Introduction
Esophageal cancer (EC) is the seventh most common
malignancy worldwide and the sixth leading cause of cancer-related
mortality. The incidence and mortality rates of EC in Eastern Asia
are significantly higher than the global average (1,2). EC is
highly invasive and associated with a poor prognosis. The two major
histological subtypes of EC are squamous cell carcinoma and
adenocarcinoma, with esophageal squamous cell carcinoma (ESCC)
accounting for ~90% of cases in China. Due to the lack of obvious
early symptoms, most patients are diagnosed at an advanced stage,
leading to a 5-year survival rate of <30% (3), and only 25–35% of patients are
eligible for curative surgery. For patients with locally advanced
disease, poor surgical tolerance or unresectable tumors, radical
radiotherapy remains the initial treatment option (4).
Patients with EC frequently experience malnutrition
due to dysphagia, and muscle loss worsens with aging (5,6).
Elderly patients are particularly susceptible to sarcopenia, and
studies have demonstrated that muscle nutritional status is closely
associated with the prognosis of various malignancies, including EC
(7–9). Therefore, selecting appropriate
clinical indicators to quantify muscle nutritional status is
crucial for the prognostic evaluation of EC. Imaging modalities
such as computed tomography (CT) and magnetic resonance imaging are
considered the gold standard for non-invasive muscle mass
assessment (10,11). The skeletal muscle index (SMI) (a
planar index that describes the area of skeletal muscle)
(cm2/m2) is a widely accepted parameter for
quantifying muscle mass and is calculated as SMI=skeletal muscle
area (cm2)/height2 (m2). SMI at
the third lumbar vertebra (L3) level best reflects overall skeletal
muscle status (9). However, as EC
is a thoracic malignancy, imaging evaluations primarily involve
chest CT scans. Therefore, using radiotherapy planning CT scans to
assess thoracic skeletal muscle parameters, such as the skeletal
muscle volume index (SMVI) (a three-dimensional index that
describes the volume of skeletal muscle), may provide a convenient
and standardized method to quantify muscle nutritional status.
The present study utilized CT-based quantification
of skeletal muscle volume at the 10th thoracic vertebra (T10)
level, combined with hematological nutritional indices, to
investigate prognostic factors in elderly patients with ESCC.
Additionally, the study proposes a clinically feasible method for
quantifying muscle nutritional status to provide a reference for
nutritional intervention and prognostic assessment.
Patients and methods
Study participants
The present study was a retrospective analysis of
data from 123 elderly patients with ESCC treated with radiotherapy
in the Department of Radiotherapy of The Affiliated Hospital of
Xuzhou Medical University (Xuzhou, China) between October 2018 and
October 2021. The inclusion criteria were as follows: i) Elderly
patients aged 60–89 years; ii) a diagnosis of ESCC confirmed
through histology or cytology records; iii) stage II–IVa disease
[American Joint Committee on Cancer (AJCC) 8th edition] (12); and iv) complete medical records
before and after treatment. Exclusion criteria: i) Patients
receiving resection; ii) patients with a second primary tumor; iii)
patients with serious underlying disease affecting the tumor
treatment; iv) patients with distant metastasis; and v) patients
missing clinical data or lost to follow-up before and after
treatment. Patients were followed up by telephone and by follow-up
visits until October 2024. The observed endpoints were overall
survival (OS) (defined as the time from the diagnosis to the death
due to any cause) and progression-free survival (PFS) (defined as
the time from diagnosis to tumor progression or death from any
cause). This study was approved by the Ethics Committee of the
Affiliated Hospital of Xuzhou Medical University (approval no.
XYFY2025-KL215-01).
Collection of clinical data
General clinical data were collected from patient
records, including sex, age, height, weight, tumor location, lesion
length, gross tumor volume (GTV), Tumor-Node-Metastasis (TNM) stage
(AJCC 8th edition), initial treatment, application of immunotherapy
(immunotherapy before PFS or OS), radiotherapy dose and ECOG score.
Hematological data included routine blood test results and albumin
levels in a morning examination within 2 weeks of primary
radiotherapy. Imaging data included radiotherapy localization
CT.
Research nutrition-related
indicators
In the thoracic region, the T10 region is located at
the junction of the thoracic and abdominal regions, and contains
both thoracic and abdominal skeletal muscles, making it a
comprehensive indicator of skeletal muscle status (13). Tissue with a CT attenuation value
between −29 and 150 Hounsfield Units (HU) is classified as skeletal
muscle (10). In the CT soft-tissue
window of the Varian radiotherapy system (Siemens Healthineers),
T10 was identified between the upper and lower intervertebral
discs, with an automated delineation of tissue within the −29 to
150 HU range, followed by manual layer-by-layer modifications to
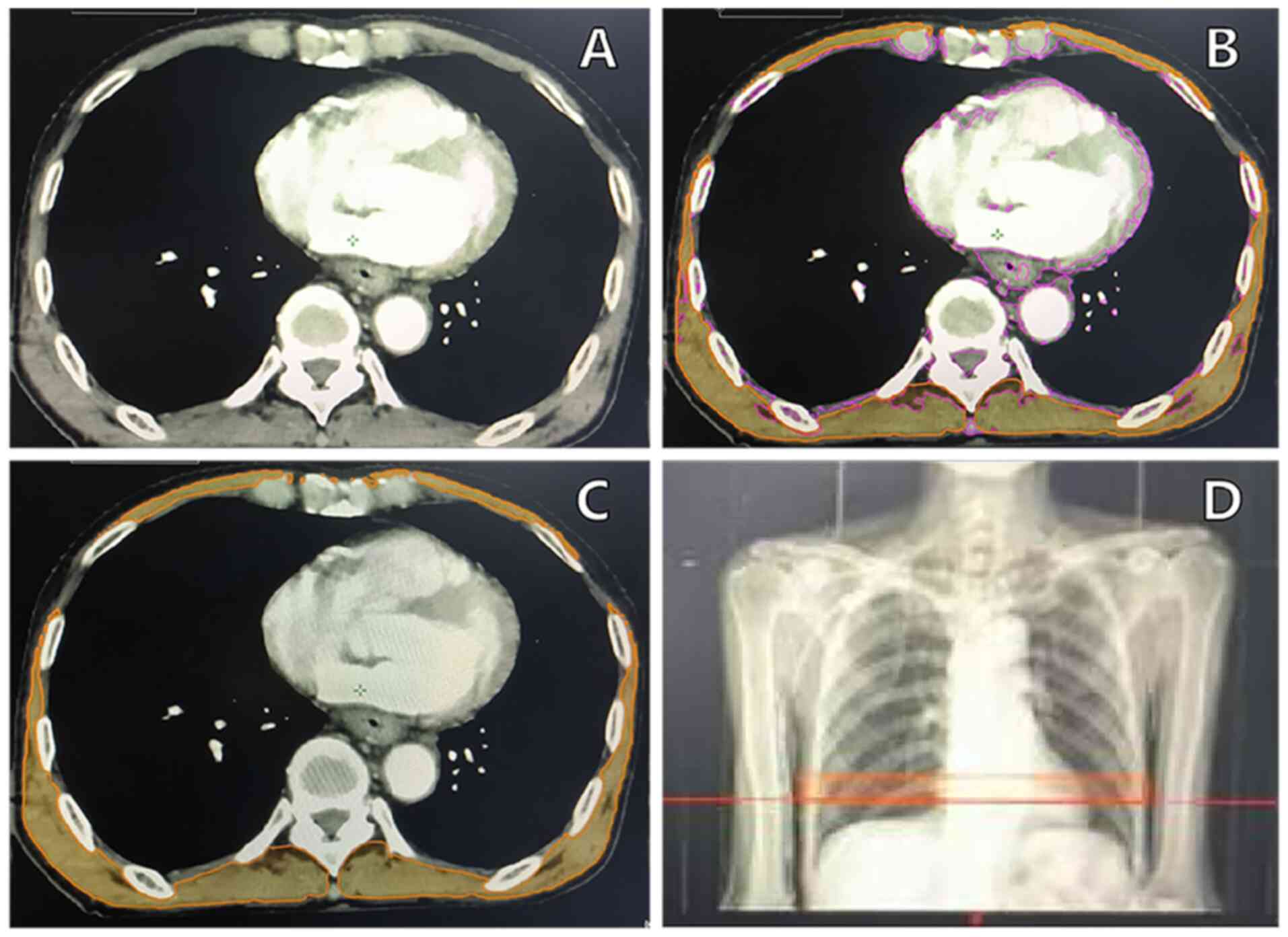
accurately outline all skeletal muscles in this segment. As shown
in Fig. 1, the skeletal muscle
volume (cm3) was measured and normalized by height
(m2) to assess skeletal muscle nutritional status,
calculated using the T10 SMVI as follows: T10 SMVI
(cm3/m2)=T10 skeletal muscle volume
(cm3)/height2 (m2). The Prognostic
Nutritional Index (PNI) and the Geriatric Nutritional Risk Index
(GNRI) were used as representative hematological nutritional
markers (14). PNI has been
extensively studied as a prognostic factor for multiple solid
tumors, including those of the digestive system (14–17),
while GNRI reflects the nutritional status of elderly patients and
has been validated in multiple disease studies as an effective
predictor of prognosis (18,19).
PNI was calculated as PNI=serum albumin (g/l) + 5 × blood
lymphocyte count (/l), while GNRI was calculated as GNRI=1.489 ×
serum albumin (g/l) + 41.7 × (weight/ideal weight), with ideal
weight determined using the Lorentz formula [22 ×
height2 (m2)]. GNRI risk classification was
divided into four categories: Normal (GNRI >98), low risk
(92≤GNRI≤98), moderate risk (82≤GNRI<92) and high risk (GNRI
<82). In this study, a GNRI score of 98 was used as the cutoff
to classify patients into a GNRI normal group (GNRI >98; n=84)
and a GNRI risk group (GNRI ≤98; n=39) (17).
Statistical analysis
Statistical analysis and data visualization were
conducted using SPSS 25.0 (IBM Corp.) and RStudio 4.2.0 (RStudio
Inc.). The optimal cutoff values for T10 SMVI, PNI and GTV were
determined using X-tile 3.6.1 (Yale University School of Medicine).
Quantitative variables following a normal distribution are
expressed as the mean ± standard deviation (x̄ ± s) and were
compared between groups using an independent sample t-test.
Categorical variables are reported as frequencies and percentages,
and were analyzed using the χ2 test for between-group
comparisons. Kaplan-Meier survival curves were generated for
survival analysis, and the log-rank test was used to assess
differences in survival outcomes between subgroups. The receiver
operating characteristic (ROC) curve was used to calculate the area
under the curve to evaluate the predictive performance of PNI, GNRI
and T10 SMVI for survival outcomes in elderly patients with ESCC.
Univariate and multivariate analyses were performed using Cox
proportional hazards regression models to estimate hazard ratios.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of general clinical
characteristics in elderly patients with ESCC undergoing
radiotherapy
A total of 85 male and 38 female patients were
included in this study. The optimal cutoff values for T10 SMVI were
determined as 52.1 cm3/m2 for males and 45.5
cm3/m2 for females, and patients were
stratified into two groups: The T10 sarcopenia group (below the
cutoff value) and the T10 non-sarcopenia group (above the cutoff
value). Patients in the T10 sarcopenia group were more likely to be
older (≥75 years), with a lower BMI, tumors located in the lower
thoracic region and advanced TNM stage, and were more likely to
have received radiotherapy alone (all P<0.05). Additionally, the
T10 sarcopenia group had a significantly lower hemoglobin level,
GNRI and serum albumin level compared with the T10 non-sarcopenia
group (all P<0.05), as shown in Tables I and II.
 | Table I.Comparison of general clinical
characteristics in elderly patients with esophageal squamous cell
carcinoma undergoing radiotherapy [n (%)] classified by the T10
skeletal muscle volume index. |
Table I.
Comparison of general clinical
characteristics in elderly patients with esophageal squamous cell
carcinoma undergoing radiotherapy [n (%)] classified by the T10
skeletal muscle volume index.
| Clinical
features | T10
sarcopenia (n=59) | T10
non-sarcopenia (n=64) | Total (n=123) | χ2 | P-value |
|---|
| Sex |
|
|
| 2.727 | 0.099 |
| Male | 45 (76.3) | 40 (62.5) | 85 (69.1) |
|
|
|
Female | 14 (23.7) | 24 (37.5) | 38 (30.9) |
|
|
| Age, years |
|
|
| 8.836 | 0.003 |
|
60-74 | 30 (50.8) | 49 (76.6) | 79 (64.2) |
|
|
|
75-89 | 29 (49.2) | 15 (23.4) | 44 (35.8) |
|
|
| BMI,
kg/m2 |
|
|
| 13.063 | 0.001 |
|
<18.5 | 11 (18.6) | 1 (1.6) | 12 (9.8) |
|
|
|
18.5-24 | 39 (66.1) | 42 (65.6) | 81 (65.9) |
|
|
|
≥24 | 9 (15.3) | 21 (32.8) | 30 (24.4) |
|
|
| Tumor location |
|
|
| 7.322 | 0.026 |
|
Upper | 13 (22.0) | 27 (42.2) | 40 (32.5) |
|
|
|
Middle | 26 (44.1) | 26 (40.6) | 52 (42.3) |
|
|
|
Lower | 20 (33.9) | 11 (17.2) | 31 (25.2) |
|
|
| TNM stage |
|
|
| 12.267 | 0.002 |
| II | 2 (3.4) | 14 (21.9) | 16 (13.0) |
|
|
|
III | 37 (62.7) | 40 (62.5) | 77 (62.6) |
|
|
|
IVa | 20 (33.9) | 10 (15.6) | 30 (24.4) |
|
|
| Lesion length,
cm |
|
|
| 0.016 | 0.898 |
| ≤7 | 39 (66.1) | 43 (67.2) | 45 (36.6) |
|
|
|
>7 | 20 (33.9) | 21 (32.8) | 41 (33.3) |
|
|
| GTV,
cm3 |
|
|
| 0.098 | 0.755 |
|
≤22.9 | 26 (44.1) | 30 (46.9) | 56 (45.5) |
|
|
|
>22.9 | 33 (55.9) | 34 (53.1) | 67 (54.5) |
|
|
| Initial
treatment |
|
|
| 8.016 | 0.018 |
| RT | 26 (44.1) | 13 (20.3) | 39 (31.7) |
|
|
|
CCRT | 25 (42.4) | 38 (59.4) | 63 (51.2) |
|
|
|
RT-AIC | 8 (13.6) | 13 (20.3) | 21 (17.1) |
|
|
 | Table II.Comparison of hematological
indicators in elderly patients with esophageal squamous cell
carcinoma undergoing radiotherapy (mean ± standard deviation). |
Table II.
Comparison of hematological
indicators in elderly patients with esophageal squamous cell
carcinoma undergoing radiotherapy (mean ± standard deviation).
| Hematological
indicator | T10
sarcopenia (n=59) | T10
non-sarcopenia (n=64) | T | P-value |
|---|
| HB, g/l | 126.47±14.28 | 131.50±11.61 | 2.149 | 0.034 |
| Albumin, g/l | 39.63±3.64 | 40.99±3.46 | 2.128 | 0.035 |
| PNI | 47.77±4.52 | 48.91±4.92 | 1.334 | 0.185 |
| GNRI | 98.99±7.70 | 105.44±8.11 | 4.514 | <0.001 |
Association between T10 SMVI and
survival prognosis in elderly patients with ESCC
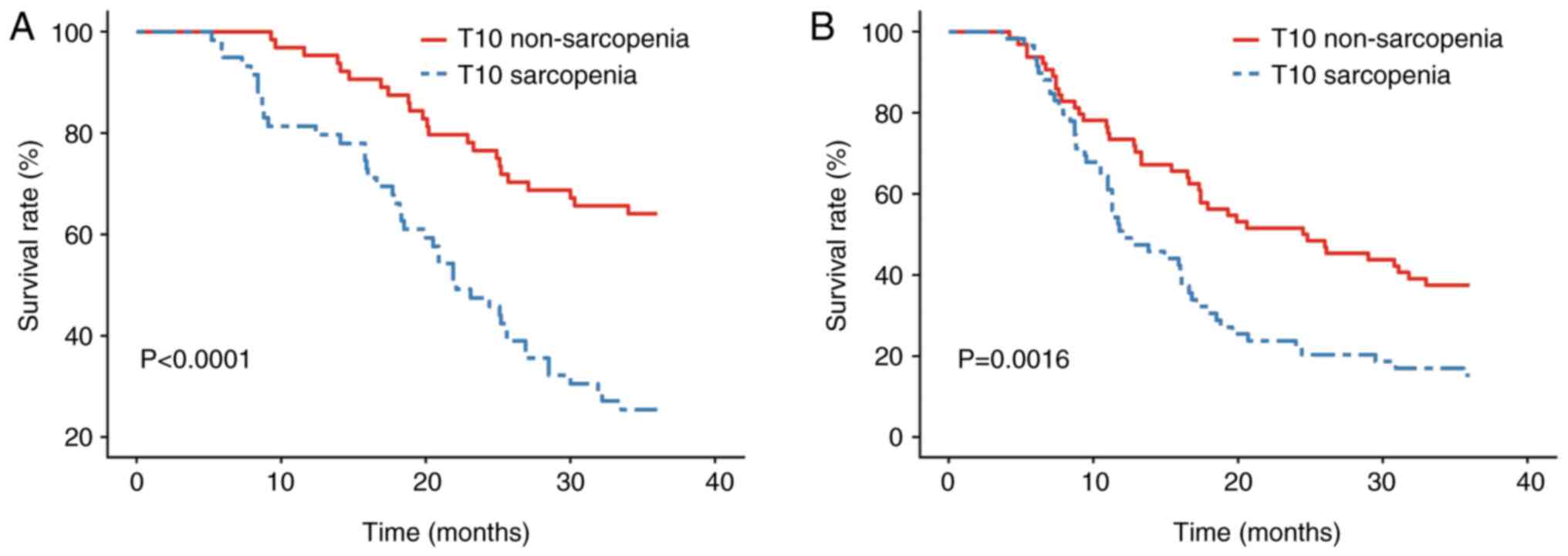
Kaplan-Meier survival curve analysis (Fig. 2A and B) and the log-rank test
demonstrated that OS and PFS times were significantly lower in the
T10 sarcopenia group than in the T10 non-sarcopenia group
(P<0.05), indicating a worse prognosis in patients with reduced
skeletal muscle volume. All patients were followed up for 36
months, during which 44 patients in the T10 sarcopenia group and 23
patients in the T10 non-sarcopenia group succumbed to the disease.
The 1-, 2- and 3-year OS rates were 81.4 vs. 95.3%, 47.5 vs. 76.6%
and 25.4 vs. 64.1%, respectively, for the T10 sarcopenia group and
T10 non-sarcopenia group. The median OS time in the T10 sarcopenia
group was 22.1 months, whereas the median OS time in the T10
non-sarcopenia group was not reached. A significant difference in
OS survival curves was observed between the two groups
(P<0.001), as shown in Fig. 2,
classified by T10 SMVI. The PFS rates in the T10 sarcopenia group
compared with those in the T10 non-sarcopenia group were 50.8 vs.
73.4%, 23.7 vs. 51.6% and 15.3 vs. 37.5% at 1, 2, and 3 years,
respectively, with a median PFS time of 12.0 vs. 24.7 months. The
PFS rate in the T10 sarcopenia group was significantly lower than
that in the T10 non-sarcopenia group (P=0.0016), as shown in
Fig. 2B.
T10 SMVI and the predictive value of
hematological nutritional indicators (PNI and GNRI) for survival
prognosis in elderly patients with ESCC
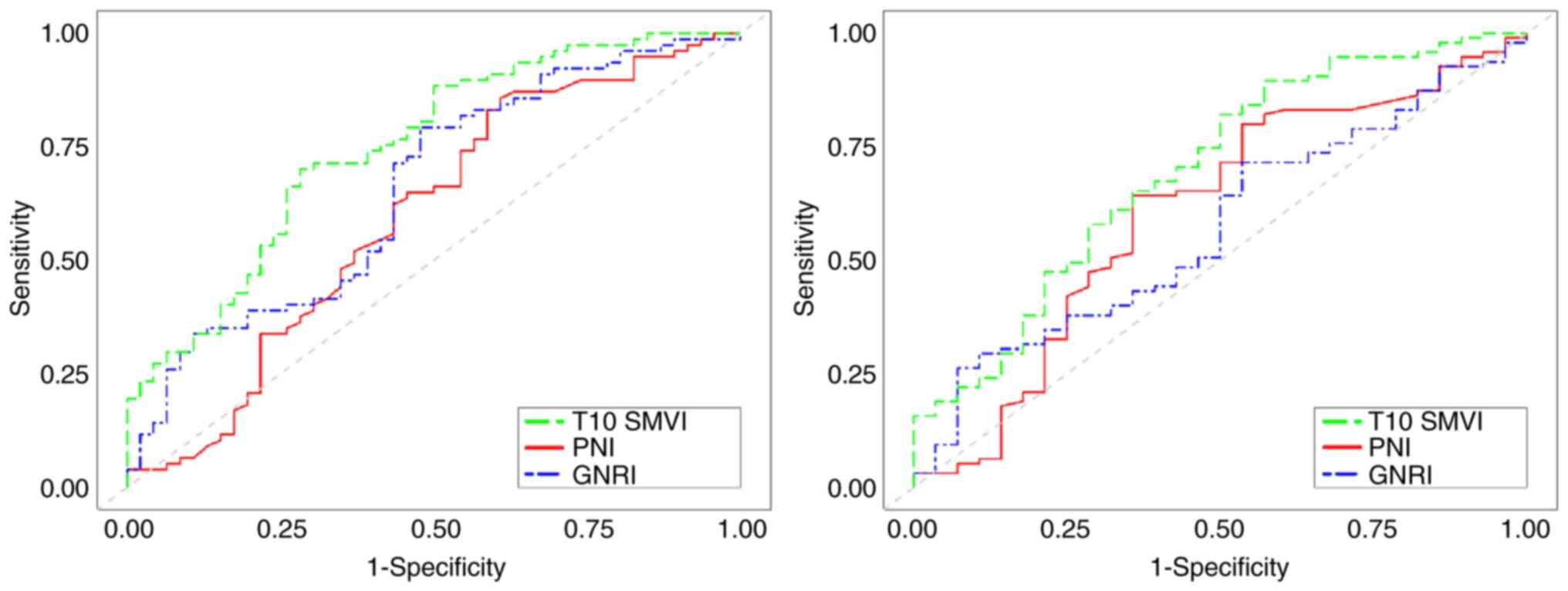
ROC curves were generated for T10 SMVI, PNI and
GNRI, as shown in Fig. 3. The
results demonstrated that T10 SMVI, PNI and GNRI were all
significant predictors of long-term survival in elderly patients
with ESCC (P<0.05), with T10 SMVI exhibiting superior predictive
value compared with PNI and GNRI, as shown in Table III.
 | Table III.Receiver operating characteristic
curve analysis of three nutritional indicators for predicting
long-term survival (OS and PFS). |
Table III.
Receiver operating characteristic
curve analysis of three nutritional indicators for predicting
long-term survival (OS and PFS).
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Indicator | AUC | 95% CI | P-value | AUC | 95% CI | P-value |
|---|
| T10 SMVI | 0.746 | 0.659-0.833 | <0.001 | 0.690 | 0.582-0.798 | 0.001 |
| PNI | 0.629 | 0.528-0.730 | 0.014 | 0.618 | 0.501-0.736 | 0.045 |
| GNRI | 0.690 | 0.596-0.784 | <0.001 | 0.624 | 0.516-0.733 | 0.035 |
Cox single-and multivariate regression
analysis
Univariate Cox proportional hazards regression
analysis of the 123 elderly patients with ESCC demonstrated that
BMI, TNM stage, GTV, initial treatment, T10 SMVI, PNI, GNRI and
immunotherapy were significantly associated with PFS and OS
(P<0.05). Further multivariate analysis identified TNM stage,
GTV, initial treatment, T10 SMVI and PNI as independent risk
factors for OS (P<0.05), while TNM stage, GTV, and initial
treatment were independent risk factors for PFS (P<0.05), as
shown in Tables IV and V. Additionally, late-stage radiotherapy
alone, advanced TNM stage, high GTV, low T10 SMVI and low PNI were
identified as independent risk factors for a shorter survival
time.
 | Table IV.Univariate and multivariate analyses
of overall survival. |
Table IV.
Univariate and multivariate analyses
of overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.847 | 0.512-1.404 | 0.520 |
|
|
|
| Age (≥75 vs. <75
years) | 1.604 | 0.991-2.596 | 0.054 |
|
|
|
| BMI (≥18.5 vs.
<18.5 kg/m2) | 0.307 | 0.156-0.604 | 0.001 | 0.866 | 0.378-1.980 | 0.732 |
| Tumor location
(upper vs. middle-lower neck) | 0.932 | 0.552-1.574 | 0.973 |
|
|
|
| TNM stage (IVa vs.
II/III) | 3.729 | 2.246-6.190 | <0.001 | 2.996 | 1.692-5.306 | <0.001 |
| Lesion length
(>7 vs. ≤7 cm) | 0.838 | 0.500-1.404 | 0.501 |
|
|
|
| GTV (>22.9 vs.
≤22.9 cm3) | 1.833 | 1.116-3.011 | 0.017 | 1.943 | 1.114-3.388 | 0.019 |
| Initial treatment
(non-RT only vs. RT only) | 0.388 | 0.239-0.629 | <0.001 | 0.527 | 0.304-0.916 | 0.023 |
| Immunotherapy (yes
vs. no) | 0.364 | 0.190-0.696 | 0.002 | 0.595 | 0.294-1.203 | 0.148 |
| Radiation dose (≥60
vs. <60 Gy) | 1.416 | 0.875-2.293 | 0.157 |
|
|
|
| T10 non-sarcopenia
group vs. T10 sarcopenia group | 0.332 | 0.200-0.552 | <0.001 | 0.467 | 0.263-0.831 | 0.010 |
| PNI (>52.0 vs.
≤52.0) | 0.357 | 0.170-0.748 | 0.006 | 0.389 | 0.178-0.848 | 0.018 |
| GNRI (>98 vs.
≤98) | 0.443 | 0.272-0.720 | 0.001 | 0.830 | 0.458-1.504 | 0.538 |
 | Table V.Univariate and multivariate analyses
of progression-free survival. |
Table V.
Univariate and multivariate analyses
of progression-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.890 | 0.572-1.386 | 0.607 |
|
|
|
| Age (≥75 vs. <75
years) | 1.492 | 0.981-2.270 | 0.062 |
|
|
|
| BMI (≥18.5 vs.
<18.5 kg/m2) | 0.478 | 0.253-0.903 | 0.023 | 0.956 | 0.439-2.081 | 0.909 |
| Tumor location
(upper vs. middle-lower neck) | 0.999 | 0.639-1.564 | 0.998 |
|
|
|
| TNM stage (IVa vs.
II/III) | 2.684 | 1.685-4.275 | <0.001 | 2.037 | 1.240-3.346 | 0.005 |
| Lesion length
(>7 vs. ≤7 cm) | 0.739 | 0.471-1.161 | 0.190 |
|
|
|
| GTV (>22.9 vs.
≤22.9 cm3) | 1.624 | 1.065-2.475 | 0.024 | 1.786 | 1.144-2.790 | 0.011 |
| Initial treatment
(non-RT only vs. RT only) | 0.437 | 0.283-0.673 | <0.001 | 0.602 | 0.371-0.979 | 0.041 |
| Immunotherapy (yes
vs. no) | 0.410 | 0.218-0.773 | 0.006 | 0.592 | 0.300-1.168 | 0.130 |
| Radiation dose (≥60
vs. <60 Gy) | 1.156 | 0.765-1.749 | 0.491 |
|
|
|
| T10 non-sarcopenia
group vs. T10 sarcopenia group | 0.514 | 0.338-0.783 | 0.002 | 0.692 | 0.433-1.106 | 0.124 |
| PNI (>52.0 vs.
≤52.0) | 0.482 | 0.277-0.842 | 0.010 | 0.559 | 0.312-1.004 | 0.051 |
| GNRI (>98 vs.
≤98) | 0.559 | 0.362-0.862 | 0.008 | 0.854 | 0.511-1.427 | 0.547 |
Discussion
The results of the present study showed that T10
SMVI, as an indicator to quantify the nutritional status of T10
segments, can effectively predict the prognosis of elderly patients
with ESCC and has higher predictive efficacy than the hematological
nutritional indicators PNI and GNRI. Specifically, the T10 SMVI
threshold value used was 52.1 cm3/m2 in men
and 45.5 cm3/m2 in women. Although there are
ethnic, regional and measurement site differences in SMI, there are
no uniform criteria or reference values across studies. The samples
in the present study are from East China and align with the L3 SMI
diagnostic reference values [40.8 cm2/m2 for
men and 34.9 cm2/m2 for women, proposed by
Zhuang et al (20) in
Shanghai]. Previous SMI studies have mostly focused on
cross-sectional area, but the present study has explored muscle
volume in depth (9–11). Compared with plane studies, volume
studies are more comprehensive and holistic.
Radiotherapy localized planning CT, as a routine
examination method for radiotherapy patients, has the advantages of
being simple, non-invasive and economical to operate, so it has
high clinical application value. Although the cross-sectional area
of muscles at the lumbar 3 level has a high correlation with the
whole body muscle level and has become the common standard for SMI
assessment (9), since CT scans in
patients with EC do not always contain the third lumbar spine,
additional abdominal CT scans not only increase radiation exposure
but also increase the economic burden on patients. Unlike prior
studies using L3-level muscle index (requiring additional abdominal
CT) (9,20), the present study innovatively
leveraged routine radiotherapy planning CT to quantify thoracic
muscle volume (T10 SMVI), eliminating extra scans and reducing
radiation and economic burdens, thus enhancing clinical utility.
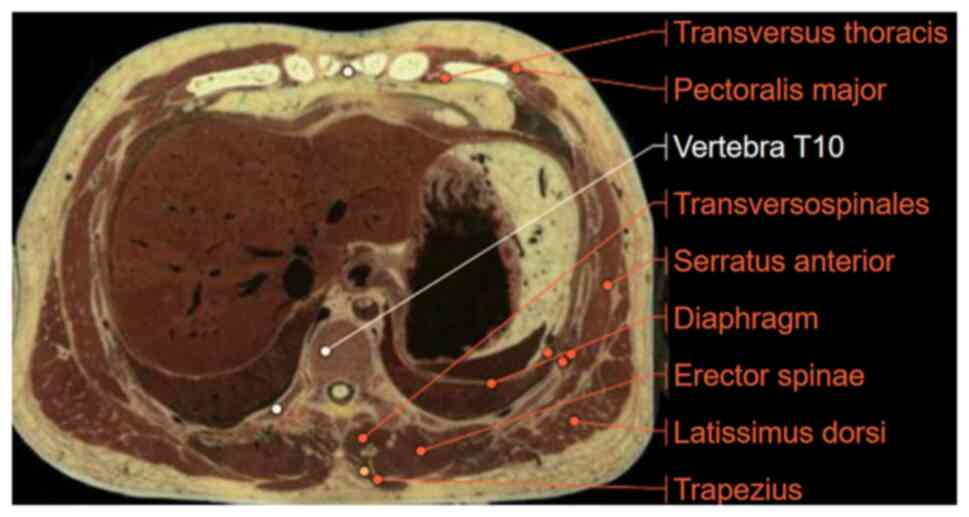
Therefore, the T10 segment was chosen as the anatomical region for
skeletal muscle quality assessment. This segment is located at the
thoracoabdominal junction and contains several key skeletal muscle
groups such as the erector spinal, latissimus dorsi, trapezius,
external abdominis, rectus abdominis and transverse
thoracoabdominal muscles (Fig. 4).
Among these, the erector spinal muscle is essential for spinal
support and serves as an early indicator reflecting whole-body
muscle wasting. Therefore, the T10 segment can comprehensively and
accurately reflect muscle mass, becoming the ideal region (13,21)
for assessing muscle nutritional status in the present study.
EC, as a chronic wasting disease, often leads to
sarcopenia in elderly patients, and is more common in male patients
>60 years old (22). The causes
of skeletal muscular dystrophy in patients with EC include: i)
Insufficient nutritional intake due to dysphagia (23). ii) Chronic inflammation promotes
protein and energy expenditure through multiple signaling pathways,
keeping the body in a negative nitrogen balance (24). iii) Neoadjuvant chemoradiotherapy
increases the risk of sarcopenia in patients with EC, which may
cause apoptosis during chemoradiotherapy to cause inflammatory
response, and activate inflammatory factors to promote muscle
breakdown or stress response (25).
iv) Patients with EC, after long-term release and chemotherapy,
often need long-term bed rest due to physical decline, being easily
fatigued, poor spirit and a lack of moderate muscle exercise
(26). Therefore, assessing the
muscle nutritional status of patients and providing corresponding
nutritional intervention and treatment programs are crucial for
patient prognosis.
In the present study, the T10 sarcopenia group had a
significantly poorer long-term prognosis compared with the T10
non-sarcopenia group. Multivariate analysis showed that T10 SMVI
was an independent risk factor for OS in elderly patients with ESCC
who underwent radiotherapy. Despite baseline differences,
multivariate analysis confirmed the independent prognostic value of
T10 SMVI after adjusting for confounders. Further analysis found
that T10 sarcopenia group patients were mostly of an advanced
prognosis, with low BMI, late TNM stage and undergoing combined
radiotherapy rather than antitumor therapy. These characteristics
further support the association between skeletal sarcopenia and a
poor prognosis, which is consistent with the association between
sarcopenia and tumor prognosis in previous studies (9,20).
Patients with sarcopenia often show malnutrition, decreased muscle
strength and reduced quality of life, the factors that may
influence prognosis by influencing patient tolerance to treatment
and tumor biological behavior. The results of the present study
suggest that muscle nutritional status not only directly affects
the prognosis, but also may indirectly influence the survival
benefit of patients by influencing treatment choice and tolerance.
Combined treatment of EC can significantly improve OS compared with
radiotherapy alone (27). In recent
years, the application of immunotherapy in EC has gradually
increased, and it has been shown to bring marked survival benefits.
The results of the present study also show that either receiving
immunotherapy in the initial treatment stage or adding
immunotherapy after progression and relapse can improve the
long-term prognosis. Although immunotherapy was statistically
significant in the univariate analyses of PFS and OS, it did not
become an independent influence in the multivariate analysis, which
may be related to differences in the sample size and treatment
regimen of the patients.
The SMI is related to the toxicity of cancer
chemotherapy, and patients with EC and a low SMI are more likely to
have acute adverse reactions above grade 3 after chemotherapy
(28). Therefore, patients in the
T10 sarcopenia group are mostly treated with radiotherapy alone,
compared with the non-sarcopenia group with chemoradiotherapy. For
patients with EC, the SMI is measured before and during treatment,
and the muscle nutritional status is assessed, which can help guide
the treatment plan and dosage, and avoid acute adverse reactions.
Good nutritional status and muscle reserve help patients better
tolerate concurrent chemotherapy and immunotherapy, thus prolonging
survival. Clinically, for patients with skeletal muscle dystrophy,
personalized nutritional support and skeletal muscle exercise can
be provided to enhance treatment tolerance and improve survival
benefits (29,30).
In conclusion, the present study quantified the
muscle nutrition status of the T10 segment and introduced the T10
SMVI to predict the prognosis of elderly patients with ESCC. The
predictive efficacy of the T10 SMVI was higher than that of the
hematological nutrition indexes PNI and GNRI. In addition, T10 SMVI
has the advantages of being economically viable, rapid to check and
standardized, and can provide a new perspective for the prognostic
evaluation of elderly patients with ESCC undergoing radiotherapy,
with potential clinical application value. Specific values are
obtained from this study, which can provide a reference for the
clinical prognosis, diagnosis and treatment plan, and nutritional
intervention. However, the present study is a single-center study
with a limited sample size, and the reference values need to be
optimized through a multi-center, large-sample survey. Future
multi-center studies should validate T10 SMVI cutoff
generalizability and explore integrated models with hematological
indices, such as PNI. Investigations on nutrition and exercise
interventions to reverse sarcopenia and improve survival outcomes
are warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GL was the guarantor of integrity of the entire
study. SL and GL were responsible for the study concept and confirm
the authenticity of all the raw data. SL and DP performed
literature research. SL, QL and DL designed the study and analyzed
the data. Data acquisition was performed by SL, QL and DL.
Statistical analysis and manuscript preparation were performed by
SL, DL, CJ and DP. The manuscript was edited by SL, QL, DL and CJ,
and reviewed by SL, QL and DL. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
The study was approved by the Clinical Research
Ethics Committee of The Affiliated Hospital of Xuzhou Medical
University (Xuzhou, China; approval no. XYFY2025-KL215-01). All
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen R, Zheng R, Zhang S, Wang S, Sun K,
Zeng H, Li L, Wei W and He J: Patterns and trends in esophageal
cancer incidence and mortality in China: An analysis based on
cancer registry data. J Natl Cancer Cent. 3:21–27. 2023.PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
3
|
He J, Chen WQ, Li ZS, Li N, Ren JS, Tian
JH, Tian WJ, Hu FL, Peng J; Expert Group of China Guideline for the
Screening, ; et al: China guideline for the screening, early
detection and early treatment of esophageal cancer (2022, Beijing).
Zhonghua Zhong Liu Za Zhi. 44:491–522. 2022.(InChinese). PubMed/NCBI
|
|
4
|
Guo Y, Wang T, Liu Y, Gu D, Li H, Liu Y,
Zhang Z, Shi H, Wang Q, Zhang R, et al: Comparison of
immunochemotherapy followed by surgery or chemoradiotherapy in
locally advanced esophageal squamous cell cancer. Int
Immunopharmacol. 141:1129392024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the Asian working group for
Sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baracos V, Caserotti P, Earthman CP,
Fields D, Gallagher D, Hall KD, Heymsfield SB, Müller MJ, Rosen AN,
Pichard C, et al: Advances in the science and application of body
composition measurement. JPEN J Parenter Enteral Nutr. 36:96–107.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao L, Liu Y, Zhang X, Nie X, Bai H, Lyu
J and Li T: Prognostic value of sarcopenia and inflammatory indices
synergy in patients with esophageal squamous cell carcinoma
undergoing chemoradiotherapy. BMC Cancer. 24:8602024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto M, Ozawa S, Koyanagi K, Kazuno A,
Ninomiya Y, Yatabe K, Higuchi T, Kanamori K and Tajima K:
Usefulness of skeletal muscle measurement by computed tomography in
patients with esophageal cancer: Changes in skeletal muscle mass
due to neoadjuvant therapy and the effect on the prognosis. Surg
Today. 53:692–701. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng K, Liu X, Li Y, Cui J and Li W:
CT-based muscle and adipose measurements predict prognosis in
patients with digestive system malignancy. Sci Rep. 14:130362024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou H, Yu D, Yan X, Dong W, Chen W and Yu
Z: The effect of low muscle mass as measured on CT scans on
clinical outcome in elderly gastric cancer patients with
nutritional risks. Parenteral & Enteral Nutrition. 28:129–134.
2021.(In Chinese).
|
|
11
|
Kazemi-Bajestani SM, Mazurak VC and
Baracos V: Computed tomography-defined muscle and fat wasting are
associated with cancer clinical outcomes. Semin Cell Dev Biol.
54:2–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen M, Li X, Chen Y, Liu P, Chen Z, Shen
M, Liu X, Lin Y, Yang R, Ni W, et al: Proposed revision of the 8th
edition AJCC clinical staging system for esophageal squamous cell
cancer treated with definitive chemo-IMRT based on CT imaging.
Radiat Oncol. 14:542019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Z, Lu Y, Huang J, Shao J, Gao Z, Zhi
H, Shen Q and Shen X: The application of thoracic vertebral cross
section skeletal muscle index in the diagnosis and prognosis of
sarcopenia in patients with advanced gastric cancer. Journal of
Wenzhou Medical University. 53:868–874. 2023.(In Chinese).
|
|
14
|
Tsukagoshi M, Araki K, Igarashi T, Ishii
N, Kawai S, Hagiwara K, Hoshino K, Seki T, Okuyama T, Fukushima R,
et al: Lower geriatric nutritional risk index and prognostic
nutritional index predict postoperative prognosis in patients with
hepatocellular carcinoma. Nutrients. 16:9402024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X and Wang Y: The prognostic
nutritional index is prognostic factor of gynecological cancer: A
systematic review and meta-analysis. Int J Surg. 67:79–86. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu
B and Wang W: Prognostic nutritional index as a prognostic
biomarker for gastrointestinal cancer patients treated with immune
checkpoint inhibitors. Front Immunol. 14:12199292023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi J, Liu T, Ge Y, Liu C, Zhang Q, Xie H,
Ruan G, Lin S, Zheng X, Chen Y, et al: Cholesterol-modified
prognostic nutritional index (CPNI) as an effective tool for
assessing the nutrition status and predicting survival in patients
with breast cancer. BMC Med. 21:5122023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bouillanne O, Morineau G, Dupont C,
Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L and
Aussel C: Geriatric nutritional risk index: A new index for
evaluating at-risk elderly medical patients. Am J Clin Nutr.
82:777–783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu X, Wu Q, Zhang Y, Zhu Y, Yang M and
Tao L: Geriatric nutritional risk index and mortality from
all-cause, cancer, and non-cancer in US cancer survivors: NHANES
2001–2018. Front Oncol. 14:13999572024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang CL, Huang DD, Pang WY, Zhou CJ,
Wang SL, Lou N, Ma LL, Yu Z and Shen X: Sarcopenia is an
independent predictor of severe postoperative complications and
long-term survival after radical gastrectomy for gastric cancer:
Analysis from a large-scale cohort. Medicine (Baltimore).
95:e31642016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derstine BA, Holcombe SA, Ross BE, Wang
NC, Su GL and Wang SC: Skeletal muscle cutoff values for sarcopenia
diagnosis using T10 to L5 measurements in a healthy US population.
Sci Rep. 8:113692018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hai S, Cao L, Wang H, Zhou J, Liu P, Yang
Y, Hao Q and Dong B: Association between sarcopenia and nutritional
status and physical activity among community-dwelling Chinese
adults aged 60 years and older. Geriatr Gerontol Int. 17:1959–1966.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meza-Valderrama D, Marco E, Dávalos-Yerovi
V, Muns MD, Tejero-Sánchez M, Duarte E and Sánchez-Rodríguez D:
Sarcopenia, malnutrition, and cachexia: Adapting definitions and
terminology of nutritional disorders in older people with cancer.
Nutrients. 13:7612021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan L, Xie W, Fu X, Lu W, Jin H, Lai J,
Zhang A, Yu Y, Li Y and Xiao W: Inflammation and sarcopenia: A
focus on circulating inflammatory cytokines. Exp Gerontol.
154:1115442021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Xie K, Xiao X, Xu P, Tang M and Li
D: Correlation between sarcopenia and esophageal cancer: A
narrative review. World J Surg Oncol. 22:272024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang YL, Tsai YF, Hsu CL, Chao YK, Hsu CC
and Lin KC: The effectiveness of a nurse-led exercise and health
education informatics program on exercise capacity and quality of
life among cancer survivors after esophagectomy: A randomized
controlled trial. Int J Nurs Stud. 101:1034182020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Wang F and Liu C: Sarcopenia
Before Concurrent Chemoradiotherapy May DevelopSevere Adverse
Reactions in Patients with Esophageal Squamous CelCarcinoma. Cancer
Research on Prevention and Treatment. 50:1203–1208. 2023.(In
Chinese).
|
|
29
|
Jordan T, Mastnak DM, Palamar N and Kozjek
NR: Nutritional therapy for patients with esophageal cancer. Nutr
Cancer. 70:23–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zitvogel L, Pietrocola F and Kroemer G:
Nutrition, inflammation and cancer. Nat Immunol. 18:843–850. 2017.
View Article : Google Scholar : PubMed/NCBI
|