Introduction
Fumarate hydratase-deficient renal cell carcinoma
(FHdRCC) is a molecularly defined renal cell carcinoma (RCC)
according to the WHO classification of urinary and male genital
tumors (1). It occurs due to the
inactivation of the FH gene. FH deficiency causes the accumulation
of fumarate in the mitochondria, which acts as an oncometabolite in
the cytoplasm, leading to RCC (2).
It is common in males and is a highly aggressive metastatic
carcinoma. However, it has rarely been reported as
dialysis-associated renal carcinoma.
RCC diagnosis relies on imaging techniques. Computed
tomography (CT) or magnetic resonance imaging (MRI) are used to
confirm RCC (3). However, it is
sometimes difficult for imaging alone to identify renal masses as
malignant tumors in cystic kidney diseases such as autosomal
dominant polycystic kidney disease (ADPKD) or acquired cystic
kidney disease (ACKD). In such cases, pathological confirmation by
biopsy is required.
Here, we report a case of FHdRCC that was developed
in a patient with ACKD who presented with fever and hematuria. This
case indicates that it is important to perform a renal mass biopsy
if its radiographic images are equivocal and that it can be
performed in the same way as percutaneous kidney biopsies.
Case report
Case presentation
A 76-year-old Japanese male started hemodialysis at
the age of 47 years with an unknown etiology. The patient had no
personal or family history of malignancy. Intermittent gross
hematuria was observed at 75 years of age. Enhanced computed
tomography (CT) and cystoscopy at another hospital showed no
malignancy but revealed polycystic kidneys. He was referred to our
hospital with persistent fever and an elevated C-reactive protein
(CRP)-suspected cyst infection in ADPKD.
The total kidney volume (TKV) was 616 ml (right: 288
ml; left: 328 ml). The cyst distribution differed from that in
ADPKD; he was diagnosed with ACKD. He was exhausted and presented
with hematuria and associated fever, which seemed to be a symptom
of cystic hemorrhage. Therefore, transcatheter embolization of the
bilateral renal arteries was performed. Only the left artery could
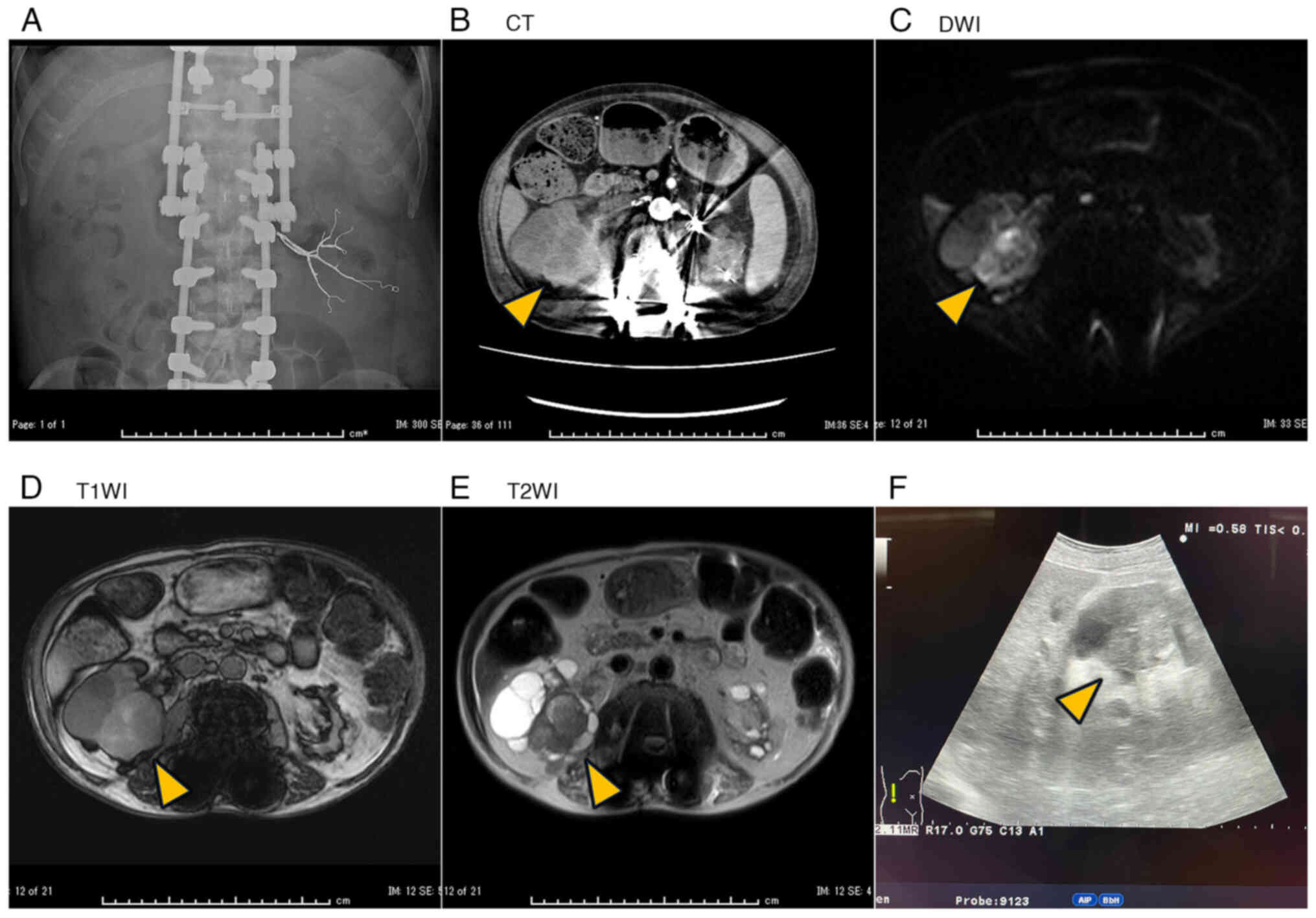
be embolized because of right arterial stenosis (Fig. 1A). Nevertheless, hematuria and fever
subsequently ceased. At three-month follow-up, his TKV had
decreased to 380 ml (right: 256 ml, left: 94.6 ml), and the left
kidney had shrunk.
Four months after embolization (one year after onset
of symptoms), he re-presented with persistent elevated CRP, fever
(temperature around 37.6°C), and hematuria over two weeks. The
patient was admitted to our department. On admission, the patient
height and weight were 165 cm and 57.4 kg, respectively. His vital
signs were stable (body temperature, 37.1°C; blood pressure, 123/64
mmHg; heart rate; 56 bpm). The patient had no abdominal or back
pain. The laboratory test findings are listed in Table I. His CRP was elevated to 8.01
mg/dl, whereas other hematological parameters, including hemoglobin
(11.0 g/dl) and serum creatinine (5.36 mg/dl, elevated as expected
in a dialysis patient), were within the anticipated ranges for a
dialysis patient. CT showed a thickened cyst wall in the right
kidney (Fig. 1B). MRI demonstrated
the same cyst, with decreased diffusion on diffusion-weighted
images, slightly high intensity on T1-weighted images, and low
intensity on T2-weighted images (Fig.
1C-E). TKV was slightly increased to 426 ml (right: 334 ml,
left: 91.5 ml), and the right side became swollen. These findings,
together with the CRP elevation, were suggestive of a cystic
infection or hemorrhage, and antibiotics were administered.
 | Table I.Laboratory findings on admission. |
Table I.
Laboratory findings on admission.
| Laboratory
values | Patient result | Reference range |
|---|
| Blood
biochemistry |
|
|
| Total
protein, g/dl | 7.3 | 6.6–8.1 |
| Albumin,
g/dl | 3.6 | 4.1–5.1 |
| Aspartate
aminotransferase, U/l | 3 | 13-30 |
| Alanine
aminotransferase, U/l | 9 | 7-13 |
| Lactate
dehydrogenase (IFCC), U/l | 239 | 124-222 |
| Alkaline
phosphatase (IFCC), U/l | 130 | 38-113 |
|
γ-glutamyl transpeptidase,
U/l | 59 | 9-32 |
| Total
bilirubin, mg/dl | 0.4 | <1.5 |
| Urea
nitrogen, mg/dl | 41.0 | 8.0–20.0 |
|
Creatinine, mg/dl | 5.36 | 0.46–0.79 |
| Uric
acid, mg/dl | 4.0 | 2.6–7.0 |
| Sodium,
mmol/l | 142 | 138-145 |
|
Potassium, mmol/l | 5.1 | 3.6–4.8 |
| Chloride,
mmol/l | 102 | 101-108 |
| Calcium,
mg/dl | 9.1 | 8.8–10.1 |
|
Phosphate, mg/dl | 4.1 | 2.7–4.6 |
| C
reactive protein, mg/dl | 8.01 | <0.14 |
| Complete blood
count |
|
|
| White
blood cell count, ×103/µl | 6.6 | 4.0–11.0 |
|
Hemoglobin, g/dl | 11.0 | 12.0–16.0 |
|
Hematocrit, % | 36.8 | 35.1–44.4 |
| Platelet
count, ×103/µl | 272 | 120-450 |
As there was little improvement in the fever and CRP
levels, we attempted cyst drainage on day 8. Examining the cyst
using echography incidentally revealed a highly echoic and solid
mass (Fig. 1F). The mass was
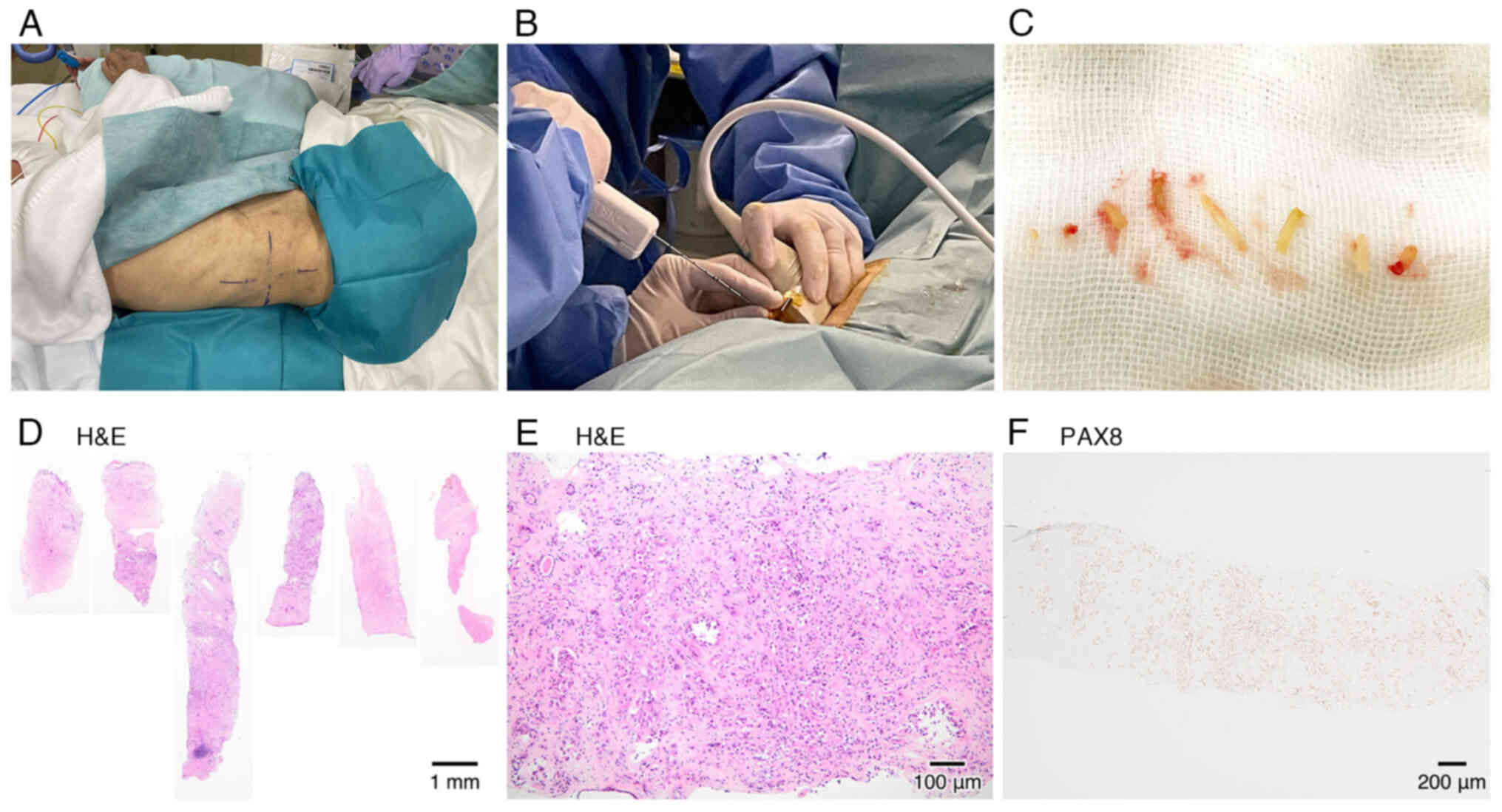
punctured using a biopsy needle gun that is used in percutaneous
kidney biopsy, while remaining in the lateral position (Fig. 2A and B). Six white hard core
biopsies were obtained without complications (Fig. 2C).
Kidney mass biopsy findings
Light microscopy of the biopsy specimen (Fig. 2D-F) revealed cribriform-pattern
invasive RCC. The tumor demonstrated a mixed cellular pattern: some
cells had prominent atypical nuclei and clear cytoplasm, while
others were small and round, with surrounding eosinophilic
macronucleoli and gritty calcification. Immunohistochemistry was
performed on formalin-fixed, paraffin-embedded sections using the
following primary antibodies: PAX8 (rabbit polyclonal, 1:2,000
dilution; cat. no. 10336-1-AP, Proteintech, distributed by Cosmo
Bio, Tokyo, Japan); GATA3 (mouse monoclonal, clone L50-823, 1:1
dilution; cat. no. 418201, Nichirei Biosciences, Tokyo, Japan). It
revealed positive nuclear PAX8 expression and negative
GATA3 expression. These findings suggest an uncommon renal
cell carcinoma, such as molecularly defined RCC.
Clinical course
The body temperature of the patient remained around
38°C, and his CRP remained around 8–19 mg/dl. As renal cancer was
detected by biopsy, we decided to perform right nephrectomy. On day
20, the patient underwent total right nephrectomy. The patient had
no severe post-operative complications and had good pain control.
On day 23, he underwent hemodialysis without difficulties and was
in a good condition during the night nurse rounds; however, 30 min
after a nurse's round, he was found in cardiac arrest.
Cardiopulmonary resuscitation was promptly performed; however, the
patient remained unresponsive and died. We considered that the
patient vomited following postoperative paralytic ileus, which led
to asphyxiation.
Nephrectomy findings
Macroscopic findings in the kidneys are shown in
Fig. 3A. The tumor lacked a
pseudocapsule and exhibited infiltrative growth. Microscopically,
the tumor had a papillary, adenoductal, and solid architecture. The
tumor cell nuclei were round and highly atypical, and the cytoplasm
was clear. Eosinophilic amorphous material was evident in the
papillary architecture. Microcalcifications were also observed
(Fig. 3B and C).
Immunohistochemistry was performed on formalin-fixed,
paraffin-embedded sections using the following primary antibodies:
alpha-methylacyl-CoA racemase (AMACR) (mouse monoclonal, clone
13H4, 1:300 dilution; cat. no. M3616, Dako, Glostrup, Denmark),
Fumarate hydratase (FH) (rabbit polyclonal, 1:500 dilution; cat.
no. ab95950, Abcam, Cambridge, UK), S-2-succinylcysteine (2SC)
(rabbit polyclonal, 1:500 dilution; cat. no. crb20005017d,
Discovery Antibodies, UK), TFE3 (mouse monoclonal, clone MRQ-37,
1:50 dilution; cat. no. 354R-16-RUO, Cell Marque, Rocklin, CA,
USA), TFEB (mouse monoclonal, clone C-6, 1:100 dilution; cat. no.
sc166736, Santa Cruz Biotechnology, Dallas, TX, USA). It was
positive for α-methylacyl-CoA racemase (AMACR), FH, and
S-2-succinylated-cystaine (2SC) (Fig.
3D-F), and was negative for TFE3 and TFEB.
Although FH staining was positive, concurrent 2SC positivity, which
accumulates with FH loss, indicates that FH preserves its
antigenicity but lacks enzymatic activity. These findings confirmed
the diagnosis of FHdRCC (staged pT3NxM0).
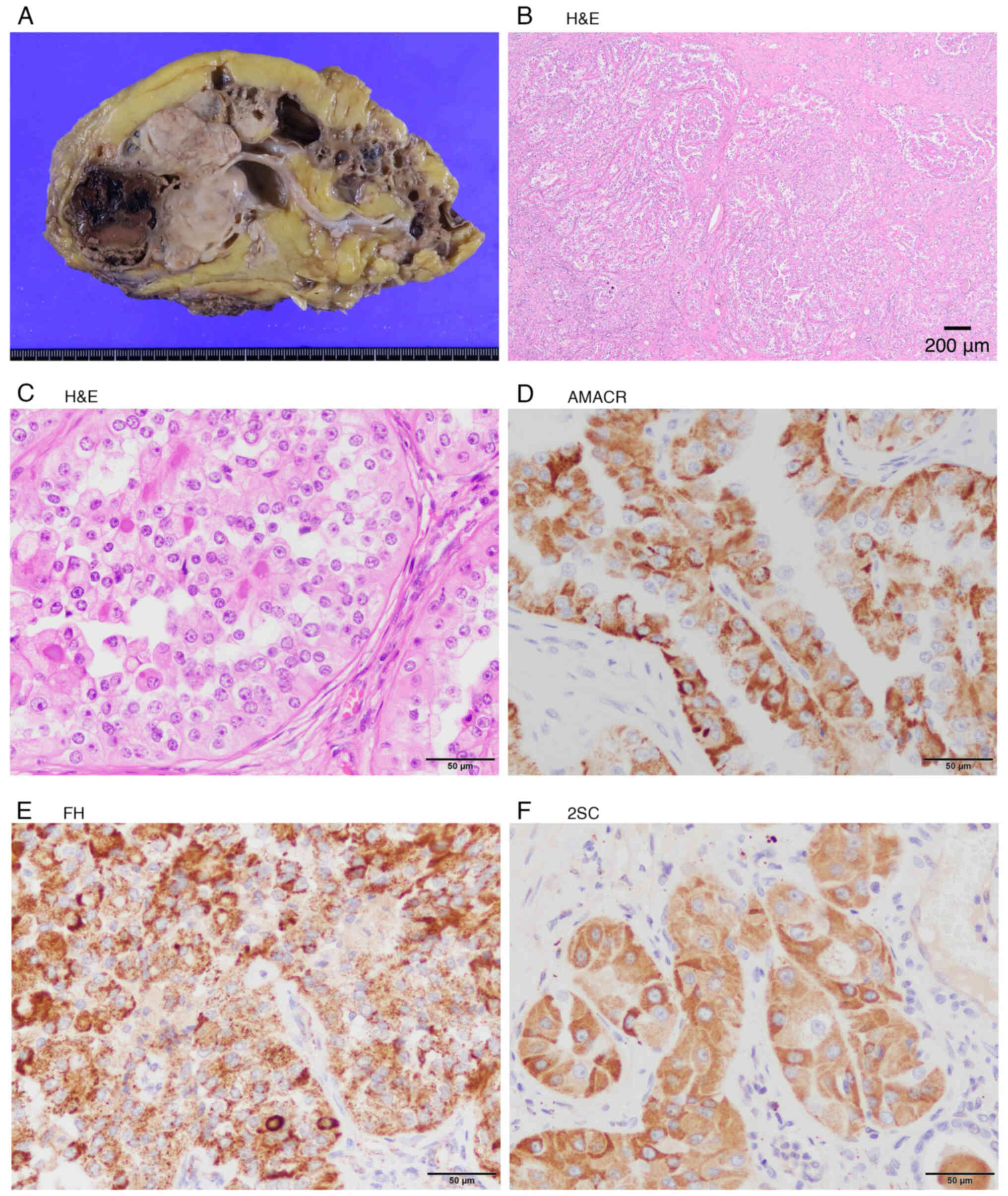 | Figure 3.Nephrectomy findings. (A) Macroscopic
finding of the kidney. A white, nodular and solid tumor
(6.5×5.6×4.5 cm) was present at the upper pole of the kidney.
Numerous small to medium-sized cysts with gelatinous contents were
observed in the kidney. (B) Low power view of the renal mass
(H&E staining). The tumor lacked a pseudo-capsule, grew
infiltratively, and exhibited papillary, adenoductal and solid
architecture. Scale bar, 200 µm. (C) High power view of the renal
mass (H&E staining). The tumor cell nuclei were round and
highly atypical, and the cytoplasm was clear. Eosinophilic and
amorphous material was evident in the papillary architecture. Some
microcalcifications were present. Scale bar, 50 µm. (D)
Immunohistochemical staining showing positive expression of AMACR.
(E) Immunohistochemical staining showing positive expression of FH.
(F) Immunohistochemical staining showing positive expression of
2SC. Scale bar, 50 µm. 2SC, S-2-succinylated-cystaine; AMACR,
α-methylacyl-CoA racemase; FH, fumarate hydratase. |
Genetic analysis
The WHO Classification states that FHdRCC is
important because it should initiate genetic counselling for the
patient's family. To determine the FH genetic mutation, we
obtained approval from the institutional ethics committee and the
patient's family for genetic analysis of stored blood samples.
Genetic testing for hereditary leiomyomatosis and renal cell cancer
(HLRCC) was performed at the Kazusa DNA Research Institute (Chiba,
Japan). Comprehensive analysis of the FH gene using targeted
next-generation sequencing did not reveal any pathogenic or likely
pathogenic variants. The patient was diagnosed with an isolated
FHdRCC.
Discussion
We encountered a rare case of FHdRCC arising from
acquired cystic kidney disease as dialysis-associated renal
carcinoma. This case highlights that imaging alone has limitations
in diagnosing malignant tumors arising in an acquired cystic
kidney. In addition, the rare carcinoma diagnosis was only achieved
after obtaining a biopsy. Therefore, this case was regarded as a
valuable model that demonstrates the importance of actively
performing biopsies for suspected masses.
FHdRCC is uncommon in dialysis-associated renal
carcinomas. In a Japanese multicenter study, ACKD-associated RCC
was found to be the most common type, followed by clear-cell RCC,
in patients undergoing long-term dialysis (4). FHdRCC has rarely been reported. Doi
et al (5) reported a
75-year-old male with FHdRCC in the left kidney 7 years before the
induction of dialysis; however, this case was not FHdRCC occurring
in a patient undergoing dialysis as that observed in our case.
ACKD-associated RCC often needs to be differentiated from FHdRCC
owing to its histopathological similarities such as abundant
granular eosinophilic cytoplasm. However, it differs by the
presence of intratumoral oxalate crystals and absence of FH
deficiency (6). Our case was
diagnosed with FHdRCC based on 2SC positivity. Pollard et al
(7) reported that FH deficiency
causes the overexpression of hypoxia-inducible factors, leading to
proliferative renal cyst development. Adam et al (8) reported that the loss of FH causes
KEAP1 succination and Nrf2 dysregulation independent of
hypoxia-inducible factors, leading to renal cysts and tumors.
Considering these reports, it is interesting to speculate that FH
deficiency may also be associated with ACKD.
Patients undergoing dialysis should undergo regular
kidney imaging assessments for RCC surveillance; however, this may
sometimes be insufficient for patients with ACKD. The standard
diagnosis of RCC is based on the use of imaging techniques.
Ultrasonography is recommended for the initial screening, while
computed CT and MRI are used to confirm RCC. The Bosniak
classification is recommended to categorize cystic renal masses
(3). Rahbari-Oskoui and O'Neill
(9) also suggested that a complex
cyst categorized as Bosniak class III or IV should prompt
contrast-enhanced CT or MRI. This classification is useful for
evaluating single cystic masses, which is similar to the Bosniac
Class IV case that was reported by Doi et al (5). However, it is difficult to evaluate
masses that emerge in multiple cysts, which were observed in our
case. Our case was considered a Bosniak class IIF, which typically
indicates a benign or indolent lesion; however, this was not the
case.
If imaging was insufficient to diagnose RCC, a
biopsy was deemed as necessary. The European Association of Urology
guidelines on RCC do not prohibit renal mass biopsy and propose
situations for renal biopsy (10).
Volpe et al (11) reported
that among 100 biopsies of renal masses, 84 were malignant, with
93% of the RCCs accurately classified and graded with 100%
concordance with surgical specimens. Although the outcome of our
case was unfortunate, we consider that the biopsy enabled a
definitive diagnosis of the rare aggressive tumor, leading to
nephrectomy.
The 2020 Kidney Biopsy guidebook in Japan also
supports the safety of renal tumor biopsy in cases of difficult
diagnosis and reports a minimal risk of serious hemorrhagic
complications or cancer cell dissemination after biopsy (12). Sawa et al (13) reported that renal biopsy which was
performed for fever of unknown origin revealed an intravascular
lymphoma. A perirenal mass biopsy was performed in the sitting
position, which led to a diagnosis of extramedullary relapse of
acute lymphoblastic leukemia (14).
Both patients are still alive without any post-biopsy
complications. Additionally, the present case demonstrates that
renal tumor biopsies can be performed in the same manner as
percutaneous kidney biopsies, even in the lateral position.
The biopsy technique was the same as that of
ultrasound-guided biopsy using a biopsy gun, which has already been
reported. However, conventional sequencing involves the suspicion
of malignancy on imaging before performing a biopsy. In contrast,
in our case, the puncture was initially intended for a presumed
cyst, but a mass was incidentally discovered during the procedure,
essentially reversing the sequence of events.
One limitation of our study is that we cannot
exclude the possibility that this diagnosis was achieved, to some
extent, by serendipity. A core needle biopsy, not limited to the
kidney, may result in an insufficient sample volume (15). In our case, we were able to obtain a
sufficient number of cores (six in total); however, if the mass had
been smaller, an accurate diagnosis might not have been possible.
Moreover, forcing the procedure may increase the risk of bleeding
and metastasis. Although obtaining a biopsy cannot be considered as
useful in all cases, a biopsy should be obtained whenever
feasible.
In conclusion, we reported FHdRCC as a type of
dialysis-associated kidney cancer. This case indicates that it is
important for patients with ACKD undergoing dialysis to undergo
imaging to detect RCC. If the findings are equivocal, a biopsy
should be performed to establish a definitive diagnosis and guide
appropriate management. Our case highlights the usefulness of
needle biopsy using the same method used by nephrologists for renal
biopsy in cases where determining the lesion nature using imaging
alone is deemed as difficult.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YO conceived the study, acquired, analyzed and
interpreted the data, and wrote the manuscript. HK contributed to
clinical interpretation and differential diagnosis, and critically
revised the manuscript. AS provided genetic counseling and
interpreted genetic testing as a genetics specialist. KK, SI, YT,
KO and YoN performed pathological evaluations, and revised the
manuscript as pathologists. KM and YuN performed the nephrectomy,
contributed to surgical interpretation and specimen acquisition,
and critically revised the manuscript as surgeons. SK, MY, TS, YU
and NS contributed to clinical investigation and data acquisition
(dialysis parameters, laboratory and imaging data), participated in
diagnostic discussions, conducted the literature review and revised
the Discussion, and critically revised the manuscript. YO and HK
confirm the authenticity of all raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
The Declaration of Helsinki and its revisions. According to the
Ethical Guidelines for Medical and Health Research involving Human
Subjects in Japan, ethical approval is not necessary for case
reports.
Patient consent for publication
Informed, voluntary and written consent for
publication was obtained from the patient's family.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
2SC
|
S-2-succinylated-cystaine
|
|
ACKD
|
acquired cystic kidney disease
|
|
ADPKD
|
autosomal dominant polycystic kidney
disease
|
|
AMACR
|
α-methylacyl-CoA racemase
|
|
CRP
|
C-reactive protein
|
|
CT
|
computed tomography
|
|
FHdRCC
|
fumarate hydratase-deficient renal
cell carcinoma
|
|
MRI
|
magnetic resonance imaging
|
|
RCC
|
renal cell carcinoma
|
|
TKV
|
total kidney volume
|
References
|
1
|
WHO Classification of Tumours Editorial
Board (WHO), . WHO classification of tumours of the urinary system
and male genital organs. 5 Edition. International agency for
research on cancer; Lyon: 2022
|
|
2
|
Lindner AK, Tulchiner G, Seeber A, Siska
PJ, Thurnher M and Pichler R: Targeting strategies in the treatment
of fumarate hydratase deficient renal cell carcinoma. Front Oncol.
12:9060142022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverman SG, Pedrosa I, Ellis JH, Hindman
NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman
SS, et al: Bosniak classification of cystic renal masses, version
2019: An update proposal and needs assessment. Radiology.
292:475–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo T, Sasa N, Yamada H, Takagi T,
Iizuka J, Kobayashi H, Yoshida K, Fukuda H, Ishihara H, Tanabe K
and Tsuzuki T: Acquired cystic disease-associated renal cell
carcinoma is the most common subtype in long-term dialyzed
patients: Central pathology results according to the 2016 WHO
classification in a multi-institutional study. Pathol Int.
68:543–549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doi K, Okugi H, Okazaki H, Ikota H and
Nakamura T: Fumarate hydratase-deficient renal cell carcinoma and
multilocular cystic renal neoplasm of low malignant potential: A
case report and literature review. Japanese J Urol. 113:42–45.
2022. View Article : Google Scholar
|
|
6
|
Przybycin CG, Harper HL, Reynolds JP,
Magi-Galluzzi C, Nguyen JK, Wu A, Sangoi AR, Liu PS, Umar S, Mehra
R, et al: Acquired cystic Disease-associated renal cell carcinoma
(ACD-RCC). Am J Surg Pathol. 42:1156–1165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollard PJ, Spencer-Dene B, Shukla D,
Howarth K, Nye E, El-Bahrawy M, Deheragoda M, Joannou M, McDonald
S, Martin A, et al: Targeted inactivation of Fh1 causes
proliferative renal cyst development and activation of the hypoxia
pathway. Cancer Cell. 11:311–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adam J, Hatipoglu E, O'Flaherty L,
Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW,
Wolhuter K, et al: Renal cyst formation in Fh1-deficient mice is
independent of the Hif/Phd pathway: Roles for fumarate in KEAP1
succination and Nrf2 signaling. Cancer Cell. 20:524–537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahbari-Oskoui F and O'Neill WC: Diagnosis
and management of acquired cystic kidney disease and renal tumors
in ESRD patients. Semin Dialysis. 30:373–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU Guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volpe A, Mattar K, Finelli A, Kachura JR,
Evans AJ, Geddie WR and Jewett MA: Contemporary results of
percutaneous biopsy of 100 small renal masses: A single center
experience. J Urol. 180:2333–2337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ubara Y, Kawaguchi T, Nagasawa T, Miura K,
Katsuno T, Morikawa T, Ishikawa E, Ogura M, Matsumura H, Kurayama
R, et al: Kidney biopsy guidebook 2020 in Japan. Clin Exp Nephrol.
25:325–364. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawa N, Ubara Y, Katori H, Hoshino J,
Suwabe T, Tagami T, Takemoto F, Miyakoshi S, Taniguchi S, Ohashi K
and Takaichi K: Renal intravascular large B-cell lymphoma localized
only within peritubular capillaries report of a case. Intern Med.
46:657–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oba Y, Koizumi R, Kageyama K, Yoshimoto M,
Kurihara S, Ikuma D, Yamaguchi K, Yamanouchi M, Suwabe T, Ishiwata
K, et al: Percutaneous perirenal mass biopsy in a sitting position
revealed extramedullary relapse of acute lymphoblastic leukemia.
Cancer Diagn Progn. 4:66–70. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto K, Nishimura S, Ito T, Oka N and
Akagi M: Limitations and usefulness of biopsy techniques for the
diagnosis of metastatic bone and soft tissue tumors. Ann Med Surg
(Lond). 68:1025812021.PubMed/NCBI
|