Introduction
Glioblastoma (GBM) is the most aggressive primary
malignant tumor of the adult central nervous system and has a poor
prognosis. Despite comprehensive standard-of-care
treatment-including maximal safe surgical resection, followed by
radiotherapy with concomitant temozolomide (TMZ), and subsequent
maintenance TMZ-progression-free survival (PFS) typically ranges
from 6.2 to 7.5 months, and overall survival (OS) remains limited
to 14.6–16.7 months (1).
Tumor-treating fields (TTFields) are non-invasive
antimitotic therapies that use low-intensity,
intermediate-frequency alternating electric fields to disrupt
mitosis and induce apoptosis in dividing cancer cells (2). In an initial phase III randomized
controlled trial involving 237 patients with recurrent GBM,
TTFields did not significantly improve PFS or OS compared to
physician-choice chemotherapy (3).
Nevertheless, TTFields were associated with a preserved quality of
life, minimal systemic adverse effects, and high patient
acceptability, leading to Food and Drug Administration approval in
2011 for use after recurrence following standard
chemoradiotherapy.
Subsequent evidence from the EF-14 trial established
the efficacy of TTFields combined with maintenance TMZ in newly
diagnosed GBM and demonstrated a significant survival benefit over
TMZ alone (4). This finding
supports the adoption of TTFields as a standard adjunctive therapy
worldwide, including insurance approval in Japan in 2019.
The optimal therapeutic benefits of TTFields are
achieved by high adherence. Post-hoc analyses from the EF-14 showed
that patients with device usage rates of at least 75% and daily
usage of ≥18 h exhibited significantly improved PFS and OS
(5). Moreover, a longer treatment
duration correlated with better outcomes; patients treated for at
least 6 months, 9 months, or longer achieved superior OS and higher
2-year survival rates than those treated for shorter durations. In
addition, real-world data indicate that patients who continued
TTFields for ≥12 months experienced a median OS improvement of
>10 months compared to those who received standard treatment
alone (6).
Conversely, patients with treatment durations
<2-3 months are commonly excluded from analyses because
therapeutic efficacy is difficult to evaluate in such cases.
Therefore, the risk factors associated with early discontinuation
of TTFields remain poorly understood.
This study identified the clinical and demographic
factors associated with early discontinuation of TTFields therapy
in adult patients with GBM, based on a retrospective analysis
conducted at a single institution.
Patients and methods
Aim
This retrospective cohort study was conducted at a
single institution to identify the clinical and molecular factors
associated with early discontinuation of TTFields therapy in
patients with GBM.
Ethical approval
The study protocol was approved by the Ethics
Committee of the University of Occupational and Environmental
Health (UOEH; Kitakyushu, Japan; approval no. CR24-117). The
GBM-specific next-generation sequencing (NGS) panel data used in
the present study were obtained from a multicenter collaborative
project approved by the Ethics Committee of Kagoshima University
(approval no. 180104-Eki; Kagoshima, Japan) and were additionally
approved by the Ethics Committee of UOEH for participation as a
collaborating site (approval no. UOEHCRB20-100). All procedures
were conducted in accordance with the principles of the Declaration
of Helsinki. Written informed consent was obtained from all
patients, in accordance with the approval of the institutional
ethics committee.
Patient data
This study included 16 consecutive adult patients
(≥18-years-old) newly diagnosed with supratentorial GBM between
September 2019 and February 2025, who initiated TTFields therapy at
the University Hospital of Occupational and Environmental Health.
Clinical and treatment-related data were retrospectively collected
from medical records. The assessed variables included age, sex,
medical history, family history, presenting symptoms, activities of
daily living (ADL), neurocognitive assessment results at TTFields
initiation, histopathological findings, extent of resection,
adjunctive therapies, pre- and post-treatment imaging findings,
average TTFields adherence rate, treatment duration, travel
distance to the hospital, and availability and type of caregivers.
All patients were diagnosed with GBM according to the World Health
Organization (WHO) classification criteria at the time of
diagnosis. All patients were treated according to the standard of
care, which included maximal safe resection followed by
radiotherapy (RT) with concurrent daily TMZ and maintenance TMZ for
6–12 months.
ADL and neurocognitive function were assessed at the
initiation of TTFields therapy. Based on a previous study (7), early discontinuation was defined as
the cessation of TTFields therapy within <3 months without
subsequent resumption.
Molecular data
Tumors were classified according to the 5th edition
of the WHO Classification of Tumors of the Central Nervous System.
The molecular profiling required for integrated diagnosis was
conducted using a GBM-specific NGS panel as previously described
(8). Our institution participated
as a collaborating center in this molecular study and obtained
ethical approval from both the central review board and the
coordinating institution (approval number: UOEHCRB20-100).
Statistical analysis
All statistical analyses were performed using EZR
version 1.54 (2020), a graphical interface for R (9). P<0.05 was considered to indicate a
statistically significant difference. Categorical variables were
analyzed using the χ2 test or Fisher's exact test, as
appropriate. The Shapiro-Wilk test was used to assess normality for
continuous variables, with P≥0.05 indicating a normal distribution.
Unpaired Student's t-test was applied to normally distributed
variables, while the Mann-Whitney U test was used for non-normally
distributed variables. Kaplan-Meier survival analysis with the
log-rank test was used to estimate PFS and OS. Hazard ratios (HRs)
were calculated using the Cox proportional hazard model. Outlier
analysis for continuous variables was performed using the
Smirnov-Grubbs test. To evaluate robustness without case exclusion
after outlier screening, Welch's t-test was used for between-group
comparisons of continuous variables. In addition, the treatment
duration and distance from the patient's residence to the hospital
were analyzed using both parametric and non-parametric tests after
normality assessment. Outlier analysis identified two extreme
values in treatment duration and one extreme value in distance from
the patient's residence to the hospital. These cases were retained
in the primary analyses and were excluded only in sensitivity
analyses, with results compared with those of the full dataset to
confirm robustness. All statistical analyses were univariate.
Multivariate analyses were not performed because of the limited
sample size and risk of model overfitting.
Results
Patient and treatment
characteristics
Overall, our analyses included 16 patients newly
diagnosed with supratentorial GBM, who were initiated on TTFields
therapy (Table I). The mean age was
57.4±14.6 years (range: 19–80), with nine males and seven females.
The Smirnov-Grubbs test was performed to identify outliers;
however, no outliers were detected. Therefore, all cases were
included in the analysis. The presenting symptoms included
hemiparesis in three patients (18.8%), symptomatic epilepsy in
three patients (18.8%), and motor aphasia in three patients
(18.8%). The surgical resection status included seven cases of
gross total resection (43.8%), five cases of subtotal resection
(31.3%), and four biopsies (25.0%). The primary caregiver was a
spouse in 11 cases (68.8%), a parent in 2 cases (12.5%), child in 4
cases (25.0%), and sibling in 1 case (6.3%). In two cases, multiple
primary caregivers were present (spouse and child); thus, each
caregiver type was counted separately. The mean distance from the
patient's residence to the hospital was 20.4±17.0 km (range:
1.2–65.0 km). The mean duration of TTFields therapy was 199.5±206.5
days. Early discontinuation, defined as cessation within 90 days
without resumption, occurred in five patients (31.3%). The primary
reason for early discontinuation was the preference of patients and
their families. The mean adherence rate during treatment was
58.5±26.5% (range: 3–96.96), while the mean OS and PFS were
484.9±234.1 days and 397.0±203.9 days, respectively.
 | Table I.Patient and treatment characteristics
(n=16). |
Table I.
Patient and treatment characteristics
(n=16).
| Characteristic | Value |
|---|
| Age, years |
|
| Mean ± SD
(range) | 57.4±14.6
(19–80) |
| Sex, n (%) |
|
| Men | 9 (56.3) |
|
Women | 7 (43.8) |
| Clinical symptom, n
(%) |
|
|
Hemiparesis | 3 (18.8) |
| Motor
aphasia | 3 (18.8) |
|
Epilepsy | 3 (18.8) |
| Higher brain
dysfunction, n (%) | 7 (43.8) |
| Attention impairment,
n (%) | 6 (37.5) |
| Resection status, n
(%) |
|
| GTR | 7 (43.8) |
| STR | 5 (31.3) |
|
Biopsy | 4 (25.0) |
| Caregiver, n (%) |
|
|
Spouse | 11 (68.8) |
|
Parent | 2 (12.5) |
|
Child | 4 (25.0) |
|
Sibling | 1 (6.3) |
| Distance to the
medical facility, km |
|
| Mean ± SD
(range) | 20.4±17.0
(1.2–65.0) |
| TTFields treatment
status |
|
| Duration
of TTFields, days, |
|
|
Mean ± SD
(range) | 199.5±206.5 (1–800) |
|
Mean ± SD
adherence rate, % (range) | 58.5±26.5
(3–96.96) |
| Mean ± SD survival
outcome, days (range) |
|
| OS | 484.9±234.1
(187–958) |
| PFS | 397.0±203.9
(143–800) |
| ADL |
|
| Mean ± SD
KPS (range) | 85.6±16.7
(50–100) |
| Mean ± SD
Barthel Index (range) | 88.9±22.5
(39–100) |
|
≥85a, n (%) | 13 (81.3) |
The molecular profiles obtained using a GBM-specific
NGS panel were available for 12 of the 16 patients (75%). Among
these, isocitrate dehydrogenase (IDH) 1/2 mutations were found in
two cases, TP53 mutations in five cases,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
mutations in three cases, epithelial growth factor receptor
amplification in two cases, and telomerase reverse transcriptase
promoter mutations in six cases (C228T: four cases; C250T: two
cases). According to the 2021 edition of the WHO classification,
the integrated diagnoses were as follows: 10 cases of GBM, IDH
wild-type; 2 cases of astrocytoma, IDH mutant, grade 4; and 4 cases
of GBM, not otherwise specified.
All ADL assessments were performed by a
multidisciplinary team of rehabilitation professionals including
physicians, physical therapists, occupational therapists, and
speech-language pathologists. At the initiation of TTFields
therapy, the mean Karnofsky performance status (KPS) was 85.6±16.7
(range: 50–100) and mean the Berthel index (BI) was 88.9±22.5
(range: 39–100). Thirteen patients (81.3%) had BI scores >85,
which is generally considered indicative of ADL independence.
Neurocognitive function was evaluated by a
multidisciplinary team including rehabilitation specialists
(Table II). All patients underwent
at least one assessment. The reasons for missing or incomplete
examinations included patient refusal, inability to continue the
assessment because of reduced endurance, and the examiner's
judgment that the test was inappropriate. In some cases, the
examiner determined that alternative higher-order cognitive
assessments would be more suitable. However, these were not
included in the current analysis because of sample size
limitations. The tests included the Mini-Mental State Examination
(MMSE) in 13 patients (81.3%), Frontal Assessment Battery (FAB) in
10 patients (62.5%), Trail Making Test (TMT) in 8 patients (50.0%),
including 1 patient with only part A; and the Rey-Osterrieth
Complex Figure Test (ROCFT) in 3 patients (18.8%). Mean scores (±
standard deviation) were as follows: MMSE, 26.8±3.6; FAB, 14.6±4.2;
TMT-A, 83.0±96.7 sec (four patients below age-based cutoff); TMT-B,
80.4±46.6 sec (two patients below cutoff); ROCFT copy score,
34.3±1.5; and memory score, 24.2±9.3. Therefore, seven patients
(43.8%) were judged to have neurocognitive dysfunction, and six
(37.5%) were considered to have attention impairment.
 | Table II.Neurocognitive test scores of patients
treated with TTFields. |
Table II.
Neurocognitive test scores of patients
treated with TTFields.
| Neurocognitive test
(n) | Score, mean ± SD
(range) |
|---|
| MMSE (13) | 26.8±3.6 (19–30) |
| FAB (10) | 14.6±4.2 (5–18) |
| TMT |
|
| Part-A
(8) | 83.0±96.7
(21.8–317) |
| Part-B
(7) | 80.4±46.6
(37.5–151) |
| ROCFT |
|
| Copy
(3) | 34.3±1.5 (33–36) |
| Memory
(3) | 24.2±9.3
(13.5–30) |
Comparative characteristics by
cohort
A comparative analysis was conducted between the
early discontinuation (n=5) and continuation (n=11) groups,
focusing on clinical characteristics, TTFields treatment status,
survival outcomes, and ADL (Table
III). The mean duration of TTFields use was significantly
shorter in the early discontinuation group than in the continuation
group (46±29.7 days vs. 269.3±210.5 days; Mann-Whitney U test,
P<0.001). Welch's t-test yielded consistent results (P=0.007).
Outlier analysis identified two extreme values; however, when these
cases were excluded (n=14), the difference remained statistically
significant (t-test, P<0.001), confirming the robustness of our
finding. No significant differences were observed in age, sex, or
baseline characteristics between groups. Regarding the distance
from the patient's residence to the hospital, Welch's t-test using
the full dataset showed no significant differences between the
groups (P=0.177). An outlier analysis identified one extreme value;
however, when this case was excluded, the Mann-Whitney U test also
indicated no significant difference (P=0.892), confirming that the
presence of this outlier did not alter the interpretation. However,
hemiparesis at TTFields therapy initiation (P=0.018) and attention
impairment (P=0.036) was significantly more frequent in the early
discontinuation group than in the continuation group. Regarding
ADL, the early discontinuation group had significantly lower
baseline KPS scores (P=0.005), with a trend similar trend to that
observed for BI scores (P=0.013). No significant differences were
observed in the overall neurocognitive test scores between the two
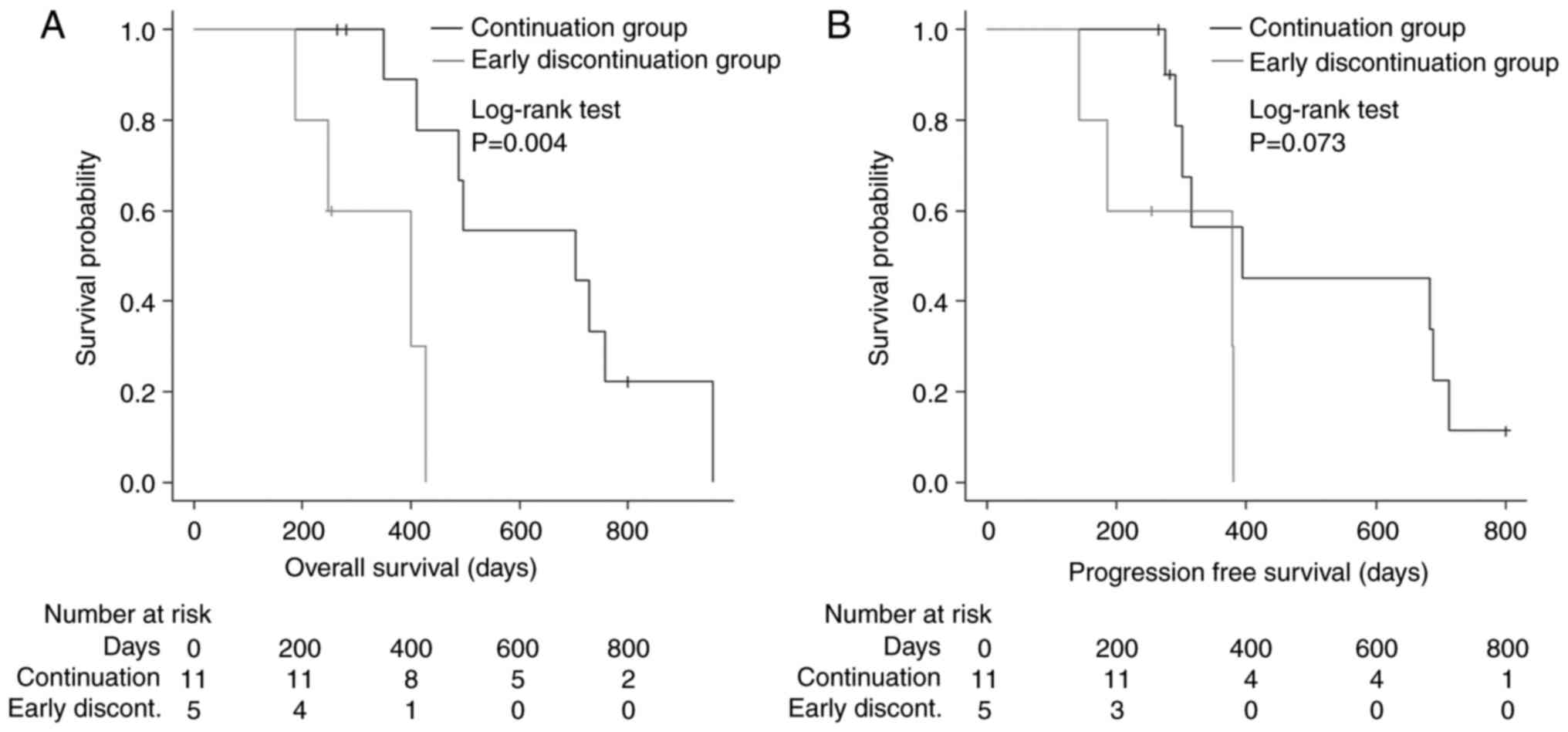
groups. Regarding survival outcomes, OS was significantly shorter
in the early discontinuation group [HR: 8.857, 95% confidence
interval (CI): 1.56–50.29, P=0.004; Fig. 1A] than in the continuation group.
Although the difference was not significant, PFS also tended to be
shorter in the early discontinuation group (HR: 3.35, 95% CI:
0.83–13.59, P=0.073; Fig. 1B) than
in the continuation group. Regarding molecular status, TP53
mutations tended to be more frequent in the treatment continuation
group than in the early discontinuation group; however, this
difference was not statistically significant (P=0.061). No
differences were observed in the molecular profiles between the two
groups (Table IV).
 | Table III.Comparison of patient characteristics
between cohorts. |
Table III.
Comparison of patient characteristics
between cohorts.
| Characteristic | Early discontinuation
group (n=5) | Continued use group
(n=11) | P-value |
|---|
| Age, years |
|
|
|
| Mean ± SD
(range) | 60.6±11.3
(42–73) | 56.0±15.5
(19–80) | 0.607 |
| Sex, n (%) |
|
| >0.999 |
| Men | 3 (60.0) | 6 (54.5) |
|
|
Women | 2 (40.0) | 5 (45.5) |
|
| Clinical symptom, n
(%) |
|
|
|
|
Hemiparesis | 3 (60.0) | 0 (0.0) | 0.018a |
| Motor
aphasia | 2 (40.0) | 1 (9.1) | 0.214 |
|
Epilepsy | 1 (20.0) | 2 (18.2) | >0.999 |
| Higher brain
dysfunction, n (%) | 4 (80.0) | 3 (27.3) | 0.106 |
| Attention
impairment, n (%) | 4 (80.0) | 2 (18.2) | 0.036a |
| Resection status, n
(%) |
|
|
|
|
GTR | 1 (20.0) | 6 (56.3) | 0.308 |
|
STR | 2 (40.0) | 3 (56.3) | >0.999 |
|
Biopsy | 2 (40.0) | 2 (56.3) | 0.547 |
| Caregiver, n
(%) |
|
|
|
|
Spouse | 5 (100) | 6 (54.5) | 0.119 |
|
Parent | 0 (0.0) | 2 (18.2) | >0.999 |
|
Child | 1 (20.0) | 3 (27.3) | >0.999 |
|
Other | 0 (0.0) | 1 (9.1) | 0.509 |
| Distance to the
medical facility, km |
|
|
|
| Mean ± SD
(range) | 21.2±10.9 (13–41) | 20.1±19.3
(1.2–65) | 0.335 |
| TTFields treatment
status |
|
|
|
|
Duration of TTFields,
days, |
|
|
|
| Mean ±
SD (range) | 46±29.7 (1–89) | 269.3±210.5
(102–800) |
<0.001b |
| Mean ±
SD adherence rate, % (range) | 55.5±33.8
(17–96.96) | 59.6±25.5
(3–86.7) | 0.800 |
| Mean ± SD survival
outcome, days (range) |
|
|
|
| OS | 303.2±93.9
(187–429) | 567.5±227.4
(265–958) | 0.004a |
|
PFS | 268.8±97.7
(143–381) | 455.3±210.3
(265–800) | 0.073 |
| ADL |
|
|
|
| Mean ±
SD KPS (range) | 66.0±15.1
(50–90) | 94.5±6.9
(80–100) | 0.005b |
| Mean ±
SD Barthel Index (range) | 65.8±30.7
(39–100) | 99.5±1.3
(96–100) | 0.013a |
|
≥85c, n (%) | 2 (40.0) | 11 (100) | 0.018a |
 | Table IV.Comparison of tumor molecular
profiles between cohorts. |
Table IV.
Comparison of tumor molecular
profiles between cohorts.
| Molecular
profile | Early
discontinuation group (n=4) | Continued use group
(n=8) | P-value |
|---|
| IDH1/2
mutation | 0 | 2 | 0.515 |
| TP53 mutation | 0 | 6 | 0.061 |
| PI3KCA
mutation | 0 | 3 | 0.491 |
| EGFR
amplification | 2 | 1 | 0.236 |
| TERT promoter
mutation | 3 | 3 | 0.545 |
|
C228T | 2 | 2 | 0.547 |
|
C250T | 1 | 1 | >0.999 |
Discussion
This single-institution retrospective study
identified hemiparesis, low baseline KPS, and attentional
impairment as significant factors associated with the early
discontinuation of TTFields therapy in patients with GBM. To the
best of our knowledge, few studies have directly examined the
predictors of early treatment termination, as most pivotal trials,
including the EF-14 trial, have excluded patients who discontinued
therapy within the first 2–3 months (5,6).
Identifying factors that may predict early withdrawal after
treatment initiation is essential to enable more appropriate
patient selection and predict the need for treatment support
interventions. Our findings provide novel insights into clinical
characteristics that may hinder treatment persistence in real-world
settings.
Previous studies have established that high
adherence and prolonged TTFields use are strongly correlated with
improved survival outcomes (5,6). In
this study, the TTFields continuation group demonstrated a
significantly prolonged OS and favorable PFS. However, determinants
of poor adherence and early treatment discontinuation remain
largely unknown.
In this cohort, the presence of hemiparesis at the
start of treatment may have contributed to treatment difficulties
due to challenges in maintaining balance during movement and
cognitive-motor interference when operating the device while
walking. Previous studies examining the transport ability of
patients with hemiparesis after stroke have reported a prolonged
unloading phase and difficulties in maintaining posture during the
transition from lifting to placing objects (10). Furthermore, in another study
involving patients with chronic stroke, even those capable of
walking short distance without assistive devices exhibited
significant reductions in walking speed when performing simple
cognitive and motor tasks, such as carrying water in a cup without
a handle or walking while performing serial subtraction, likely due
to cognitive-motor interference (11).
Similarly, impaired attention has been associated
with difficulties in continuing TTFields treatment. Such deficits
may reduce a patient's ability to comply with the complex routines
required for effective TTFields. This observation aligns with
previous reports showing that attention deficits are associated
with reduced treatment adherence in patients with higher brain
function disorders. Conditions characterized by attention deficit
and executive dysfunction, such as attention-deficit hyperactivity
disorder, are associated with impaired diabetes management and low
treatment adherence rates (12).
Although the association between higher brain dysfunction and
treatment adherence is well-established, evidence specific to
wearable treatment devices remains limited. A review of continuous
positive airway pressure, another wearable treatment device with
results comparable to TTFields, reported that for patients with
mild cognitive impairment and Alzheimer's disease, device setup and
continued use require attention, memory, and executive function;
moreover, support or caregiver involvement is important when
adherence declines (13).
In this study, no significant differences were
observed between the two groups in neurocognitive evaluation test
score, which were associated with attention and executive function.
This may be attributed to the small sample size, which prevented
multivariate analysis from being performed. Therefore, our results
should be interpreted with caution. In addition, the type of
neuropsychological tests administered and the lack of data may have
influenced our results. Nevertheless, these findings suggest that
impaired attention may be an important factor that hinders the
continuation of TTFields treatment.
In addition, molecular profiles were available for
12 of the 16 patients. A trend toward a higher frequency of TP53
mutations was observed in the continued use group, although the
difference was not statistically significant. TP53 has been
primarily investigated for its role in tumor proliferation,
treatment responsiveness, and prognosis (14). To date, no direct association
between TP53 mutations and functional indicators such as the KPS or
the BI has been established. Moreover, reports on the prognostic
significance of TP53 mutations in glioblastoma remain controversial
(15). Given the limited sample
size of our cohort, these findings should be interpreted with
caution, and confirmation in large-scale studies is warranted.
A low baseline functional status, as reflected by
KPS, likely compounded these difficulties, making sustained
treatment less feasible. These findings are consistent with the
inclusion criteria of the EF-14 trial, which required a KPS ≥70,
and with the National comprehensive network guidelines, which
recommend adjuvant treatment for patients with good performance
status, defined as KPS ≥60 (16).
Our cohort did not include patients with sensory
aphasia, as they have been reported to contribute to TTFields
discontinuation. However, motor aphasia was not identified as a
contributing factor in this study (17). These results may have been
influenced by the limited sample size and should, therefore, be
interpreted with caution.
These observations underscore the importance of
early identification of patients at high risk of treatment
discontinuation. In such cases, proactive interventions, including
enhanced caregiver involvement, structured educational programs,
and frequent follow-up visits to monitor device usage, are
warranted. Moreover, comprehensive welfare support such as the
provision of in-home nursing services, assistive devices, and
transportation assistance may alleviate both physical and
logistical barriers to treatment continuity. Integrating these
supportive measures into standard clinical practice potentially
improves adherence and thereby enhances therapeutic benefits,
particularly in patients with neurological deficits or cognitive
impairment.
This study had several limitations. The small sample
size and single-center design limited the generalizability of our
findings. The small cohort also reduced the statistical power of
the analysis and may have resulted in unstable estimates of effect
sizes. Therefore, our results should be interpreted as exploratory
rather than confirmatory. The retrospective nature of this study
may have introduced selection and information biases, and
unmeasured confounding factors cannot be excluded. Neurocognitive
assessments were not performed uniformly across all patients, and
molecular profiling data were unavailable for a cohort subset.
Furthermore, treatment adherence and discontinuation may have been
influenced by unmeasured psychosocial or socioeconomic factors that
were not captured in this analysis. Despite these limitations, the
consistency of the associations observed between functional
deficits and early discontinuation suggests that these factors
warrant further investigation. Future research should focus on
validating these findings through larger, prospective, multicenter
studies that incorporate standardized neurocognitive testing,
comprehensive molecular profiling, and detailed psychosocial
assessments to better elucidate the determinants of treatment
persistence.
In conclusion, our findings highlight specific
clinical features, including hemiparesis, attentional impairment,
and reduced KPS status, which may predispose patients with GBM to
early discontinuation of TTFields therapy. Addressing these
challenges through targeted clinical and welfare support strategies
may improve treatment persistence and maximize the survival
benefits of TTFields. Future studies should explore the effects of
these interventions on patient adherence and long-term
outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The GBM-specific NGS panel
data generated in the present study are not publicly available due
to institutional and ethical restrictions imposed by the governance
policy of the multicenter consortium but may be requested from the
corresponding author.
Authors' contributions
KSu designed the study, collected and analyzed the
clinical data, and drafted the manuscript. KSu, SN and JY confirm
the authenticity of all the raw data. KSa, SN, RM, TS, YN and JY
contributed to the study design, acquisition and analysis of
clinical data, and critically reviewed the manuscript for important
intellectual content. NH, TA, AT and RH were responsible for the
acquisition and analysis of molecular data, and contributed to data
interpretation. All authors read and approved the final version of
the manuscript, and agree to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the University of Occupational and Environmental Health (UOEH)
(approval number: CR24-117). The glioblastoma-specific
next-generation sequencing panel data used in the present study
were obtained from a multicenter collaborative project approved by
the Ethics Committee of Kagoshima University (approval no.
180104-Eki) and were additionally approved by the Ethics Committee
of UOEH for participation as a collaborating site (approval no.
UOEHCRB20-100). All procedures were performed in accordance with
the ethical standards of the Institutional Research Committee and
the 1964 Declaration of Helsinki and its later amendments. Patient
participation consent was obtained through an opt-out procedure
approved by the institutional ethics committee.
Patient consent for publication
Not applicable, as this study did not include any
individual patient images or identifiable data requiring consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C,
Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirson ED, Dbalý V, Tovaryš F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilbert MR, Wang M, Aldape KD, Stupp R,
Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT,
et al: Dose-dense temozolomide for newly diagnosed glioblastoma: A
randomized phase III clinical trial. J Clin Oncol. 31:4085–4091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with Tumor-treating fields plus
temozolomide vs. temozolomide alone for glioblastoma: A randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toms SA, Kim CY, Nicholas G and Ram Z:
Increased compliance with tumor treating fields therapy is
prognostic for improved survival in the treatment of glioblastoma:
A subgroup analysis of the EF-14 phase III trial. J Neurooncol.
141:467–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ballo MT, Conlon P, Lavy-Shahaf G, Kinzel
A, Vymazal J and Rulseh AM: Association of Tumor Treating Fields
(TTFields) therapy with survival in newly diagnosed glioblastoma: A
systematic review and meta-analysis. J Neurooncol. 164:1–9. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pena LC, Anderson C, Agarwal MS, Galvan E,
Kelly W and Wagner TD: Retrospective review of the factors limiting
optune initiation in GBM patients. Int J Radiat Oncol. 117:e932023.
View Article : Google Scholar
|
|
8
|
Higa N, Akahane T, Yokoyama S, Yonezawa H,
Uchida H, Takajo T, Kirishima M, Hamada T, Matsuo K, Fujio S, et
al: A tailored next-generation sequencing panel identified distinct
subtypes of wildtype IDH and TERT promoter glioblastomas. Cancer
Sci. 111:3902–3911. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parry R, Macias Soria S, Pradat-Diehl P,
Marchand-Pauvert V, Jarrassé N and Roby-Brami A: Effects of hand
configuration on the grasping, holding, and placement of an
instrumented object in patients with hemiparesis. Front Neurol.
10:2402019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goh LY, Tan IO, Yang LC and Ng SSM:
Effects of cognitive and motor tasks on the walking speed of
individuals with chronic stroke. Medicine (Baltimore).
96:e62322017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dehnavi AZ, Zhang-James Y, Draytsel D,
Carguello B, Faraone SV and Weinstock RS: Association of ADHD
symptoms with type 2 diabetes and cardiovascular comorbidities in
adults receiving outpatient diabetes care. J Clin Transl
Endocrinol. 32:1003182023.PubMed/NCBI
|
|
13
|
DeVettori G, Troxel WM, Duff K and Baron
KG: Positive airway pressure adherence among patients with
obstructive sleep apnea and cognitive impairment: A narrative
review. Sleep Med. 111:28–35. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Dube C, Gibert MJ, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 pathway in glioblastoma. Cancers (Basel). 10:2972018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park AK, Kim P, Ballester LY, Esquenazi Y
and Zhao Z: Subtype-specific signaling pathways and genomic
aberrations associated with prognosis of glioblastoma. Neuro Oncol.
21:59–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
NCCN Clinical Practice Guidelines in
Oncology, . Central Nervous System Cancers. Version 2.2021.
National Comprehensive Cancer Network; Plymouth Meeting, PA:
Available at:. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdfAugust
18–2025
|
|
17
|
Takido Y, Ohka F, Deguchi S, Motomura K,
Mitsuya K, Aoki K, Shiba Y, Takeuchi K, Nagata Y, Yamaguchi J, et
al: Distant parenchymal recurrence during long-term use of TTFields
treatment for glioblastoma. Int J Clin Oncol. 30:1309–1318. 2025.
View Article : Google Scholar : PubMed/NCBI
|