Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer, with diagnoses made using clinical examination,
colonoscopy with biopsy, pelvic magnetic resonance imaging (MRI)
for local staging and computed tomography (CT) to detect distant
metastases (1,2). According to GLOBOCAN 2022, rectal
cancer accounted for 729,833 new cases globally, with an
age-standardized incidence rate (ASR) of 7.1/100,000, and caused
343,817 deaths, corresponding to an ASR of 3.1/100,000 (3,4). CRC)
represents 9–10% of all cancer diagnoses and ~9% of cancer-related
deaths worldwide (3). Established
risk factors for CRC include dietary and lifestyle exposures such
as high intake of processed and red meat, obesity and excess body
fat, alcohol consumption, tobacco use, low fiber intake, diets rich
in processed foods and sugar-sweetened beverages, and physical
inactivity (5–7). Non-modifiable factors include advanced
age, family history of CRC, hereditary syndromes such as Lynch
syndrome (hereditary nonpolyposis colorectal cancer) and familial
adenomatous polyposis (FAP), as well as inflammatory bowel
diseases, notably ulcerative colitis and Crohn's disease (8). Prognosis depends on tumor stage, lymph
node involvement, metastatic spread, histological grade and
response to neoadjuvant therapy, with early-stage disease generally
associated with improved outcomes (9,10).
Treatment is stage-specific: Early tumors may be managed with
surgery alone, whereas locally advanced cases often require
neoadjuvant chemoradiotherapy followed by surgery (11,12).
Fluoropyrimidines, such as 5-fluorouracil and capecitabine, are
commonly used in chemoradiotherapy regimens; they exert their
antitumor effects primarily by inhibiting thymidylate synthase,
thereby disrupting DNA synthesis, and by incorporating into RNA and
DNA, leading to cytotoxicity in rapidly dividing tumor cells
(13–15).
Fluoropyrimidines are commonly used chemotherapeutic
agents for treating several types of solid tumor such as
gastrointestinal, head and neck, and breast cancers (16). These agents are primarily
metabolized by dihydropyrimidine dehydrogenase (DPD) (17). Fluoropyrimidine-based regimens with
oxaliplatin or irinotecan are the standard treatment for colorectal
cancer. DPD is the key enzyme in fluoropyrimidine metabolism, with
DPYD gene variants causing DPD deficiency and increasing
toxicity risk (18). Complete DPD
deficiency is rare, occurring in 0.1–0.5% of the general population
(19,20). Partial DPD deficiency is more
common; heterozygous variant carrier rates have been reported in
3–8% of cases. Certain polymorphisms, particularly in the DPYD
gene, are associated with partial loss of enzyme activity (21,22).
Treatment-related mortality in patients with unrecognized DPD
deficiency who receive fluoropyrimidines has been reported to be
around 0.2–0.5% (19,23). The risk of fatal toxicity is
increased 5–10-fold, particularly in carriers of severe DPYD
variants (24). The presence of
DPYD variants was reported to be significantly associated
with increased treatment-related mortality [odds ratio, 34.86; 95%
confidence interval (CI), 13.96–87.05; P<0.05] (25).
The present study aimed to present a case of rectal
cancer treated with the CAPOX regimen (capecitabine and
oxaliplatin) and developing severe fluoropyrimidine toxicity due to
the DPYD mutation.
Case report
A mass was detected in the mid-rectum during a
colonoscopy performed on a 35-year-old male patient at Harran
University Medical Faculty Hospital (Sanliurfa, Turkey, in November
2023 due to rectal bleeding (Fig.
S1). The patient was admitted for advanced examination and
treatment with a preliminary diagnosis of rectal cancer. The biopsy
sample was fixed in 10% neutral formalin solution at room
temperature for 18–24 h, embedded in paraffin and sectioned at a
thickness of 2.5 µm. The sections were stained with hematoxylin and
eosin using a Leica Autostainer XL. The preparations were examined
under a light microscope (Olympus BX43). Glandular structures
exhibiting cellular and structural atypia were observed, and the
findings were consistent with adenocarcinoma. Abdominal and pelvic
MRI and thoracic CT revealed locally advanced rectal cancer
(Fig. S2). Therefore, the patient
was started on neoadjuvant CAPOX chemotherapy (Oxaliplatin 130
mg/m2 IV on day 1 + capecitabine 1,000 mg/m2
orally twice daily on days 1–14, repeated every 21 days). By the
14th day of capecitabine treatment, the patient presented with a
fever (38.5°C), grade 3 oral mucositis, abdominal pain and grade 3
diarrhea. Laboratory findings revealed grade 3 neutropenia, grade 3
thrombocytopenia (according to Common Terminology Criteria for
Adverse Events v6.0) (26),
elevated international normalized ratio (INR) and grade 1
hyperbilirubinemia. Admission laboratory results are presented in
Table I. DPD enzyme deficiency was
suspected. Serological tests for hepatitis A, B, C, toxoplasmosis,
rubella, cytomegalovirus, herpes virus and other agents, and
brucella were negative. Stool examination showed no parasitic
infections, and cultures were negative for rotavirus, adenovirus,
Giardia, Cryptosporidium, Shigella, Salmonella, E. coli, V.
cholerae, Y. enterocolitica, E. histolytica and C.
difficile toxins A/B. Blood, urine and stool cultures were
sterile.
 | Table I.Laboratory test results. |
Table I.
Laboratory test results.
| Test | Result | Normal range |
|---|
| Leukocyte,
×103/µl | 2.6 | 4.0–10.0 |
| Neutrophil,
×103/µl | 1.0 | 1.6–6.9 |
| Lymphocyte,
×103/µl | 1.6 | 1.0–2.9 |
| Hemoglobin, g/dl | 15.1 | 12.0–18.1 |
| Thrombocyte,
×103/ul | 67.0 | 142.0–424.0 |
| Glucose, mg/dl | 113.0 | 74.0–106.0 |
| Urea, mg/dl | 38.5 | 19.0–50.0 |
| Creatinine,
mg/dl | 1.0 | 0.7–1.3 |
| ALT, U/l | 17.0 | 10.0–49.0 |
| AST, U/l | 21.0 | 0.0–34.0 |
| ALP, U/l | 43.0 | 46.0–116.0 |
| GGT, U/l | 16.0 | 0.0–73.0 |
| Total bilirubin,
mg/dl | 1.4 | 0.3–1.2 |
| Direct bilirubin,
mg/dl | 0.5 | 0.0–0.3 |
| Albumin, g/dl | 4.0 | 3.2–4.8 |
| Sodium, mmol/l | 133.0 | 132.0–146.0 |
| Potassium,
mmol/l | 3.7 | 3.5–5.5 |
| Calcium, mg/dl | 8.0 | 8.7–10.4 |
| Creatinine kinase,
U/l | 38.0 | 32.0–294.0 |
| Lactate
dehydrogenase, U/l | 209.0 | 120.0–246.0 |
| Serum reactive
protein, mg/dl | 0.3 | 0.0–0.5 |
| INR | 2.2 | 0.8–1.2 |
| Prothrombin time,
sec | 25.0 | 10.5–15.5 |
| Activated prothrombin
time, sec | 124.0 | 22.0–36.0 |
At admission, treatment included granulocyte
colony-stimulating factor (G-CSF), piperacillin-tazobactam,
metronidazole, oral loperamide, glutamine and intravenous (IV) 0.9%
NaCl. On day 5 of hospitalization, severe diarrhea (>20
episodes/day) led to acute kidney injury (creatinine, 1.44 mg/dl),
electrolyte imbalances and hemodynamic instability, requiring ICU
transfer. Oral nystatin was started for suspected candidiasis.
Despite treatment, deep neutropenia and diarrhea persisted,
requiring continuous IV fluids, loperamide and octreotide infusion.
Furthermore, the cytopenia was closely followed. Grade 4
neutropenia (0.38×103/µl) presented on hospital day 2,
with a nadir of 0.002×103/µl by day 8. With daily
filgrastim, neutrophil counts normalized by day 17. Grade 4
thrombocytopenia (31×103/µl) developed by day 3,
reaching 5×103/µl on day 9 despite platelet
transfusions. Platelet counts recovered by day 24. Anemia
(hemoglobin, 10.5 g/dl) was detected on day 8 and a transfusion was
required. The recovery sequence was as follows: Neutropenia,
thrombocytopenia and finally, anemia. Moreover, by day 4, an
ongoing fever (38.4°C) and elevated C-reactive protein (CRP) (36.8
mg/dl) prompted escalation to meropenem, linezolid and oral
vancomycin administration for suspected typhlitis, along with
fluconazole prophylaxis. Persistent fever and CRP elevation led to
caspofungin initiation on day 12. On day 14, herpetic lip lesions
were treated with ganciclovir. The patient received 18 units of
platelets, 8 units of fresh frozen plasma and 4 units of
erythrocytes. Due to ongoing abdominal symptoms, imaging was
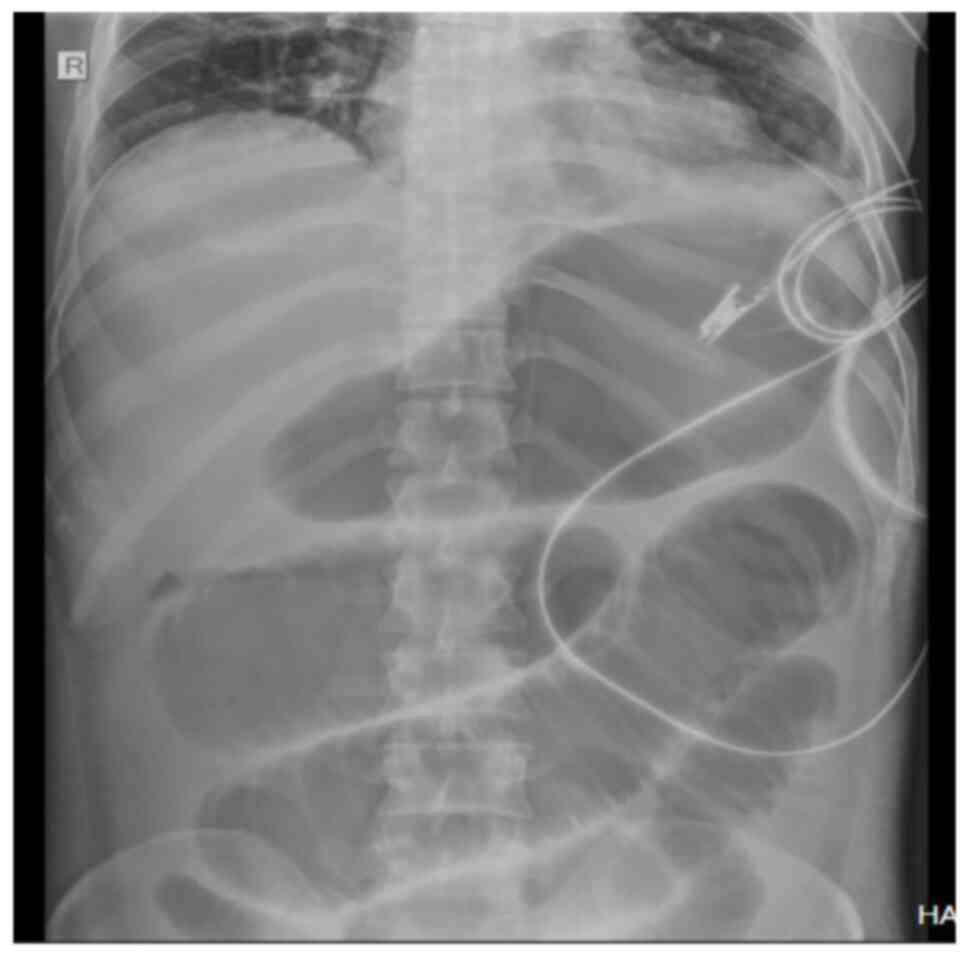
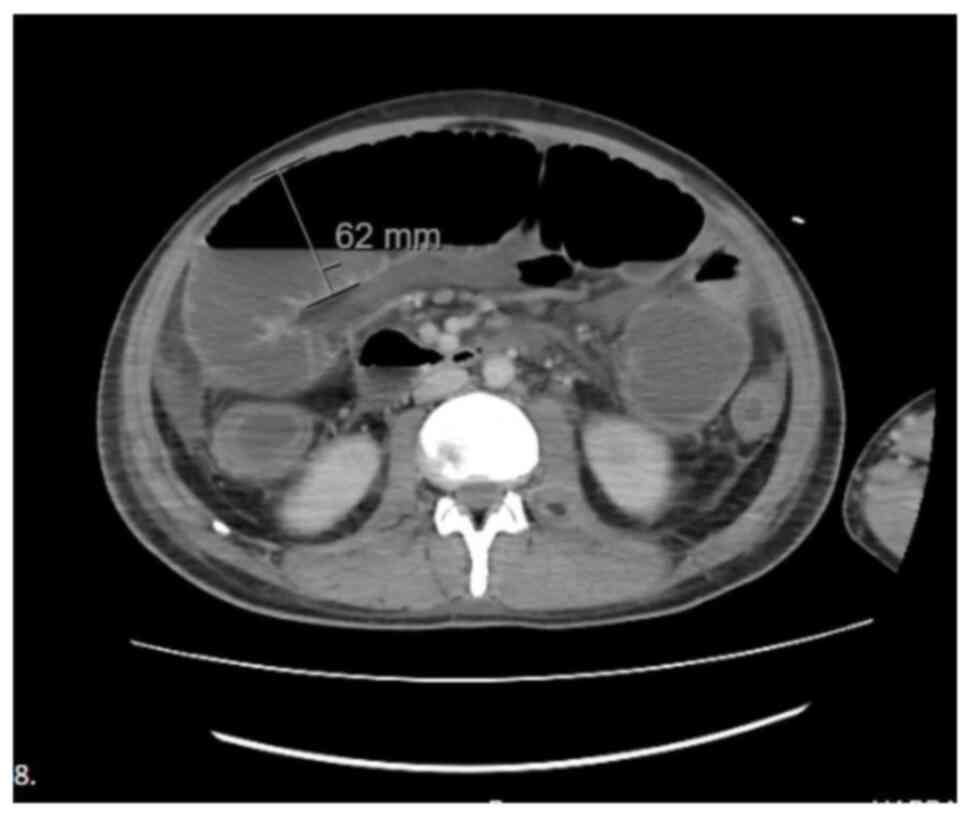
performed which revealed toxic megacolon (Figs. 1 and 2). Oral food intake was stopped, and
parenteral nutrition was initiated.
Genetic testing was performed using Sanger
sequencing to determine DPD deficiency, which was detected as
heterozygous c.2846A>T (p.Asp949Val) in Exon 22 (Fig. S3). The fever resolved by day 15,
diarrhea gradually subsided after day 20, and toxic megacolon
improved (Fig. S4). Neutropenia,
thrombocytopenia, renal dysfunction, INR and bilirubin levels
normalized during follow-up. By day 28, all antibiotic, antifungal
and antiviral treatments were discontinued as infection markers
normalized. The patient achieved full clinical recovery and was
discharged. Laboratory follow-up results are presented in Table II.
 | Table II.Laboratory results of the patient
during follow-up. |
Table II.
Laboratory results of the patient
during follow-up.
|
|
Day |
|---|
|
|
|
|---|
| Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 14 | 15 | 16 | 17 | 18 | 20 | 21 | 23 | 24 | 26 | 29 | 30 |
|---|
| Leukocyte,
103/µl | 2.6 | 1.3 | 0.8 | 0.7 | 0.6 | 0.4 | 0.5 | 0.6 | 0.5 | 0.6 | 0.7 | 1.7 | 2.3 | 3.3 | 4.5 | 7.2 | 9.9 | 11.5 | 12.9 | 12.2 | 10.2 | 6.2 | 5.1 | 4.9 |
| Neutrophil,
103/µl | 0.9 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.6 | 0.7 | 1.6 | 2.5 | 3.5 | 4.5 | 6.7 | 8.0 | 7.6 | 3.2 | 2.2 | 1.7 |
| Platelet,
103/µl | 67.0 | 58.0 | 31.0 | 12.0 | 25.0 | 38.0 | 25.0 | 15.0 | 5.0 | 27.0 | 20.0 | 22.0 | 19.0 | 50.0 | 31.0 | 25.0 | 49.0 | 59.0 | 50.0 | 98.0 | 144.0 | 192.0 | 285.0 | 381.0 |
| Hgb, g/dl | 15.0 | 15.0 | 16.0 | 15.5 | 15.0 | 13.2 | 12.1 | 10.5 | 9.4 | 8.1 | 10.0 | 9.5 | 9.9 | 7.9 | 10.6 | 11.2 | 11.2 | 11.2 | 11.3 | 10.9 | 10.2 | 9.1 | 10.4 | 10.5 |
| Creatinine,
mg/dl | 1.0 | 0.8 | 1.0 | 1.1 | 1.4 | 2.0 | 2.6 | 2.2 | 1.7 | 1.4 | 1.0 | 0.9 | 0.7 | 0.7 | 0.6 | 0.7 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 |
| ALT, U/l | 17.0 | 17.0 | 15.0 | 13.0 | 17.0 | 19.0 | 18.0 | 22.0 | 23.0 | 15.0 | 21.0 | 19.0 | 23.0 | 41.0 | 42.0 | 43.0 | 43.0 | 52.0 | 54.0 | 48.0 | 43.0 | 28.0 | 25.0 | 21.0 |
| Total bilirubin,
mg/dl | 1.4 | 1.7 | 3.1 | 4.6 | 6.2 | 5.5 | 6.2 | 5.8 | 5.6 | 5.7 | 6.1 | 5.4 | 5.2 | 3.8 | 4.2 | 4.4 | 4.1 | 3.7 | 4.3 | 3.2 | 2.2 | 1.8 | 1.3 | 0.9 |
| Direct bilirubin,
mg/dl | 0.5 | 0.9 | 0.9 | 3.4 | 4.6 | 4.3 | 5.0 | 4.9 | 4.5 | 4.4 | 4.7 | 3.7 | 3.5 | 2.5 | 2.9 | 2.7 | 2.4 | 2.3 | 1.9 | 1.4 | 1.3 | 0.8 | 0.5 | 0.7 |
| Albumin, g/dl | 4.0 | 3.9 | 3.6 | 2.7 | 2.6 | 2.9 | 2.9 | 2.5 | 2.5 | 2.6 | 2.3 | 2.3 | 2.3 | 2.0 | 2.6 | 2.5 | 2.5 | 2.5 | 2.8 | 2.8 | 2.7 | 2.5 | 2.3 | 2.7 |
| Sodium, mmol/l | 133.0 | 136.0 | 131.0 | 120.0 | 119.0 | 122.0 | 124.0 | 137.0 | 148.0 | 155.0 | 152.0 | 148.0 | 147.0 | 140.0 | 135.0 | 133.0 | 134.0 | 132.0 | 132.0 | 131.0 | 133.0 | 134.0 | 138.0 | 137.0 |
| Potassium,
mmol/l | 3.7 | 4.0 | 4.1 | 4.0 | 4.1 | 3.9 | 3.8 | 4.2 | 3.8 | 3.7 | 3.3 | 2.8 | 3.8 | 3.6 | 4.0 | 4.1 | 3.6 | 3.8 | 4.1 | 4.0 | 3.8 | 3.5 | 3.8 | 3.6 |
| LDH, U/l | 209.0 | 159.0 | 210.0 | 164.0 | 188.0 | 164.0 | 172.0 | 204.0 | 241.0 | 231.0 | 245.0 | 315.0 | 444.0 | 430.0 | 406.0 | 541.0 | 326.0 | 303.0 | 298.0 | 268.0 | 345.0 | 173.0 | 239.0 | 276.0 |
| CRP, mg/dl | 0.2 | 0.5 | 5.6 | 36.8 | 40.8 | 37.4 | 42.4 | 35.0 | 37.0 | 35.5 | 39.0 | 42.0 | 44.0 | 23.0 | 18.0 | 16.0 | 13.0 | 7.9 | 6.2 | 5.0 | 4.4 | 3.2 | 3.5 | 0.7 |
| INR | 2.1 | 1.0 | - | - | 1.8 | 1.9 | 1.8 | - | 2.2 | 1.8 | 1.9 | - | 1.7 | 1.3 | 1.2 |
|
|
| 1.2 |
|
|
|
|
|
| PT, sec | 25.0 | 12.0 | - | - | 21.0 | 22.0 | 24.0 | - | 26.0 | 22.0 | 23.0 | - | 19.0 | 17.0 | 15.0 |
|
|
| 14.0 |
|
|
|
|
|
| APT, sec | 124.0 | 19.0 | - | - | 26.0 | 38.0 | 28.0 | - | 27.0 | 28.0 | 27.0 | - | 28.0 | 26.0 | 25.0 |
|
|
|
|
|
|
|
|
|
In February 2024, a follow-up abdominal MRI revealed
a partial radiological response (Fig.
S5). Based on this finding, the multidisciplinary tumor board
recommended a low anterior resection, which was performed at the
Department of General Surgery, Koç University Hospital, Istanbul,
Turkey, Histopathological examination of the surgical specimen
confirmed a partial tumor response (data not shown). Adjuvant
chemotherapy was not administered due to the development of severe
DPD deficiency. The patient was followed up with abdominal MRI and
thoracic CT scans at 3-month intervals. The latest imaging
modalities revealed no evidence of disease recurrence as of June
2025 (Fig. S6).
Discussion
DPD deficiency is a life-threatening complication of
fluoropyrimidine-based chemotherapy (27). In a meta-analysis of eight cohort
studies (n=7,365), four DPYD variants [c.1905+1G>A,
c.2846A>T, c.1679T>G and c.1129–5923C>G (HapB3)] were
notably associated with severe fluoropyrimidine-associated
toxicity, with relative risks of 2.9 (95% CI, 1.8–4.6), 3.0 (95%
CI, 2.2–4.1), 4.4 (95% CI, 2.1–9.3) and 1.6 (95% CI, 1.3–2.0),
respectively (19). In a DPD
deficiency study, c.2846A>T (Asp949Val) was associated with
increased toxicity in 1.4% (30/2,116) of patients, who presented
with neutropenia, nausea, vomiting, diarrhea and infection
(28). Another study reported that
all 10 patients with the 2846A>T variant were heterozygous, and
6/10 experienced grade 3–4 toxicities within the first two cycles
(29). In the present case,
heterozygosity at c.2846A>T was identified, and the patient
developed grade 4 neutropenia, nausea, vomiting and diarrhea during
the initial cycles of treatment, similar to previously reported
cases. These toxicities typically begin with nonspecific symptoms
such as neutropenic fever, mucositis and diarrhea (30), and in the patient in the present
case, these emerged by day 14 of therapy. Previous research
indicates that symptom onset generally occurs between days 10–24,
with gastrointestinal involvement being common (31). Clinically, oncologists should remain
vigilant for possible DPD deficiency in patients who present with
high-grade fluoropyrimidine-related toxicities, particularly in the
early phase of treatment.
As the patient in the present case received CAPOX,
we hypothesized that the clinical spectrum was attributable to
oxaliplatin toxicity; however, the patient did not exhibit any
neuropathy or cold paresthesias that could be associated with acute
oxaliplatin toxicity. There was no acute hemolytic anemia
associated with oxaliplatin due to predominance of direct
bilirubin. Similarly, no elevations in alanine aminotransferase or
aspartate aminotransferase were observed, suggesting acute liver
toxicity associated with oxaliplatin. Indeed, genetic testing
identified DPD deficiency, and the current clinical condition was
attributed to this deficiency.
The present patient demonstrated worsening of
symptoms despite aggressive treatment. The neutrophil count of the
patient in the present case declined from 950 to 50/µl by day 3,
reducing to 2/µl on day 8 despite G-CSF therapy. This profound
neutropenia necessitated early broad-spectrum antibiotics.
Therefore, piperacillin-tazobactam and metronidazole were
initiated, later escalating to meropenem, linezolid and antifungal
coverage. Despite extensive microbiological testing, no causative
pathogen was identified. A previous report indicated fatal
infection such as cytomegalovirus enterocolitis (32).
Severe diarrhea is another hallmark of severe
fluoropyrimidine-associated toxicity. In the present case, the
patient's diarrhea symptoms progressed from grade 3 to 4, requiring
intensive care hospitalization. Despite loperamide and intravenous
hydration, symptoms persisted, requiring high-dose octreotide
infusion. This led to abdominal distension, ileus and toxic
megacolon, and the diarrhea continued. Octreotide was suspected to
contribute to these complications.
Furthermore, slight fluctuations in certain
laboratory parameters (leukocyte, neutrophil, platelet, total and
direct bilirubin) were observed; however, these were considered to
be minimal with no notable clinical impact as the variations were
within acceptable clinical and laboratory limits (Table II). The clinical condition of the
patient remained stable despite these changes. Additionally, a
search of the literature revealed no research specifically
addressing such fluctuations in laboratory values in patients with
DPD deficiency.
Currently, routine DPYD genetic testing is
not standard in several countries, including Turkey; however, the
European Medicines Agency recommends pre-treatment screening to
adjust dosing and prevent toxicity (24). In most regions, testing is performed
retrospectively, potentially underestimating mortality (33). According to Clinical
Pharmacogenetics Implementation Consortium (CPIC) guidelines,
fluoropyrimidine dosing should be based on DPD activity assessed by
uracil concentration and the dihydrouracil to uracil (UH2:Ura)
ratio. Standard dosing is appropriate for normal DPD activity
(uracil, <16 ng/ml;, UH2:Ura, >10). In partial DPD deficiency
(uracil, ≥16 ng/ml and/or UH2:Ura, <10), a 50% dose reduction is
recommended. In complete DPD deficiency, fluoropyrimidines should
be avoided due to high toxicity risk (20).
Despite having a heterozygous DPYD mutation, the
clinical course was severe, although severe diarrhea continued,
toxic megacolon developed, and the patient was discharged with full
recovery with long-term supportive treatment.
Moreover, there are certain limitations in the
present case. Firstly, DPD activity measurement was not performed
using the UH2:Ura ratio recommended for fluoropyrimidine dose
modification according to the CPIC guidelines, and therefore
adjuvant chemotherapy treatment was not administered. Secondly, a
single case cannot clearly establish causality, estimate risk
magnitude, or offer generalizable clinical recommendations based on
case series.
In conclusion, patients with suspected DPD
deficiency should be managed as immunosuppressed individuals, akin
to stem cell transplant recipients. Intensive monitoring,
broad-spectrum antimicrobial therapy, antifungals and aggressive
supportive care, including blood transfusions and ICU admission,
are crucial to improve outcomes in these high-risk patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ST was the major contributor to writing the
manuscript. ST and OK analysed the data and conceived and design of
the study. ST and OK confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
National Comprehensive Cancer Network
(NCCN), . Clinical practice guidelines in oncology: Rectal Cancer.
Version 2.2024.
|
|
2
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Dilawari RA, Engstrom
PF, et al: Rectal cancer, version 2.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:1129–1161. 2022.
View Article : Google Scholar
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
4
|
International Agency for Research on
Cancer (IARC), . Global cancer observatory: Cancer today. IARC;
Lyon: 2022
|
|
5
|
World cancer research fund/American
ınstitute for cancer research, . Diet, nutrition, physical activity
and colorectal cancer. Continuous Update Project Expert Report.
2018.
|
|
6
|
Bouvard V, Loomis D, Guyton KZ, Grosse Y,
Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H and Straif K;
International Agency for Research on Cancer Monograph Working
Group, : Carcinogenicity of consumption of red and processed meat.
Lancet Oncol. 16:1599–1600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Botteri E, Borroni E, Sloan EK, Bagnardi
V, Bosetti C, Peveri G, Santucci C, Specchia C, van den Brandt P,
Gallus S and Lugo A: Smoking and colorectal cancer risk: An overall
and dose-response meta-analysis. Ann Oncol. 31:545–559. 2020.
|
|
8
|
Keum N and Giovannucci E: Global burden of
colorectal cancer: Emerging trends, risk factors and prevention
strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative versus postoperative chemoradiotherapy for
rectal cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D; ESMO Guidelines Committee, :
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl):iv22–iv40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Gijn W, Marijnen CAM, Nagtegaal ID,
Kranenbarg EM, Putter H, Wiggers T, Rutten HJT, Påhlman L,
Glimelius B and van de Velde CJH; Dutch Colorectal Cancer Group, :
Preoperative radiotherapy combined with total mesorectal excision
for resectable rectal cancer: 12-year follow-up of the multicentre,
randomised Dutch TME trial. Lancet Oncol. 12:575–582. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC; EORTC Radiotherapy Group Trial 22921, : Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, et al: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Houghton JA, Tillman DM and Harwood FG:
Ratio of 5-fluorouracil incorporation into RNA and DNA of human
colon carcinoma cells determines cytotoxicity. Cancer Res.
55:611–617. 1995.
|
|
16
|
Gross E, Busse B, Riemenschneider M,
Neubauer S, Seck K, Klein HG, Kiechle M, Lordick F and Meindl A:
Strong association of a common dihydropyrimidine dehydrogenase gene
polymorphism with fluoropyrimidine-related toxicity in cancer
patients. PLoS One. 3:e40032008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thorn CF, Marsh S, Carrillo MW, McLeod HL,
Klein TE and Altman RB: PharmGKB summary: Fluoropyrimidine
pathways. Pharmacogenet Genomics. 21:237–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paulsen NH, Vojdeman F, Andersen SE,
Bergmann TK, Ewertz M, Plomgaard P, Hansen MR, Esbech PS, Pfeiffer
P, Qvortrup C and Damkier P: DPYD genotyping and dihydropyrimidine
dehydrogenase (DPD) phenotyping in clinical oncology. A clinically
focused minireview. Basic Clin Pharmacol Toxicol. 131:325–346.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meulendijks D, Henricks LM, Sonke GS,
Deenen MJ, Froehlich TK, Amstutz U, Largiadèr CR, Jennings BA,
Marinaki AM, Sanderson JD, et al: Clinical relevance of DPYD
variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as
predictors of severe fluoropyrimidine-associated toxicity: A
systematic review and meta-analysis of individual patient data.
Lancet Oncol. 16:1639–1650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amstutz U, Henricks LM, Offer SM,
Barbarino J, Schellens JHM, Swen JJ, Klein TE, McLeod HL, Caudle
KE, Diasio RB and Schwab M: Clinical pharmacogenetics
ımplementation consortium (CPIC) guideline for dihydropyrimidine
dehydrogenase genotype and fluoropyrimidine dosing: 2017 update.
Clin Pharmacol Ther. 103:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deenen MJ, Meulendijks D, Cats A,
Sechterberger MK, Severens JL, Boot H, Smits PH, Rosing H,
Mandigers CM, Soesan M, et al: Upfront genotyping of DPYD*2A to
individualize fluoropyrimidine therapy: A safety and cost analysis.
J Clin Oncol. 34:227–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lunenburg CATC, van der Wouden CH,
Nijenhuis M, Crommentuijn-van Rhenen MH, de Boer-Veger NJ, Buunk
AM, Houwink EJF, Mulder H, Rongen GA, van Schaik RHN, et al: Dutch
pharmacogenetics working group (DPWG) guideline for the gene-drug
interaction of DPYD and fluoropyrimidines. Eur J Hum Genet.
28:508–517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henricks LM, Lunenburg CATC, de Man FM,
Meulendijks D, Frederix GWJ, Kienhuis E, Creemers GJ, Baars A,
Dezentjé VO, Imholz ALT, et al: DPYD genotype-guided dose
individualisation of fluoropyrimidine therapy in patients with
cancer: A prospective safety analysis. Lancet Oncol. 19:1459–1467.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
European Medicines Agency (EMA), .
Fluorouracil and fluorouracil-related substances (capecitabine,
tegafur and flucytosine): Direct healthcare professional
communication on pre-treatment testing to identify DPD-deficient
patients at increased risk of severe toxicity. EMA; Amsterdam:
2020
|
|
25
|
de Moraes FCA, de Almeida Barbosa AB, Sano
VKT, Kelly FA and Burbano RMR: Pharmacogenetics of DPYD and
treatment-related mortality on fluoropyrimidine chemotherapy for
cancer patients: a meta-analysis and trial sequential analysis. BMC
Cancer. 24:12102024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Cancer Institute (NCI), . Common
Terminology Criteria for Adverse Events (CTCAE). Version v6.0.U.S.
Department of Health and Human Services; 2025
|
|
27
|
Morel A, Boisdron-Celle M, Fey L,
Lainé-Cessac P and Gamelin E: Identification of a novel mutation in
the dihydropyrimidine dehydrogenase gene in a patient with a lethal
outcome following 5-fluorouracil administration and the
determination of its frequency in a population of 500 patients with
colorectal carcinoma. Clin Biochem. 40:11–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morel A, Boisdron-Celle M, Fey L, Soulie
P, Craipeau MC, Traore S and Gamelin E: Clinical relevance of
different dihydropyrimidine dehydrogenase gene single nucleotide
polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther.
5:2895–2904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Madi A, Fisher D, Maughan ST, Colley JP,
Meade AM, Maynard J, Humphreys V, Wasan H, Adams RA, Idziaszczyk S,
et al: Pharmacogenetic analyses of 2183 patients with advanced
colorectal cancer; potential role for common dihydropyrimidine
dehydrogenase variants in toxicity to chemotherapy. Eur J Cancer.
102:31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grothey A, Sobrero AF, Shields AF, Yoshino
T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, et al:
Duration of adjuvant chemotherapy for stage III colon cancer. N
Engl J Med. 378:1177–1188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagiwara Y, Yamamoto Y, Inagaki Y,
Tomisaki R, Tsuji M, Fukuda S, Fukuda S, Onoda T, Suzuki H, Niisato
Y, et al: Severe gastrointestinal disorder due to capecitabine
associated with dihydropyrimidine dehydrogenase deficiency: A case
report and literature review. Intern Med. 61:2449–2455. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inoue F, Yano T, Nakahara M, Okuda H,
Amano H, Yonehara S and Noriyuki T: Cytomegalovirus enterocolitis
in a patient with dihydropyrimidine dehydrogenase deficiency after
capecitabine treatment: A case report. Int J Surg Case Rep.
56:55–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ragia G, Maslarinou A, Atzemian N, Biziota
E, Koukaki T, Ioannou C, Balgkouranidou I, Kolios G, Kakolyris S,
Xenidis N, et al: Implementing pharmacogenetic testing in
fluoropyrimidine-treated cancer patients: DPYD genotyping to guide
chemotherapy dosing in Greece. Front Pharmacol. 14:12488982023.
View Article : Google Scholar : PubMed/NCBI
|