Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors in children and young adults, and its
occurrence and development are influenced by various factors. In
recent years, advancements in the understanding of OS and the
refinement of therapeutic approaches have led to an increase in the
5-year survival rate for patients with tumors, now ranging from 60
to 80% (1). Nevertheless, the
mortality rate associated with OS remains elevated compared with
other malignant bone tumors, particularly among patients with
metastatic or recurrent OS, where the long-term survival rate
frequently falls below 30% (2).
OS tumors develop within a multifaceted and
dynamically evolving tumor microenvironment (TME) that encompasses
bone cells, stromal cells, vascular cells, immune cells and a
mineralized extracellular matrix (ECM). The intricate interactions
between OS cells and their surrounding microenvironment are
critical in influencing various aspects of tumor biology, including
progression, apoptosis, invasion, metastasis, angiogenesis and
responses to therapeutic interventions (3). The TME is not a passive bystander but
an active participant in oncogenesis; it provides critical support
for tumor cell growth and dissemination and promotes the
maintenance of the malignant phenotype through key mechanisms such
as immune evasion, induction of angiogenesis and metabolic
reprogramming (4).
Specific components of the OS TME, including
osteocytes, tumor-associated immune cells, bone marrow (BM)
mesenchymal stem cells (MSCs) and the ECM, interact to create a
hypoxic and vascularized niche conducive to proliferation and
metastasis (5). Recognition of the
pivotal role of the TME has driven the development of novel
therapeutic strategies. For instance, inhibitors targeting
immunosuppressive cells or immune checkpoints have achieved
breakthroughs in certain solid tumors and are being explored in OS
clinical trials (6). Additionally,
approaches such as targeting cytokines secreted by
cancer-associated fibroblasts (CAFs) or blocking abnormal
angiogenesis are under investigation as potential combination
therapies to enhance traditional treatment efficacy (7). Although still in the early stages,
these TME-targeted therapies hold promise to overcome
chemoresistance and improve survival.
The present review addresses the multiple roles of
the TME in OS development, examining how its various components
influence tumor proliferation, metastasis and treatment response;
it also discusses the emerging paradigm of targeting the TME,
highlighting the need for further research into its heterogeneity
to disrupt the pro-tumorigenic cycle and offer new hope for
patients with OS.
TME of OS
OS tumors arise within a complex and dynamic
microenvironment that comprises both cellular and non-cellular
components. This environment consists of a heterogeneous population
of bone cells, which includes osteoblasts (OBs), osteoclasts and
osteocytes, alongside stromal cells such as MSCs and fibroblasts.
Furthermore, it encompasses vascular cells, including endothelial
cells and pericytes, as well as immune cells, which comprise BM
cells and lymphocytes, in addition to a mineralized ECM (8).
Under typical physiological conditions, the
interactions among bone, vascular and stromal cells occur through
paracrine signaling and cellular communication, which are essential
for maintaining bone homeostasis (8). BM cells represent the primary cell
type within TME of OS (8). Recent
single-cell analyses have uncovered a multitude of ligand-receptor
interactions among OS tumors, BM and OBs, leading to the
identification of 21 ligand-receptor gene pairs that exhibit a
significant correlation with survival outcomes (9). The TME not only fosters a conducive
environment for the proliferation of tumor cells but also secretes
various factors, including cytokines, chemokines and growth
factors, which facilitate tumor cell metastasis (8). Fig. 1
illustrates the complex composition of the OS TME, encompassing
major cellular components such as osteocytes, stromal cells and
immune cells, alongside non-cellular elements including the ECM,
vascular networks and hypoxic conditions; it also depicts
interactions between tumor cells and select components within the
OS TME.
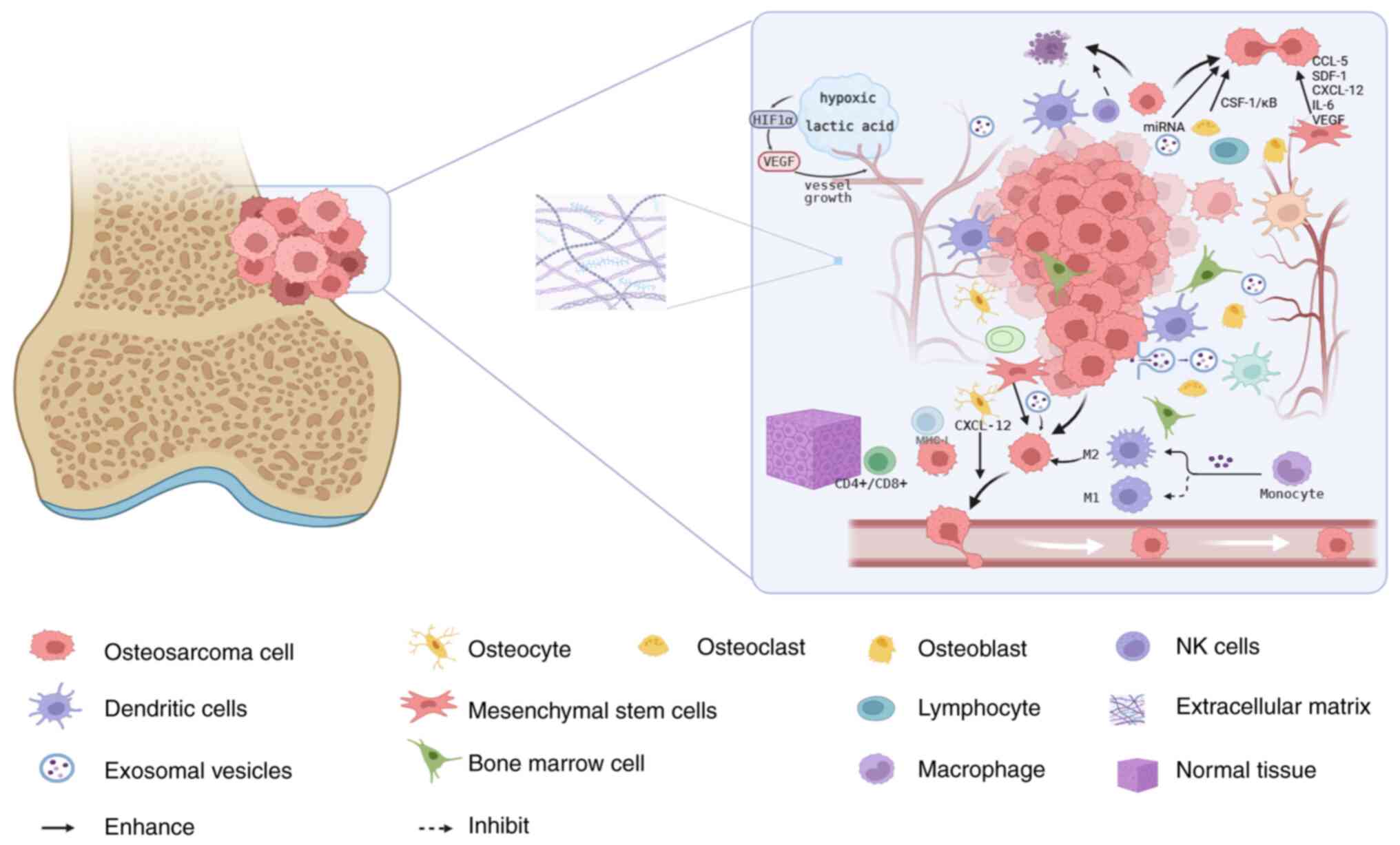 | Figure 1.Microenvironment of osteosarcoma is
composed of cellular and non-cellular components, including bone
cells (osteoblasts, osteoclasts and osteocytes), stromal cells
(mesenchymal stem cells and fibroblasts), immune cells (bone marrow
and lymphocytes), mineralized ECM, rich microcirculation vessels
and a hypoxic environment. Other components include CTCs, EVs and
various signaling molecules. The tumor microenvironment not only
provides a favorable growth environment for tumor cells, but also
secretes various factors, including various cytokines, chemokines
and growth factors, promoting tumor cell metastasis. The figure was
created by BioRender. OSc, osteosarcoma cell; Ocytes, osteocyte;
OCs, osteoclast; OBs, osteoblast; TAMs, tumor-associated
macrophages; DCs, dendritic cells; MST, mesenchymal stem cell;
Lymph, lymphocyte; ECM, extracellular matrix; EV, exosomal
vesicles; BMC, bone marrow cell; NT, normal tissue; MSC,
multipotent stem cell; CTCs, circulating tumor cells. |
Osteocytes
Osteocytes constitute an essential element of the
bone microenvironment, which encompasses three principal categories
of bone cells: OBs, osteoclasts and osteocytes. These cells
interact with stromal and immune cells by secreting a variety of
cytokines and chemokines that facilitate tumor growth, invasion and
metastasis. OBs originate from multipotent MSCs, and their
development can be augmented by particular cytokines, including
interferon (IFN)-γ, interleukin (IL)-12 and IL-13 (8). Conversely, their function may be
inhibited by factors including tumor necrosis factor (TNF)-α,
TNF-β, IL-1, IFN-α, IL-4 and IL-7 (10). OBs also have a notable role in
influencing the formation, differentiation or apoptosis of
osteoclasts through various signaling pathways, including the
osteoprotegerin/receptor activator of nuclear factor-κB ligand
(RANKL)/receptor activator of nuclear factor-κB (RANK),
RANKL/leucine-rich repeats containing G protein-coupled receptor
4/RANK, Ephrin 2/ephB4 and Fas/FasL pathways (11). Osteocytes can secrete a variety of
soluble factors, including growth differentiation factor 15, TGF-β,
chemokines CXC-motif chemokine ligand (CXCL) 1 and CXCL2, as well
as vascular endothelial growth factor (VEGF). They additionally
synthesize RANKL, colony-stimulating factor 1 (CSF-1),
high-mobility group box 1 (HMGB1) and IL-11, all of which promote
osteoclastogenesis and the process of bone resorption (12). In OS, osteocytes activate the
CXCL12/CXCR4 signaling axis by producing CXCL12, which is linked to
tumor metastasis. Osteoclasts are integral to the initiation and
metastasis of OS (13). Among the
cytokines involved, CSF-1 and soluble RANKL are crucial for the
differentiation and activation of OBs). CSF-1 is responsible for
the regulation of both the proliferation and survival of
pre-osteoclasts (14). By contrast,
RANKL, which is synthesized by OBs and various other bone cells
(15), interacts with its receptor
RANK located on the surface of osteoclast precursors (16). This interaction regulates the
differentiation and maturation of osteoclast precursors via
paracrine signaling mechanisms. In the context of OS, heightened
osteoclast activity plays a significant role in the proliferation
of OS cells and the degradation of bone tissue. This mechanism
leads to the liberation of pro-tumorigenic factors, such as
insulin-like growth factor 1 (IGF-1) and from the bone matrix,
thereby promoting tumor proliferation and metastasis (17).
Tumor-associated immune cells
Within the microenvironment of OS, monocytes and
macrophages constitute 70–80% of the total BM cell population
(18). Various cell types,
including tumor-associated macrophages (TAMs), T cells, B cells,
natural killer (NK) cells and BM MSCs, present in the tumor immune
microenvironment significantly influence tumor development,
invasion and metastasis. Recent investigations into TAMs have
emerged as a focal point of research.
TAMs constitute the most prevalent immune cell
population within the OS microenvironment, and account for >50%
of all infiltrating immune cells, alongside other cell types
including dendritic cells (DCs), lymphocytes and myeloid-derived
cells. Collectively, these cells form the primary components of the
OS immune microenvironment (19).
Research indicates that TAMs are prevalent in most high-grade OS
biopsy specimens, and an increase in TAMs is associated with
reduced metastasis and extended survival (20); however, the underlying mechanisms
remain unclear. Immune cells within the TME can either promote or
inhibit OS, contingent upon the immune environment and the specific
cell types involved (21). For
instance, certain immune cells, including T cells, B cells and NK
cells, can exert antitumor effects, while others, such as
myeloid-derived suppressor cells (MDSCs), M2 macrophages and
regulatory T cells (Tregs), may facilitate tumor growth and
metastasis. Consequently, elucidating the intricate mechanisms of
interaction between OS and immune cells is essential for advancing
research into novel immunotherapies (17).
TAMs
TAMs originate from peripheral monocytes and are
recruited to the tumor site by various chemokines and cytokines,
including C-C motif ligand 2, CSF-1 and VEGF. Upon entering the
tumor, TAMs can exhibit two primary phenotypes: The
pro-inflammatory M1 phenotype and the immunosuppressive M2
phenotype. The M1 phenotype is associated with protective responses
and is typically linked to a favorable prognosis in patients with
OS (22). Conversely, the M2
phenotype can be induced by various stimuli within the immune
microenvironment, particularly through cytokines IL-4 and IL-13
that signal via the signal transducer and activator of the
transcription 6 (STAT6) pathway, as well as IL-10 and
glucocorticoids. M2-type TAMs secrete a range of cytokines that
promote the migration and invasion of OS cells while inhibiting the
immune function of T lymphocytes (23). M1 and M2 TAMs can undergo
interconversion under the influence of key cytokines and signaling
pathways, such as the Th2 cytokines (IL-4 and IL-13) via the STAT6
pathway, as well as IL-10 and TGF-β. This macrophage polarization
is dynamic. Research has demonstrated that polarized M2-type TAMs
play a significant role in the proliferation and metastasis of OS.
For example, M2-type TAMs can facilitate the migration and invasion
of OS cells by triggering an autocrine signaling loop that
activates HMGB1 expression within the tumor cells. Furthermore,
HMGB1 has been shown to encourage the polarization of M1-type TAMs
into M2-type TAMs, thereby establishing a positive feedback
mechanism that exacerbates the development and progression of OS
(24).
T cells
T cells are integral to both cellular and humoral
immunity and comprise a wide array of functionally specialized
subsets, including T helper cells (Th1, Th2, Th9, Th17, Th22 and
follicular helper T cells), cytotoxic T lymphocytes (CTLs) and
Tregs. This diversity is crucial for orchestrating the antitumor
immune response in OS, with the composition and balance of these
infiltrating T cell subsets determining the immunological outcome.
In OS, tumor-infiltrating lymphocytes are predominantly located in
areas expressing human leukocyte antigen class I, while
CD4+ and CD8+ T cells primarily aggregate at
the interface between lung metastases and normal tissue (25). Studies have indicated that the
density of T cells in metastatic lesions is significantly greater
than that in primary and recurrent lesions, suggesting that T cells
may also serve as potential prognostic indicators (26,27).
Analysis of biopsy tissues and peripheral blood from patients with
primary OS has revealed that T cell levels in biopsy specimens
exceed those in peripheral blood, suggesting that the immune
microenvironment within tumor lesions is suppressive, potentially
inhibiting T cell immune activity through TAMs, further suggesting
that T cells may serve as an auxiliary biomarker for clinical
diagnosis (28). Furthermore, the
depletion of CD163+ macrophages, a hallmark
immunosuppressive subset in the OS TME, has been shown to enhance T
cell growth and pro-inflammatory factor production in vitro.
Therefore, targeting these cells represents a promising approach to
reprogram the immunosuppressive OS TME and potentiate antitumor
immunity (28). The prognostic
value of T cells is well-established across various cancer types,
with studies confirming that specific T-cell signatures, such as
the CD8 T-cell signature, are robust predictors of patient survival
(29,30). Furthermore, prognostic models based
on T-cell-related genes have been successfully constructed for
cancer types such as hepatocellular carcinoma (29,30).
B cells
B cells can be classified into three categories:
Naive B cells, memory B cells and effector B cells/plasma cells,
with the latter being the primary source of antibodies. Regulatory
B cells, a subset of B cells, exert immunosuppressive effects by
inhibiting CD4+ T cells, CTLs, macrophages and DCs
through the secretion of inhibitory cytokines such as IL-10, TGF-β
and IL-35, as well as the expression of membrane surface regulatory
molecules such as FasL and CD1d (31). Regulatory B cells also promote the
conversion of T cells into T lymphocytes, thereby diminishing the
antitumor immune response (32)].
Current research on B cells in OS remains limited; however, a
recent pan-cancer immune-infiltration analysis showed that patients
with high B-cell abundance exhibit a significantly improved overall
survival, and the proportion of activated B cells
(CD19+CD27+) correlates positively with
metastasis-free survival in OS (33). Thus, the presence of effector B
cells may serve as a favorable prognostic indicator. Antibodies
play a crucial role in the regulation of tumor growth and
metastasis through various mechanisms, including antibody-dependent
cell-mediated cytotoxicity, regulatory effects, complement
activation, tumor cell receptor blockade and alterations in tumor
cell adhesion (34,35). Nonetheless, some studies have also
indicated that certain antibodies may bind to antigens on the
surface of tumor cells, thereby obstructing their cytotoxic effects
(32,36).
NK cells
NK cells are a type of innate lymphocyte
characterized by the expression of the intracellular transcription
factor E4 BP 4+ and are identified as CD3−
CD19− CD56+ CD16+. NK cells have
been shown to not only directly eliminate tumor cells but also
regulate tumor progression and metastasis (37,38).
They can induce tumor cell death in the TME by releasing perforin,
granzyme and TNF-α, as well as expressing FasL (39). The programmed cell death protein-1
(PD-1)/programmed cell death ligand 1 (PD-L1) axis plays a role in
modulating the antitumor effects of NK cells. Research has
demonstrated that blocking the PD-1/PD-L1 axis with PD-L1
antibodies enhances the cytotoxic activity of NK cells against
human OS cells by inhibiting NK cell toxicity through the secretion
of granzyme B (40). Comprehensive
analyses of immune infiltration in the OS microenvironment have
revealed that male patients exhibit a higher presence of NK cells
compared with female patients (41). Additionally, it has been observed
that NK cells are suppressed in the OS microenvironment, with
increased expression of TGF-β (42). This suppression may involve the
inhibition of the activating receptor natural killer group 2 member
D and a reduction in perforin release by NK cells, thereby
promoting angiogenesis, bone remodeling and cellular migration.
BM MSCs
BM MSCs are classified as multipotent stem cells
that significantly contribute to the development of OS tumors
through the modulation of immune responses and the facilitation of
cell fusion and differentiation (43). MSCs and OBs are regarded as
potential precursors to OS cells (33). Research suggests that MSCs play a
crucial role in mediating the bidirectional crosstalk between OS
tumor cells and the TME through the secretion of a variety of
cytokines, chemokines, ILs and other signaling molecules (44). MSCs are actively involved in the
paracrine signaling mechanisms of OS tumor cells, influencing
multiple facets of tumor behavior, such as angiogenesis,
proliferation, invasion, metastasis, immune modulation and
resistance to chemotherapy (45).
Furthermore, MSCs facilitate the growth, metastasis, and
angiogenesis of OS by releasing an array of chemokines, including
CCL5, stromal-derived factor 1, CXCL12, IL-6 and VEGF (45).
MSC-derived extracellular vesicles have been shown
to enhance the proliferation, invasion and migration of OS cells
via the metastasis-associated lung adenocarcinoma transcript
1/microRNA (miR)-143/Neurensin-2/Wnt/β-catenin signaling pathway,
as well as through miR-655-mediated β-catenin signaling (46). Research indicates that TGF-β is
significantly upregulated in patients with OS (47). Furthermore, OS cells enhance the
secretion of extracellular vesicles that contain TGF-β. This
process subsequently stimulates the release of IL-6, which is
activated by signal transducer and activator of transcription 3
(STAT3) from MSCs, thereby facilitating lung metastasis (48). Additionally, single-cell RNA
sequencing has uncovered a considerable heterogeneity among MSCs
associated with OS (18).
MSCs facilitate the proliferation and metastasis of
OS cells through two primary non-immune mechanisms. First, the
interaction between OS cells and MSCs is mediated by IL-8 and
aquaporin 1. Second, aberrant gene expression, including
retinoblastoma, c-Myc, TP53, KRas and Indian Hedgehog, contributes
to the transformation of MSCs into OS cells (49). Furthermore, studies have
demonstrated that when MSCs are located within the microenvironment
of OS cells, they have the capacity to differentiate into CAFs
(50,51). This differentiation notably
contributes to the increased proliferation, migration and invasion
of OS cells. Under the influence of MSCs, OS cells are capable of
inducing the migration and invasion of endothelial cells, which in
turn promotes angiogenesis (52).
From an immunological perspective, MSCs secrete anti-inflammatory
factors and inhibit pro-inflammatory substances, thereby assisting
OS cells in evading immune surveillance, particularly through
autocrine or paracrine exosomal mechanisms. Lagerwei et al
(53) demonstrated that MSCs
suppress T cell proliferation and immune responses by releasing
exosomal vesicles (EVs) containing miRNA/RNA and proteins.
Additionally, Zhang et al (54) reported that MSC-derived EVs express
TGF-β and TGF-β1. Moreover, MSC-derived exosomes can enhance OS
tumorigenesis and metastasis through the induction of autophagy, as
supported by evidence from other studies (55,56).
Circulating tumor cells (CTCs)
OS cells are present not only within the tumor
tissue but also in the bloodstream, where they are identified as
CTCs (57). CTCs demonstrate the
capacity to circumvent localized therapeutic approaches, including
surgical resection and radiation therapy, and can remain present in
small numbers during systemic treatments. This persistence
ultimately facilitates the metastasis and recurrence of OS
(57,58). Current research suggests that CTCs
play a unique role within the immune microenvironment associated
with OS. For instance, Zhang et al (59) demonstrated that the inhibition of
IL-6 can suppress the proliferation of OS cells and decrease the
presence of CTCs. In vitro studies revealed that IL-6
activates the Janus kinase (JAK)/STAT3 and mitogen-activated
protein kinase/extracellular signal-regulated kinase 1/2 (ERK1/2)
signaling pathways. While both pathways facilitate the
proliferation of OS cells, only the JAK/STAT3 pathway is implicated
in promoting cell migration (59–61). A
clinical study has shown that the level of IL-6 in OS samples is
significantly upregulated compared with normal bone tissue,
indicating that IL-6 plays an important role in the progression of
OS (62). Furthermore, MSCs have
been shown to enhance OS proliferation and metastasis through the
secretion of IL-6 (63,64). Liu et al (65) further identified that IL-8 also
contributes to the progression of OS. Their research involved
isolating and culturing CTCs from patients, revealing that IL-8
promotes tumor growth and lung metastasis in both in vitro
and in vivo models, indicating that targeting IL-8 may yield
antitumor effects. Although the precise mechanisms governing the
interaction between CTCs and tumor immunity remain unclear, CTCs
may represent promising targets for therapeutic intervention and
serve as biomarkers for predicting clinical outcomes.
Exosomes
Exosomes are a key subtype of EVs. EVs are
membrane-bound structures that are released into the ECM following
the fusion of intracellular multivesicular bodies with the cell
membrane. These vesicles encapsulate a diverse array of
biomolecules, including long non-coding RNAs, miRNAs, proteins,
lipids and metabolites. Notably, cancer cells tend to produce a
greater quantity of exosomes compared with normal cells, and these
exosomes are implicated in various aspects of tumor biology,
including tumor initiation, progression, immune evasion and drug
resistance (66). Evidence suggests
that EVs play a critical role in the onset, advancement and
metastasis of OS (15).
Specifically, exosomes can facilitate tumor growth by influencing
endothelial cells to enhance angiogenesis, while also mediating
intercellular interactions and participating in the regulation of
cellular functions and the transmission of biological information,
which in turn affects the expression and secretion of specific
cytokines (67,68). Furthermore, exosomes can modify
signaling pathways in recipient cells, thereby promoting the
metastasis of cancer cells (69).
Concurrently, exosomes can activate multiple signaling pathways
that lead to the development of drug resistance in previously
drug-sensitive cells or assist cancer cells in expelling cytotoxic
agents (70). Research has also
indicated that exosomes play a role in modulating the immune
response, aiding tumor cells in evading immune surveillance and
facilitating cancer metastasis. For instance, exosomes have been
shown to promote lung metastasis in OS by releasing PD-L1 (9). Recent studies have advanced our
understanding of the role of exosomal miRNAs in OS (71,72).
For instance, in the context of OS, it has been demonstrated that
exosomal miRNAs, such as miR-148a-3p, can stimulate the release of
pro-angiogenic factors and induce angiogenesis by influencing the
activity of osteoclasts and endothelial cells (73). Additionally, exosomes can enhance
the invasiveness of OS through immune modulation; the surface of
exosomes is adorned with tumor-associated antigens that interact
with antigen-presenting cells, thereby inducing tumor-specific
cytotoxic T cell immune responses (74). Moreover, it has been observed that
exosomes secreted by metastatic OS cells can induce M2 polarization
via TGF-β2, further promoting tumor invasion and metastasis
(75). In a study, Shimbo et
al (76) encapsulated synthetic
therapeutic miRNA-143 within exosomes and administered it into the
OS microenvironment, resulting in a significant reduction in OS
cell migration. The investigation of exosomes has emerged as a
prominent area of research and ongoing advancements in this field
have the potential to yield novel breakthroughs in the clinical
management of OS.
ECM
The ECM is a complex, mesh-like structure comprised
of collagen, proteoglycans, glycoproteins and glycosaminoglycans,
including hyaluronic acid. This matrix not only supplies essential
nutrients and structural support to tumor cells but also plays a
critical role in the construction and remodeling of the ECM,
thereby influencing the physical and chemical characteristics of
the TME (77). Cytokines and the
ECM serve as pivotal mediators within this microenvironment,
capable of modulating various biological behaviors of tumor cells,
including proliferation, apoptosis, invasion and metastasis
(78). OS is known to produce a
large amount of ECM, which significantly impacts tumor invasiveness
and the response to therapeutic interventions (66). Specific ECM components, such as
collagen, fibronectin and laminin, are implicated in aberrant
signaling pathways and structural irregularities that facilitate
sarcoma growth and metastasis through diverse mechanisms, including
integrin-mediated signaling activation, the promotion of EMT and
the enhancement of cell migration and invasion (79). Furthermore, the ECM has the capacity
to regulate the IGF axis, which is instrumental in modulating OS
growth and conferring resistance to conventional chemotherapy
agents (66). Targeting the ECM in
the treatment of OS presents promising potential; for instance,
ECM-like hydrogels can be utilized for the delivery of therapeutic
agents, thereby offering a novel platform for OS treatment and bone
regeneration (80). Additionally,
ECM-associated factors, such as neurotrophic EGF-like molecule 1,
have emerged as promising candidates for novel therapeutic
strategies aimed at impeding OS progression (20). Evidence suggests that the
invasiveness of OS can be mitigated by targeting the underlying
mechanisms of ECM degradation and angiogenesis (14,81).
Therapeutic strategies include suppressing key enzymes such as
matrix metalloproteinases (MMPs; including MMP-2 and MMP-9) to
prevent ECM breakdown and employing monoclonal antibodies or
small-molecule inhibitors against pro-angiogenic factors such as
VEGF to block new blood vessel formation (14,81).
The interplay between the ECM and CAFs is also known to foster the
development of OS (82). This
cooperative relationship drives disease progression by creating a
stiffened, pro-fibrotic microenvironment that supports tumor cell
survival, proliferation and invasion. The critical nature of this
interplay is evidenced by studies showing that therapeutic
strategies designed to destroy CAFs and disrupt the ECM can
effectively suppress OS tumor growth (82). Moreover, ECM components, including
EVs, hold potential as biomarkers for the diagnosis and prognosis
of OS (83). Engineered AttIL 12-T
cells, which are tumor-specific T cells with IL-12 anchored to
their cell membrane, have demonstrated enhanced efficacy by
targeting CAFs within the ECM, disrupting the tumor stroma and
promoting T cell infiltration, indicating promising avenues for
future research (82). This
engineering approach involves uniformly tethering the IL-12
cytokine onto the surface of the adoptively transferred T cells,
which allows for dose-controlled and localized delivery of IL-12
directly to the tumor site (82).
In conclusion, the significant role of the ECM in OS is
increasingly becoming a focal point for therapeutic strategies.
Vascular microenvironment
Tumor-associated blood vessels constitute a critical
element of the TME. The establishment of a robust vascular network
is critical for the growth and metastasis of tumors, as it provides
essential oxygen and nutrients while also facilitating the spread
of cancer cells. The presence of environmental stressors, such as
hypoxia and acidosis, disrupts the balance between pro-angiogenic
and anti-angiogenic factors, resulting in an increased expression
of pro-angiogenic elements, including hypoxia-inducible factors
(HIF) and VEGF, which together enhance tumor angiogenesis (84). Although the specific mechanisms
governing neovascularization in OS are not yet fully understood, it
is noteworthy that OS typically develops near the epiphyseal
regions of long bones, where H-type endothelial cells known to
promote angiogenesis are abundant (85). This suggests a potential involvement
of these cells in the neovascularization process associated with
OS. VEGF is crucial in modulating the growth, differentiation and
development of new blood vessels within endothelial cells, which in
turn has a notable impact on tumor proliferation and migration
(86). By enhancing tumor
angiogenesis, VEGF guarantees the supply of vital oxygen and
nutrients to tumor cells, thereby supporting the processes of tumor
initiation and progression (86,87).
Notably, VEGF expression, particularly its isoform VEGF-A, is
correlated with advanced tumor stages and metastatic potential
(88). Compared with normal bone
tissue, the expression of its receptor, VEGFR-2, is markedly
elevated in OS, with high levels of VEGFR-2 expression associated
with unfavorable prognostic outcomes (89). Gene amplification of VEGF,
especially VEGF-A, has been documented in patients with OS and
corroborated at the protein level. Empirical research has
established a positive association between increased expression of
VEGF and both tumor stage and the occurrence of metastasis
(90). Consequently, a significant
increase in vascular density may serve as a biomarker
distinguishing primary OS tumors in patients with metastasis from
those with non-metastatic disease. Furthermore, tumor-derived
exosomes facilitate intercellular communication and angiogenesis by
transporting pro-angiogenic factors and angiogenesis-related miRNAs
(91). Tumor vascular endothelial
cells have consistently been recognized as a vital target for
anticancer therapies. Therefore, the application of anti-angiogenic
agents, particularly those directed against VEGF, has the potential
to selectively inhibit neovascularization and enhance
progression-free survival in patients with cancer (92). Bevacizumab, an antibody targeting
VEGF, has demonstrated notable efficacy in clinical settings when
administered in conjunction with chemotherapy agents or immune
checkpoint inhibitors (93,94). Research indicates that the VEGFR-2
tyrosine kinase inhibitor Apatinib can attenuate the activity of
the Y chromosome sex-determining region box transcription factor-2
via the STAT3 signaling pathway, thereby mitigating
doxorubicin-induced chemoresistance in OS (95). However, prolonged anti-angiogenic
treatment may induce tumor hypoxia and promote invasive behavior,
potentially leading to therapeutic resistance (96).
Hypoxia and the TME of OS
The rapid expansion of tumors beyond the oxygen
supply capacity of surrounding blood vessels results in localized
hypoxia, which is characterized by diminished oxygen levels and
elevated lactate concentrations within the TME (97). This hypoxic and acidic milieu
fosters the expression of angiogenic factors, enabling invasive OS
cells to utilize vascular mimicry for the formation of angiogenic
microchannels (98). Hypoxia
triggers a range of cellular mechanisms, primarily governed by the
transcription factor HIF-1α. Under normoxic conditions, HIF-1α
undergoes rapid degradation; however, in hypoxic environments, it
remains active, facilitating processes such as tumor growth,
invasiveness, angiogenesis, metastasis and resistance to therapy
(99,100). Empirical studies have demonstrated
that in rapidly proliferating tumor tissues, HIF-1 facilitates a
metabolic shift in hypoxic tumor cells from the more efficient
oxidative phosphorylation to the less efficient glycolytic pathway,
thereby sustaining energy production (101). This metabolic adaptation, referred
to as the Warburg effect, leads to an increased production of
lactate, which modifies the TME and promotes the proliferation,
invasion and migration of tumor cells (102).
Research has indicated a direct correlation between
lactate levels in tumors and the incidence of distant metastasis,
suggesting that lactate accumulation may serve as a predictive
biomarker for tumor metastasis and patient survival rates (103). Beyond being a mere metabolic
byproduct, lactate functions as a signaling molecule. Zhang et
al (104) identified
lactylation as a significant epigenetic modification capable of
regulating the transcription of numerous oncogenes and tumor
suppressor genes, thereby revealing a universal mechanism of
metabolic regulation that broadly influences tumor progression.
Additionally, Lee et al (105) discovered a hypoxia-regulated
protein, N-myc downstream regulated gene-3 (NDRG-3), which operates
independently of HIF-1α. In the TME, reduced oxygen availability
and hypoxia-induced glycolysis, which results in lactate
production, can enhance the expression of NDRG-3 protein through
the activation of the Raf/ERK signaling pathway. This process
subsequently facilitates tumor angiogenesis and cellular
proliferation.
Subsequent research has demonstrated that hypoxic
conditions can expedite tumor progression by influencing the immune
response, cytokine production, growth factors and ILs, thereby
promoting tumor immune evasion (106). Specifically, in hypoxic
environments, immune responses are diminished due to a decrease in
the infiltration and functionality of CD8+ T cells, as
well as the impaired maturation and activity of DCs and NK cells.
Additionally, there is an induction of M2 polarization in TAM,
along with an increase in the activity of Tregs and MDSCs (107). Furthermore, lactic acid plays a
role in tumor immune resistance by facilitating M2 polarization,
enhancing the activity of CD8+ T cells and elevating
PD-1 expression in Tregs (108,109).
He et al (110) demonstrated the inhibition of OS
metastasis through the application of nanomaterials designed to
deliver oxidants that degrade β-catenin within the
HIF-1α/Bcl-2/adenovirus E1B 19-kDa interacting protein
3/LC3B-mediated mitochondrial autophagy pathway. Zheng et al
(111) found that hypoxia
significantly influences metabolic reprogramming; their research
indicated that the inhibition of long non-coding RNA DLGAP 1-AS 2
can suppress aerobic glycolysis via the miR-451a/hexokinase 2 axis,
thereby impeding OS progression. In conclusion, the hypoxic
microenvironment in OS is garnering increasing attention for its
pivotal role in orchestrating tumor progression, metastasis, immune
regulation, metabolic alterations and therapeutic responses.
Comprehensive research into its complexities presents promising
opportunities for the development of innovative treatment
strategies for OS.
Translational challenges and future
directions in OS TME research
Despite extensive research regarding TME
heterogeneity, including insights gained from animal models
(112) and findings from in
vitro experiments (113,114), effectively translating these
findings into clinical practice remains challenging. First, studies
have shown that the TME of OS exhibits high heterogeneity, with
significant variations observed across patients, subtypes and even
different regions within the same tumor, rendering a
one-size-fits-all targeted strategy impractical (21,112).
This heterogeneity extends to the dynamic nature of the TME,
demanding therapeutic approaches capable of adapting to evolving
tumor characteristics over time (21). Furthermore, there is a current lack
of preclinical models that accurately mimic the human OS TME
(112). Traditional animal
xenograft models, along with cell line cultures, often fail to
reproduce the full complexity of human-specific cell-cell
interactions and the immune microenvironment, limiting the
translational potential of laboratory discoveries (115,116).
Moreover, therapies targeting the TME may induce
unintended systemic toxicity or immune-related adverse events. This
necessitates particularly cautious evaluation in adolescent and
pediatric patients, as this predominant OS patient population is
especially vulnerable due to their ongoing physical development,
which can affect organ function, drug metabolism and the long-term
impact of treatments on growth and fertility (117). Existing clinical trials primarily
focus on single-target therapies, overlooking the synergistic
effects of multi-signaling networks within the TME, which may limit
therapeutic efficacy. The potential for developing resistance to
targeted therapies poses another significant challenge, as cancer
cells can adapt and find alternative pathways for survival.
Furthermore, the complexity of TME interactions means targeting one
component may have unintended effects on others, potentially
leading to treatment resistance or other adverse reactions
(21). Developing reliable
biomarkers to monitor the TME and predict treatment response
remains an active research area. Identifying these biomarkers is
crucial for achieving more personalized and effective treatment
regimens.
In summary, while the TME presents a promising
target for OS therapy, addressing its heterogeneity, developing
accurate preclinical models, managing potential toxicity and
overcoming resistance mechanisms are key challenges that must be
overcome to successfully translate these insights into clinical
practice. Future efforts must prioritize multi-omics integration
analyses, develop humanized organoids or patient-derived xenograft
models and advance personalized therapeutic strategies targeting
both the TME and tumor cells simultaneously. Only through such a
comprehensive approach can true lab-to-bedside translation be
achieved.
In conclusion, the TME represents a highly intricate
network system characterized by the coexistence and interaction of
various cellular and non-cellular components, which are integral to
the proliferation, invasion and metastasis processes in OS. Within
the TME, diverse cell types, including TAMs, MSCs and
immunosuppressive T cells, contribute to the invasive progression
of OS through the secretion of numerous cytokines and the
activation of associated signaling pathways. Concurrently, the high
metabolic activity, extensive vascular network and hypoxic
conditions of the tumor further intensify its invasiveness and
metastatic capabilities. At present, the treatment of OS has
encountered significant challenges, primarily the limited
improvement in survival outcomes for patients with metastatic or
recurrent disease, the high degree of inter- and intra-tumoral
heterogeneity that complicates targeted therapy and the plateaued
efficacy of conventional chemotherapy regimens (118). Future advancements are anticipated
to involve the application of single-cell sequencing and spatial
transcriptomics to conduct in-depth analyses of TME heterogeneity,
thereby elucidating critical regulatory mechanisms (119,120). Moreover, the development of
targeted therapies and immunotherapeutic strategies that
specifically address the TME is a promising avenue for research
(121,122). Additionally, the integration of
artificial intelligence and multi-omics data analysis is expected
to yield innovative approaches for the personalized treatment of OS
(123). Through interdisciplinary
collaboration, efforts to disrupt the malignant cycle perpetuated
by the TME may offer new therapeutic prospects for patients with
OS.
Acknowledgements
Not applicable.
Funding
This work was supported by a project of the Department of
Education of Yunnan Province (grant no. 2025J0346) and an
intramural project of the Second Affiliated Hospital of Kunming
Medical University (grant no. 2022yk05).
Availability of data and materials
Not applicable.
Authors' contributions
TS, JK and QW designed the study. TS, JK and JS
performed the literature search and analysis. TS and JK wrote the
draft; TS and JS prepared the figure. QW, JS and XH critically
revised the manuscript. XH conceived the intellectual framework.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao X, Wu Q, Gong X, Liu J and Ma Y:
Osteosarcoma: A review of current and future therapeutic
approaches. Biomed Eng Online. 20:242021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moukengue B, Lallier M, Marchandet L,
Baud'huin M, Verrecchia F, Ory B and Lamoureux F: Origin and
therapies of osteosarcoma. Cancers (Basel). 14:35032022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsagozis P, Gonzalez-Molina J, Georgoudaki
AM, Lehti K, Carlson J, Lundqvist A, Haglund F and Ehnman M:
Sarcoma tumor microenvironment. Adv Exp Med Biol. 1296:319–348.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei L and Wang J: Tumor-associated
macrophages promote pre-metastatic niche formation in ovarian
cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 40:1138–1145. 2024.(In
Chinese). PubMed/NCBI
|
|
5
|
Fang J, Lu Y, Zheng J, Jiang X, Shen H,
Shang X, Lu Y and Fu P: Exploring the crosstalk between endothelial
cells, immune cells, and immune checkpoints in the tumor
microenvironment: new insights and therapeutic implications. Cell
Death Dis. 14:5862023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devaraji M and Varghese Cheriyan B:
Immune-based cancer therapies: Mechanistic insights, clinical
progress, and future directions. J Egypt Natl Canc Inst. 37:622025.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malone MK, Smrekar K, Park S, Blakely B,
Walter A, Nasta N, Park J, Considine M, Danilova LV, Pandey NB, et
al: Cytokines secreted by stromal cells in TNBC microenvironment as
potential targets for cancer therapy. Cancer Biol Ther. 21:560–569.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng J, Peng Y, Wang D, Ayesha K and Chen
S: The interaction between osteosarcoma and other cells in the bone
microenvironment: From mechanism to clinical applications. Front
Cell Dev Biol. 11:11230652023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen F, Liu J, Yang T, Sun J, He X, Fu X,
Qiao S, An J and Yang J: Analysis of intercellular communication in
the osteosarcoma microenvironment based on single cell sequencing
data. J Bone Oncol. 41:1004932023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amarasekara DS, Kim S and Rho J:
Regulation of osteoblast differentiation by cytokine networks. Int
J Mol Sci. 22:28512021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anloague A and Delgado-Calle J:
Osteocytes: New kids on the block for cancer in bone therapy.
Cancers(Basel). 15:26452023.PubMed/NCBI
|
|
13
|
Behzatoglu K: Osteoclasts in tumor
biology: Metastasis and epithelial-mesenchymal-myeloid transition.
Pathol Oncol Res. 27:6094722021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Q, Liu J, Wu B, Wang X, Jiang Y and
Zhu D: Role of extracellular vesicles in osteosarcoma. Int J Med
Sci. 19:1216–1226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jerez S, Araya H, Hevia D, Irarrázaval CE,
Thaler R, van Wijnen AJ and Galindo M: Extracellular vesicles from
osteosarcoma cell lines contain miRNAs associated with cell
adhesion and apoptosis. Gene. 710:246–257. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X and Teitelbaum SL: Osteoclasts: New
insights. Bone Res. 1:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang G, Jian A, Zhang Y and Zhang X: A
New signature of sarcoma based on the tumor microenvironment
benefits prognostic prediction. Int J Mol Sci. 24:29612023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Yang D, Yang Q, Lv X, Huang W,
Zhou Z, Wang Y, Zhang Z, Yuan T, Ding X, et al: Single-cell RNA
landscape of intratumoral heterogeneity and immunosuppressive
microenvironment in advanced osteosarcoma. Nat Commun. 11:63222020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cersosimo F, Lonardi S, Bernardini G,
Telfer B, Mandelli GE, Santucci A, Vermi W and Giurisato E:
Tumor-associated macrophages in osteosarcoma: From mechanisms to
therapy. Int J Mol Sci. 21:52072020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin Q, Gomez-Salazar M, Tower RJ, Chang L,
Morris CD, McCarthy EF, Ting K, Zhang X and James AW: NELL1
regulates the matrisome to promote osteosarcoma progression. Cancer
Res. 82:2734–2747. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y
and Xu J: Immune microenvironment in osteosarcoma: Components,
therapeutic strategies and clinical applications. Front Immunol.
13:9075502022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Ding L, Mei J, Hu Y, Kong X, Dai S,
Bu T, Xiao Q and Ding K: Dual roles and therapeutic targeting of
tumor-associated macrophages in tumor microenvironments. Signal
Transduct Target Ther. 10:2682025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Q, Liang X, Ren T, Huang Y, Zhang H,
Yu Y, Chen C, Wang W, Niu J, Lou J and Guo W: The role of
tumor-associated macrophages in osteosarcoma
progression-therapeutic implications. Cell Oncol (Dordr).
44:525–539. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou C, Lu M, Lei Z, Dai S, Chen W, Du S,
Jin Q, Zhou Z and Li H: HMGB1 positive feedback loop between cancer
cells and tumor-associated macrophages promotes osteosarcoma
migration and invasion. Lab Invest. 103:1000542023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ligon JA, Choi W, Cojocaru G, Fu W, Hsiue
EH, Oke TF, Siegel N, Fong MH, Ladle B, Pratilas CA, et al:
Pathways of immune exclusion in metastatic osteosarcoma are
associated with inferior patient outcomes. J Immunother Cancer.
9:e0017722021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Judge SJ, Darrow MA, Thorpe SW, Gingrich
AA, O'Donnell EF, Bellini AR, Sturgill IR, Vick LV, Dunai C,
Stoffel KM, et al: Analysis of tumor-infiltrating NK and T cells
highlights IL-15 stimulation and TIGIT blockade as a combination
immunotherapy strategy for soft tissue sarcomas. J Immunother
Cancer. 8:e0013552020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kleinberger M, Çifçi D, Paiato C, Tomasich
E, Mair MJ, Steindl A, Spiró Z, Carrero ZI, Berchtold L,
Hainfellner J, et al: Density and entropy of immune cells within
the tumor microenvironment of primary tumors and matched brain
metastases. Acta Neuropathol Commun. 13:342025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu X, Liu M, Yang J, Que Y and Zhang X:
Panobinostat enhances NK cell cytotoxicity in soft tissue sarcoma.
Clin Exp Immunol. 209:127–139. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shigehara K, Kawai N, Shirosaki T, Ebihara
Y, Murai A, Takaya A, Tokita S, Sasaki K, Shijubou N, Kubo T, et
al: The size of CD8+ infiltrating T cells is a prognostic marker
for esophageal squamous cell carcinoma. Sci Rep. 15:266382025.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Huang H, Huang Z, Chen J, Liu Y,
Wu Y, Li A, Ge J, Fang Z, Xu B, et al: Prognostic values of
tissue-resident CD8+T cells in human hepatocellular
carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol.
21:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinker GS, Vitiello GAF, Ferreira WAS,
Chaves AS, Cordeiro de Lima VC and Medina TDS: B cell orchestration
of anti-tumor immune responses: A matter of cell localization and
communication. Front Cell Dev Biol. 9:6781272021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao Y, Kong L, Zhai Y, Hou W, Wang J, Liu
Y, Wang C, Zhao W, Ji H and He P: Comprehensive analysis of
TRIM56′s prognostic value and immune infiltration in pan-cancer.
Sci Rep. 15:136732025. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lo Nigro C, Macagno M, Sangiolo D,
Bertolaccini L, Aglietta M and Merlano MC: NK-mediated
antibody-dependent cell-mediated cytotoxicity in solid tumors:
Biological evidence and clinical perspectives. Ann Transl Med.
7:1052019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Capuano C, Pighi C, Battella S, De
Federicis D, Galandrini R and Palmieri G: Harnessing CD16-mediated
NK cell functions to enhance therapeutic efficacy of
tumor-targeting mAbs. Cancers (Basel). 13:25002021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rodríguez-Nava C, Ortuño-Pineda C,
Illades-Aguiar B, Flores-Alfaro E, Leyva-Vázquez MA, Parra-Rojas I,
Del Moral-Hernández O, Vences-Velázquez A, Cortés-Sarabia K and
Alarcón-Romero LDC: Mechanisms of action and limitations of
monoclonal antibodies and single chain fragment variable (scFv) in
the treatment of cancer. Biomedicines. 11:16102023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khodayari H, Khodayari S, Ebrahimi E,
Hadjilooei F, Vesovic M, Mahmoodzadeh H, Saric T, Stücker W, Van
Gool S, Hescheler J and Nayernia K: Stem cells-derived natural
killer cells for cancer immunotherapy: Current protocols,
feasibility, and benefits of ex vivo generated natural killer cells
in treatment of advanced solid tumors. Cancer Immunol Immunother.
70:3369–3395. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin E, Bak SH, Park T, Kim JW, Yoon SR,
Jung H and Noh JY: Understanding NK cell biology for harnessing NK
cell therapies: Targeting cancer and beyond. Front Immunol.
14:11929072023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prager I and Watzl C: Mechanisms of
natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol.
105:1319–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang ML, Chen L, Li YJ and Kong DL:
PD-L1/PD-1 axis serves an important role in natural killer
cell-induced cytotoxicity in osteosarcoma. Oncol Rep. 42:2049–2056.
2019.PubMed/NCBI
|
|
41
|
Yang H, Zhao L, Zhang Y and Li FF: A
comprehensive analysis of immune infiltration in the tumor
microenvironment of osteosarcoma. Cancer Med. 10:5696–5711. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lazarova M and Steinle A: Impairment of
NKG2D-mediated tumor immunity by TGF-β. Front Immunol. 10:26892019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang X, Ma Z, Zhu G, Lu Y and Yang J: New
perspective into mesenchymal stem cells: Molecular mechanisms
regulating osteosarcoma. J Bone Oncol. 29:1003722021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Antoon R, Overdevest N, Saleh AH and
Keating A: Mesenchymal stromal cells as cancer promoters. Oncogene.
43:3545–3555. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng Y, Wang G, Chen R, Hua Y and Cai Z:
Mesenchymal stem cells in the osteosarcoma microenvironment: Their
biological properties, influence on tumor growth, and therapeutic
implications. Stem Cell Res Ther. 9:222018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu G, Xia Y, Zhao Z, Li A, Li H and Xiao
T: LncRNA XIST from the bone marrow mesenchymal stem cell derived
exosome promotes osteosarcoma growth and metastasis through
miR-655/ACLY signal. Cancer Cell Int. 22:3302022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen C, Xie L, Ren T, Huang Y, Xu J and
Guo W: Immunotherapy for osteosarcoma: Fundamental mechanism,
rationale, and recent breakthroughs. Cancer Lett. 500:1–10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tu B, Du L, Fan QM, Tang Z and Tang TT:
STAT3 activation by IL-6 from mesenchymal stem cells promotes the
proliferation and metastasis of osteosarcoma. Cancer Lett.
325:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deng Q, Li P, Che M, Liu J, Biswas S, Ma
G, He L, Wei Z, Zhang Z, Yang Y, et al: Activation of hedgehog
signaling in mesenchymal stem cells induces cartilage and bone
tumor formation via Wnt/β-catenin. Elife. 8:e502082019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Feng L, Chen Y and Jin W: Research
progress on cancer-associated fibroblasts in osteosarcoma. Oncol
Res. 33:1091–1103. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Duan W, Niu X, Liu Y and Tian W:
Rab27b-mediated CAFs derived exosomal miR-22-3p suppresses
ferroptosis and promotes cisplatin resistance in osteosarcoma. Cell
Signal. Sep 17–2025.(Epub ahead of print). View Article : Google Scholar
|
|
52
|
Lin L, Huang K, Guo W, Zhou C, Wang G and
Zhao Q: Conditioned medium of the osteosarcoma cell line U2OS
induces hBMSCs to exhibit characteristics of carcinoma-associated
fibroblasts via activation of IL-6/STAT3 signalling. J Biochem.
168:265–271. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lagerweij T, Pérez-Lanzón M and Baglio SR:
A preclinical mouse model of osteosarcoma to define the
extracellular vesicle-mediated communication between tumor and
mesenchymal stem cells. J Vis Exp. 569322018.PubMed/NCBI
|
|
54
|
Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma
C and Wang H: Exosomes originating from MSCs stimulated with TGF-β
and IFN-γ promote Treg differentiation. J Cell Physiol.
233:6832–6840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ruksha T and Palkina N: Role of exosomes
in transforming growth factor-β-mediated cancer cell plasticity and
drug resistance. Explor Target Antitumor Thery. 6:10023222025.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Su Z, Fang X and Duan H: The paradoxical
role of stem cells in osteosarcoma: From pathogenesis to
therapeutic breakthroughs. Front Oncol. 15:16434912025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang C, Liu C, Xia C and Fu L: Clinical
applications of circulating tumor cells in metastasis and therapy.
J Hematol Oncol. 18:802025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fu Y, Xu Y, Liu W, Zhang J, Wang F, Jian
Q, Huang G, Zou C, Xie X, Kim AH, et al: Tumor-informed deep
sequencing of ctDNA detects minimal residual disease and predicts
relapse in osteosarcoma. EClinicalMedicine. 73:1026972024.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y, Ma Q, Liu T, Guan G, Zhang K,
Chen J, Jia N, Yan S, Chen G, Liu S, et al: Interleukin-6
suppression reduces tumour self-seeding by circulating tumour cells
in a human osteosarcoma nude mouse model. Oncotarget. 7:446–458.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tian H, Cao J, Li B, Nice EC, Mao H, Zhang
Y and Huang C: Managing the immune microenvironment of
osteosarcoma: The outlook for osteosarcoma treatment. Bone Res.
11:112023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Thuya WL, Cao Y, Ho PC, Wong AL, Wang L,
Zhou J, Nicot C and Goh BC: Insights into IL-6/JAK/STAT3 signaling
in the tumor microenvironment: Implications for cancer therapy.
Cytokine Growth Factor Rev. 85:26–42. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dou B, Chen T, Chu Q, Zhang G and Meng Z:
The roles of metastasis-related proteins in the development of
giant cell tumor of bone, osteosarcoma and Ewing's sarcoma. Technol
Health Care. 29:91–101. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Paino F, La Noce M, Di Nucci D, Nicoletti
GF, Salzillo R, De Rosa A, Ferraro GA, Papaccio G, Desiderio V and
Tirino V: Human adipose stem cell differentiation is highly
affected by cancer cells both in vitro and in vivo: Implication for
autologous fat grafting. Cell Death Dis. 8:e25682017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Perrot P, Rousseau J, Bouffaut AL, Rédini
F, Cassagnau E, Deschaseaux F, Heymann MF, Heymann D, Duteille F,
Trichet V and Gouin F: Safety concern between autologous fat graft,
mesenchymal stem cell and osteosarcoma recurrence. PLoS One.
5:e109992010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu T, Ma Q, Zhang Y, Wang X, Xu K, Yan K,
Dong W, Fan Q, Zhang Y and Qiu X: Self-seeding circulating tumor
cells promote the proliferation and metastasis of human
osteosarcoma by upregulating interleukin-8. Cell Death Dis.
10:5752019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tzanakakis GN, Giatagana EM, Berdiaki A,
Spyridaki I, Hida K, Neagu M, Tsatsakis AM and Nikitovic D: The
role of IGF/IGF-IR-signaling and extracellular matrix effectors in
bone sarcoma pathogenesis. Cancers (Basel). 13:24782021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duan SL, Fu WJ, Jiang YK, Peng LS, Ousmane
D, Zhang ZJ and Wang JP: Emerging role of exosome-derived
non-coding RNAs in tumor-associated angiogenesis of tumor
microenvironment. Front Mol Biosci. 10:12201932023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li Y, Jiang D, Zhang ZX, Zhang JJ, He HY,
Liu JL, Wang T, Yang XX, Liu BD, Yang LL, et al: Colorectal cancer
cell-secreted exosomal miRNA N-72 promotes tumor angiogenesis by
targeting CLDN18. Am J Cancer Res. 13:3482–3499. 2023.PubMed/NCBI
|
|
69
|
Ning XY, Ma JH, He W and Ma JT: Role of
exosomes in metastasis and therapeutic resistance in esophageal
cancer. World J Gastroenterol. 29:5699–5715. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pan W, Miao Q, Yin W, Li X, Ye W, Zhang D,
Deng L, Zhang J and Chen M: The role and clinical applications of
exosomes in cancer drug resistance. Cancer Drug Resist.
7:432024.PubMed/NCBI
|
|
71
|
Tian W, Niu X, Feng F, Wang X, Wang J, Yao
W and Zhang P: The promising roles of exosomal microRNAs in
osteosarcoma: A new insight into the clinical therapy. Biomed
Pharmacother. 163:1147712023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen CC and Benavente CA: Exploring the
impact of exosomal cargos on osteosarcoma progression: Insights
into therapeutic potential. Int J Mol Sci. 25:5682024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li X, Rong K, Huang Y, Zhao Z, Xu C, Lin
L, Zhang Y, Yan Y, Huang W, Zhang Y, et al: Suppression of
miR-148a-3p can promote bone healing by enhancing
angiogenesis-osteogenesis coupling through the PI3K/Akt pathway.
FASEB J. 39:e709302025. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pu F, Chen F, Zhang Z, Liu J and Shao Z:
Information transfer and biological significance of neoplastic
exosomes in the tumor microenvironment of osteosarcoma. Onco
Targets Ther. 13:8931–8940. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wolf-Dennen K, Gordon N and Kleinerman ES:
Exosomal communication by metastatic osteosarcoma cells modulates
alveolar macrophages to an M2 tumor-promoting phenotype and
inhibits tumoricidal functions. Oncoimmunology. 9:17476772020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shimbo K, Miyaki S, Ishitobi H, Kato Y,
Kubo T, Shimose S and Ochi M: Exosome-formed synthetic microRNA-143
is transferred to osteosarcoma cells and inhibits their migration.
Biochem Biophys Res Commun. 445:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hu Q, Zhu Y, Mei J, Liu Y and Zhou G:
Extracellular matrix dynamics in tumor immunoregulation: From tumor
microenvironment to immunotherapy. J Hematol Oncol. 18:652025.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pattabiram S, Gangadaran P, Dhayalan S,
Chatterjee G, Reyaz D, Prakash K, Arun R, Rajendran RL, Ahn BC and
Aruljothi KN: Decoding the tumor microenvironment: Insights and new
targets from single-cell sequencing and spatial transcriptomics.
Curr Issues Mol Biol. 47:7302025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cui J, Dean D, Hornicek FJ, Chen Z and
Duan Z: The role of extracelluar matrix in osteosarcoma progression
and metastasis. J Exp Clin Cancer Res. 39:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tian H, Wu R, Feng N, Zhang J and Zuo J:
Recent advances in hydrogels-based osteosarcoma therapy. Front
Bioeng Biotechnol. 10:10426252022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu Y, Li K, Peng Y, Zhang Z, Pu F, Shao Z
and Wu W: Tumor microenvironment in osteosarcoma: From cellular
mechanism to clinical therapy. Genes Dis. 12:1015692025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hu J, Lazar AJ, Ingram D, Wang WL, Zhang
W, Jia Z, Ragoonanan D, Wang J, Xia X, Mahadeo K, et al: Cell
membrane-anchored and tumor-targeted IL-12 T-cell therapy destroys
cancer-associated fibroblasts and disrupts extracellular matrix in
heterogenous osteosarcoma xenograft models. J Immunother Cancer.
12:e0069912024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Loi G, Stucchi G, Scocozza F, Cansolino L,
Cadamuro F, Delgrosso E, Riva F, Ferrari C, Russo L and Conti M:
Characterization of a bioink combining extracellular matrix-like
hydrogel with osteosarcoma cells: Preliminary results. Gels.
9:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fan J, Xie Y, Liu D, Cui R, Zhang W, Shen
M and Cao L: Crosstalk between H-type vascular endothelial cells
and macrophages: A potential regulator of bone homeostasis. J
Inflamm Res. 18:2743–2765. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee C, Kim MJ, Kumar A, Lee HW, Yang Y and
Kim Y: Vascular endothelial growth factor signaling in health and
disease: From molecular mechanisms to therapeutic perspectives.
Signal Transduct Target Ther. 10:1702025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shah FH, Nam YS, Bang JY, Hwang IS, Kim
DH, Ki M and Lee HW: Targeting vascular endothelial growth
receptor-2 (VEGFR-2): Structural biology, functional insights, and
therapeutic resistance. Arch Pharm Res. 48:404–425. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Corre I, Verrecchia F, Crenn V, Redini F
and Trichet V: The osteosarcoma microenvironment: A complex but
targetable ecosystem. Cells. 9:9762020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lammli J, Fan M, Rosenthal HG, Patni M,
Rinehart E, Vergara G, Ablah E, Wooley PH, Lucas G and Yang SY:
Expression of vascular endothelial growth factor correlates with
the advance of clinical osteosarcoma. Int Orthop. 36:2307–2313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Y, Lin S, Xie X, Zhu H, Fan T and Wang
S: Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma
tumor angiogenesis and autophagy through miR-153 and ATG5. Am J
Transl Res. 13:4211–4223. 2021.PubMed/NCBI
|
|
92
|
Assi T, Watson S, Samra B, Rassy E, Le
Cesne A, Italiano A and Mir O: Targeting the VEGF pathway in
osteosarcoma. Cells. 10:12402021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang C, Duan L, Zhao Y, Wang Y and Li Y:
Efficacy and safety of bevacizumab combined with temozolomide in
the treatment of glioma: A systematic review and meta-analysis of
clinical trials. World Neurosurg. 193:447–460. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Han X, Qin S, Liu S and Li Z:
Intracavitary perfusion with bevacizumab plus cisplatin versus
cisplatin alone for malignant pleural effusion in lung cancer
patients: A meta-analysis of randomized controlled trials. World J
Surg Oncol. 23:2782025. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lowery CD, Blosser W, Dowless M, Renschler
M, Perez LV, Stephens J, Pytowski B, Wasserstrom H, Stancato LF and
Falcon B: Anti-VEGFR2 therapy delays growth of preclinical
pediatric tumor models and enhances anti-tumor activity of
chemotherapy. Oncotarget. 10:5523–5533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Haibe Y, Kreidieh M, El Hajj H, Khalifeh
I, Mukherji D, Temraz S and Shamseddine A: Resistance mechanisms to
anti-angiogenic therapies in cancer. Front Oncol. 10:2212020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhou J, Lan F, Liu M, Wang F, Ning X, Yang
H and Sun H: Hypoxia inducible factor-1α as a potential therapeutic
target for osteosarcoma metastasis. Front Pharmacol.
15:13501872024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ren K, Zhang J, Gu X, Wu S, Shi X, Ni Y,
Chen Y, Lu J, Gao Z, Wang C and Yao N: Migration-inducing gene-7
independently predicts poor prognosis of human osteosarcoma and is
associated with vasculogenic mimicry. Exp Cell Res. 369:80–89.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang QJ, Liu CH, Wang K, Mao XY, Dong Y,
Zang MY, Zhang W, Yu QS and Hao L: Hypoxia-induced HIF-1α/VASN
promotes bladder cancer progression. Sci Rep. 15:216352025.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zeng X, Liu S, Yang H, Jia M, Liu W and
Zhu W: Synergistic anti-tumour activity of ginsenoside Rg3 and
doxorubicin on proliferation, metastasis and angiogenesis in
osteosarcoma by modulating mTOR/HIF-1α/VEGF and EMT signalling
pathways. J Pharm Pharmacol. 75:1405–1417. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Vander Heiden MG and DeBerardinis RJ:
Understanding the intersections between metabolism and cancer
biology. Cell. 168:657–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Huang Y, Fan H and Ti H: Tumor
microenvironment reprogramming by nanomedicine to enhance the
effect of tumor immunotherapy. Asian J Pharm Sci.
19:1009022024.PubMed/NCBI
|
|
103
|
Zhu L, Lin Z, Wang K, Gu J, Chen X, Chen
R, Wang L and Cheng X: A lactate metabolism-related signature
predicting patient prognosis and immune microenvironment in ovarian
cancer. Front Endocrinol (Lausanne). 15:13724132024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lee DC, Sohn HA, Park ZY, Oh S, Kang YK,
Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, et al: A lactate-induced
response to hypoxia. Cell. 161:595–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15:772022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mortezaee K and Majidpoor J: The impact of
hypoxia on immune state in cancer. Life Sci. 286:1200572021.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang
J, Wilhelm J, Li S, Song J, Li W, et al: Lactate increases stemness
of CD8 + T cells to augment anti-tumor immunity. Nat Commun.
13:49812022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kumagai S, Koyama S, Itahashi K,
Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono
H, et al: Lactic acid promotes PD-1 expression in regulatory T
cells in highly glycolytic tumor microenvironments. Cancer Cell.
40:201–218.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
He G, Nie JJ, Liu X, Ding Z, Luo P, Liu Y,
Zhang BW, Wang R, Liu X, Hai Y and Chen DF: Zinc oxide
nanoparticles inhibit osteosarcoma metastasis by downregulating
β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact
Mater. 19:690–702. 2023.PubMed/NCBI
|
|
111
|
Zheng C, Li R, Zheng S, Fang H, Xu M and
Zhong L: The knockdown of lncRNA DLGAP1-AS2 suppresses osteosarcoma
progression by inhibiting aerobic glycolysis via the miR-451a/HK2
axis. Cancer Sci. 114:4747–4762. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Z, Ma L, Xu J and Jiang C: Editorial:
Genetic and cellular heterogeneity in tumors. Front Cell Dev Biol.
12:15195392024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Oh JM, Park Y, Lee J and Shen K:
Microfabricated organ-specific models of tumor microenvironments.
Annu Rev Biomed Eng. 27:307–333. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yu Z, Gan Z, Wu W, Sun X, Cheng X, Chen C,
Cao B, Sun Z and Tian J: Photothermal-triggered extracellular
matrix clearance and dendritic cell maturation for enhanced
osteosarcoma immunotherapy. ACS Appl Mater Interfaces.
16:67225–67234. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rodrigues J, Sarmento B and Pereira CL:
Osteosarcoma tumor microenvironment: The key for the successful
development of biologically relevant 3D in vitro models. In Vitro
Model. 1:5–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Liu X, Fang J, Huang S, Wu X, Xie X, Wang
J, Liu F, Zhang M, Peng Z and Hu N: Tumor-on-a-chip: From
bioinspired design to biomedical application. Microsyst Nanoeng.
7:502021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Spalato M and Italiano A: The safety of
current pharmacotherapeutic strategies for osteosarcoma. Expert
Opin Drug Saf. 20:427–438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lu J, Tang H, Chen L, Huang N, Hu G, Li C,
Luo K, Li F, Liu S, Liao S, et al: Association of survivin positive
circulating tumor cell levels with immune escape and prognosis of
osteosarcoma. J Cancer Res Clin Oncol. 149:13741–13751. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nasir I, McGuinness C, Poh AR, Ernst M,
Darcy PK and Britt KL: Tumor macrophage functional heterogeneity
can inform the development of novel cancer therapies. Trends
Immunol. 44:971–985. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sharma A, Raut SS, Shukla A, Gupta S,
Mishra A and Singh A: From tissue architecture to clinical
insights: Spatial transcriptomics in solid tumor studies. Semin
Oncol. 52:1523892025. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Alnaqbi H, Becker LM, Mousa M, Alshamsi F,
Azzam SK, Emini Veseli B, Hymel LA, Alhosani K, Alhusain M, Mazzone
M, et al: Immunomodulation by endothelial cells: Prospects for
cancer therapy. Trends Cancer. 10:1072–1091. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li Y, Huang Z and Yin Y: Heterogeneity of
tumor-associated macrophages in colorectal cancer: Origins,
classification, and immunotherapeutic implications. Pathol Res
Pract. 272:1560822025. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu C, Yang J, Xiong H, Cui X, Zhang Y, Gao
M, He L, Fang Q, Han C, Liu W, et al: Machine learning and
multi-omics analysis reveal key regulators of proneural-mesenchymal
transition in glioblastoma. Sci Rep. 15:197312025. View Article : Google Scholar : PubMed/NCBI
|















