUC II is a novel enzyme-targeting
radiosensitization therapy developed at Kochi University and is the
world's first radiosensitizer designed for intratumoral injection
(1–3). This treatment is compatible with
conventional linear accelerators (linacs) used in most radiotherapy
facilities. The combination of H2O2 and HA in
KORTUC inactivates the antioxidant enzyme peroxidase within tumor
tissues. This inactivation results in oxygen generation, increased
oxygen tension, and enhanced X-ray-induced free-radical effects.
Due to the limited peroxidase activity in cancer cells,
intracellular accumulation of H2O2 occurs,
promoting apoptosis via the mitochondrial and lysosomal pathways
(5,6).
Through this mechanism, KORTUC transforms
radiation-resistant cells into radiation-sensitive cells,
significantly enhancing the efficacy of low-LET radiation, such as
X-rays (4,7,8). When
H2O2 is injected alone, it causes severe
local pain and rapid diffusion of oxygen, thereby preventing
sustained oxygen tension. However, the addition of HA not only
reduces pain but also sustains intratumoral oxygenation for up to
24 h (5,9). As both components are naturally
occurring substances in the human body-H2O2
in saliva and hyaluronate in connective tissues-KORTUC is
considered a highly safe radiosensitization method.
This study demonstrated favorable local control and
OS with KORTUC-BCT. For early-stage (0-IIA) breast cancer, the
3-year local control and DFS rates were 100 and 94%, respectively.
Ogawa et al previously reported a 5-year progression-free
survival rate of 97.1% in patients with stage I–II disease
(1). Historically, radiation
monotherapy has been used for inoperable or older patients with
breast cancer, often with conventional fractionation, yielding
modest results; 3-year local control rates range from 45 to 57%
(10–12).
Techniques such as radiofrequency ablation (RFA)
have reported complete pathological responses of 30–100% (mostly
over 60%) after resection (13–18),
and high-intensity focused ultrasound (HIFU) has shown complete
necrosis rates between 17 and 100% (14,19–22).
However, these methods often require subsequent surgery for
confirmation and are limited in scope.
For stage IIB-IIIC cases, the 3-year local control
rate was 72.9%, although the DFS rate was lower at 48.2%, with
distant metastasis occurring in more than half of the advanced
cases. Among patients with T2b or higher tumors, approximately 80%
refused chemotherapy and 40% refused hormone therapy (HT). Given
the systemic nature of breast cancer, local treatments alone, such
as KORTUC, are insufficient for advanced cases. Chemotherapy
notably improved the 3-year DFS rate, from 38.8% (without
chemotherapy) to 75% (with chemotherapy). Although this difference
was not statistically significant due to the small sample size, the
trend suggests that combining chemotherapy with local treatment is
preferable. Although we did not perform an analysis by molecular
subtype in this study, we carefully explained to patients with
high-grade tumors or high recurrence risk that, despite good local
control, distant metastases may still occur and strongly
recommended systemic therapy in conjunction with KORTUC
treatment.
Remarkably, the 3-year control rate for metastatic
lymph nodes within the irradiation field was 100%. HA has high
lymphatic affinity, and studies in rats have shown rapid migration
(within 5 min) to adjacent lymph nodes (23,24).
This supports the hypothesis that KORTUC injected into the tumor
may migrate to the lymph nodes and enhance radiosensitization.
Distant metastasis occurred in 8 patients, involving 12 sites,
primarily in stage IIA or higher. The sites included the bone
(n=4), liver (n=3), lungs (n=1), brain (n=1), ovary (n=1), muscle
(n=1), and contralateral lymph nodes (n=1), predominantly via
hematogenous spread. The potential for the abscopal effect, a
systemic antitumor response induced by localized radiation, is of
particular interest. KORTUC has demonstrated such effects through
immune activation in murine models, especially when combined with
PD-1 blockade (25). However, its
abscopal efficacy in the clinical setting has not yet been
proven.
This study highlights the potential of KORTUC as an
alternative for patients who refuse standard therapies, respecting
individual values, and enabling personalized treatment. For
patients seeking cosmetic preservation or avoiding surgery, the
KORTUC-BCT offers a promising nonsurgical option. Other
non-surgical modalities, such as RFA and HIFU (21,26–28),
have limitations; RFA requires large-bore needle insertion under
local anesthesia, and HIFU is suitable only for small (<10 mm),
superficial tumors with adequate fat tissue, making KORTUC
potentially more beneficial.
This study has several limitations. A sample size
was relatively small, which may limit the generalizability of the
findings. The retrospective design of the study inherently carries
the risk of selection bias and limits the ability to establish
causal relationships. Although early outcomes appear promising, the
follow-up period was relatively short for a breast cancer study,
making it difficult to assess long-term efficacy and safety. To
validate the clinical utility of KORTUC II, further prospective
studies with larger patient cohorts and longer follow-up periods
are warranted.
Effective administration of KORTUC requires skill,
and the tumor location and morphology affect the technique.
However, the optimal dose remains undefined, necessitating further
quantitative studies. Currently, dosing is based on tumor size;
however, the future integration of imaging analysis and AI-based
prediction models is anticipated. KORTUC demonstrated remarkable
local control beyond that achieved with radiation therapy alone.
Its efficacy has been reported for other solid tumors, including
cervical, pancreatic, and rectal cancers (17,29–31).
With proper techniques and attention paid to the risks of
intravascular injection, it remains a safe radiosensitizer. A phase
II trial for advanced breast cancer is ongoing in the UK, and phase
I trials for cervical and rectal cancers are scheduled to begin
this year. KORTUC may also be a viable treatment option for
patients with primary breast cancer who have declined standard
therapies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AH, TS and KeN designed the study and wrote the
manuscript. AH, TS and MN analyzed and interpreted the patient's
clinical data. AH, TS, JI, TI, MM, KKo, TO, AK, YY, ST and HY
performed the KORTUC treatment and statistical analysis. KaN, HA,
KY, KKi and MI contributed to collecting the relevant literature
and performing the data analysis. AH and TS confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present KORTUC II clinical study was approved by
the Ethics Committee of Osaka Medical and Pharmaceutical University
(trial no. 1973, May 10, 2010; UMIN Clinical Trials Registry,
UMIN000003734, June 10, 2010). All the patients provided written
informed consent after receiving a detailed explanation of the
procedure.
Patient consent for publication
The patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogawa Y, Kubota K, Aoyama N, Yamanishi T,
Kariya S, Hamada N, Nogami M, Nishioka A, Onogawa M and Miyamura M:
Non-surgical breast-conserving treatment (KORTUC-BCT) using a new
radio-sensitization method (KORTUC II) for patients with stage I or
II breast cancer. Cancers (Basel). 7:2277–2289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takaoka T, Shibamoto Y, Matsuo M, Sugie C,
Murai T, Ogawa Y, Miyakawa A, Manabe Y, Kondo T, Nakajima K, et al:
Biological effects of hydrogen peroxide administered intratumorally
with or without irradiation in murine tumors. Cancer Sci.
108:1787–1792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme targeting radiosensitization treatment, Kochi Oxydol
Radiation Therapy for Unresectable Carcinomas, type II (KORTUC II).
Int J Oncol. 34:609–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI
|
|
5
|
Nimalasena S, Anbalagan S, Box C, Yu S,
Boult JKR, Bush N, Howell L, Sinnett V, Murphy W, Yarnold J, et al:
Tumour reoxygenation after intratumoural hydrogen peroxide (KORTUC)
injection: A novel approach to enhance radiosensitivity. BJC Rep.
2:782024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kariya S, Sawada K, Kobayashi T, Karashima
T, Shiuin T, Nishioka A and Ogawa Y: Combination treatment of
hydrogen peroxide and X-rays induces apoptosis in human prostate
cancer PC-3 cells. Int J Radiat Oncol Biol Phys. 75:449–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogawa Y, Kobayashi T, Nishioka A, Kariya
S, Ohnishi T, Hamasato S, Seguchi H and Yoshida S: Reactive oxygen
species-producing site in hydrogen peroxide-induced apoptosis of
human peripheral T cells: Involvement of lysosomal membrane
destabilization. Int J Mol Med. 13:383–388. 2024.
|
|
8
|
Ogawa Y, Kobayashi T, Nishioka A, Kariya
S, Ohnishi T, Hamasato S, Harumichi S and Yoshida S: Reactive
oxygen species-producing site in radiation and hydrogen
peroxide-induced apoptosis of human peripheral T cells: Involvement
of lysosomal membrane destabilization. Int J Mol Med. 13:655–660.
2024.
|
|
9
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a new
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nimalasena S, Gothard L, Anbalagan S,
Allen S, Sinnett V, Mohammed K, Kothari G, Musallam A, Lucy C, Yu
S, et al: Intratumoral hydrogen peroxide with radiation therapy in
locally advanced breast cancer: Results from a Phase 1 clinical
trial. Int J Radiat Oncol Biol Phys. 108:1019–1029. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arriagada R, Mouriesse H, Sarrazin D,
Clark RM and Deboer G: Radiotherapy alone in breast cancer. I.
Analysis of tumor parameters, tumor dose and local control: The
experience of the Gustave-Roussy Institute and the Princess
Margaret Hospital. Int J Radiat Oncol Biol Phys. 11:1751–1757.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bedwinek J, Rao DV, Perez C, Lee J and
Fineberg B: Stage III and localized stage IV breast cancer:
Irradiation alone vs irradiation plus surgery. Int J Radiat Oncol
Biol Phys. 8:31–36. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pediconi F, Marzocca F, Marincola BC and
Napoli A: MRI-guided treatment in the breast. J Magn Reson Imaging.
48:1479–1488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito T, Oura S, Nagamine S, Takahashi M,
Yamamoto N, Yamamichi N, Earashi M, Doihara H, Imoto S, Mitsuyama S
and Akazawa K: Radiofrequency ablation of breast cancer: A
retrospective study. Clin Breast Cancer. 18:495–500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oura S, Tamaki T, Hirai I, Yoshimasu T,
Ohta F, Nakamura R and Okamura Y: Radiofrequency ablation therapy
in patients with breast cancers two centimeters or less in size.
Breast Cancer. 14:48–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinoshita T, Iwamoto E, Tsuda H and Seki
K: Radiofrequency ablation as local therapy for early breast
carcinomas. Breast Cancer. 18:10–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto N, Fujimoto H, Nakamura R, Arai
M, Yoshii A, Kaji S and Itami M: Pilot study of radiofrequency
ablation therapy without surgical excision for T1 breast cancer:
Evaluation with MRI and vacuum-assisted core needle biopsy and
safety management. Breast Cancer. 18:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palussière J, Henriques C, Mauriac L,
Asad-Syed M, Valentin F, Brouste V, Mathoulin-Pélissier S, de Lara
CT and Debled M: Radiofrequency ablation as a substitute for
surgery in elderly patients with nonresected breast cancer: Pilot
study with long-term outcomes. Radiology. 264:597–605. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roknsharifi S, Wattamwar K, Fishman MDC,
Ward RC, Ford K, Faintuch S, Joshi S and Dialani V: Image-guided
microinvasive percutaneous treatment of breast lesions: Where do we
stand? Radiographics. 41:945–966. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmitz AC, Gianfelice D, Daniel BL, Mali
WPTM and van der Bosch MAAJ: Image-guided focused ultrasound
ablation of breast cancer: Current status, challenges, and future
directions. Eur Radiol. 18:1431–1441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gianfelice D, Khiat A, Boulanger Y, Amara
M and Belblidia A: Feasibility of magnetic resonance imaging-guided
focused ultrasound surgery as an adjunct to tamoxifen therapy in
high-risk surgical patients with breast carcinoma. J Vasc Intervent
Radiol. 14:1275–1282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furusawa H, Namba K, Thomsen S, Akiyama F,
Bendet A, Tanaka C, Yasuda Y and Nakahara H: Magnetic
resonance-guided focused ultrasound surgery of breast cancer:
Reliability and effectiveness. J Am Coll Surg. 203:54–63. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida H: A Novel Hyaluronan-degrading
machinery mediated by HYBID (KIAA1199): New Aspects of Hyaluronan
metabolism. Kagaku to Seibutsu. 55:119–127. 2017. View Article : Google Scholar
|
|
24
|
David GJ: The lymphatic endothelial
Hyaluronan receptor LYVE-1. Glycoforum. 8:A22004.
|
|
25
|
Kemmotsu N, Zhu L, Nagasaki J, Otani Y,
Ueda Y, Dansako H, Fang Y, Date I and Togashi Y: Combination
therapy with hydrogen peroxide and irradiation promotes an abscopal
effect in mouse models. Cancer Sci. 114:3848–3856. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jolesz FA: MRI-guided focused ultrasound
surgery. Ann Rev Med. 60:417–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manenti G, Bolacchi F, Perretta T, Cossu
E, Pistolese CA, Buonomo OC, Bonanno E, Oriandi A and Simonetti G:
Small breast cancers: In vivo percutaneous US-guided radiofrequency
ablation with dedicated cool-tip radiofrequency system. Radiology.
251:339–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimbo T, Yoshida K, Nakata M, Kobata K,
Ogawa T, Kihara K, Sato C, Hori A, Takeno S, Yoshioka H, et al:
KORTUC, a novel hydrogen peroxide-based radiosensitizer for the
enhancement of brachytherapy in patients with unresectable
recurrent uterine cervical cancer. Oncol Lett. 26:3782023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishioka A, Ogawa Y, Miyatake K, Tadokoro
M, Nogami M, Hamada N, Kubota K, Kariya S, Kohsaki T, Saibara T, et
al: Safety and efficacy of image-guided enzyme-targeting
radiosensitization and intraoperative radiotherapy for locally
advanced unresectable pancreatic cancer. Oncol Lett. 8:404–408.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Usui K and Saito AI: Radiosensitization
treatment using hydrogen peroxide for inoperable rectal cancer. Mol
Clin Oncol. 21:682024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Obata S, Ishimaru Y, Miyagi S, Nakatake M,
Kuroiwa A, Ohta Y, Kan T, Kanegae S, Inoue Y, Nishizato R and
Miyazaki K: Actual practice of Kochi oxydol radiation therapy for
unresectable carcinomas by intra-tumoral administration of hydrogen
peroxide as a radiosensitizer. Mol Clin Oncol. 16:682022.
View Article : Google Scholar : PubMed/NCBI
|