Introduction
Cervical cancer, a major global health burden, is
primarily driven by persistent high-risk human papillomavirus (HPV)
infection, with HPV 16 and HPV 18 as key oncogenic drivers.
According to the latest GLOBOCAN 2022 global cancer statistics,
there were an estimated 662,301 new cases and 348,874 deaths from
cervical cancer worldwide (1).
Recent epidemiological reports have shown an increasing incidence
among younger women, particularly those aged 30–44 years, with an
annual rise of ~1–2% in certain populations (2,3).
Although HPV infection is common, only a minority progress to
malignancy, highlighting the crucial role of the tumor immune
microenvironment (TIME), in determining disease outcome.
The TIME, comprising immune cells, cytokines,
stromal elements and tumor cells (4,5),
orchestrates tumor progression, immune evasion and therapeutic
response. Although the TIME is influenced by stromal remodeling,
cytokine signaling and microbial interactions, the present review
primarily emphasizes HPV-mediated immune evasion and its
implications for immunotherapy, while briefly covering secondary
mechanisms. Recent advances, including spatial multi-omics and
microbiome studies, have revealed novel insights into TIME
heterogeneity and HPV-driven immunosuppression, offering novel
therapeutic avenues (6,7).
Accordingly, the present review focused on three
main objectives: i) To collate current knowledge of the cervical
cancer TIME; ii) to clarify HPV-driven immune evasion mechanisms;
and iii) to highlight progress, challenges and future prospects of
immunotherapy. By prioritizing HPV-related immune dysregulation,
the present study aimed to provide a focused and clinically
relevant overview that may inform the development of personalized
treatment strategies. In summary, the present review is organized
into three major sections: First, an overview of the cervical
cancer TIME; second, the mechanisms by which HPV oncoproteins
mediate immune evasion; and third, recent advances and remaining
challenges in immunotherapeutic strategies. The novelty of this
article lies in its comprehensive synthesis of HPV etiology, immune
microenvironment remodeling and biomarker interactions, offering an
updated perspective that bridges molecular mechanisms with
therapeutic innovation.
Cervical cancer TIME: Architectural and
functional features
Overview of the TIME
The cervical cancer TIME is a dynamic network of
immune cells [such as T lymphocytes, tumor-associated macrophages
(TAMs), natural killer (NK) cells and myeloid-derived suppressor
cells (MDSCs)] (8), cytokines (such
as IL-10 and TGF-β), chemokines and stromal components [such as
cancer-associated fibroblasts (CAFs)] and tumor vasculature
composed of endothelial cells and newly formed vessels. Recent
advances in spatial multi-omics have revealed the heterogeneity of
immune cell clusters and their spatial relationships with tumor and
stromal cells, uncovering mechanisms of immune evasion and therapy
resistance, including in cervical cancer (9–12).
Immune cell dynamics in the tumor
microenvironment
The TIME hosts diverse immune cell populations with
distinct antitumor or protumor functions, thereby shaping disease
progression and clinical outcomes (13,14).
Cytotoxic CD8+ T cells and NK cells mediate direct tumor
elimination (15,16), whereas regulatory T cells (Tregs),
TAMs and MDSCs promote immune evasion and tumor progression
(13,16). Understanding the balance between
these opposing forces provides the foundation for subsequent
discussion on HPV-mediated immune escape and immunotherapeutic
interventions.
T lymphocytes
CD8+ T cells are cytotoxic effectors, but
often display functional exhaustion characterized by programmed
death-1 (PD-1) and T-cell immunoglobulin and mucin-domain
containing-3 (TIM-3) upregulation, resulting in impaired antitumor
activity (17,18). CD4+ subsets play dual
roles: T helper (Th)1 cells enhance IFN-γ, Th2 cells promote
immunosuppression and Th17 cells exert context-dependent effects
through angiogenesis or T-cell recruitment (19). Tregs are elevated in cervical cancer
and promote immune suppression through cytokines such as IL-10 and
TGF-β, contributing to disease progression (20–22).
TAMs
Derived from circulating monocytes, TAMs polarize
into M1 (antitumor) or M2 (protumor) subsets. In cervical cancer,
tumor-derived cytokines favor M2 polarization, which supports
angiogenesis, invasion and metastasis, and is linked to poor
outcomes (23–26).
NK cells
NK cells contribute to immune surveillance, but
their activity is impaired in cervical cancer through mechanisms
such as indoleamine 2,3-dioxygenase (IDO)-mediated tryptophan
depletion, leading to reduced proliferation and cytotoxicity
(27–29). Increased NK infiltration
post-chemotherapy has been associated with improved prognosis.
MDSCs
Elevated levels of granulocytic and monocytic MDSCs
correlate with tumor burden, recurrence and immune suppression,
positioning them as potential biomarkers of progression (30–33).
Immunosuppressive mediators
Key cytokines and checkpoints reinforce
immunosuppression within the TIME. IL-10 impairs antigen-presenting
cell (APC) maturation and promotes M2 polarization (26,34).
TGF-β suppresses the cytotoxic activity of CD8+ T cells
and NK cells, drives Treg differentiation, and contributes to
stromal fibrosis, thereby restricting immune infiltration (35–39).
VEGF facilitates aberrant angiogenesis and recruits Tregs and M2
TAMs, generating a hypoxic, tumor-promoting environment (40,41).
PD-1 and its ligand, programmed death-ligand 1 (PD-L1), are
overexpressed on T cells and tumor cells, respectively, leading to
T-cell exhaustion and disease progression (42,43).
These cytokines and checkpoint pathways collectively amplify the
immunosuppressive network initiated by Tregs and exhausted T cells,
further shaping the cervical cancer TIME. Additionally, stromal
elements such as CAFs contribute to immune evasion by remodeling
the extracellular matrix, secreting TGF-β and VEGF, which further
enhance angiogenesis and immune suppression (44–46).
Collectively, both cellular and stromal mediators cooperate to
establish an immunosuppressive microenvironment that favors HPV
persistence and cervical carcinogenesis.
HPV-mediated immune evasion mechanisms
Persistent infection with high-risk HPV relies on a
coordinated set of immune evasion strategies orchestrated by the
viral oncoproteins E5, E6 and E7. These proteins target multiple
components of both innate and adaptive immunity, enabling viral
persistence and promoting malignant transformation (47–49).
E5-mediated antigen presentation
suppression
HPV E5 downregulates surface expression of major
histocompatibility complex class I (MHC-I) molecules on infected
keratinocytes, thereby limiting recognition by CD8+ T
cells while selectively preserving human leukocyte antigen (HLA)-C
and HLA-E to avoid elimination by NK cells (47,50).
Through these actions, E5 impairs immune surveillance during the
early stages of HPV infection.
E6 and E7 interference with antiviral
signaling
E6 and E7 disrupt innate immune recognition pathways
that normally trigger antiviral responses. E6 promotes degradation
of p53 and inhibits interferon regulatory factor (IRF)-3 and STAT1
signaling, thereby suppressing type I interferon production
(49,51,52).
E7 inactivates the retinoblastoma protein and downregulates the
activity of interferon regulatory factors IRFs (IRF1 and IRF3),
thereby suppressing antiviral gene transcription and impairing
dendritic cell maturation (49,51,52).
In addition, E7 blocks the stimulator of interferon genes
(STING)-cyclic GMP-AMP synthase (cGAS) pathway, impairing cytosolic
DNA sensing and further attenuating interferon-β production
(51,52).
Cytokine and immune checkpoint
modulation
HPV infection reshapes the local immune milieu
toward an immunosuppressive phenotype. E6 and E7 upregulate the
immunosuppressive cytokines IL-10 and TGF-β, enhancing Treg
differentiation and suppressing cytotoxic lymphocyte activity
(53,54). E6 and E7 also induce PD-L1
expression on cervical epithelial and tumor cells, leading to
T-cell exhaustion (55,56). These events synergize with the
pre-existing immunosuppressive tumor microenvironment to sustain
HPV persistence and drive immune escape.
To visualize these interrelated mechanisms, Fig. 1 provides an overview of HPV-mediated
suppression of innate immunity, disruption of antigen presentation
and promotion of an immunosuppressive tumor microenvironment.
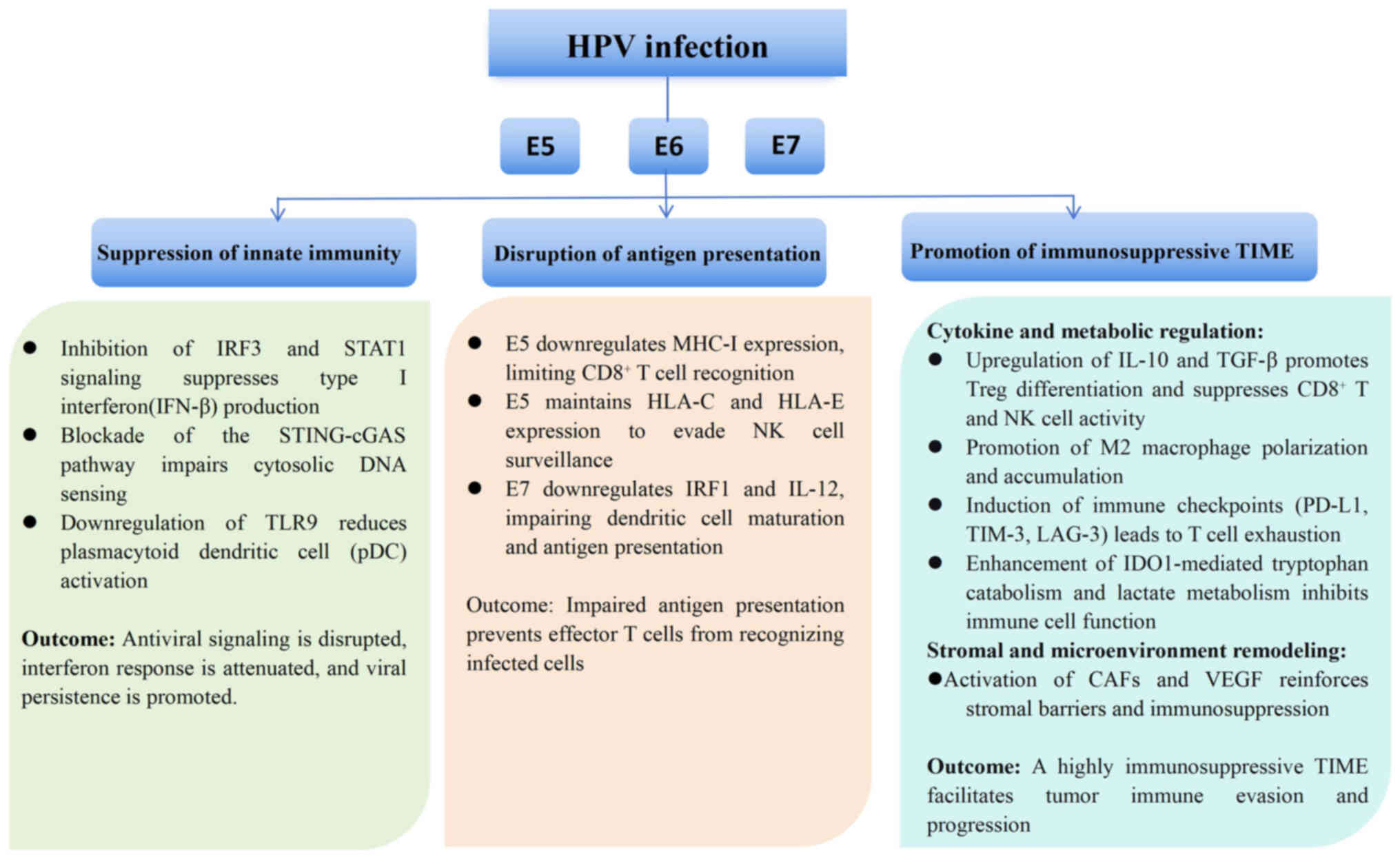 | Figure 1.Mechanisms of HPV-mediated immune
evasion in cervical cancer. HPV oncoproteins E5, E6 and E7
orchestrate multi-level immune evasion. E6 and E7 inhibit the IRF3,
STAT1 and the STING-cGAS signaling pathways, suppressing type I
interferon signaling. E5 downregulates MHC-I while maintaining
HLA-C/E to avoid NK cells. E6 and E7 impair DC maturation and
antigen presentation. Together, these oncoproteins remodel the TIME
by upregulating IL-10, TGF-β and PD-L1, inducing Treg and M2
macrophage accumulation, enhancing IDO1-mediated tryptophan
catabolism and promoting immune tolerance. Collectively, these
processes establish an immunosuppressive TIME that facilitates
viral persistence and tumor progression (38–49,64).
IDO, indoleamine 2,3-dioxygenase; STING, stimulator of interferon
genes; HPV, human papillomavirus; IRF3, interferon regulatory
factor-3; cGAS, cyclic GMP-AMP synthase; MHC-1, major
histocompatibility complex class I; HLA, human leukocyte antigen;
NK, natural killer; PD-L1, programmed death-ligand 1; Treg,
regulatory T cell; TIME, tumor immune microenvironment; pDC,
plasmacytoid dendritic cell. |
Epigenetic and metabolic
reprogramming
HPV infection induces widespread epigenetic
alterations that contribute to immune evasion. Viral oncoproteins
E6 and E7 interact with host chromatin modifiers, leading to DNA
methylation and histone modification of key immune regulatory genes
such as Toll-like receptor-9, cGAS and STING (57,58).
These changes silence antiviral signaling and antigen presentation
pathways, thereby reducing innate immune detection. In addition,
HPV-driven methylation of the promoter regions of HLA and
interferon-stimulated genes further impairs adaptive immune
activation (59,60).
HPV also reprograms cellular metabolism to create a
microenvironment that supports immune suppression and tumor growth.
E6 and E7 upregulate glycolytic enzymes and enhance lactate
production, leading to local acidification and inhibition of
cytotoxic T lymphocytes and NK cells (61–63).
Metabolic byproducts such as adenosine and kynurenine accumulate,
engaging adenosine A2A and aryl hydrocarbon receptors on immune
cells, which suppress effector T-cell function and promote Treg
expansion (59,64). These metabolic shifts cooperate with
cytokine and checkpoint-mediated pathways to reinforce an
immunosuppressive milieu conducive to viral persistence and tumor
progression.
In summary, HPV exploits multiple, interconnected
mechanisms-including impaired antigen presentation, disrupted
interferon signaling, epigenetic silencing and metabolic rewiring-
to escape host immune surveillance and establish a chronically
immunosuppressive tumor microenvironment.
Advances and challenges in
immunotherapy
Immunotherapy has emerged as a promising approach to
overcome HPV-driven immune evasion in cervical cancer. Building on
advances in other malignancies, various strategies-including immune
checkpoint inhibitors, therapeutic vaccines and adoptive cell
therapies-have been investigated in cervical cancer, with
encouraging but heterogeneous results (61,65,66).
Despite notable progress, major challenges such as primary and
acquired resistance, lack of robust predictive biomarkers, and the
profound influence of the TIME remain unresolved. The following
section summarizes recent advances, highlights key clinical data,
and discusses ongoing challenges and future perspectives.
Immune checkpoints inhibitors
(ICIs)
PD-1/PD-L1 inhibitors represent the most advanced
immunotherapy for cervical cancer. The phase III KEYNOTE-826 trial
(ClinicalTrials.gov, NCT03635567) enrolled patients with
persistent, recurrent or metastatic disease. In the PD-L1 combined
positive score (CPS) ≥1 population, pembrolizumab combined with
chemotherapy ± bevacizumab significantly improved clinical outcomes
compared with placebo plus chemotherapy. Median progression-free
survival (PFS) was 10.4 vs. 8.2 months (hazard ratio, 0.62), median
overall survival (OS) was 28.6 vs. 16.5 months (hazard ratio,
0.60), and the objective response rates (ORR) were 80 vs. 68%,
respectively (67). Similarly, the
phase I/II CheckMate 358 trial (ClinicalTrials.gov, NCT02488759)
evaluated nivolumab monotherapy in patients with recurrent or
metastatic cervical cancer previously treated with platinum-based
chemotherapy. In this single-arm study, nivolumab achieved an ORR
of 26%, with durable responses observed in PD-L1-positive tumors
(68). In addition, the phase III
EMPOWER-Cervical1/GOG-3016/ENGOT-cx9 trial (ClinicalTrials.gov,
NCT03257267) evaluated cemiplimab vs. investigator's-choice
chemotherapy in patients with recurrent or metastatic cervical
cancer who had progressed after platinum-based therapy. Cemiplimab
significantly improved OS (12.0 vs. 8.5 months; HR 0.69, 95% CI
0.56–0.84; P=0.00011) and achieved a higher ORR (16.4 vs. 6.3%)
compared with chemotherapy, representing another effective
second-line immunotherapeutic option (69). While ICIs represent a major step
forward, the ORR remains modest, and most patients eventually
experience disease progression.
Therapeutic vaccines
Therapeutic vaccines targeting HPV E6 and E7
oncoproteins aim to induce tumor-specific T-cell immunity. DNA
vaccines such as VGX-3100 and GX-188E have shown safety,
immunogenicity and HPV-specific T cell activation in clinical
studies, with VGX-3100 showing efficacy in HPV-related precancerous
lesions (59,64). However, their effectiveness in
advanced cervical cancer remains limited, highlighting the
immune-suppressive TIME as a major barrier. Ongoing trials are
exploring vaccine-ICI combinations to enhance antitumor activity
(NCT04287868, NCT03946358, NCT06686043).
Adoptive cell therapy (ACT)
ACT strategies, including tumor-infiltrating
lymphocytes (TILs) and engineered T cells, have demonstrated
encouraging activity in cervical cancer. In the phase II single-arm
C-145-04 trial (NCT03108495), the TIL product LN-145 achieved an
ORR of 44% in heavily pretreated patients with recurrent or
metastatic disease, with durable complete responses and median
duration of response not reached at 12 months (70). An early-phase trial of T cell
receptor-engineered T cells targeting HPV16 E6/E7 antigens
(NCT03356795) is underway, though efficacy and safety require
further validation (71,72). ACT represents a promising option for
refractory cases but faces challenges in manufacturing, cost and
scalability.
A summary of key immunotherapeutic modalities,
including checkpoint inhibitors, therapeutic vaccines and adoptive
cell therapies, is provided in Table
I to highlight their mechanisms of action, clinical trial
status and efficacy outcomes.
 | Table I.Current immunotherapy strategies for
cervical cancer and key clinical outcomes. |
Table I.
Current immunotherapy strategies for
cervical cancer and key clinical outcomes.
| Modality | Representative
agent/strategy | Mechanism of
action | Key trials | Clinical
outcomes | (Refs.) |
|---|
| PD-1 inhibitor | Pembrolizumab | Blocks PD-1/PD-L1
interaction, restores T-cell activity | KEYNOTE-826
(NCT03635567, phase III) | ORR 80 vs. 68%; PFS
10.4 vs. 8.2 months; OS 28.6 vs. 16.5 months (pembrolizumab +
chemotherapy ± bevacizumab vs. placebo + chemotherapy ±
bevacizumab) | (67) |
|
| Nivolumab | Blocks PD-1
signaling | CheckMate 358
(NCT02488759, phase I/II) | ORR 26% in
pretreated patients; durable response in PD-L1-positive tumors
(single-arm study, no control group) | (68) |
|
| Cemiplimab | PD-L1 blockade | EMPOWER-Cervical 1
(NCT03257267, phase III) | OS 12.0 vs. 8.5
months; HR 0.69; ORR 16.4 vs. 6.3% (cemiplimab vs.
investigator's-choice chemotherapy) | (69) |
| Therapeutic
vaccine | VGX-3100 (DNA
vaccine) | Plasmid DNA vaccine
encoding HPV16/18 E6/E7 antigens; induces HPV-specific
CD8+ and CD4+ T-cell responses | Phase II | Histological
regression in CIN2/3; limited efficacy in advanced cancer | (64) |
|
| GX-188E (DNA
vaccine) | DNA vaccine
encoding HPV E6/E7 fusion antigens to induce HPV-specific
CD8+ cytotoxic and CD4+ helper T-cell
responses via MHC-I/II pathways | Phase II | ORR 18%; enhanced
with pembrolizumab | (60) |
| Adoptive cell
therapy | LN-145 (TIL
therapy) | Expansion of
patient-derived TILs | Single-arm phase II
(C-145-04, NCT03108495) | ORR 44% in heavily
pretreated recurrent/metastatic cervical cancer; durable complete
responses; median DOR not reached | (70) |
|
| CAR-T/TCR-T
cells | Redirects T cells
against HPV antigens | Early-phase
trials | Preliminary safety
and immune activation; further efficacy validation required | (71,72) |
Challenges and biomarkers
Resistance to immunotherapy arises from multiple
mechanisms, including upregulation of alternative checkpoints
(e.g., TIM-3 and lymphocyte-activation gene-3), stromal barriers
formed by CAFs and dense extracellular matrix that limit immune
cell infiltration, metabolic reprogramming (e.g., IDO1 and
adenosine) and the immunomodulatory role of the vaginal microbiome
(73). Predictive biomarkers for
immunotherapy resistance, such as PD-L1 CPS (67,74),
tumor mutational burden (TMB) (75), microsatellite instability and HPV
genotype (75–77), have been studied, but none alone is
able to sufficiently stratify patients. Emerging evidence suggests
that integrated biomarker approaches-combining PD-L1, TMB and
immune cell infiltration profiles-may improve prediction of patient
responses and, therefore, guide precision immunotherapy (66,78–80).
HPV etiology and immunotherapy
efficacy
HPV status strongly influences the tumor immune
landscape and clinical response to immunotherapy. HPV-positive
cervical cancers exhibit higher neoantigen load, increased
CD8+ T-cell infiltration and elevated PD-L1 expression
compared with HPV-negative counterparts, features associated with
improved responsiveness to ICIs (61,78).
However, HPV genotype-specific differences exist: Tumors driven by
HPV16 tend to show stronger cytotoxic immune activation and
improved immunotherapy outcomes, whereas HPV18 and mixed infections
are linked to a more immunosuppressive milieu characterized by
abundant Tregs and M2 macrophages (65,81).
Moreover, persistent HPV E6/E7 oncoprotein expression maintains
chronic antigenic stimulation, which can both prime immune
recognition and promote T-cell exhaustion (66). Therefore, integrating HPV genotype
and viral gene-expression profiles into biomarker evaluation may
refine patient selection and optimize immunotherapeutic
efficacy.
To provide an overview of currently available and
emerging biomarkers for immunotherapy response in cervical cancer,
Table II summarizes their
biological basis, detection methods and clinical implications.
 | Table II.Predictive biomarkers for cervical
cancer immunotherapy. |
Table II.
Predictive biomarkers for cervical
cancer immunotherapy.
| Biomarker | Mechanism/role | Clinical
evidence | Limitations | (Refs.) |
|---|
| PD-L1 CPS | Indicates T cell
inflamed TIME, predicts ICI benefit | KEYNOTE-826: Higher
CPS improved ORR/OS | Heterogeneous
expression; not fully predictive | (67,74) |
| TMB | High mutation load
increases neoantigen presentation | FDA approval of
pembrolizumab for TMB-high tumors | Rare in cervical
cancer; cut-off values debated | (75) |
| MSI-H/dMMR | dMMR leads to the
accumulation of neoantigens | Pembrolizumab
active in MSI-H tumors (pan-cancer) | Very low prevalence
in cervical cancer (<3%) | (76,77) |
| HPV genotype | Viral oncoproteins
are immunogenic targets | Basis for
vaccine/TCR therapy | Not all genotypes
equally immunogenic | (75–77) |
| TIL density | Reflects host
antitumor immunity | High
CD8+ TILs improved prognosis, improved ICI response | Variable assessment
methods | (79) |
| Microbiome
composition | Influences mucosal
immunity and ICI response | Emerging evidence
in gynecological cancers | Limited
cervical-specific data | (73) |
Future directions
Future research should prioritize biomarker-driven
precision immunotherapy. Rational combination regimens-including
ICIs with therapeutic vaccines, ACT or oncolytic viruses- are under
active investigation and may overcome resistance. Novel approaches
such as bispecific antibodies, microbiome modulation and metabolic
checkpoint inhibitors hold additional promise (82–84).
The integration of spatial multi-omics technologies with immune
monitoring will deepen understanding of TIME heterogeneity and
inform the design of next-generation immunotherapies. Ultimately,
translating these insights into rational, biomarker-guided
strategies is key to improving durable benefit for patients with
cervical cancer.
Conclusion and future perspectives
The present review highlights the central role of
the cervical cancer TIME in modulating disease progression, with
HPV oncoproteins E5, E6 and E7 orchestrating a multifaceted immune
escape strategy. Advances in immunotherapy, particularly ICIs,
therapeutic vaccines and adoptive cell therapies, have
significantly reshaped the therapeutic landscape, yet their
clinical efficacy remains limited to a subset of patients.
Key challenges include primary and acquired
resistance, heterogeneous biomarker expression, stromal and
metabolic barriers, and the influence of the vaginal microbiome.
Future efforts should prioritize biomarker-driven precision
approaches, combining PD-L1, TMB and immune infiltration profiles
with emerging markers such as microbiome signatures and spatial
transcriptomics. Integration of multi-omics technologies will allow
for a more comprehensive understanding of the TIME and
identification of novel targets.
Furthermore, rational design of combination
regimens-including checkpoint inhibitor combinations, oncolytic
viruses, bispecific antibodies and vaccines-represents a promising
direction to overcome resistance. By emphasizing HPV-mediated
immune evasion as a unifying framework, the present review provides
a focused perspective that not only synthesizes existing knowledge
but also highlights opportunities for innovation in personalized
immunotherapy for cervical cancer. In conclusion, a deeper
understanding of HPV-mediated immune evasion and the cervical
cancer TIME will be essential to guide the development of
next-generation, personalized immunotherapeutic strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Author's contributions
XZ and RA wrote the original draft manuscript. XL
wrote, reviewed and edited the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent for
publication
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z, Liu P, Yin A, Zhang B, Xu J, Chen Z,
Zhang Z, Zhang Y, Wang S, Tang L, et al: Global landscape of
cervical cancer incidence and mortality in 2022 and predictions to
2030: The urgent need to address inequalities in cervical cancer.
Int J Cancer. 157:288–297. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
3
|
Shahmoradi Z, Damgacioglu H, Clarke MA,
Wentzensen N, Montealegre J, Sonawane K and Deshmukh AA: Cervical
cancer incidence among US women, 2001–2019. JAMA. 328:2267–2269.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Visser KE and Joyce JA: The evolving
tumor microenvironment: From cancer initiation to metastatic
outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karin N: The development and homing of
myeloid-derived suppressor cells: From a two-stage model to a
multistep narrative. Front Immunol. 11:5575862020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Y, Liu J, Beeraka NM, Manogaran P,
Ramachandrappa HVP, Naga LDY, Suhail SM, Pradeepkumar B, Sinelnikov
MY, Venkata GM, et al: Inflammation and stem cell stochasticity of
HPV-induced cervical cancer: Epigenetics based biomarkers through
microbiome and metabolome for personalized medicine: A systematic
review. Curr Med Chem. 32:2390–2408. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudela E, Liskova A, Samec M, Koklesova L,
Holubekova V, Rokos T, Kozubik E, Pribulova T, Zhai K, Busselberg
D, et al: The interplay between the vaginal microbiome and innate
immunity in the focus of predictive, preventive, and personalized
medical approach to combat HPV-induced cervical cancer. EPMA J.
12:199–220. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma XY, Lu LL and Sun PF: Research progress
on the immune microenvironment in cervical cancer. Int J Oncol
(China). 50:47–50. 2023.(In Chinese).
|
|
9
|
Wu Y, Cheng Y, Wang X, Fan J and Gao Q:
Spatial omics: Navigating to the golden era of cancer research.
Clin Transl Med. 12:e6962022. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Mauro F and Arbore G: Spatial
dissection of the immune landscape of solid tumors to advance
precision medicine. Cancer Immunol Res. 12:800–813. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Q, Tian X, Li F, Yu X, Gong W, Chen Y,
Wang J and Yang S, Zhang S, Zhang Q and Yang S: Integrated
multi-omics analysis of single-cell and spatial transcriptomics
reveals distinct hpv-associated immune microenvironment features
and prognostic signatures in cervical cancer. Front Immunol.
16:16126232025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Z, Zhou Y, Liu Z, Nie W, Cao H, Li S,
Zhu L, Lin G, Ding Y, Jiang Y, et al: Deciphering the tumor immune
microenvironment: Single-cell and spatial transcriptomic insights
into cervical cancer fibroblasts. J Exp Clin Cancer Res.
44:1942025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Liu M, An Y, Gao C, Wang T, Zhang
Z, Zhang G, Li S, Li W, Li M and Wang G: Targeting immune
microenvironment in cervical cancer: Current research and advances.
J Transl Med. 23:8882025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grant G and Ferrer CM: The role of the
immune tumor microenvironment in shaping metastatic dissemination,
dormancy, and outgrowth. Trends Cell Biol. Jul 4–2025.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu J, Fu L, Wu R, Che L, Liu G, Ran Q, Xia
Z, Liang X and Zhao G: Immunocytes in the tumor microenvironment:
Recent updates and interconnections. Front Immunol. 16:15179592025.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Yu X, Han X, Lian C, Wang Z, Shao S,
Shao F, Wang H, Ma S and Liu J: Innate immune cells in tumor
microenvironment: A new frontier in cancer immunotherapy. iScience.
27:1107502024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou R, Gu R, Yu X, Hu Y, Yu J, Xue X and
Zhu X: Characteristics of infiltrating immune cells and a
predictive immune model for cervical cancer. J Cancer.
12:3501–3514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maskey N, Thapa N, Maharjan M, Shrestha G,
Maharjan N, Cai H and Liu S: Infiltrating CD4 and CD8 lymphocytes
in HPV infected uterine cervical milieu. Cancer Manag Res.
11:7647–7655. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anvar MT, Rashidan K, Arsam N,
Rasouli-Saravani A, Yadegari H, Ahmadi A, Asgari Z, Vanan AG,
Ghorbaninezhad F and Tahmasebi S: Th17 cell function in cancers:
Immunosuppressive agents or anti-tumor allies? Cancer Cell Int.
24:3552024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ao C and Zeng K: The role of regulatory T
cells in pathogenesis and therapy of human papillomavirus-related
diseases, especially in cancer. Infect Genet Evol. 65:406–413.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Zhang HL, Nie ZY, Wang Z, Kang Y,
Yang XS and Yuan F: The disease stage-associated imbalance of
Th1/Th2 and Th17/Treg in uterine cervical cancer patients and their
recovery with the reduction of tumor burden. BMC Womens Health.
20:1262020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Zhan J, Guan Z, Lin X, Li T, Li
M, Zhang C and Zhong L: The prognostic value of Th17/treg cell in
cervical cancer: A systematic review and meta-analysis. Front
Oncol. 14:14421032024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Q, Steger A, Mahner S, Jeschke U and
Heidegger H: The formation and therapeutic update of
tumor-associated macrophages in cervical cancer. Int J Mol Sci.
20:33102019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu SY, Wu QY, Zhang CX, Wang Q, Ling J,
Huang XT, Sun X, Yuan M, Wu D and Yin HF: miR-20a inhibits the
killing effect of natural killer cells to cervical cancer cells by
downregulating RUNX1. Biochem Biophys Res Commun. 505:309–316.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Wang H, Liu Y, Tao T, Zeng Z,
Zhou Y and Wang M: Nocardia rubra cell-wall skeleton influences the
development of cervical carcinoma by promoting the antitumor effect
of macrophages and dendritic cells. Cancer Med. 11:1249–1268. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi Y, Lee D, Kim NY, Seo I, Park NJY and
Chong GO: Role of tumor-associated macrophages in cervical cancer:
Integrating classical perspectives with recent technological
advances. Life (Basel). 14:4432024.PubMed/NCBI
|
|
27
|
Guo L and Hua K: Cervical cancer: Emerging
immune landscape and treatment. Onco Targets Ther. 13:8037–8047.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gutiérrez-Hoya A and Soto-Cruz I: NK cell
regulation in cervical cancer and strategies for immunotherapy.
Cells. 10:31042021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Venancio PA, Consolaro MEL, Derchain SF,
Boccardo E, Villa LL, Maria-Engler SS, Campa A and Discacciati MG:
Indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase
expression in HPV infection, SILs, and cervical cancer. Cancer
Cytopathol. 127:586–597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding
J, Zhu J, Wei H and Zhao K: Circulating and tumor-infiltrating
myeloid-derived suppressor cells in patients with colorectal
carcinoma. PLoS One. 8:e571142013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang Y, Lü B, Zhao P and Lü W: Increased
circulating GrMyeloid-derived suppressor cells correlated with
tumor burden and survival in locally advanced cervical cancer
patients. J Cancer. 10:1341–1348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dysthe M and Parihar R: Myeloid-derived
suppressor cells in the tumor microenvironment. Adv Exp Med Biol.
1224:117–140. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L, Liu H, Guo H, Wu Q, Yu S, Qin Y,
Wang G, Wu Q, Zhang R, Wang L, et al: Circulating and
tumor-infiltrating myeloid-derived suppressor cells in cervical
carcinoma patients. Oncol Lett. 15:9507–9515. 2018.PubMed/NCBI
|
|
34
|
Bermudez-Morales VH, Gutierrez LX,
Alcocer-Gonzalez JM, Burguete A and Madrid-Marina V: Correlation
between IL-10 gene expression and HPV infection in cervical cancer:
A mechanism for immune response escape. Cancer Invest.
26:1037–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zimmer N, Trzeciak ER, Graefen B, Satoh K
and Tuettenberg A: GARP as a therapeutic target for the modulation
of regulatory T cells in cancer and autoimmunity. Front Immunol.
13:9284502022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haque S and Morris JC: Transforming growth
factor-β: A therapeutic target for cancer. Hum Vaccin Immunother.
13:1741–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yi M, Li T, Niu M, Wu Y, Zhao Z and Wu K:
TGF-β: A novel predictor and target for anti-PD-1/PD-L1 therapy.
Front Immunol. 13:10613942022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wilson EB, El-Jawhari JJ, Neilson AL, Hall
GD, Melcher AA, Meade JL and Cook GP: Human tumour immune evasion
via TGF-β blocks NK cell activation but not survival allowing
therapeutic restoration of anti-tumour activity. PLoS One.
6:e228422011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
George N, Bhandari P, Shruptha P, Jayaram
P, Chaudhari S and Satyamoorthy K: Multidimensional outlook on the
pathophysiology of cervical cancer invasion and metastasis. Mol
Cell Biochem. 478:2581–2606. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu C, Gu L, Liu Z, Li J, Yao M and Fang
C: Correlation between vascular endothelial growth factor pathway
and immune microenvironment in head and neck squamous cell
carcinoma. BMC Cancer. 21:8362021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol. 59:455–467.
2018.PubMed/NCBI
|
|
42
|
Zheng D, Hou X, Yu J and He X:
Combinatorial strategies with PD-1/PD-L1 immune checkpoint blockade
for breast cancer therapy: Mechanisms and clinical outcomes. Front
Pharmacol. 13:9283692022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Zhu W, Zhang X, Qu Q and Zhang L:
Expression and clinical significance of programmed death-1 on
lymphocytes and programmed death ligand-1 on monocytes in the
peripheral blood of patients with cervical cancer. Oncol Lett.
14:7225–7231. 2017.PubMed/NCBI
|
|
44
|
Feng X, Meng X, Tang D, Guo S, Liao Q,
Chen J, Xie Q, Liu F, Fang Y, Sun C, et al: Reversal of the
immunosuppressive tumor microenvironment via platinum-based
neoadjuvant chemotherapy in cervical cancer. Cancer Pathog Ther.
2:38–49. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ping Q, Yan R, Cheng X, Wang W, Zhong Y,
Hou Z, Shi Y, Wang C and Li R: Cancer-associated fibroblasts:
Overview, progress, challenges, and directions. Cancer Gene Ther.
28:984–999. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Walch-Rückheim B, Ströder R, Theobald L,
Pahne-Zeppenfeld J, Hegde S, Kim YJ, Bohle RM, Juhasz-Böss I,
Solomayer EF and Smola S: Cervical cancer-instructed stromal
fibroblasts enhance IL23 expression in dendritic cells to support
expansion of Th17 cells. Cancer Res. 79:1573–1586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Freitas AC, de Oliveira THA, Barros MR
Jr and Venuti A: hrHPV E5 oncoprotein: Immune evasion and related
immunotherapies. J Exp Clin Cancer Res. 36:712017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan Y, Cai X, Shen F and Ma F: HPV
post-infection microenvironment and cervical cancer. Cancer Lett.
497:243–254. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lo Cigno I, Calati F, Girone C, Catozzo M
and Gariglio M: High-risk HPV oncoproteins E6 and E7 and their
interplay with the innate immune response: Uncovering mechanisms of
immune evasion and therapeutic prospects. J Med Virol.
96:e296852024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Galazka K, Opławski M, Windorbska W,
Skret-Magierlo J, Koper K, Basta P, Mach P, Dutch-Wicherek M, Mazur
A and Wicherek L: The immunohistochemical analysis of antigens such
as RCAS1 and B7H4 in the cervical cancer nest and within the
fibroblasts and macrophages infiltrating the cancer
microenvironment. Am J Reprod Immunol. 68:85–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ronco LV, Karpova AY, Vidal M and Howley
PM: Human papillomavirus 16 E6 oncoprotein binds to interferon
regulatory factor-3 and inhibits its transcriptional activity.
Genes Dev. 12:2061–2072. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park JS, Kim EJ, Kwon HJ, Hwang ES,
Namkoong SE and Um SJ: Inactivation of interferon regulatory
factor-1 tumor suppressor protein by HPV E7 oncoprotein.
Implication for the E7-mediated immune evasion mechanism in
cervical carcinogenesis. J Biol Chem. 275:6764–6769. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Manzo-Merino J, del-Toro-Arreola S,
Rocha-Zavaleta L, Peralta-Zaragoza Ó, Jiménez-Lima R and
Madrid-Marina V: Immunology of cervical cancer. Rev Invest Clin.
72:188–197. 2020.PubMed/NCBI
|
|
54
|
Ovestad IT, Gudlaugsson E, Skaland I,
Malpica A, Munk AC, Janssen EA and Baak JP: The impact of
epithelial biomarkers, local immune response and human
papillomavirus genotype in the regression of cervical
intraepithelial neoplasia grades 2–3. J Clin Pathol. 64:303–307.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hasan UA, Zannetti C, Parroche P, Goutagny
N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M,
Taylor-Papadimitriou J, et al: The human papillomavirus type 16 E7
oncoprotein induces a transcriptional repressor complex on the
Toll-like receptor 9 promoter. J Exp Med. 210:1369–1387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hasan UA, Bates E, Takeshita F, Biliato A,
Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T,
et al: TLR9 expression and function is abolished by the cervical
cancer-associated human papillomavirus type 16. J Immunol.
178:3186–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Campo MS, Graham SV, Cortese MS, Ashrafi
GH, Araibi EH, Dornan ES, Miners K, Nunes C and Man S: HPV-16 E5
down-regulates expression of surface HLA class I and reduces
recognition by CD8 T cells. Virology. 407:137–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Torres-Poveda K, Bahena-Román M,
Madrid-González C, Burguete-García AI, Bermúdez-Morales VH,
Peralta-Zaragoza O and Madrid-Marina V: Role of IL-10 and TGF-β1 in
local immunosuppression in HPV-associated cervical neoplasia. World
J Clin Oncol. 5:753–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sen P, Ganguly P and Ganguly N: Modulation
of DNA methylation by human papillomavirus E6 and E7 oncoproteins
in cervical cancer. Oncol Lett. 15:11–22. 2018.PubMed/NCBI
|
|
60
|
Jiménez-Wences H, Peralta-Zaragoza O and
Fernández-Tilapa G: Human papilloma virus, DNA methylation and
microRNA expression in cervical cancer (review). Oncol Rep.
31:2467–2476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shamseddine AA, Burman B, Lee NY, Zamarin
D and Riaz N: Tumor immunity and immunotherapy for HPV-related
cancers. Cancer Discov. 11:896–1912. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yadav C, Yadav R, Chabbra R, Nanda S,
Ranga S, Kadian L and Ahuja P: Overview of genetic and epigenetic
regulation of human papillomavirus and apoptosis in cervical
cancer. Apoptosis. 28:683–701. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McLaughlin-Drubin ME, Crum CP and Münger
K: Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B
histone demethylase expression and causes epigenetic reprogramming.
Proc Natl Acad Sci USA. 108:2130–2135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dey T and Agrawal S: Immunotherapy in
cervical cancer: an innovative approach for better treatment
outcomes. Explor Target Antitumor Ther. 6:10022962025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ogasawara A and Hasegawa K: Recent
advances in immunotherapy for cervical cancer. Int J Clin Oncol.
30:434–448. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Colombo N, Dubot C, Lorusso D, Caceres MV,
Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E,
Yañez E, et al: Pembrolizumab for persistent, recurrent, or
metastatic cervical cancer. N Engl J Med. 385:1856–1867. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Naumann RW, Hollebecque A, Meyer T, Devlin
MJ, Oaknin A, Kerger J, López-Picazo JM, Machiels JP, Delord JP,
Evans TRJ, et al: Safety and efficacy of nivolumab monotherapy in
recurrent or metastatic cervical, vaginal, or vulvar carcinoma:
Results from the phase I/II CheckMate 358 trial. J Clin Oncol.
37:2825–2834. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tewari KS, Monk BJ, Vergote I, Miller A,
de Melo AC, Kim HS, Kim YM, Lisyanskaya A, Samouëlian V, Lorusso D,
et al: Survival with cemiplimab in recurrent cervical cancer. N
Engl J Med. 386:544–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Stevanović S, Helman SR, Wunderlich JR,
Langhan MM, Doran SL, Kwong MLM, Somerville RPT, Klebanoff CA,
Kammula US, Sherry RM, et al: A phase II study of
tumor-infiltrating lymphocyte therapy for human
papillomavirus-associated epithelial cancers. Clin Cancer Res.
25:1486–1793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nagarsheth NB, Norberg SM, Sinkoe AL,
Adhikary S, Meyer TJ, Lack JB, Warner AC, Schweitzer C, Doran SL,
Korrapati S, et al: TCR-engineered T cells targeting E7 for
patients with metastatic HPV-associated epithelial cancers. Nat
Med. 27:419–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Draper LM, Kwong ML, Gros A, Stevanović S,
Tran E, Kerkar S, Raffeld M, Rosenberg SA and Hinrichs CS:
Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered
T cells directed against E6. Clin Cancer Res. 21:4431–4439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Łaniewski P, Barnes D, Goulder A, Cui H,
Roe DJ, Chase DM and Herbst-Kralovetz MM: Linking cervicovaginal
immune signatures, HPV and microbiota composition in cervical
carcinogenesis in non-hispanic and hispanic women. Sci Rep.
8:75932018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chung HC, Ros W, Delord JP, Perets R,
Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L,
Zeigenfuss S, et al: Efficacy and safety of pembrolizumab in
previously treated advanced cervical cancer: Results from the phase
II KEYNOTE-158 study. J Clin Oncol. 37:1470–1478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet, Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017.PO.17.00073. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Eksteen C, Riedemann J, van der Merwe FH,
Botha MH and Engelbrecht AM: Advancing personalized medicine in
LMICs: Predictive indicators for cervical cancer immunotherapy
response. Semin Oncol. 52:1523522025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Stevanović S, Draper LM, Langhan MM,
Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry
RM, Kammula US, et al: Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted
tumor-infiltrating T cells. J Clin Oncol. 33:1543–1550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cristescu R, Mogg R, Ayers M, Albright A,
Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al:
Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based
immunotherapy. Science. 362:eaar35932018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ling J, Sun Q, Tian Q, Shi H, Yang H and
Ren J: Human papillomavirus 16 E6/E7 contributes to immune escape
and progression of cervical cancer by regulating miR-142-5p/PD-L1
axis. Arch Biochem Biophys. 731:1094492022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Romero D: Cadonilimab is effective and
safe in recurrent cervical cancer. Nat Rev Clin Oncol. 22:22025.
View Article : Google Scholar
|
|
83
|
De Jaeghere EA, Hamerlinck H, Tuyaerts S,
Lippens L, Van Nuffel AMT, Baiden-Amissah R, Vuylsteke P, Henry S,
Trinh XB, van Dam PA, et al: Associations of the gut microbiome
with outcomes in cervical and endometrial cancer patients treated
with pembrolizumab: Insights from the phase II PRIMMO trial.
Gynecol Oncol. 191:275–286. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gore M, Kabekkodu SP and Chakrabarty S:
Exploring the metabolic alterations in cervical cancer induced by
HPV oncoproteins: From mechanisms to therapeutic targets. Biochim
Biophys Acta Rev Cancer. 1880:1892922025. View Article : Google Scholar : PubMed/NCBI
|















