Introduction
Globally, ovarian cancer is the eighth most common
cancer among women and the second leading cause of death from
gynecological cancer. In 2020, it accounted for an estimated 3.7%
of cases and 4.7% of cancer deaths (1,2).
Ovarian cancer is associated with a poor prognosis because 70% of
cases are diagnosed at stage III or IV disease (3). Brain metastasis from ovarian cancer is
extremely rare, accounting for less than 1% of all ovarian cancers
(4). The most common histological
type of such brain metastasis from ovarian cancer is serous
carcinoma. Brain metastasis from clear cell ovarian cancer is
particularly uncommon, and its pathology remains unclear (5).
Few cases of ovarian cancer metastasizing to the
brain have been reported, with the average period from initial
diagnosis to brain metastasis being approximately 19.6 months; the
median survival time after diagnosis of brain metastasis is
reported to be less than 6 months (6,7).
Because the prognosis of this type of brain metastasis is poor, few
reports regarding the course of the disease and its treatment
exist, and no consistent consensus regarding treatment has been
established. Given the uniqueness of metastasis of ovarian cancer
to the brain and the difficulty in treating repeated metastases,
the best treatment options for ovarian cancer and the various
outcomes must be considered. Herein, we report a rare case of brain
metastasis that occurred more than 20 years after ovarian cancer
was initially diagnosed.
The patient provided written informed consent prior
to receiving chemotherapy or radiotherapy in our hospital and
consent to publish.
Case report
In February 2025, a 76-year-old woman, gravida 2
para 2, developed sudden confusion and dizziness caused by brain
metastases of ovarian cancer after undergoing chemotherapy for lung
metastases of ovarian cancer. When she was 57 years old, she
presented to a clinic with abdominal swelling, which had been
present for 6 months. The patient was first introduced to our
hospital with suspected ovarian cancer in July 2005. She had a
history of hypertension and diabetes, which were controlled with
medication. Magnetic resonance imaging (MRI) and computed
tomography (CT) scans showed a massive ovarian tumor without
distant metastasis. In 2005, the patient underwent abdominal
hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy,
pelvic lymphadenectomy, and para-aortic lymphadenectomy. Based on
the pathological findings of the specimens obtained during the
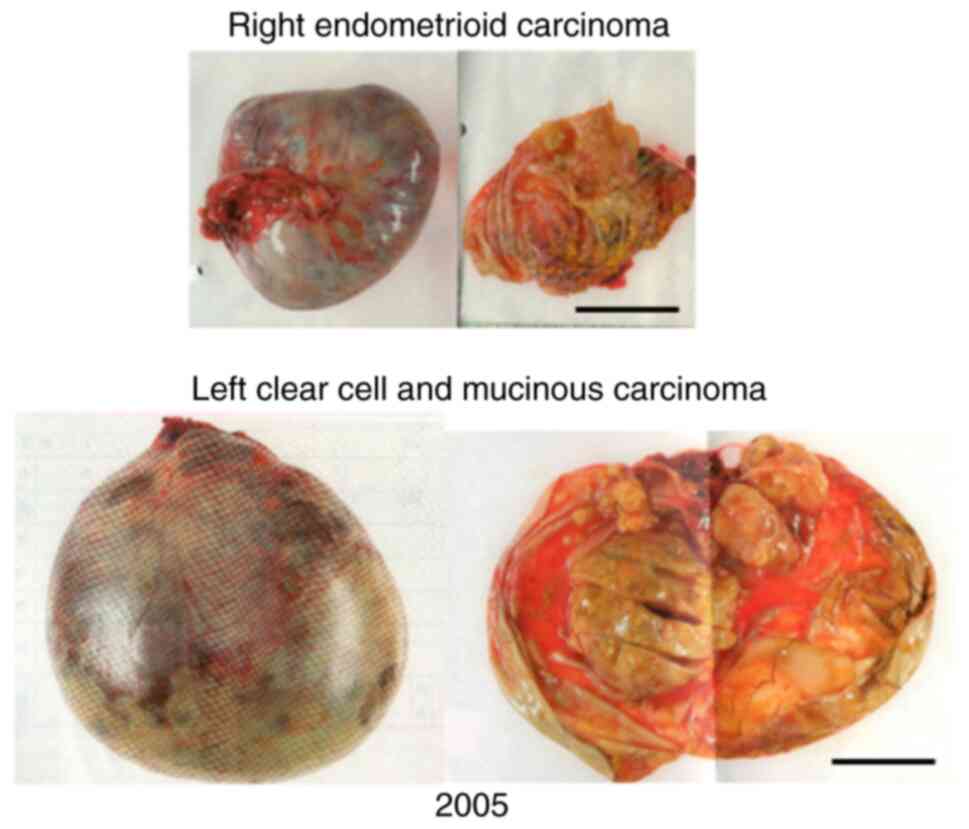
surgery, the final diagnosis was ovarian cancer, including clear
cell and mucinous carcinomas in the left ovary and endometrioid
carcinoma in the right ovary (Fig.
1). Six cycles of paclitaxel-carboplatin chemotherapy were
administered as adjuvant therapy.
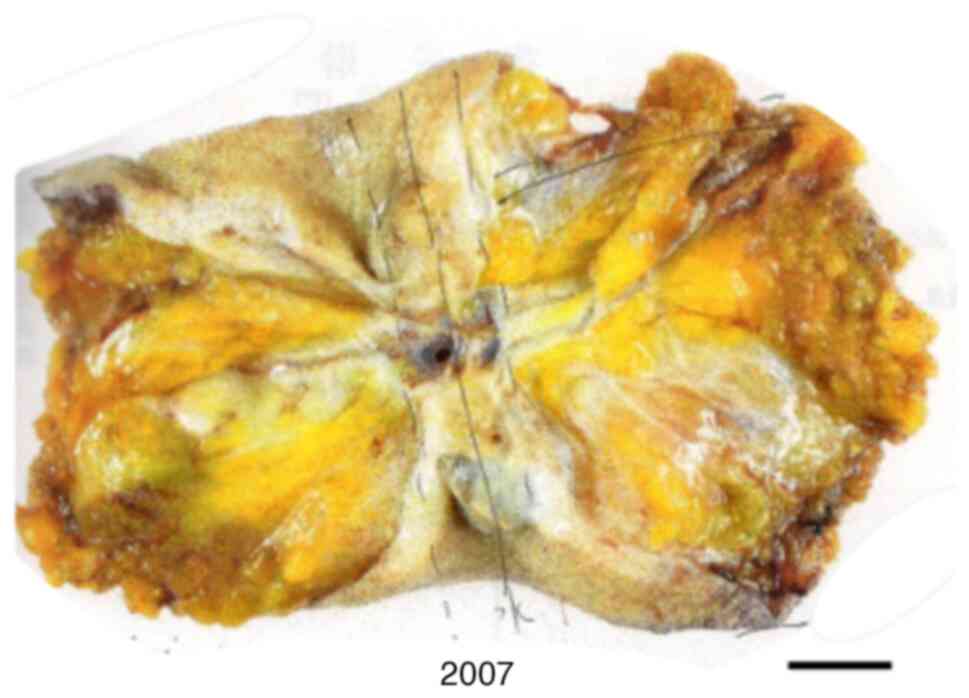
In 2007, the patient was diagnosed with an umbilical
tumor and underwent umbilical tumor resection. The resulting
diagnosis was recurrent umbilical clear cell carcinoma (Fig. 2).
In 2011, the patient developed swelling in her right
inguinal lymph node. In February 2012, she underwent a resection
and was diagnosed with recurrent endometrioid cancer. Postoperative
radiotherapy (60 Gy in 30 fractions) was administered from March to
April 2012. In March 2012, the patient presented with an enlarged
lymph node in the left groin, and resection was performed in
September 2013. The lesion was diagnosed as recurrent endometrioid
carcinoma. Postoperative radiotherapy (50 Gy in 25 fractions) was
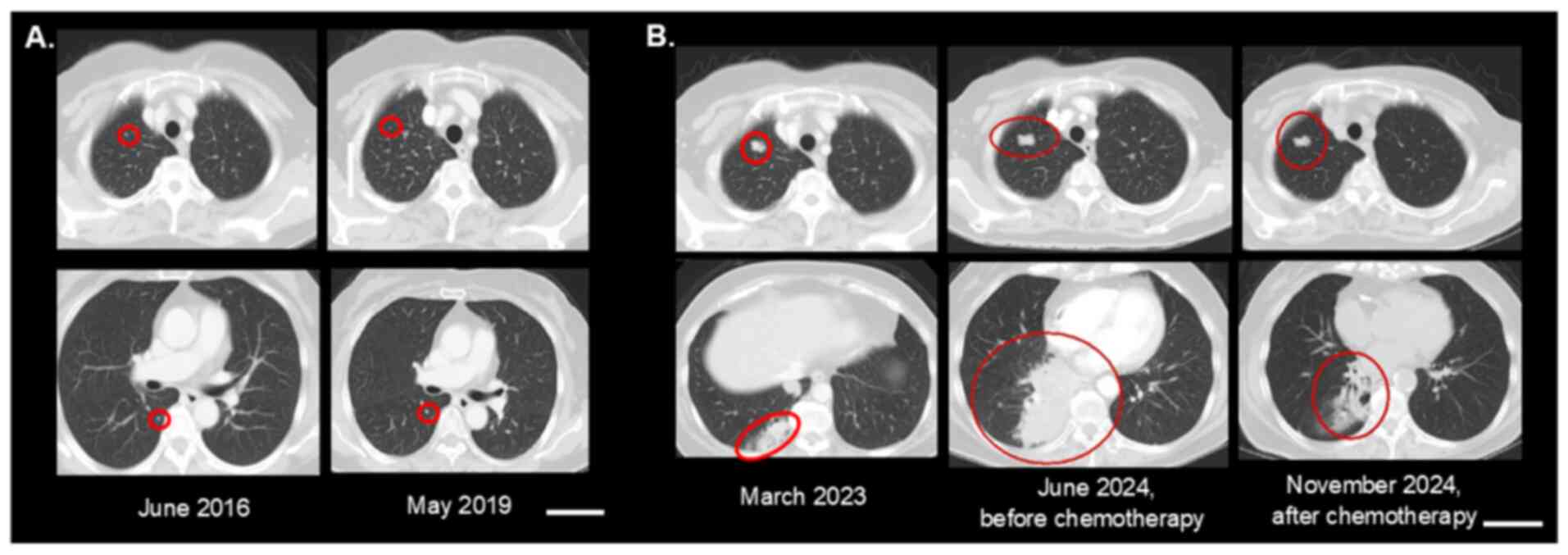
administered from August to October 2013. In June 2016, CT revealed
suspected lung metastasis; however, because the sizes of the
lesions remained constant for several years, the patient was placed
under observation (Fig. 3A).
In September 2019, recurrent metastasis was again
noted in the left inguinal lymph node. Considering that the
previous radiotherapy had enabled relatively long-term control of
the disease, we performed radiotherapy at the same site to achieve
local control. Intensity-modulated radiotherapy at 48 Gy in 12
fractions was administered with a 12 Gy boost in 4 fractions; the
total dosage administered was 60 Gy in 16 fractions.
In August 2023, the metastases in the lung grew to a
maximum diameter of 2 cm (Fig. 3B),
and chemotherapy was initially planned. However, against the
treatment guidelines of our institution, the patient requested and
underwent palliative radiotherapy (35 Gy in 10 fractions) at
another hospital.
The metastases in the lower lobe of the lung grew
larger and spread further, and the patient returned to our hospital
in May 2024 with a severe cough. Biopsy of the lung and regional
subcarinal lymph nodes revealed a clear cell carcinoma, which was
diagnosed as metastasis from ovarian cancer. To accurately
determine the pathological condition, we attempted homologous
recombination deficiency testing on ovarian surgical specimens;
however, given that the pathological specimens were from 20 years
ago, their processing was not sufficiently accurate to allow
homologous recombination deficiency testing. Instead, BRCA analysis
was performed on a blood sample collected in May 2024. The analysis
was commissioned from Myriad Genetic Laboratories, Inc. Genomic DNA
was extracted from whole blood, and polymerase chain reaction
amplification and Sanger sequencing were subsequently performed to
check for mutations in the BRCA1/2 genes. No BRCA1/2
mutations were detected.
Paclitaxel-carboplatin chemotherapy was resumed from
June to November 2024; the tumor size was partially reduced, and
the severe cough disappeared (Fig.
3B). The patient underwent treatment with a poly adenosine
diphosphate (ADP) ribose polymerase inhibitor (olaparib; 600
mg/day) from January 2025 as maintenance therapy for
platinum-sensitive recurrent ovarian cancer. In February 2025, she
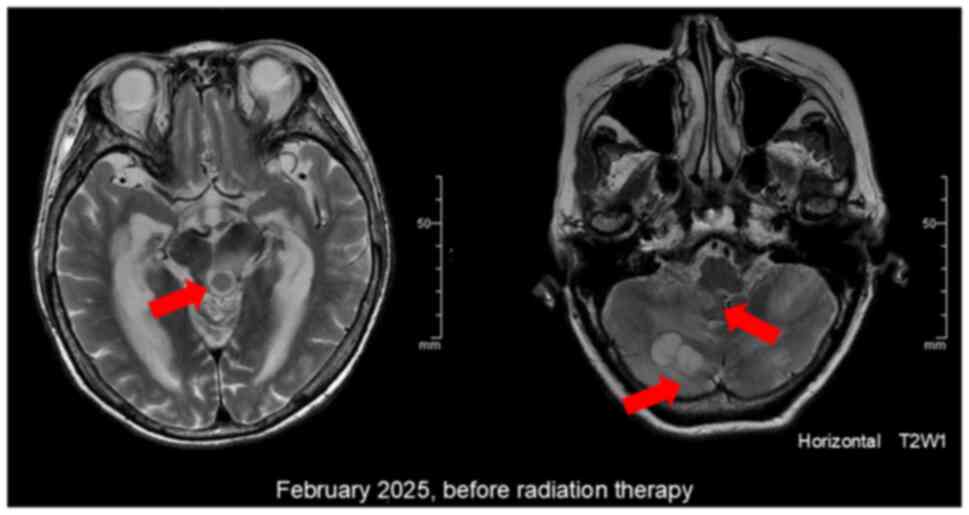
developed sudden confusion and dizziness. CT and MRI scans showed
significant multiple brain metastases and enlargement of the lung
metastases (Fig. 4). The patient
had impaired consciousness and difficulty moving; consequently, her
ability to perform activities of daily living was significantly
reduced. The patient's overall condition was poor, and she was
unable to tolerate invasive tests such as biopsy of the brain tumor
and collection of cerebrospinal fluid. MRI revealed multiple
lesions in the cerebellum, cerebral parenchyma, basal ganglia, and
brainstem, accompanied by extensive cerebral edema surrounding the
lesions. The lesions demonstrated low to isointense signal on
T1-weighted imaging and mildly hyperintense signal on T2-weighted
imaging. The patient also presented with multiple pulmonary
metastases, which were enlarging, consistent with ovarian cancer.
Based on the imaging and clinical findings, a diagnosis of multiple
cerebral metastases from ovarian cancer was made (8).
The invasiveness of standard therapies for brain
metastasis, such as surgical resection or stereotactic
radiosurgery, would have been intolerable in this patient.
Consequently, palliative therapy was chosen, and palliative whole
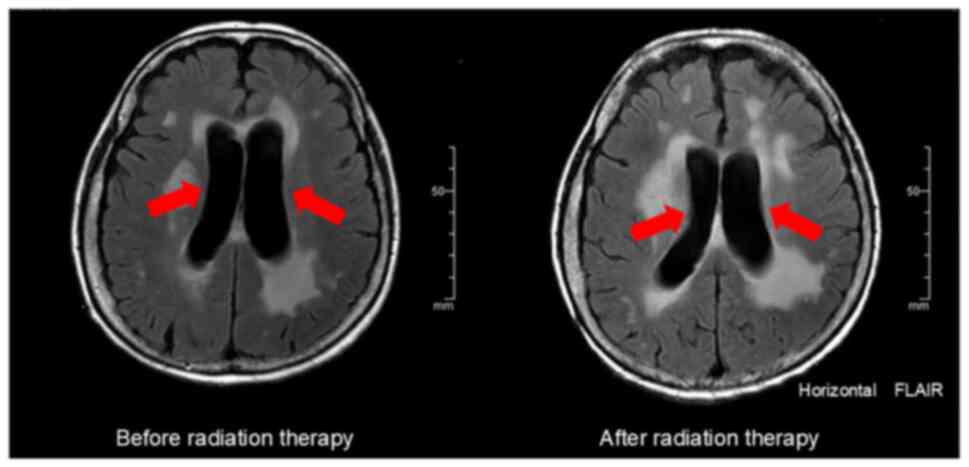
brain radiotherapy (30 Gy in 10 fractions) was administered.
Although only slight improvement in the hydrocephalus was observed
on MRI, the patient's clinical symptoms improved (Fig. 5). Her sense of orientation improved,
enabling her to understand her circumstances. Additionally, her
stability improved, allowing her to remain seated in bed or in a
wheelchair, as well as to perform tasks such as holding a spoon to
eat.
The patient was transferred to a nursing home. To
date, 6 months after the transfer, the patient is still alive.
Discussion
Cases of metastasis to the brain of an ovarian
cancer that was initially diagnosed 20 years earlier are extremely
rare. The incidence of ovarian cancer metastasizing to the brain is
estimated to be only 1 to 3% (4).
In a Surveillance, Epidemiology, and End Results-based study
involving 2,418 cases, 35 (1.6%) patients developed metastases in
the brain, 13 (0.54%) developed combined metastases in the lung and
brain, and only 3 (0.12%) developed metastases in the lung and/or
brain only (9). One clinical report
of eight cases indicated a median interval of 19.6 months (range,
0.1-61.6 months) from the initial diagnosis of ovarian cancer to
the detection of brain metastases (6). A previous review of 38 clinical series
comprising 521 patients with central nervous system metastases from
ovarian carcinoma, and spanning the years 1978 to 2011, reported an
average interval of 24.3 months (range, 11–46 months). The shortest
recorded interval was 0 months and the longest 291 months (4,7,10–44).
Considering the reports to date, an interval of 20 years from the
initial diagnosis of ovarian cancer to brain metastasis is
extremely long.
The histology of the ovarian cancer with brain
metastases observed in the current case was also rare. In previous
studies, the most common histological type associated with brain
metastases was high-grade serous carcinoma (77.6%), whereas clear
cell carcinoma accounted for only 5.2% (5). In general, brain metastasis from
ovarian cancer is associated with a poor prognosis (45). One study reported a median overall
survival of 8.3 months (range, 1–28 months) following the diagnosis
of brain metastasis (46).
First-line chemotherapy drugs, such as paclitaxel and platinum, are
reportedly unable to cross the blood-brain barrier, making the
treatment of brain metastases difficult (47–49).
Particularly, several studies have shown that conventional
platinum-based chemotherapy regimens yield a poorer prognosis in
patients with clear cell carcinoma than in patients with serous
subtypes (50–53).
Clear cell carcinoma has a low sensitivity to
platinum-based chemotherapy. The findings of an in vitro
study suggest that the low proliferation of such carcinomas may
contribute to cisplatin resistance (54,55).
Indeed, the Ki-67 labeling index was found to be significantly
lower in clear cell carcinoma than in serous adenocarcinoma
(54,55). In the present case, the patient's
return to the hospital likely coincided with increased tumor
activity in a typically slow-growing clear cell carcinoma. The
delayed resumption of chemotherapy for lung metastases may have
enabled residual disease to progress and ultimately metastasize to
the brain.
Although the use of poly ADP-ribose polymerase
inhibitors has shown efficacy in overcoming the blood-brain barrier
in animal models of brain metastasis from ovarian cancer (47,56,57),
the use of these agents was ineffective in the present case. Among
the poly ADP-ribose polymerase inhibitors, olaparib, used in the
present case, has limited brain permeability, resulting in
restricted exposure of brain tumors to the drug. Additionally,
olaparib demonstrates minimal activity in the central nervous
system (58–60). Therefore, the efficacy of poly
ADP-ribose polymerase inhibitors for treating brain metastasis may
be constrained, necessitating further research. The delayed
initiation of chemotherapy may also have contributed to the
inability of the inhibitor to control disease progression.
The current case showed an unusual course of brain
metastasis 20 years after the initial diagnosis. The unusual course
may have arisen because multiple histological subtypes coexisted,
thereby complicating the clinical picture, especially after
numerous lines of treatment were administered. Accurate
pathological diagnosis via biopsy is essential for tailoring
treatment strategies based on the tumor characteristics. The
administration of radiotherapy was a second possible contributing
factor. Radiotherapy was initiated at the patient's request, but
outside the guidelines of the National Comprehensive Cancer Network
(version 3, 2024) (61). Several
reports have indicated that radiotherapy can alter the biology and
microenvironment of the tumor, potentially exacerbating disease
progression through mechanisms such as cytokine modulation and
changes in cell division (62–64).
Although palliative radiotherapy may be appropriate for symptomatic
lung metastases when chemotherapy is contraindicated, our patient
was asymptomatic at the time of radiotherapy. Thus, chemotherapy
should have been considered as the initial approach. The limitation
of this report is that the findings from a single case cannot be
generalized. Nevertheless, the disease course reported in this case
provides a foundation for the development of various treatment
strategies and new treatment possibilities.
Currently, no standardized treatment strategy exists
for ovarian cancer with brain metastasis. Some studies report a
median survival time of 4.5 months (range, 1.1-28.7 months)
following cranial radiation and dexamethasone treatment (6), whereas others report a median survival
of 6.4 months (range, 1–28 months) (7). In cases of isolated, solitary brain
metastasis, surgical resection followed by whole brain radiotherapy
is often recommended. For multiple brain metastases, whole brain
radiotherapy with or without systemic chemotherapy is typically
administered. From initial treatment for ovarian tumors to
recurrence in the umbilicus, lymph nodes, and lungs, we sought
optimal curative therapy through surgery, radiation therapy, and
chemotherapy, aiming to prevent recurrence. However, curative
treatment was not an option for this multiple brain metastasis.
Although curative treatment was not feasible, administering
treatment that improved the patient's level of consciousness and
provided her with more time to spend with her family may have
offered some benefit to her quality of life.
With advances in treatment options and imaging
techniques, the long-term prognosis for patients with ovarian
cancer has improved; similarly, the capability of detecting brain
metastasis has improved (65).
Molecular profiling and next-generation sequencing have recently
been proposed as tools for guiding the choice of personalized
medical therapy for recurrent, heterogeneous ovarian cancers.
Multi-gene panel testing is widely used in the field of
gynecological cancer (66,67). Although testing for BRCA gene
mutations is the mainstream method for determining sensitivity to
poly ADP-ribose polymerase inhibitors in advanced ovarian cancer
(68,69), multi-gene panel testing can expand
treatment options by identifying rare cancers, cancers of unknown
primary origin, recurrent ovarian cancer, and drug-resistant
recurrent ovarian cancer (70–72).
Therapeutic decisions regarding ovarian cancer need to be based on
pathological findings, with the long-term and genetic perspectives
carefully considered.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CI, KM, MT, KN and YK treated the patient. CI and AT
contributed to the conception and design of the report. CI
contributed to data acquisition and wrote the manuscript. KM, KN,
and YK confirm the authenticity of all the raw data. AT supervised
the report. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication, authorizing the use of their imaging, pathological and
clinical data for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADP
|
adenosine diphosphate
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
References
|
1
|
Webb PM and Jordan SJ: Global epidemiology
of epithelial ovarian cancer. Nat Rev Clin Oncol. 21:389–400. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI
|
|
3
|
Roett MA and Evans P: Ovarian cancer: An
overview. Am Fam Physician. 80:609–616. 2009.PubMed/NCBI
|
|
4
|
Geisler JP and Geisler HE: Brain
metastases in epithelial ovarian carcinoma. Gynecol Oncol.
57:246–249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchetti C, Ferrandina G, Cormio G,
Gambino A, Cecere S, Lorusso D, De Giorgi U, Bogliolo S, Fagotti A,
Mammoliti S, et al: Brain metastases in patients with EOC:
Clinico-pathological and prognostic factors. A multicentric
retrospective analysis from the MITO group (MITO 19). Gynecol
Oncol. 143:532–538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahat Z, Cakmak VA and Cakir E: Brain
metastasis from ovarian carcinoma: Analysis of eight cases from a
single radiotherapy center. Taiwan J Obstet Gynecol. 59:711–717.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piura E and Piura B: Brain metastases from
ovarian carcinoma. ISRN Oncol. 2011:5274532011.PubMed/NCBI
|
|
8
|
Pope WB: Brain metastases: Neuroimaging.
Handb Clin Neurol. 149:89–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L and Zhang J: Survival analysis of
ovarian cancer patients with distant metastasis after chemotherapy:
A SEER-based study. Indian J Cancer. 15:10.4103/ijc.IJC_175_20.
2023.PubMed/NCBI
|
|
10
|
Ogawa K, Yoshii Y, Aoki Y, Nagai Y,
Tsuchida Y, Toita T, Kakinohana Y, Tamaki W, Iraha S, Adachi G, et
al: Treatment and prognosis of brain metastases from gynecological
cancers. Neurol Med Chir (Tokyo). 48:57–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mayer RJ, Berkowitz RS and Griffiths CT:
Central nervous system involvement by ovarian carcinoma: A
complication of prolonged survivial with metastatic disease.
Cancer. 41:776–783. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larson DM, Copeland LJ, Moser RP, Malone
JM Jr, Gershenson DM and Wharton JT: Central nervous system
metastases in epithelial ovarian carcinoma. Obstet Gynecol.
68:746–750. 1986.PubMed/NCBI
|
|
13
|
Kolomainen DF, Larkin JM, Badran M, A'Hern
RP, King DM, Fisher C, Bridges JE, Blake PR, Barton DP, Shepherd
JH, et al: Epithelial ovarian cancer metastasizing to the brain: A
late manifestation of the disease with an increasing incidence. J
Clin Oncol. 20:982–986. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YL, Cheng WF, Hsieh CY and Chen CA:
Brain metastasis as a late manifestation of ovarian carcinoma. Eur
J Cancer Care (Engl). 20:44–49. 2011.PubMed/NCBI
|
|
15
|
Barker GH, Orledge J and Wiltshaw E:
Involvement of the central nervous system in patients with ovarian
carcinoma. Br J Obstet Gynaecol. 88:690–694. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budd GT, Webster KD, Reimer RR, Martimbeau
P and Livingston RB: Treatment of advanced ovarian cancer with
cisplatin, adriamycin, and cyclophosphamide: Effect of treatment
and incidence of intracranial metastases. J Surg Oncol. 24:192–195.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein M, Steiner M, Klein B, Beck D, Atad
J, Kuten A, Robinson E and Goldsher D: Involvement of the central
nervous system by ovarian carcinoma. Cancer. 58:2066–2069. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dauplat J, Nieberg RK and Hacker NF:
Central nervous system metastases in epithelial ovarian carcinoma.
Cancer. 60:2559–2562. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziegler J, Gliedman P, Fass D, Beckman M,
Neophytides A and Steinfeld A: Brain metastases from ovarian
cancer. J Neurooncol. 5:211–215. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ross WM, Carmichael JA and Shelley WE:
Advanced carcinoma of the ovary with central nervous system
relapse. Gynecol Oncol. 30:398–406. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardy JR and Harvey VJ: Cerebral
metastases in patients with ovarian cancer treated with
chemotherapy. Gynecol Oncol. 33:296–300. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piura B, Glezerman M, Galper Y, Segal S
and Cohen Y: Brain metastases in epithelial ovarian carcinoma; two
case reports. Eur J Obstet Gynecol Reprod Biol. 36:203–208. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plaxe SC, Dottino PR, Lipsztein R, Dalton
J and Cohen CJ: Clinical features and treatment outcome of patients
with epithelial carcinoma of the ovary metastatic to the central
nervous system. Obstet Gynecol. 75:278–281. 1990.PubMed/NCBI
|
|
24
|
LeRoux PD, Berger MS, Elliott JP and
Tamimi HK: Cerebral metastases from ovarian carcinoma. Cancer.
67:2194–2199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez GC, Soper JT, Berchuck A, Oleson
J, Dodge R, Montana G and Clarke-Pearson DL: Improved palliation of
cerebral metastases in epithelial ovarian cancer using a combined
modality approach including radiation therapy, chemotherapy, and
surgery. J Clin Oncol. 10:1553–1560. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bruzzone M, Campora E, Chiara S, Giudici
S, Merlini L, Simoni C, Mammoliti S, Rubagotti A and Rosso R:
Cerebral metastases secondary to ovarian cancer: Still an unusual
event. Gynecol Oncol. 49:37–40. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salvati M and Cervoni L: Solitary cerebral
metastasis from ovarian carcinoma: Report of 4 cases. J Neurooncol.
19:75–77. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cormio G, Maneo A, Parma G, Pittelli MR,
Miceli MD and Bonazzi C: Central nervous system metastases in
patients with ovarian carcinoma. A report of 23 cases and a
literature review. Ann Oncol. 6:571–574. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki M, Tsukagoshi S, Ohwada M, Koumura
Y and Sato I: A patient with brain metastasis from ovarian cancer
who showed complete remission after multidisciplinary treatment.
Gynecol Oncol. 74:483–486. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaminsky-Forrett MC, Weber B, Conroy T and
Spaëth D: Brain metastases from epithelial ovarian carcinoma. Int J
Gynecol Cancer. 10:366–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanderson A, Bonington SC, Carrington BM,
Alison DL and Spencer JA: Cerebral metastasis and other cerebral
events in women with ovarian cancer. Clin Radiol. 57:815–819. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anupol N, Ghamande S, Odunsi K, Driscoll D
and Lele S: Evaluation of prognostic factors and treatment
modalities in ovarian cancer patients with brain metastases.
Gynecol Oncol. 85:487–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pothuri B, Chi DS, Reid T, Aghajanian C,
Venkatraman E, Alektiar K, Bilsky M and Barakat RR: Craniotomy for
central nervous system metastases in epithelial ovarian carcinoma.
Gynecol Oncol. 87:133–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar L, Barge S, Mahapatra AK, Thulkar S,
Rath GK, Kumar S, Mishra R, Dawar R and Singh R: Central nervous
system metastases from primary epithelial ovarian cancer. Cancer
Control. 10:244–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen ZR, Suki D, Weinberg JS, Marmor E,
Lang FF, Gershenson DM and Sawaya R: Brain metastases in patients
with ovarian carcinoma: Prognostic factors and outcome. J
Neurooncol. 66:313–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tay SK and Rajesh H: Brain metastases from
epithelial ovarian cancer. Int J Gynecol Cancer. 15:824–829. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pectasides D, Aravantinos G, Fountzilas G,
Kalofonos C, Efstathiou E, Karina M, Pavlidis N, Farmakis D,
Economopoulos T and Dimopoulos MA: Brain metastases from epithelial
ovarian cancer. The Hellenic cooperative oncology group (HeCOG)
experience and review of the literature. Anticancer Res.
25:3553–3558. 2005.PubMed/NCBI
|
|
38
|
D'Andrea G, Roperto R, Dinia L, Caroli E,
Salvati M and Ferrante L: Solitary cerebral metastases from ovarian
epithelial carcinoma: 11 cases. Neurosurg Rev. 28:120–123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen PG, Lee SY, Barnett GH, Vogelbaum MA,
Saxton JP, Fleming PA and Suh JH: Use of the radiation therapy
oncology group recursive partitioning analysis classification
system and predictors of survival in 19 women with brain metastases
from ovarian carcinoma. Cancer. 104:2174–2180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kastritis E, Efstathiou E, Gika D, Bozas
G, Koutsoukou V, Papadimitriou C, Pissakas G, Dimopoulos MA and
Bamias A: Brain metastases as isolated site of relapse in patients
with epithelial ovarian cancer previously treated with platinum and
paclitaxel-based chemotherapy. Int J Gynecol Cancer. 16:994–999.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim TJ, Song S, Kim CK, Kim WY, Choi CH,
Lee JH, Lee JW, Bae DS and Kim BG: Prognostic factors associated
with brain metastases from epithelial ovarian carcinoma. Int J
Gynecol Cancer. 17:1252–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee YK, Park NH, Kim JW, Song YS, Kang SB
and Lee HP: Gamma-knife radiosurgery as an optimal treatment
modality for brain metastases from epithelial ovarian cancer.
Gynecol Oncol. 108:505–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sehouli J, Pietzner K, Harter P, Münstedt
K, Mahner S, Hasenburg A, Camara O, Wimberger P, Boehmer D,
Buehling KJ, et al: Prognostic role of platinum sensitivity in
patients with brain metastases from ovarian cancer: Results of a
German multicenter study. Ann Oncol. 21:2201–2205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cormio G, Loizzi V, Falagario M, Lissoni
AA, Resta L and Selvaggi LE: Changes in the management and outcome
of central nervous system involvement from ovarian cancer since
1994. Int J Gynaecol Obstet. 114:133–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Keskin S, Küçücük S, Ak N, Atalar B, Sarı
M, Sozen H, Ibis K, Topuz S and Saip P: Survival impact of optimal
surgical cytoreduction in recurrent epithelial ovarian cancer with
brain metastasis. Oncol Res Treat. 42:101–106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gadducci A, Tana R, Teti G, Fanucchi A,
Pasqualetti F, Cionini L and Genazzani AR: Brain recurrences in
patients with ovarian cancer: Report of 12 cases and review of the
literature. Anticancer Res. 27:4403–4409. 2007.PubMed/NCBI
|
|
47
|
Zhang Z, Xu M, Sakandar A, Du X, He H, He
W, Li D and Wen Q: Successful treatment of a patient with brain
metastasis from ovarian cancer with BRCA wild type using niraparib:
A case report and review of the literature. Front Oncol.
12:8731982022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heimans JJ, Vermorken JB, Wolbers JG,
Eeltink CM, Meijer OW, Taphoorn MJ and Beijnen JH: Paclitaxel
(Taxol) concentrations in brain tumor tissue. Ann Oncol. 5:951–953.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fortin D, Gendron C, Boudrias M and Garant
MP: Enhanced chemotherapy delivery by intraarterial infusion and
blood-brain barrier disruption in the treatment of cerebral
metastasis. Cancer. 109:751–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takano M, Tsuda H and Sugiyama T: Clear
cell carcinoma of the ovary: Is there a role of histology-specific
treatment? J Exp Clin Cancer Res. 31:532012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
O'Brien ME, Schofield JB, Tan S, Fryatt I,
Fisher C and Wiltshaw E: Clear cell epithelial ovarian cancer
(mesonephroid): bad prognosis only in early stages. Gynecol Oncol.
49:250–254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Omura GA, Brady MF, Homesley HD, Yordan E,
Major FJ, Buchsbaum HJ and Park RC: Long-term follow-up and
prognostic factor analysis in advanced ovarian carcinoma: The
gynecologic oncology group experience. J Clin Oncol. 9:1138–1150.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Goff BA, de la Cuesta RS, Muntz HG,
Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer
BE and Fuller AF Jr: Clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy in stage III disease. Gynecol Oncol.
60:412–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Itamochi H, Kigawa J, Akeshima R, Sato S,
Kamazawa S, Takahashi M, Kanamori Y, Suzuki M, Ohwada M and
Terakawa N: Mechanisms of cisplatin resistance in clear cell
carcinoma of the ovary. Oncology. 62:349–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Itamochi H, Kigawa J and Terakawa N:
Mechanisms of chemoresistance and poor prognosis in ovarian clear
cell carcinoma. Cancer Sci. 99:653–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cabitza E, Pirola M, Baldessari C,
Bernardelli G, Zunarelli E, Pipitone S, Vitale MG, Nasso C,
Molinaro E, Oltrecolli M, et al: Cerebellar metastasis of ovarian
cancer: A case report. J Med Case Rep. 17:5532023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alizzi Z, Roxburgh P, Cartwright D,
McLaren A, Park S, Jones R, Greening S, Hudson E, Green C, Gray S,
et al: Description of a retrospective cohort of epithelial ovarian
cancer patients with brain metastases: Evaluation of the role of
PARP inhibitors in this setting. J Clin Med. 12:24972023.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sun K, Mikule K, Wang Z, Poon G,
Vaidyanathan A, Smith G, Zhang ZY, Hanke J, Ramaswamy S and Wang J:
A comparative pharmacokinetic study of PARP inhibitors demonstrates
favorable properties for niraparib efficacy in preclinical tumor
models. Oncotarget. 9:37080–37096. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Q, Zhang F, Gao H and Xu Y:
Successful treatment of a patient with brain metastases from
endometrial cancer using Niraparib: A case report. Ann Palliat Med.
10:818–827. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Proskuriakova E, Aryal B, Khan S, Sanchez
D, Moss J and Khosla P: Niraparib maintenance therapy for brain
metastasis in ovarian endometrioid adenocarcinoma with peritoneal
carcinomatosis: A comprehensive case study and literature review.
Cureus. 16:e613552024.PubMed/NCBI
|
|
61
|
Liu J, Berchuck A, Backes FJ, Cohen J,
Grisham R, Leath CA, Martin L, Matei D, Miller DS, Robertson S, et
al: NCCN Guidelines® insights: Ovarian cancer/fallopian
tube cancer/primary peritoneal cancer, version 3.2024. J Natl Compr
Canc Netw. 22:512–519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Olivares-Urbano MA, Griñán-Lisón C,
Marchal JA and Núñez MI: CSC radioresistance: A therapeutic
challenge to improve radiotherapy effectiveness in cancer. Cells.
9:16512020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vilalta M, Rafat M and Graves EE: Effects
of radiation on metastasis and tumor cell migration. Cell Mol Life
Sci. 73:2999–3007. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tommelein J, De Vlieghere E, Verset L,
Melsens E, Leenders J, Descamps B, Debucquoy A, Vanhove C, Pauwels
P, Gespach CP, et al: Radiotherapy-Activated cancer-associated
fibroblasts promote tumor progression through paracrine igf1r
activation. Cancer Res. 78:659–670. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pietzner K, Oskay-Oezcelik G, El Khalfaoui
K, Boehmer D, Lichtenegger W and Sehouli J: Brain metastases from
epithelial ovarian cancer: Overview and optimal management.
Anticancer Res. 29:2793–2798. 2009.PubMed/NCBI
|
|
66
|
Mukai Y and Ueno H: Establishment and
implementation of cancer genomic medicine in Japan. Cancer Sci.
112:970–977. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Naito Y, Aburatani H, Amano T, Baba E,
Furukawa T, Hayashida T, Hiyama E, Ikeda S, Kanai M, Kato M, et al:
Clinical practice guidance for next-generation sequencing in cancer
diagnosis and treatment (edition 2.1). Int J Clin Oncol.
26:233–283. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Oda K, Tanikawa M, Sone K, Mori-Uchino M,
Osuga Y and Fujii T: Recent advances in targeting DNA repair
pathways for the treatment of ovarian cancer and their clinical
relevance. Int J Clin Oncol. 22:611–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
DiSilvestro P, Banerjee S, Colombo N,
Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet
A, Leary A, et al: Overall survival with maintenance olaparib at a
7-year follow-up in patients with newly diagnosed advanced ovarian
cancer and a BRCA mutation: The SOLO1/GOG 3004 trial. J Clin Oncol.
41:609–617. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yaghmour G, Prouet P, Wiedower E, Jamy OH,
Feldman R, Chandler JC, Pandey M and Martin MG: Genomic alterations
in neuroendocrine cancers of the ovary. J Ovarian Res. 9:522016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Meagher NS, Schuster K, Voss A, Budden T,
Pang CNI, deFazio A, Ramus SJ and Friedlander ML: Does the primary
site really matter? Profiling mucinous ovarian cancers of uncertain
primary origin (MO-CUP) to personalise treatment and inform the
design of clinical trials. Gynecol Oncol. 150:527–533. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Du ZH, Bi FF, Wang L and Yang Q:
Next-generation sequencing unravels extensive genetic alteration in
recurrent ovarian cancer and unique genetic changes in
drug-resistant recurrent ovarian cancer. Mol Genet Genomic Med.
6:638–647. 2018. View Article : Google Scholar : PubMed/NCBI
|