Introduction
Lung cancer is the leading cause of
cancer-associated morbidity and mortality globally, with ~2.1
million new cases and 800,000 mortalities annually (1). Non-small cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases (2). Despite recent advancements in early
detection techniques (low-dose computed tomography screening) and
treatment options, survival rates have only slightly improved and
the overall prognosis for patients with metastatic lung cancer
remains poor (3–6). Brain metastases (BM) are a common
metastatic pattern in lung cancer; at initial diagnosis, 10% of
patients with lung cancer present with BM and during the course of
the disease, 40–60% of patients with lung adenocarcinoma will
develop BM (7). BM poses a notable
clinical challenge, as it often leads to rapid neurological
deterioration and markedly impacts the quality of life of patients
(8). The presence of BM in patients
with lung adenocarcinoma markedly complicates treatment decisions
and therapeutic outcomes.
Patients with epidermal growth factor receptor
(EGFR) mutations are at a cumulative risk of BM as high as 70%
(9). This risk is particularly
pronounced in EGFR-mutant lung adenocarcinoma, where resistance to
first- and second-generation EGFR-tyrosine kinase inhibitors (TKIs;
such as gefitinib/erlotinib and afatinib/dacomitinib, respectively)
often results in disease progression and the development of BM. The
molecular mechanisms underlying this progression are not fully
understood; however, they are considered to involve a combination
of tumor heterogeneity, blood-brain barrier penetration and changes
in treatment response (10–12).
BM markedly impacts both quality of life and
prognosis. Common symptoms, including headaches, seizures,
cognitive dysfunction and motor deficits, complicate symptom
management and survival prediction (13,14).
The present study aimed to stratify the prognosis of patients with
lung cancer with BM and compare the predictive accuracy of various
prognostic models in a specific cohort: Patients with locally
advanced lung adenocarcinoma, EGFR mutations and BM development
following radical radiotherapy. In this cohort, accurate prediction
of patient outcomes is key to tailoring treatment plans and
improving survival rates. The use of prognostic models
incorporating clinical, molecular and radiological factors can
potentially guide clinical decisions and enhance patient care in
the future.
Patients and methods
Patients
A retrospective analysis was performed on patients
with lung adenocarcinoma with BM admitted to Cangzhou Hospital of
Integrated Traditional Chinese and Western Medicine-Hebei Province
(Cangzhou, China), between January 2016 and December 2022. The
inclusion criteria were as follows: i) Pathologically confirmed
lung adenocarcinoma; ii) locally advanced stage (inoperable or
surgery-intolerant at diagnosis, re-staged according to the 8th
edition of the AJCC TNM, IIIA, IIIB, IIIC) with initial treatment
consisting of radical radiotherapy (either synchronous or
sequential) (15); iii) BM
diagnosed via magnetic resonance imaging (MRI) of the brain, with
the brain as the first site of distant metastasis recurrence after
completion of radical radiotherapy. At initial diagnosis (prior to
radical treatment), no evidence of distant metastasis (M0 stage)
was present in any patient (16);
iv) exon 19 deletion (19DEL) or exon 21 point mutation (L858R
mutation) identified by genetic testing; and v) complete medical
records. The exclusion criteria were as follows: i) Patients lost
to follow-up; and ii) Patients with other primary malignant tumors.
A total of 340 patients with lung adenocarcinoma and BM were
initially screened for eligibility during the present study period.
The patient selection process is detailed in Fig. S1. Briefly, 80 patients were
excluded for the following reasons: i) 32 had other EGFR mutation
types or were EGFR wild-type; ii) 25 had other primary malignant
tumors; iii) 15 had incomplete medical records or were lost to
follow-up; and iv) 8 did not receive radical radiotherapy as
initial treatment. In total, 260 patients met all inclusion
criteria and constituted the present study cohort for analysis.
Definition of key variables and
molecular testing
Synchronous and metachronous BM
BM detected at the initial diagnosis of lung
adenocarcinoma or within 6 months thereafter were classified as
synchronous. BM developing >6 months after the initial diagnosis
were classified as metachronous (17).
Adjudication of primary tumor
control
‘Primary tumor control’ was defined as the absence
of progression (for example, complete response, partial response or
stable disease) at the primary pulmonary site, assessed via
contrast-enhanced CT of the chest. This assessment was performed at
the time of BM diagnosis, referencing baseline imaging obtained
prior to the initiation of radical radiotherapy (18).
Determination of EGFR mutation
status
EGFR mutation status (19DEL and exon 21 L858R point
mutation) was determined from tumor tissue samples obtained via
biopsy at initial diagnosis. Mutations were identified using a
commercially available and clinically validated Amplification
Refractory Mutation System PCR kit (AmoyDx® EGFR
Mutation Detection Kit). All genetic testing was performed in the
central laboratory of Cangzhou Hospital of Integrated Traditional
Chinese and Western Medicine-Hebei Province (Cangzhou, China) prior
to the administration of any EGFR-TKI therapy.
Timing of EGFR-TKI therapy associated
with BM
The majority of patients (95%) initiated EGFR-TKI
therapy after BM diagnosis. The timing of TKI initiation (before or
after BM diagnosis) was recorded and included as a binary covariate
in the initial univariate survival analysis. As it was not
significantly associated with overall survival (OS; P>0.1), it
was not included in the final multivariable model.
Classification of EGFR-TKIs
In the present study, EGFR-TKIs were categorized by
generation. First-generation TKIs included gefitinib and erlotinib,
second-generation TKIs included afatinib and third-generation TKIs
referred to osimertinib. The term ‘first- or second-generation
TKIs’ refers to the group of patients who did not receive
third-generation TKI as part of their initial treatment regimen.
The timing of TKI therapy associated with cranial radiotherapy was
categorized. Concurrent therapy was defined as TKI initiation
within 30 days before or after the start of radiotherapy.
Brain metastasis scoring system
model
The recursive partitioning analysis (RPA) scoring
system (19) classifies patients
into three groups: i) Grade I for patients ≤65 years old, with a
Karnofsky Performance Status (KPS) score ≥70, controlled primary
tumor and no extracranial metastases (20); ii) grade III for patients with a KPS
score <70; and iii) grade II for all others. The basic score for
BM (BSBM) system (21) utilizes
three prognostic factors: KPS score (50–70 and 70–100), primary
tumor control and presence or absence of extracranial metastases,
assigning values of 1 and 0, respectively. The total score ranges
from 0 (worst) to 3 (best). The Diagnosis-Specific Graded
Prognostic Assessment (DS-GPA) system (22) incorporates four factors: Age
(>60, 50–60 and <60), KPS score (<70, 70–80 and >80),
extracranial metastases (present and absent) and number of BM
(>3, 2–3 and 1), with values assigned as 0, 0.5 and 1,
respectively, yielding a total score between 0 and 4. The lung
cancer-associated molecular (Lung-mol) GPA system (23) includes five factors: i) Age (≥70 and
<70 years); ii) KPS score (<70, 80 and 90–100); iii)
extracranial metastases (present and absent); iv) number of BM
(>4 and 1–4); and v) genetic status (EGFR/anaplastic lymphoma
kinase (ALK)−, EGFR/ALK+). These factors are
assigned values of 0, 0.5 and 1, respectively, with total scores
ranging from 0 to 4. All 260 patients were scored using the RPA,
BSBM, DS-GPA and Lung-mol GPA systems, followed by survival
comparisons to evaluate the predictive accuracy of each model.
Treatment after brain metastasis
Upon diagnosis of BM, treatment strategies were
individualized based on a multidisciplinary team discussion. The
primary approach involved systemic therapy with EGFR-TKIs and local
intracranial treatment. Local treatment for BM included
stereotactic radiosurgery (SRS) for patients with limited (1–4)
metastases or whole-brain radiotherapy (WBRT) for those with more
extensive or diffuse metastases. Supportive care, including
corticosteroids for peritumoral edema, was administered as
clinically indicated. The use of corticosteroids (primarily
dexamethasone) adhered to a standardized institutional protocol to
manage symptomatic BM or notable peritumoral edema, with a rapid
tapering strategy following clinical and radiological improvement
(24). The KPS score used in all
analyses was assessed after the initial stabilization of cerebral
edema and neurological symptoms, typically at the first planned
follow-up visit (4–6 weeks after BM diagnosis), to best reflect the
baseline functional status of the patient independent of acute
interventions. Following progression on first-line EGFR-TKIs,
subsequent treatment lines were administered at the discretion of
the physician, which could include switching to a third-generation
EGFR-TKI (if not initially used) based on repeat genetic testing,
combination chemotherapy or continued local therapy for new
lesions. Data on the type of intracranial radiotherapy (SRS vs.
WBRT) and subsequent systemic therapy lines were collected and
analyzed. In the present study cohort, the majority of patients
(177/260, 68.1%) received concurrent EGFR-TKI therapy and cranial
radiotherapy (Table I).
 | Table I.Baseline characteristics of the
present study cohort (n=260). |
Table I.
Baseline characteristics of the
present study cohort (n=260).
| Characteristic | Number of patients
(%) |
|---|
| Age, years |
|
|
<60 | 152 (58.5) |
|
≥60 | 108 (41.5) |
| Sex |
|
|
Male | 122 (46.9) |
|
Female | 138 (53.1) |
| KPS |
|
|
<80 | 98 (37.7) |
|
≥80 | 162 (62.3) |
| Smoking
history |
|
| No | 140 (53.8) |
|
Yes | 120 (46.2) |
| EGFR mutation
type |
|
| Exon 19
deletion | 139 (53.5) |
|
L858R | 121 (46.5) |
|
EGFR-TKIs |
|
| Generation
I/II | 136 (52.3) |
| Generation III | 124 (47.7) |
| BM, n |
|
|
1-3 | 188 (72.3) |
|
>3 | 72 (27.7) |
| Extracerebral
metastasis |
|
| No | 162 (62.3) |
|
Yes | 98 (37.7) |
| Primary tumor
control |
|
| No | 172 (66.2) |
|
Yes | 88 (33.8) |
| Cranial RT |
|
| No | 41 (15.8) |
|
Yes | 219 (84.2) |
Follow-up and evaluation
Patients were followed up via telephone or
outpatient review from August 1, 2023. Follow-up occurred every 3
months for the first 2 years and every 6 months thereafter. At each
visit, patients underwent neurological assessments, brain MRI and
routine blood tests to monitor disease progression and
treatment-related side effects (such as rash, diarrhea, fatigue and
radionecrosis). OS was recorded from the diagnosis of BM to
mortality or the follow-up cut-off. Progression-free survival (PFS)
was also assessed, with a focus on changes in BM and extracranial
progression.
Statistical analysis
Statistical analysis was performed using SPSS
(version 27.0; IBM Corp.) and GraphPad Prism software (version 8.0;
Dotmatics), with AUC comparisons for each scoring system performed
using MedCalc software (version 23.0; MedCalc Software Ltd.). The
present study endpoint was OS, defined as the time from BM
diagnosis to mortality from any cause. The median follow-up time
was calculated using the reverse Kaplan-Meier method (25). Survival analysis was performed using
the Kaplan-Meier method and log-rank test. Categorical data are
presented as frequencies (percentages), and continuous data were
presented as mean ± standard deviation or median (interquartile
range). Variables for the multivariable Cox regression model were
selected based on two criteria: i) Established clinical relevance
as prognostic factors in lung cancer with BM, and ii) a
significance level of P<0.1 in the univariate analysis. This
approach aimed to minimize the risk of overfitting while reducing
the omission of potential clinical confounders. The candidate
variables meeting these criteria were age, KPS score, EGFR-TKI
generation, presence of extracranial metastases and primary tumor
control.
The ‘Enter’ method was used, wherein all selected
variables were simultaneously entered into the model to avoid the
instability associated with stepwise selection. Multicollinearity
among the included variables was assessed using the variance
inflation factor (VIF), with all VIF values being <2.0,
indicating no significant collinearity. The proportional hazards
(PH) assumption for all variables in the final model was analyzed
using Schoenfeld residuals (global test P=0.124). Furthermore, the
scaled Schoenfeld residuals for each variable were visually
inspected and indicated no clear pattern over time, confirming that
the PH assumption was not violated. Therefore, no corrective
measures, such as stratification or time-dependent covariates, were
necessary.
Due to the retrospective nature of the present
study, a pre-hoc sample size calculation was not performed and the
cohort included all consecutive eligible patients during the
present study period. However, a post hoc power analysis was
conducted using G*Power software (version 3.1.9.7; Heinrich Heine
University) for the primary multivariable Cox model. With a sample
size of 260, α level of 0.05 and the observed hazard ratio (HR) for
EGFR-TKI generation (HR=7.155), the achieved statistical power was
>99%, indicating sufficient power to detect this clinically
significant effect. The Cox proportional hazards regression model
was used for multifactorial analysis to identify statistically
significant prognostic factors. The AUC for each scoring system was
derived from receiver operating characteristic (ROC) curves. The
predictive capabilities of the four scoring systems for survival
were compared at 1, 2 and 3 years in patients with BM. Statistical
significance of differences in the AUC between the Lung-mol GPA and
other models was analyzed using the DeLong test. The calibration of
the prognostic models was assessed with calibration plots and
quantitatively using the Hosmer-Lemeshow goodness-of-fit test
(26). To quantify the incremental
predictive benefit of the Lung-mol GPA over other models, the
present study calculated the Continuous Net Reclassification
Improvement (NRI) (27).
Between-group comparisons of categorical baseline characteristics
in Table SI were performed using
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characterization
The present study included 260 patients, with 108
aged ≥60 years and 152 aged <60 years. Of these, 122 were men
and 138 were women. The basic characteristics of the 260 patients
are detailed in Table I.
Baseline characteristics stratified by
first-line TKI generation
To assess the comparability of key patient groups,
baseline characteristics were stratified according to the
generation of the first-line EGFR-TKI administered. As detailed in
Table SI, the 136 patients who
received first- or second-generation TKIs and the 124 patients who
received third-generation TKIs were well balanced across all
recorded prognostic factors, including age, sex, KPS score, smoking
history, EGFR mutation type, number of BM, presence of extracranial
metastases, primary tumor control status and receipt of cranial
radiotherapy (all P>0.05). This balance strengthens the validity
of comparing outcomes between these treatment strategies.
Analysis of factors influencing
prognosis
As of the follow-up cut-off date, the median
follow-up time for the entire cohort, calculated using the reverse
Kaplan-Meier method, was 58.0 months (95% CI, 54.2–61.8). The
median OS for the 260 patients was 28.7 months (95% CI, 26.0–31.4).
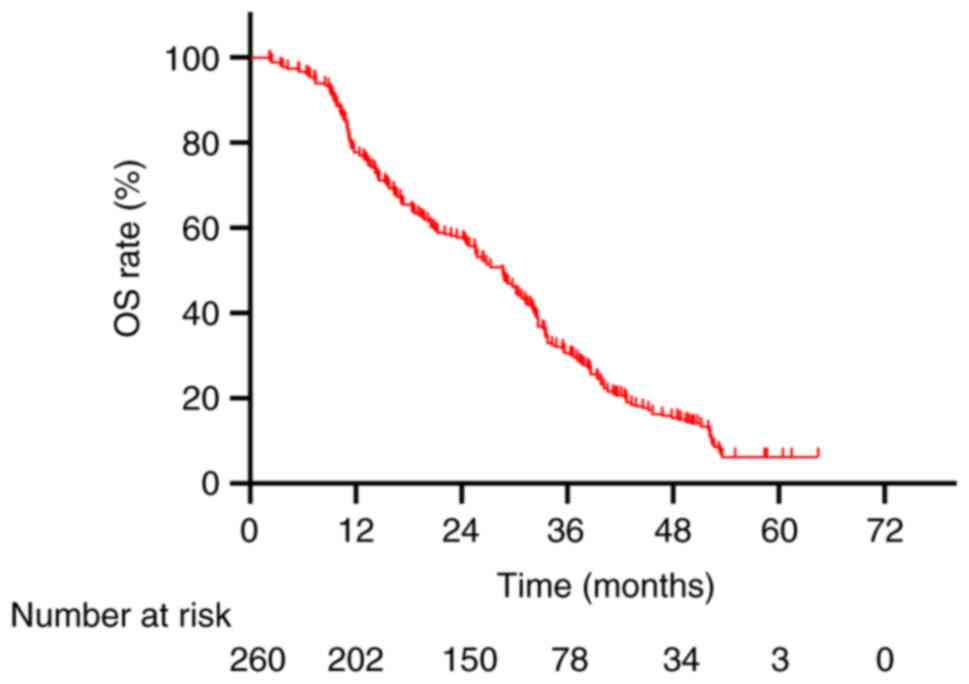
The 1-, 2- and 3-year OS rates were 77.7, 57.7 and 30.0%,
respectively (Fig. 1). Univariate
analysis demonstrated that age, KPS score, type of EGFR-TKIs used,
presence of extracranial metastasis and primary tumor control were
significantly associated with patient prognosis (P<0.05;
Table II). The multivariate Cox
analysis identified a KPS score <80, treatment with first- or
second-generation EGFR-TKIs and the presence of extracranial
metastases as independent prognostic risk factors for patients with
BM secondary to radical radiotherapy for EGFR-mutated lung
adenocarcinoma (Table II).
 | Table II.Univariate and multivariate Cox
regression analysis for overall survival. |
Table II.
Univariate and multivariate Cox
regression analysis for overall survival.
|
|
| Univariate
analysis | Multifactorial Cox
analysis |
|---|
|
Characteristica | Number of patients
(%) |
|
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<60 | 152 (58.5) | 1.000 | - | - | 1 | - | - |
|
≥60 | 108 (41.5) | 1.614 | 1.238–2.104 |
<0.001b | 1.154 | 0.854–1.557 | 0.351 |
| Sex |
|
|
|
|
|
|
|
|
Male | 122 (46.9) | 1 | - | - | - | - | - |
|
Female | 138 (53.1) | 1.002 | 0.773–1.299 | 0.986 | - | - | - |
| KPS |
|
|
|
|
|
|
|
|
≥80 | 162 (62.3) | 1 | - | - | 1 | - | - |
|
<80 | 98 (37.7) | 6.845 | 4.967–9.434 |
<0.001b | 2.706 | 1.825–4.012 |
<0.001b |
| Smoking
history |
|
|
|
|
|
|
|
| No | 140 (53.8) | 1 | - | - | - | - | - |
|
Yes | 120 (46.2) | 1.11 | 0.857–1.439 | 0.429 | - | - | - |
| EGFR mutation
type |
|
|
|
|
|
|
|
| Exon 19
deletion | 139 (53.5) | 1 | - | - | - | - | - |
|
L858R | 121 (46.5) | 1.129 | 0.871–1.463 | 0.364 | - | - | - |
| EGFR-TKIs |
|
|
|
|
|
|
|
|
Generation III | 124 (47.7) | 1 | - | - | 1 | - | - |
|
Generation I/II | 136 (52.3) | 10.277 | 7.407–14.258 |
<0.001b | 7.155 | 4.950–10.344 |
<0.001b |
| BM, n |
|
|
|
|
|
|
|
|
1-3 | 188 (72.3) | 1 | - | - | - | - | - |
|
>3 | 72 (27.7) | 1.283 | 0.950–1.734 | 0.104 | - | - | - |
| Extracerebral
metastasis |
|
|
|
|
|
|
|
| No | 162 (62.3) | 1 | - | - | 1 | - | - |
|
Yes | 98 (37.7) | 6.436 | 4.644–8.920 |
<0.001b | 2.296 | 1.543–3.418 |
<0.001b |
| Primary tumor
control |
|
|
|
|
|
|
|
|
Yes | 88 (33.8) | 1 | - | - | 1 | - | - |
| No | 172 (66.2) | 1.45 | 1.093–1.923 | 0.01b | 1.021 | 0.757–1.378 | 0.889 |
| Cranial RT |
|
|
|
|
|
|
|
|
Yes | 219 (84.2) | 1 | - | - | - | - | - |
| No | 41 (15.8) | 1.068 | 0.753–1.515 | 0.711 | - | - | - |
| Radiotherapy
type |
|
|
|
|
|
|
|
|
SRS | 158 (60.8) | 1 | - | - | - | - | - |
|
WBRT | 61 (23.4) | 1.228 | 0.865–1.908 | 0.137 | - | - | - |
| Receipt of any
subsequent systemic therapy |
|
|
|
|
|
|
|
|
Yes | 201 (77.3) | 1 | - | - | - | - | - |
| No | 59 (22.7) | 1.196 | 0.769–2.014 | 0.283 | - | - | - |
Sensitivity and subgroup analyses for
post-BM treatments
Acknowledging that subsequent therapies may
influence survival analysis, additional statistical evaluations
were performed. First, a sensitivity analysis was performed by
incorporating ‘Radiotherapy_type’ (SRS vs. WBRT) as a covariate
into the primary multivariable Cox model for the entire cohort. The
results confirmed that the three key independent prognostic
factors: KPS <80 (HR, 2.706; 95% CI, 1.825–4.012; P<0.001),
presence of extracranial metastasis (HR, 2.296; 95% CI,
1.543–3.418; P<0.001) and use of first-/second-generation
EGFR-TKIs (HR, 7.155; 95% CI, 4.950–10.344; P<0.001) remained
highly significant, with minimal change in their HRs (Table II). Second, to isolate the
prognostic effect of clinical factors from the influence of
first-line third-generation TKI use, a subgroup analysis was
performed. A separate multivariable Cox regression was performed
exclusively for the 136 patients treated with
first-/second-generation TKIs. As shown in Table III, within this subgroup, KPS
<80 (HR, 5.415; 95% CI, 1.269–9.640; P<0.001) and presence of
extracranial metastasis (HR, 3.484; 95% CI, 0.813–5.747;
P<0.001) remained strong independent predictors of worse
survival, whereas the number of BM and age were not retained in the
final model. Recognizing that subsequent treatment lines could
confound the survival analysis, the patterns of subsequent systemic
therapy between the first-/second-generation and third-generation
TKI groups were compared. Patients in the first-/second-generation
TKI group received a greater median number of subsequent therapy
lines (2 vs. 1) and were more likely to receive at least one
subsequent line of treatment (85.3 vs. 68.5%; P<0.001), with the
majority (71.3%) receiving a third-generation TKI upon progression
(Supplementary Table SII). A
sensitivity analysis adjusting for ‘Receipt of any subsequent
systemic therapy’ in the multivariable Cox model confirmed that the
association between first-line TKI generation and OS remained
robust.
 | Table III.Multivariable Cox regression analysis
of overall survival in the subgroup of patients receiving first- or
second-generation EGFR-TKIs as first-line therapy (n=136). |
Table III.
Multivariable Cox regression analysis
of overall survival in the subgroup of patients receiving first- or
second-generation EGFR-TKIs as first-line therapy (n=136).
|
| Univariate
analysis | Multifactorial Cox
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | - | - | - | - | - | - |
|
<60 | 1.000 | - | - | 1 | - | - |
|
≥60 | 1.502 | 1.022–2.207 | 0.038a | 1.101 | 0.873–1.938 | 0.598 |
| KPS | - | - | - | - | - | - |
|
≥80 | 1 | - | - | 1 | - | - |
|
<80 | 7.298 | 2.195–14.455 |
<0.001a | 5.415 | 1.269–9.640 |
<0.001a |
| Extracranial
metastasis | - | - | - | - | - | - |
| No | 1 | - | - | 1 | - | - |
|
Yes | 6.351 | 2.229–13.539 |
<0.001a | 3.484 | 0.813–5.747 |
<0.001a |
| Number of brain
metastases | - | - | - | - | - | - |
|
1-3 | 1 | - | - | 1 | - | - |
|
>3 | 1.521 | 1.021–2.266 | 0.039a | 1.132 | 0.869–2.042 | 0.485 |
| Primary tumor
control | - | - | - | - | - | - |
|
Yes | 1.000 | - | - | 1.000 | - | - |
| No | 1.802 | 0.542–2.886 | 0.006a | 1.324 | 0.427–2.368 | 0.157 |
Predictive role of brain metastasis
scoring systems
Survival curves for the four scoring systems are
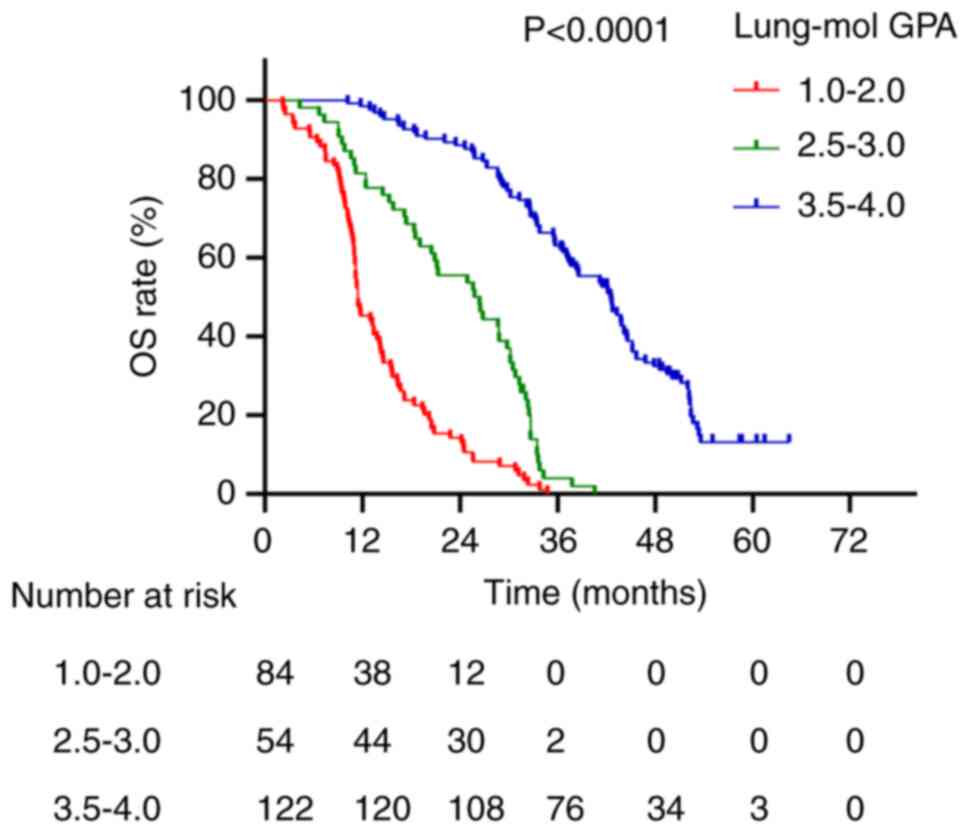
presented in Figs. 2, Fig. 3, Fig.
4, Fig. 5. The median survival
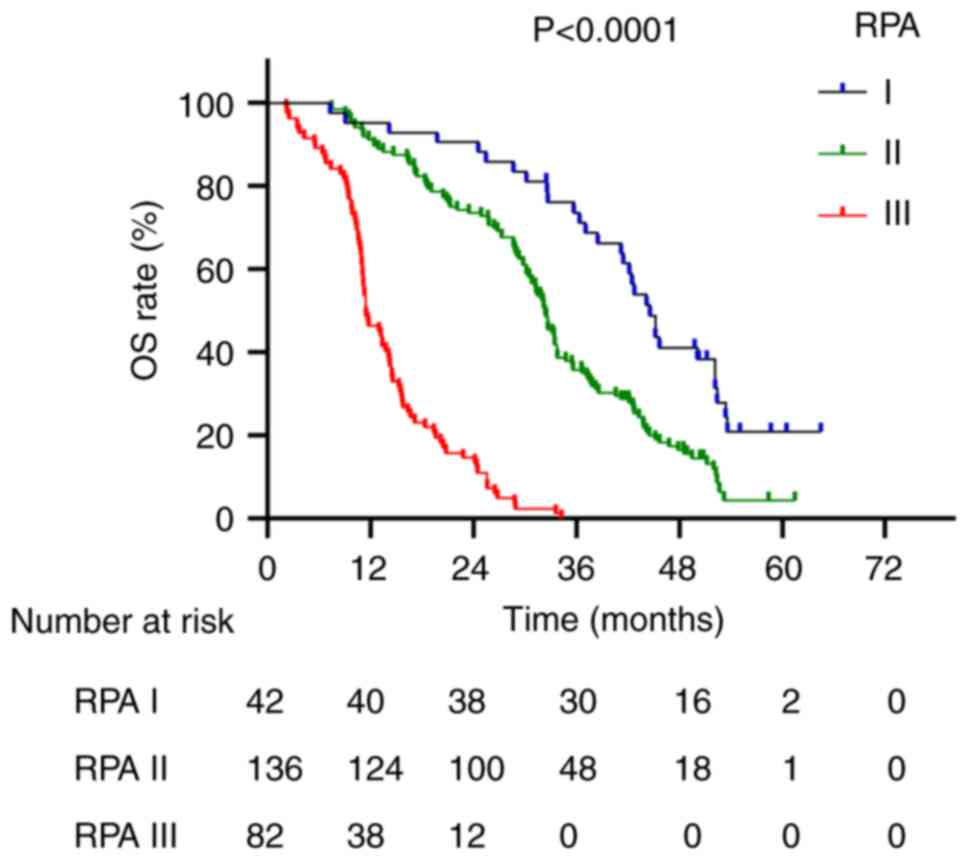
times for patients with RPA grades I, II and III were 44.6, 32.4
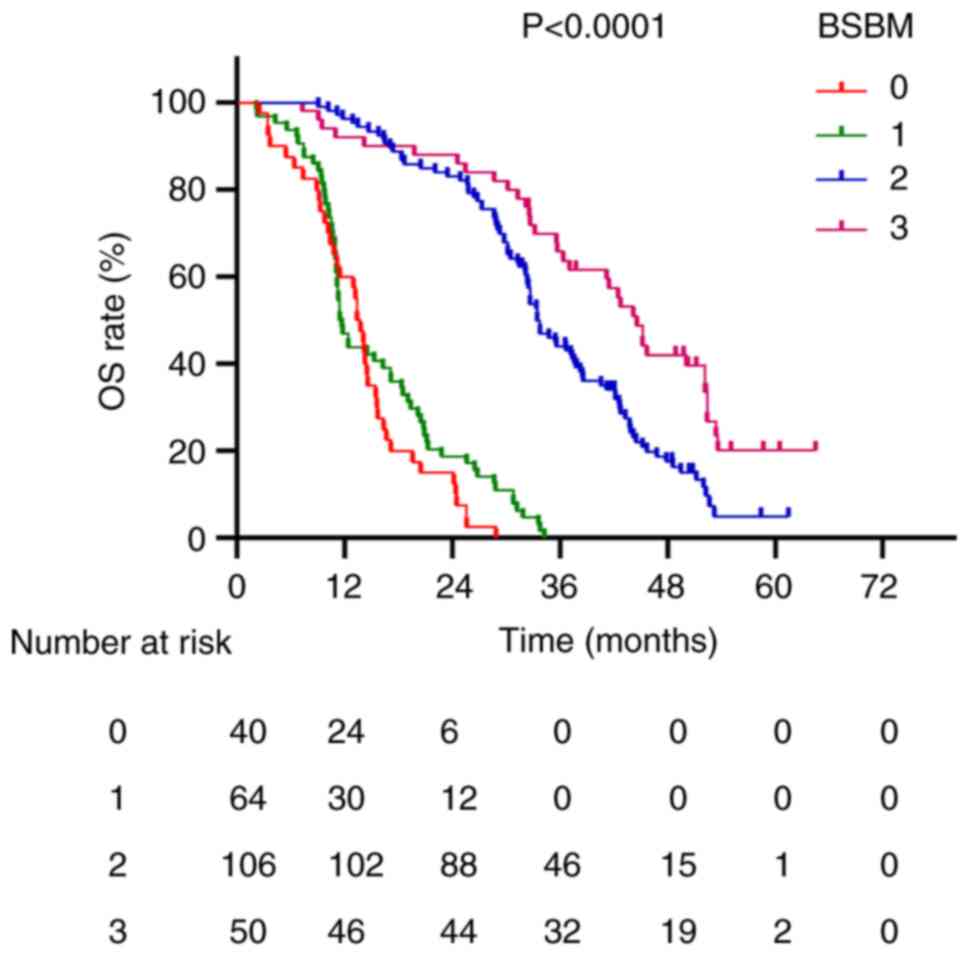
and 11.5 months, respectively (P<0.0001; Fig. 2). For the BSBM scoring system,
median survival times for scores of 0, 1, 2 and 3 were 13.6, 11.6,
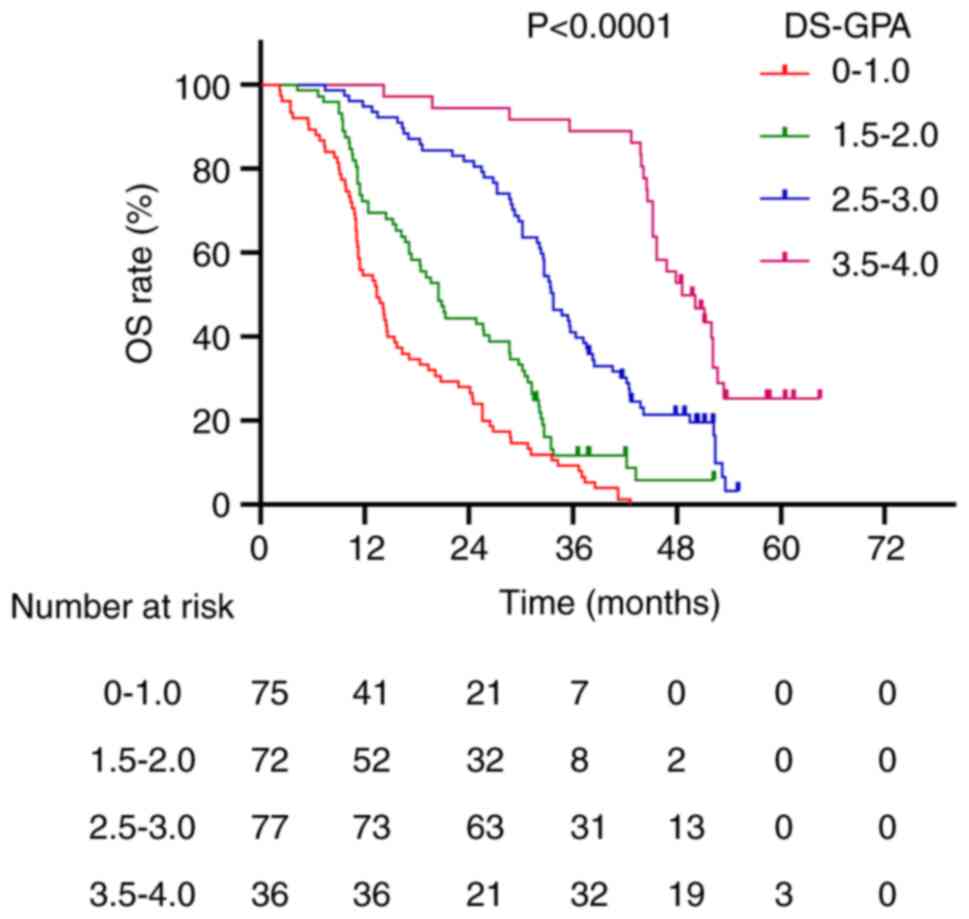
33.5 and 44.6 months, respectively (P<0.0001; Fig. 3). Patients with DS-GPA scores of
0–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0 had median survival times of
13.4, 20.5, 33.8 and 48.6 months, respectively (P<0.0001;
Fig. 4). For the Lung-mol GPA
system, median survival times for scores of 1.0–2.0, 2.5–3.0 and
3.5–4.0 were 11.5, 26.4 and 42.5 months, respectively (P<0.0001;
Fig. 5).
Comparison of the predictive power of
brain metastasis scoring systems
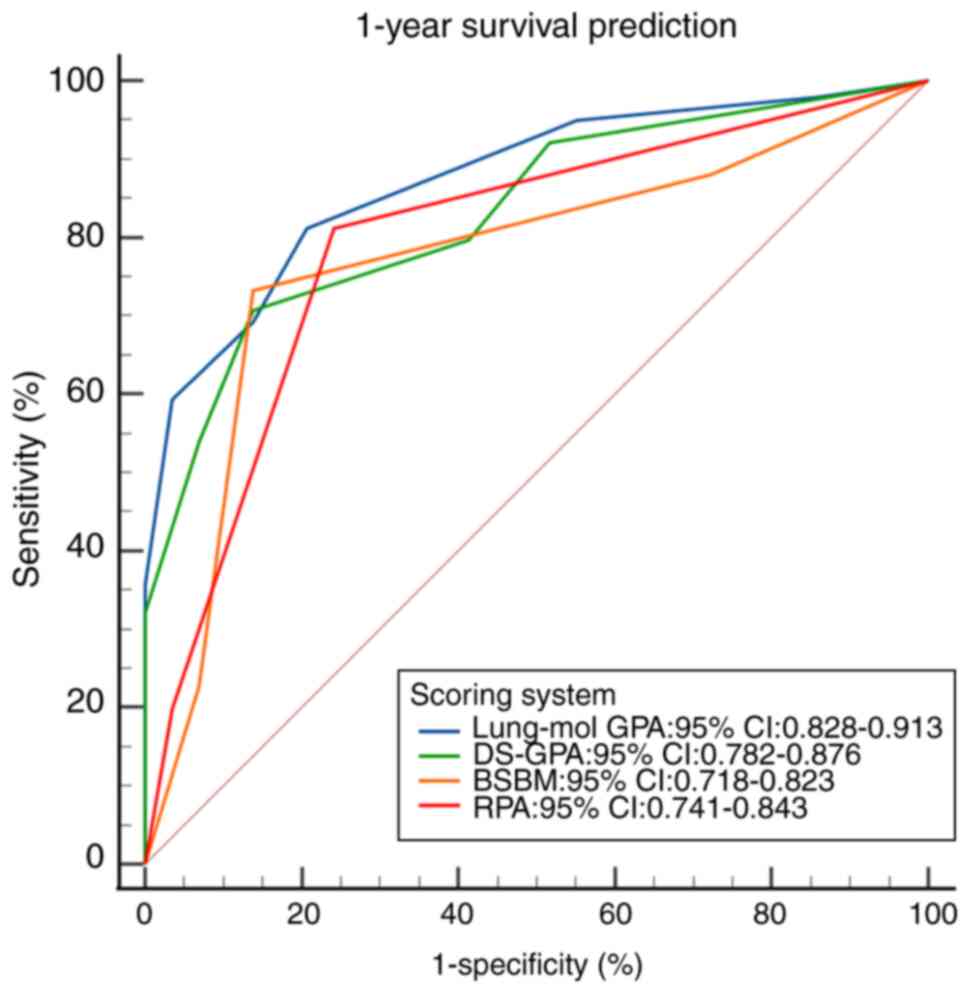
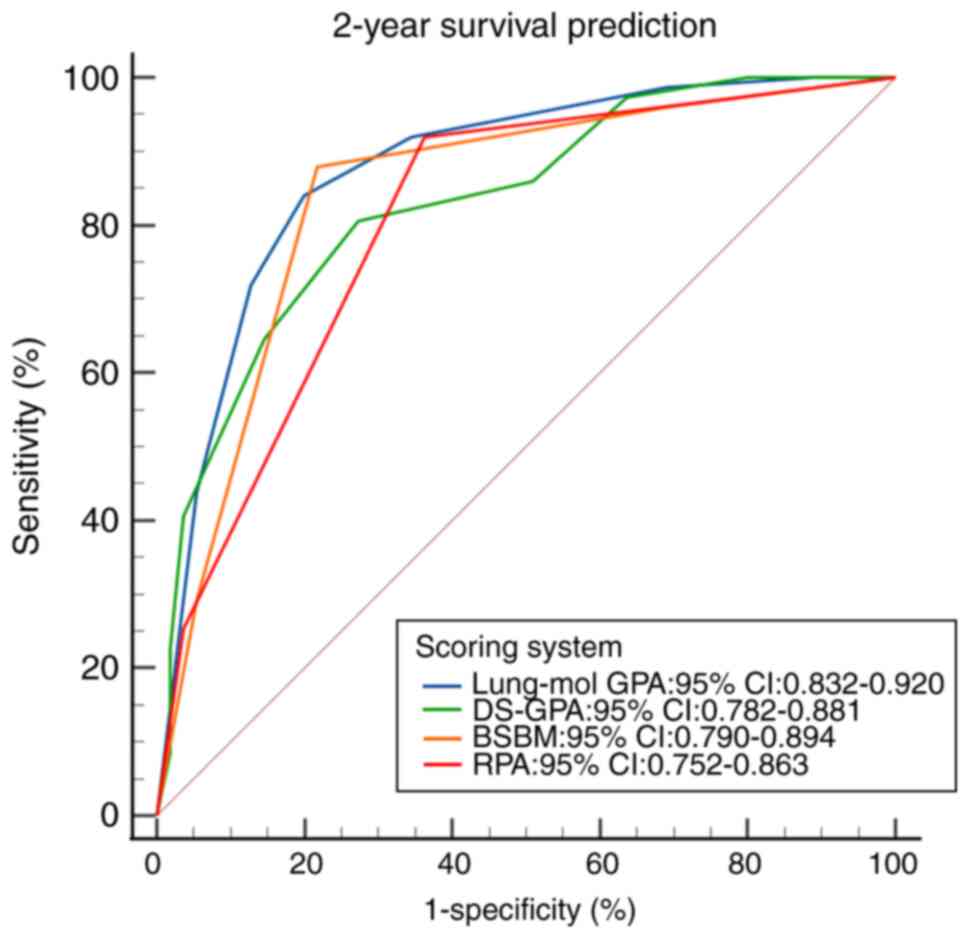
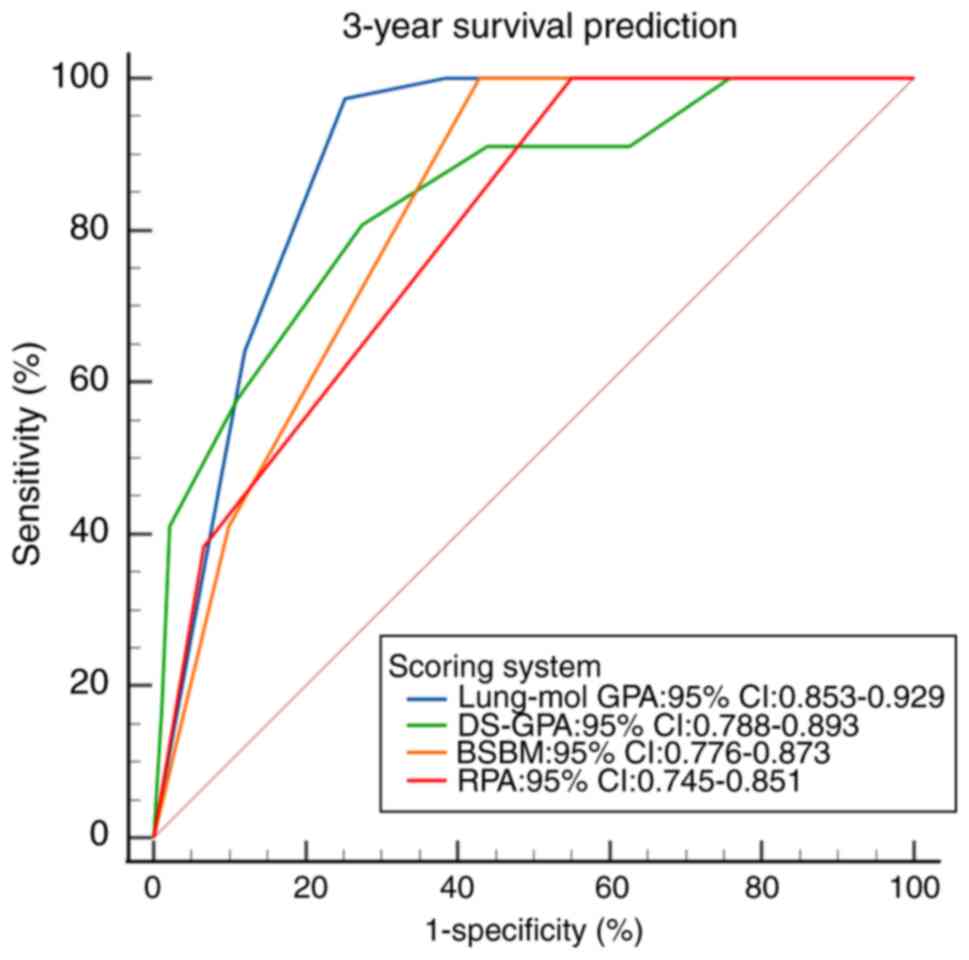
The ROC curves for the four scoring systems
predicting 1-, 2- and 3-year survival demonstrated statistical
significance (all P<0.001; Fig.
6, Fig. 7, Fig. 8). The area under the ROC curve (AUC)
comparison revealed that the Lung-mol GPA system exhibited the
highest AUC values: 0.875 (95% CI, 0.828–0.913; P<0.001), 0.876
(95% CI, 0.832–0.920; P<0.001) and 0.891 (95% CI, 0.853–0.929;
P<0.001). These values were significantly higher compared with
those of the other three scoring systems, with a statistically
significant difference (P<0.05; Table IV).
 | Table IV.Prognostic performance of the scoring
systems for 1-, 2- and 3-year OS. |
Table IV.
Prognostic performance of the scoring
systems for 1-, 2- and 3-year OS.
|
| 1-year OS | 2-year OS | 3-year OS |
|---|
|
|
|
|
|
|---|
| Model | AUC | 95% CI | P-value | AUC | 95% CI | P-value | AUC | 95% CI | P-value |
|---|
| Lung-mol GPA | 0.875 | 0.828–0.913 |
<0.001a | 0.876 | 0.832–0.920 | <0.001 | 0.891 | 0.853–0.929 |
<0.001a |
| DS-GPA | 0.833 | 0.782–0.876 |
<0.001a | 0.832 | 0.782–0.881 | <0.001 | 0.841 | 0.788–0.893 |
<0.001a |
| BSBM | 0.773 | 0.718–0.823 |
<0.001a | 0.842 | 0.790–0.894 | <0.001 | 0.824 | 0.776–0.873 |
<0.001a |
| RPA | 0.795 | 0.741–0.843 |
<0.001a | 0.808 | 0.752–0.863 | <0.001 | 0.798 | 0.745–0.851 |
<0.001a |
Comprehensive comparison of prognostic
models
The comparison of AUC values for predicting 1-, 2-
and 3-year survival, along with the results of the DeLong test, is
shown in Table V. The Lung-mol GPA
model consistently demonstrated significantly increased
discriminatory ability compared with the other three models across
all time points (all P<0.05). The calibration plot for the
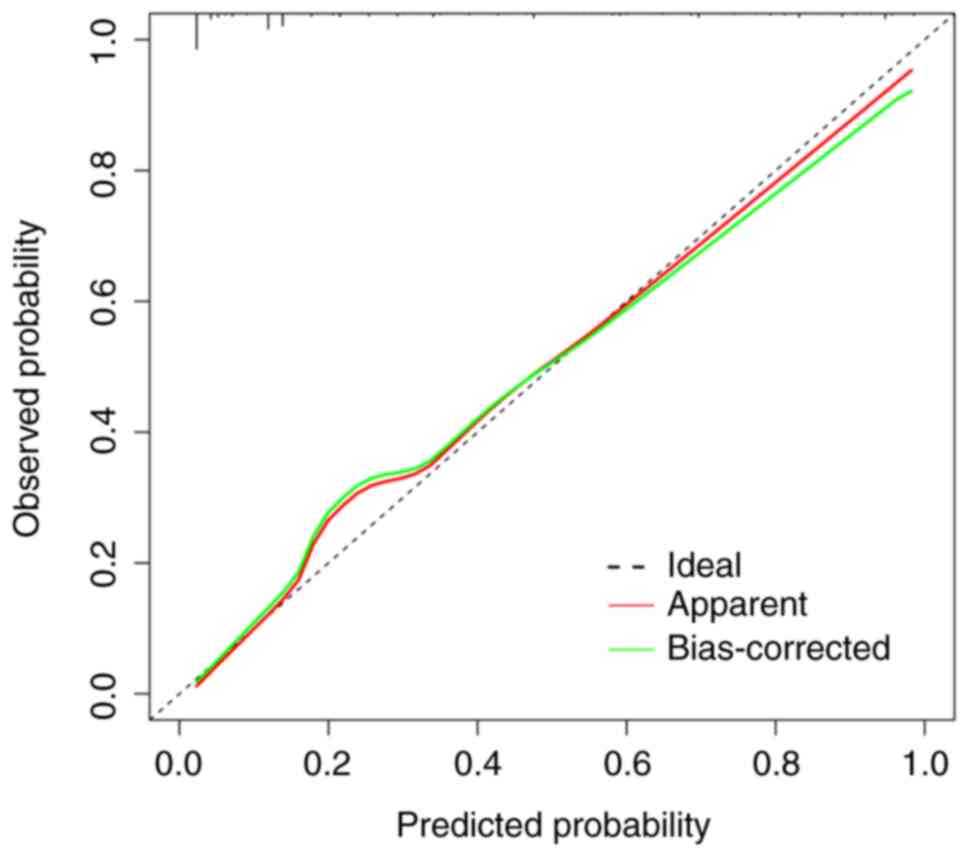
Lung-mol GPA model at 2 years is presented in Fig. 9. Visual inspection revealed strong
agreement between predicted and observed outcomes, which was
quantitatively supported by a non-significant Hosmer-Lemeshow test
(χ2=6.952; P=0.443; Table
SIII), indicating no statistically significant deviation from
perfect calibration. NRI analysis was performed to assess the
improvement provided by the Lung-mol GPA. For 2-year survival
prediction, the NRI for upgrading to the Lung-mol GPA model from
the DS-GPA model was 0.325 (95% CI, 0.152–0.498; P<0.001;
Table SIII), indicating a
significant and clinically relevant improvement in the accurate
reclassification of patient risk.
 | Table V.Pairwise comparison of the Lung-mol
GPA model with other prognostic systems using the DeLong test. |
Table V.
Pairwise comparison of the Lung-mol
GPA model with other prognostic systems using the DeLong test.
| Comparison | Time point,
year | AUC (Lung-mol
GPA) | AUC (other
model) | AUC difference | 95% CI for
difference | Z statistic | P-value |
|---|
| Lung-mol GPA
vs. | 1 | 0.875 | 0.833 | 0.042 | 0.007–0.076 | 2.373 | 0.018a |
| DS-GPA | 2 | 0.876 | 0.832 | 0.044 | 0.009–0.079 | 2.461 | 0.014a |
|
| 3 | 0.891 | 0.841 | 0.050 | 0.002–0.097 | 2.062 | 0.039a |
| Lung-mol GPA
vs. | 1 | 0.875 | 0.773 | 0.102 | 0.059–0.143 | 4.766 |
<0.001a |
| BSBM | 2 | 0.876 | 0.842 | 0.034 | 0.000–0.068 | 1.970 | 0.049a |
|
| 3 | 0.891 | 0.824 | 0.067 | 0.022–0.111 | 2.944 | 0.003a |
| Lung-mol GPA
vs. | 1 | 0.875 | 0.795 | 0.080 | 0.030–0.129 | 3.194 | 0.001a |
| RPA | 2 | 0.876 | 0.808 | 0.068 | 0.027–0.109 | 3.281 | 0.001a |
|
| 3 | 0.891 | 0.798 | 0.093 | 0.046–0.139 | 3.951 |
<0.001a |
Discussion
The prognosis for patients with BM from lung cancer
remains poor, despite advances in diagnostic and therapeutic
techniques that have improved the detection of BM and extended
survival (28,29). Previous studies investigating
prognostic factors and validating scoring systems for NSCLC BM
included a heterogeneous patient population (30–32),
encompassing those who received multiple treatments, such as
surgery, radiotherapy and chemotherapy, before the development of
BM, as well as those diagnosed with BM at initial presentation who
did not undergo treatment. The varied prognoses at different
treatment stages and the inclusion of these diverse patient groups
may have introduced notable biases into the study outcomes. To
address this, the present study specifically focused on patients
with inoperable, locally advanced lung adenocarcinoma secondary to
BM following radical radiotherapy at initial diagnosis and EGFR
mutation, thereby eliminating potential confounding factors.
The present study retrospectively analyzed the
prognostic factors of patients with BM secondary to radical
radiotherapy for EGFR-mutant lung adenocarcinoma and evaluated the
effectiveness of the RPA, DS-GPA, BSBM and Lung-mol GPA scoring
systems. The median OS for the 260 patients in the present study
was 28.7 months. This contrasts with the findings of Li et
al (33), who applied the same
scoring systems to Chinese patients with BM from EGFR-mutated lung
cancer, reporting a median survival time of 24.0 months. It is key
to note that the associations identified in the present
retrospective analysis, while robust within the present study
cohort, should be interpreted as observational and do not imply
causality. The findings require validation in prospective,
multi-institutional settings to establish their generalizability.
In the present study, prognostic factors influencing patient
outcomes were analyzed and demonstrated that the KPS score, the
presence of extracranial metastases and the type of EGFR-TKIs used
were independently significant in the multivariable Cox analysis.
The KPS score and the presence of extracranial metastases, key
indices in prior prognostic models (19,21–23),
were also independently significant in the present study. Lower KPS
scores were associated with worse patient outcomes. Zhang et
al (34) indicated that
patients with KPS scores of 80–100 had improved OS compared with
those patients with KPS <80. To the best of our knowledge, all
BM prediction models have associated KPS with survival duration
(35,36). The present study findings aligned
with the established role of KPS and further supported that a KPS
<80 is an independent prognostic risk factor for patients with
BM from EGFR-mutated lung adenocarcinoma.
Patients with concurrent extracranial metastases
often have a larger tumor burden, higher malignancy and worse
therapeutic outcomes, typically indicating a worse prognosis
(37,38). Higaki et al (39) identified concurrent extracranial
metastases as an independent risk factor affecting prognosis in a
study of 294 patients with BM from EGFR-mutated NSCLC. This finding
supported the inclusion of extracranial metastasis as a criterion
in multiple prediction models (40). Furthermore, in the present study
cohort, the use of third-generation EGFR-TKIs was associated with
significantly improved patient survival and emerged as an
independent prognostic factor in the present study model. In the
FLAURA study, first-line treatment with osimertinib in patients
with EGFR gene-sensitive mutation-positive NSCLC BM demonstrated a
median PFS markedly increased to that of first-generation
EGFR-TKIs. The domestic AENEAS and FURLONG studies also reported
that third-generation EGFR-TKIs provided a notable PFS advantage
over first/second-generation treatments (41–43).
Beyond its prognostic accuracy, the Lung-mol GPA
model holds considerable potential in guiding clinical
decision-making in the future. The present study findings suggested
that risk stratification using this model could potentially inform
the tailoring of therapeutic strategies. For example, patients
classified into the most favorable prognostic group (Lung-mol GPA
3.5–4.0) have a median survival >40 months. In the Lung-mol GPA
3.5–4.0 group, there is strong justification to implement
aggressive local control strategies, such as SRS, aimed at
achieving long-term intracranial disease control while minimizing
neurocognitive toxicity. By contrast, for patients in the poorest
prognostic group (Lung-mol GPA 1.0–2.0), with a median survival of
<12 months, clinical focus may justifiably shift towards
optimizing systemic therapy, ensuring optimal symptom control and
discussing goals of care, as the potential benefits of intensive
local interventions may be limited, to the best of our knowledge.
Thus, the Lung-mol GPA scoring system can potentially serve as a
valuable tool in adjusting treatment intensity according to
individual patient prognosis in the future.
The validation of the RPA, DS-GPA, BSBM and Lung-mol
GPA brain metastasis scoring systems was conducted in the present
study. Survival curve analysis of these systems indicated that all
four models effectively differentiate patient prognostic outcomes.
However, no significant difference in prognosis was observed
between patients scoring 0 and 1 in the BSBM system. This may be
attributed to the relatively small number of cases with scores of 0
and 1, which were insufficient to demonstrate a significant
difference. By contrast, significant prognostic differences were
observed in the remaining scoring systems when compared within
their respective groups. Further comparison of the prognostic
predictive ability of the four scoring systems was performed using
ROC curves, which revealed that the AUC of the Lung-mol GPA model
was the highest, significantly surpassing the other three models.
This suggested that the Lung-mol GPA model may be the most accurate
prognostic prediction tool for this specific patient population in
the present study. Li et al (33) also compared prognostic scoring
systems in patients with EGFR-mutant lung cancer with BM and
concluded that the Lung-mol GPA model was notably enhanced compared
with the other three systems.
The present study had several limitations inherent
to its retrospective and single-center design. First, the lack of
systematically collected data on patient-reported outcomes,
neurocognitive function and treatment-related adverse events (for
example, as graded by the Common Terminology Criteria for Adverse
Events) precludes a comprehensive risk-benefit analysis of the
treatment strategies. Similarly, the absence of a centralized
neuroradiological review to determine intracranial objective
response rates prevented an analysis of how the depth of
intracranial response associates with survival. Second, although
efforts were made to control for confounding factors using
sensitivity and subgroup analyses, residual bias due to unmeasured
or imperfectly adjusted factors cannot be excluded. Notably, while
the present study accounted for the receipt of subsequent therapy,
data on the precise timing, sequence and efficacy of subsequent
treatment lines were not fully available. Furthermore, although
corticosteroid use was similar between groups at baseline,
unmeasured variations in the total duration and tapering schedules
of corticosteroids may potentially influence both performance
status and survival. Third, the generalizability of the present
study findings may be limited by the exclusive focus on patients
with lung adenocarcinoma with a specific disease trajectory (brain
as the first site of metastasis after radical radiotherapy). Thus,
the results may not be directly applicable to other NSCLC
histological subtypes or patients with different patterns of
disease recurrence. Lastly, the present study was designed to
validate composite prognostic models rather than to assess the
individual prognostic weight of each potential variable. Therefore,
the present analysis was not powered to explore the specific roles
of factors such as the number of BM or the EGFR mutation subtype
(19DEL vs. L858R), which warrant investigation in future,
larger-scale studies.
In summary, within a well-defined cohort of patients
with EGFR-mutant lung adenocarcinoma and BM, the present
retrospective analysis identified that a lower KPS score, the
presence of extracranial metastases and the use of
earlier-generation EGFR-TKIs were independently associated with
worse OS. Among the prognostic models assessed, the Lung-mol GPA
scoring system exhibited notably increased predictive accuracy for
survival in this patient population. These findings suggested that
the Lung-mol GPA model may potentially enhance prognostic
stratification in this clinical context. However, due to the
inherent limitations of the retrospective design, the present study
results should be interpreted as hypothesis-generating regarding
prognostic associations rather than as evidence of causality. The
external validity of the present study conclusions requires
validation in larger, prospective, multi-institutional studies
incorporating patient-centered endpoints, such as quality of life
and neurocognitive function, alongside survival in the future.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Self-funded Project under
the Key Research and Development Program of Cangzhou City (grant
no. 23244102170).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JM completed the data analysis and paper writing. XY
was responsible for the research design and guided the revision of
the paper. JH contributed to the present study design and provided
essential data analysis support. YZ and LG recruited the enrolled
cases and contributed to the acquisition and interpretation of the
data. JM and XY confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the local Ethics
Committee of the Cangzhou Hospital of Integrated Traditional
Chinese and Western Medicine-Hebei Province (approval no.
CZX2024180; Cangzhou, China). Each patient provided written
informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
BM
|
brain metastases
|
|
BSBM
|
basic score for BM
|
|
DS-GPA
|
diagnosis-specific graded prognostic
assessment
|
|
EGFR
|
epidermal growth factor receptor
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
KPS
|
Karnofsky Performance Status
|
|
Lung-mol GPA
|
lung cancer-associated molecular
graded prognostic assessment
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
RPA
|
recursive partitioning analysis
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI
|
|
2
|
Wang B, Guo H, Xu H, Yu H, Chen Y and Zhao
G: Research progress and challenges in the treatment of central
nervous system metastasis of non-small cell lung cancer. Cells.
10:26202021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mulshine JL, Pyenson B, Healton C, Aldige
C, Avila RS, Blum T, Cham M, de Koning HJ, Fain SB, Field JK, et
al: Paradigm shift in early detection: Lung cancer screening to
comprehensive CT screening. Eur J Cancer. 218:1152642025.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Yan B and He S: Advances and
challenges in the treatment of lung cancer. Biomed Pharmacother.
169:1158912023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLouth LE, Gabbard J, Levine BJ, Golden
SL, Lycan TW, Petty WJ and Weaver KE: Prognostic awareness,
palliative care use, and barriers to palliative care in patients
undergoing immunotherapy or chemo-immunotherapy for metastatic lung
cancer. J Palliat Med. 26:831–836. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seute T, Leffers P, ten Velde GP and
Twijnstra A: Neurologic disorders in 432 consecutive patients with
small cell lung carcinoma. Cancer. 100:801–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maldonado F, Gonzalez-Ling A, Oñate-Ocaña
LF, Cabrera-Miranda LA, Zatarain-Barrón ZL, Turcott JG,
Flores-Estrada D, Lozano-Ruiz F, Cacho-Díaz B and Arrieta O:
Prophylactic cranial irradiation in patients with high-risk
metastatic non-small cell lung cancer: Quality of life and
neurocognitive analysis of a randomized phase II study. Int J
Radiat Oncol Biol Phys. 111:81–92. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rangachari D, Yamaguchi N, VanderLaan PA,
Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE,
Huberman MS and Costa DB: Brain metastases in patients with
EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung
Cancer. 88:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu F, Fan J, He Y, Xiong A, Yu J, Li Y,
Zhang Y, Zhao W, Zhou F, Li W, et al: Single-cell profiling of
tumor heterogeneity and the microenvironment in advanced non-small
cell lung cancer. Nat Commun. 12:25402021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim ZF and Ma PC: Emerging insights of
tumor heterogeneity and drug resistance mechanisms in lung cancer
targeted therapy. J Hematol Oncol. 12:1342019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan K, Concannon K, Li J, Zhang J, Heymach
JV and Le X: Emerging therapeutics and evolving assessment criteria
for intracranial metastases in patients with oncogene-driven
non-small-cell lung cancer. Nat Rev Clin Oncol. 20:716–732. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pike LRG, Miao E, Boe LA, Patil T, Imber
BS, Myall NJ, Pollom EL, Hui C, Qu V, Langston J, et al: Tyrosine
kinase inhibitors with and without up-front stereotactic
radiosurgery for brain metastases from EGFR and ALK oncogene-driven
non-small cell lung cancer (TURBO-NSCLC). J Clin Oncol.
42:3606–3617. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jünger ST, Schödel P, Ruess D, Ruge M,
Brand JS, Wittersheim M, Eich ML, Schmidt NO, Goldbrunner R, Grau S
and Proescholdt M: Timing of development of symptomatic brain
metastases from non-small cell lung cancer: Impact on symptoms,
treatment, and survival in the era of molecular treatments. Cancers
(Basel). 12:36182020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lancia A, Merizzoli E and Filippi AR: The
8th UICC/AJCC TNM edition for non-small cell lung cancer staging:
Getting off to a flying start? Ann Transl Med. 7 (Suppl
6):S2052019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moon SW, Choi SY and Moon MH: Effect of
invasive mucinous adenocarcinoma on lung cancer-specific survival
after surgical resection: A population-based study. J Thorac Dis.
10:3595–3608. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Zhang X, Wang Y, Jin Y, Song Y and
Wang T: Clinicopathological characteristics and prognosis of
synchronous brain metastases from non-small cell lung cancer
compared with metachronous brain metastases. Front Oncol.
14:14007922024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams TM, Miller E, Welliver M,
Brownstein J, Otterson G, Owen D, Haglund K, Shields P, Bertino E,
Presley C, et al: A phase 2 trial of primary tumor stereotactic
body radiation therapy boost before concurrent chemoradiation for
locally advanced non-small cell lung cancer. Int J Radiat Oncol
Biol Phys. 120:681–694. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frappaz D, Bonneville-Levard A, Ricard D,
Carrie S, Schiffler C, Xuan KH and Weller M: Assessment of
Karnofsky (KPS) and WHO (WHO-PS) performance scores in brain tumour
patients: The role of clinician bias. Support Care Cancer.
29:1883–1891. 2021.PubMed/NCBI
|
|
21
|
Lorenzoni J, Devriendt D, Massager N,
David P, Ruíz S, Vanderlinden B, Van Houtte P, Brotchi J and
Levivier M: Radiosurgery for treatment of brain metastases:
Estimation of patient eligibility using three stratification
systems. Int J Radiat Oncol Biol Phys. 60:218–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Summary
report on the graded prognostic assessment: An accurate and facile
diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol. 30:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sperduto PW, Yang TJ, Beal K, Pan H, Brown
PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, et al:
Estimating survival in patients with lung cancer and brain
metastases: An update of the graded prognostic assessment for lung
cancer using molecular markers (lung-molGPA). JAMA Oncol.
3:827–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho A, Gatterbauer B, Chen Y, Jankowski T,
Haider L, Tögl S, Kapfhammer I, Schreder M, Kirchbacher K,
Zöchbauer-Müller S, et al: Effect of cumulative dexamethasone dose
on the outcome of patients with radiosurgically treated brain
metastases in the era of modern oncological therapy. J Neurosurg.
143:384–395. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gillespie BW, Chen Q, Reichert H,
Franzblau A, Hedgeman E, Lepkowski J, Adriaens P, Demond A,
Luksemburg W and Garabrant DH: Estimating population distributions
when some data are below a limit of detection by using a reverse
Kaplan-Meier estimator. Epidemiology. 21 (Suppl 4):S64–S70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Surjanovic N, Lockhart RA and Loughin TM:
A generalized Hosmer-Lemeshow goodness-of-fit test for a family of
generalized linear models. Test (Madr). 33:589–608. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jewell ES, Maile MD, Engoren M and Elliott
M: Net reclassification improvement. Anesth Analg. 122:818–824.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schröder C, Windisch P, Lütscher J,
Zwahlen DR and Förster R: Validation and discussion of clinical
practicability of the 2022 graded prognostic assessment for NSCLC
adenocarcinoma patients with brain metastases in a routine clinical
cohort. Front Oncol. 13:10425482023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinese Association for Clinical
Oncologists; Medical Oncology Branch of Chinese International
Exchange and Promotion Association for Medical and Healthcare, .
Clinical practice guideline for brain metastases of lung cancer in
China (2021 version). Zhonghua Zhong Liu Za Zhi. 43:269–281.
2021.(In Chinese). PubMed/NCBI
|
|
30
|
Rades D, Hansen HC, Schild SE and Janssen
S: A new diagnosis-specific survival score for patients to be
irradiated for brain metastases from non-small cell lung cancer.
Lung. 197:321–326. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sperduto PW, De B, Li J, Carpenter D,
Kirkpatrick J, Milligan M, Shih HA, Kutuk T, Kotecha R, Higaki H,
et al: Graded prognostic assessment (GPA) for patients with lung
cancer and brain metastases: Initial report of the small cell lung
cancer GPA and update of the non-small cell lung cancer GPA
including the effect of programmed death ligand 1 and other
prognostic factors. Int J Radiat Oncol. 114:60–74. 2022. View Article : Google Scholar
|
|
32
|
Sperduto PW, Mesko S, Li J, Cagney D,
Aizer A, Lin NU, Nesbit E, Kruser TJ, Chan J, Braunstein S, et al:
Survival in patients with brain metastases: Summary report on the
updated diagnosis-specific graded prognostic assessment and
definition of the eligibility quotient. J Clin Oncol. 38:3773–3784.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li R, Zhang SR, Liu XF, Zhang JW, Zhao JY,
Bai P and Zhang XC: Prognostic factors for non-small cell lung
cancer patients with central nervous system metastasis with
positive driver genes. Zhonghua Yi Xue Za Zhi. 103:1202–1209.
2023.PubMed/NCBI
|
|
34
|
Zhang M, Tong J, Ma W, Luo C, Liu H, Jiang
Y, Qin L, Wang X, Yuan L, Zhang J, et al: Predictors of lung
adenocarcinoma with leptomeningeal metastases: A 2022
targeted-therapy-assisted molGPA model. Front Oncol. 12:9038512022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mulvenna PM, Holt T and Stephens R:
Response to ‘Diagnosis-specific prognostic factors, indexes, and
treatment outcomes for patients with newly diagnosed brain
metastases: A multi-institutional analysis of 4,259 patients’. (Int
J Radiat Oncol Biol Phys 2010:77:655-661). Int J Radiat Oncol Biol
Phys. 81:11942011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto M, Kawabe T, Higuchi Y, Sato Y,
Barfod BE, Kasuya H and Urakawa Y: Validity of three recently
proposed prognostic grading indexes for breast cancer patients with
radiosurgically treated brain metastases. Int J Radiat Oncol Biol
Phys. 84:1110–1115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Winslow N, Boyle J, Miller W, Wang Y,
Geoffroy F and Tsung AJ: Development of brain metastases in
non-small-cell lung cancer: High-risk features. CNS Oncol.
13:23958042024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmid S, Garcia M, Zhan L, Cheng S, Khan
K, Chowdhury M, Sabouhanian A, Herman J, Walia P, Strom E, et al:
Outcomes with non-small cell lung cancer and brain-only metastasis.
Heliyon. 10:e370822024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Higaki H, Nishioka K, Otsuka M, Nishikawa
N, Shido M, Minatogawa H, Nishikawa Y, Takashina R, Hashimoto T,
Katoh N, et al: Brain metastases in Japanese NSCLC patients:
Prognostic assessment and the use of osimertinib and immune
checkpoint inhibitors-retrospective study. Radiat Oncol. 18:252023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang L, Wang Z, Duan H, He Z, Lu J, Jiang
X, Hu H, Li C, Yu C, Zhong S, et al: Survival benefits of
radiotherapy and surgery in lung cancer brain metastases with poor
prognosis factors. Curr Oncol. 30:2227–2236. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohe Y, Imamura F, Nogami N, Okamoto I,
Kurata T, Kato T, Sugawara S, Ramalingam SS, Uchida H, Hodge R, et
al: Osimertinib versus standard-of-care EGFR-TKI as first-line
treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J
Clin Oncol. 49:29–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastaticnon-small-cell lung cancer with EGFR
exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y,
Liu C, Zhu S, Zhang X, Li Y, et al: Central nervous system efficacy
of furmonertinib (AST2818) versus gefitinib as first-line treatment
for EGFR-mutated NSCLC: Results from the FURLONG study. J Thorac
Oncol. 17:1297–1305. 2022. View Article : Google Scholar : PubMed/NCBI
|