In the past decades, the application of certain new
research methods and technologies have led to the proposal of new
treatments for cancer, mainly neoadjuvant chemotherapy and targeted
immunotherapy. Whilst they have often shown improved efficacy in
the initial clinical application, they are associated with drug
resistance and side effects (1).
Therefore, seeking complementary and alternative treatments from
traditional herbal medicine has become one new approach in the
treatment of cancer. For example, astragalin has attracted
widespread attention due to its wide availability, affordable cost,
potent anticancer activity and high safety profile. Moreover,
astragalin and its derivatives are considered to have definite
cytotoxicity (2).
Regarding the anticancer effects of astragalin,
previous studies have reported effects of astragalin on specific
cancers through in vitro and ex vivo experiments, or
network pharmacological analyses (16–18).
However, unlike previous literature reports on this topic, the
present paper summarizes the anticancer effects of the natural
compound Astragalin in several types of cancers, systematically
outlining the specific mechanisms of the cancer-inhibiting effects
of Astragalin, and suggesting future research directions for
Astragalin. The present paper presents a comprehensive and
systematic review of the mechanism of action of astragalin and its
derivatives in several cancers, with an in-depth exploration to
elucidate the potential and possibilities of astragalin for
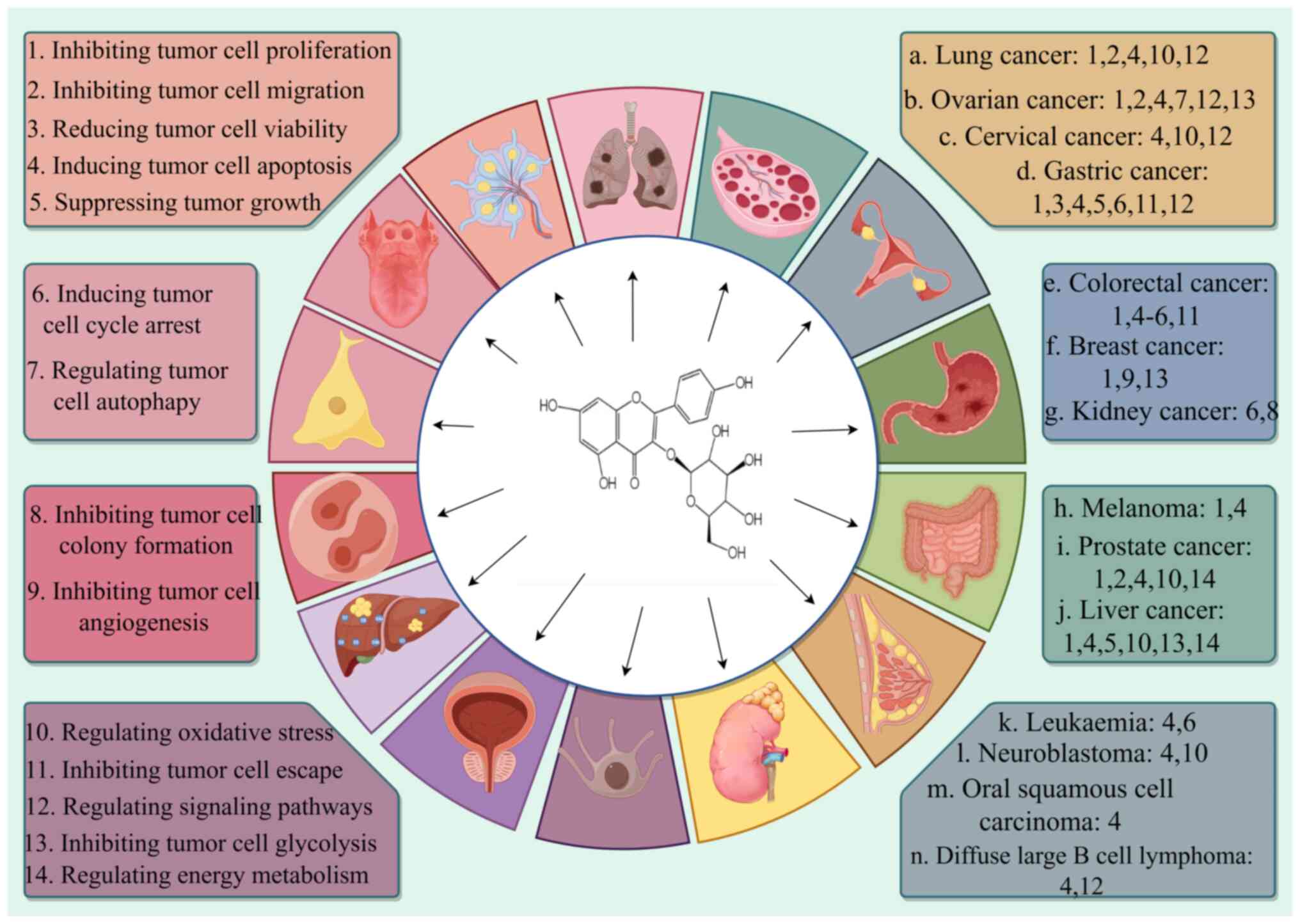
anticancer use. Table I summarizes
the mechanism of action of astragalin on several cancers and
Fig. 2 presents the specific types
of anticancer mechanisms of astragalin.
Lung cancer poses a serious health and economic
burden to the global population, with the number of new lung cancer
cases expected to reach ~3.8 million by 2050 (19). Issues with current lung cancer
treatment include high morbidity and mortality rates, late
diagnosis, limited therapeutic options, restrictions on targeted
therapies, economic and resource constraints, tumor heterogeneity
and drug resistance (20),
nutritional and quality of life issues (21), and research and clinical
translational challenges (22).
Therefore, the option of developing novel therapeutic agents with
high safety margins may improve this situation.
Among the existing herbal medicine developments,
astragalin is expected to be a safe and reliable novel ingredient
due to its potent potency to inhibit the activity of lung cancer
cells, impede cell migration, proliferation, invasion and induce
apoptosis. Astragalin belongs to the class of lotus leaf flavonoids
(LLF), and in vitro studies (23,24)
have reported that high concentrations (500 µg/ml) of LLF can
notably induce apoptosis in human lung cancer A549 cells (25). LLF increases the content of reactive
oxygen species and the level of the oxidative end-product
malondialdehyde, and at the same time, reduces superoxide dismutase
activity. It upregulates several apoptotic factors including
caspase-3 and caspase-9, and inhibits the expression of related
factors [such as B-cell lymphoma-2 (Bcl-2) and nuclear factor
erythroid 2-related factor 2], thereby achieving an anti-lung
cancer effect (25). Astragalin
acts on the JAK/STAT pathway to regulate the expression of
apoptotic factors and related genes in a dose-dependent manner,
causing damage to cellular DNA and augmenting reactive oxygen
species to promote apoptosis and inhibit migration and invasion
(26). Moreover, in vivo
studies (27,28) have reported that astragalin may
induce cell death via the aspartase, ERK-1/2, AKT and NF-κB
signaling pathways, a process that may be associated with an
increased Bax:Bcl-2 ratio (29).
Network pharmacological studies (30,31)
have also reported that astragalin treatment of lung adenocarcinoma
may exert its unique inhibitory effects on cell proliferation,
migration and apoptosis through the microRNA
(miRNA/miR)-140-3p/3-phosphoinositide dependent protein kinase 1
axis (32). Finally, astragalin has
been reported to act on the lung cancer A549 cell line with potent
cytotoxicity, activating effector caspase-3 and inducing apoptosis
(33).
Astragalin has been reported to inhibit cell
proliferation and migration and induce apoptosis in ovarian cancer
cells by regulating signaling pathways, affecting the expression of
specific cytokines and inhibiting the glycolytic pathway. This
effect is associated with the upregulation of prolyl hydroxylase
domain-containing protein 2 (PHD2) expression and inhibition of
hypoxia-inducible factor 1α (HIF-1α) (38,39).
PHD2 can act as an oxygen sensor and improve perfusion and
oxidization of tumor cells (40),
and participates in the programming of macrophage glycolysis
(41). HIF-1α is stabilized by
binding to hypoxia-responsive element in the anaerobic state,
activating key enzymes such as pyruvate dehydrogenase kinase 1
(PDK1) and pyruvate kinase type M2, and inhibiting the
tricarboxylic acid cycle, thus shifting glucose metabolism from
oxidative phosphorylation to anaerobic glycolysis (42–44).
This metabolic reprogramming enhances cell survival. Astragalin
inhibits the phosphorylation of related proteins, whereas
3-methyladenine or bafilomycin A1, which act as autophagy
inhibitors, can reverse this process. Astragalin can also regulate
cellular autophagy by targeting the PI3K/AKT/mTOR signaling pathway
(45), a classic signaling pathway
in ovarian cancer tumorigenesis, proliferation and progression
(46). Thus, astragalin may exert
its unique biological effects on ovarian cancer cells.
Cervical cancer ranks second among malignant
neoplastic diseases in women and has a high mortality rate
(47). Due to the high resistance
to platinum-based chemical drugs, this disease is now often treated
with radiotherapy (48); however,
this can cause damage to the mucosa of the vagina and urinary
tract, which can affect patient quality of life (49). Therefore, it is necessary to explore
new therapeutic methods with low toxicity and high safety.
It is conceivable that if astragalin is applied in
conjunction with radiotherapy drugs, it may be able to exert
neuroprotective effects locally, reduce the discomfort of vaginal
and urinary tract mucosa, and improve the quality of life of
patients. Astragalin inhibits cell proliferation and accelerates
the process of apoptosis in cervical cancer cells by regulating
signaling pathways and cellular oxygenation (50). Network pharmacological analysis has
reported that, in HeLa cells, astragalin can exert regulatory
effects on signaling proteins such as EGFR, STAT3 and cyclin D1,
affecting the ErbB and forkhead box protein O signaling pathways.
This induces an increase in reactive oxygen species and notably
inhibits the proliferation and promoting apoptosis in a manner that
is linearly associated with concentration (51). Moreover, astragalin, has been
reported to exhibit clear anti-cervical cancer activity (52).
Gastric cancer is a great challenge to global public
health with the fifth highest global incidence rate, the third
highest mortality rate and a high rate of metastasis (53). The molecular and phenotypic
heterogeneity of gastric cancer is particularly high (54), and >70% of patients with gastric
cancer are diagnosed at an intermediate-to-advanced stage, missing
the optimal time for treatment, with limited efficacy of
chemotherapy, targeted therapies and immunotherapy, and issues of
drug resistance and side effects (55,56).
Astragalin inhibits the activity of the drug
metabolizing enzyme cytochrome P450 family 1 subfamily B member 1
and, in vivo, it inhibits estradiolation to produce the
carcinogenic metabolite 4-hydroxy-estradiol (76). Furthermore, astragalin inhibits
breast cancer cell activity and proliferation and angiogenesis
(18,77). Astragalin can act on AKT, zinc
finger E-box binding homeobox 1, VEGF and matrix metallopeptidase
(MMP)9 targets to inhibit cell motility and exert anti-angiogenic
effects (18). It downregulates the
protein expression levels of glucose transporter protein (GLUT)-1,
lactate dehydrogenase A and hexokinase 2, activates AMP-activated
protein kinase (AMPK) and inhibits mTOR activity, reduces glucose
uptake, inhibits glycolysis, and inhibits cell proliferation
through the AMPK/mTOR pathway (77). Activation of the estrogen receptor
is associated with the development of >50% of invasive breast
cancers (78), and astragalin,
which exerts therapeutic effects comparable with those of hormones
with a high margin of safety, may offer new hope for clinical
application (79). Moreover,
astragalin may mainly interact with human estrogen receptor α
receptors to exert anticancer effects (80), and it can also bind to human
epidermal growth factor receptor-2 (HER2) and become a potential
inhibitor of HER2 (81). Astragalin
may also be an effective therapeutic agent for metastasized
advanced breast cancer, and this biological effect may be achieved
by reducing the expression of TNF-α, which impairs the expression
of MMP-9 (82,83).
Melanoma is an aggressive metastatic cancer, with a
5-year survival rate of only 4.6% (90). Global melanoma incidence and
mortality in 2040 will be >150% of what it was in 2020 (91). In recent years, immunotherapy and
targeted therapies have achieved a certain degree of efficacy as
the first-line recommended drugs (92); however, there is a substantial risk
of toxicity and drug resistance (93).
Astragalin is cytotoxic and capable of inducing
apoptosis. Mitogen- and stress-activated protein kinase 1 (MSK1) is
an enzyme involved in several cancer processes, and in melanoma,
high levels of MSK1 are often accompanied by high expression of
CREB, and increased activity of the MSK1-CREB pathway promotes cell
proliferation and metastasis (94).
Astragalin directly binds to and inhibits p38 MAPK activity,
antagonizing MSK1 phosphorylation and high levels of γ-H2AX in a
time- and dose-dependent manner, thereby reducing precancerous skin
lesions (95). Astragalin has also
been reported to exert toxic effects on melanocytic tumor A375P and
SK-MEL-2 cells, increasing the levels of positive cells and sub-G1
populations, activating cleaved poly(ADP-ribose) polymerase,
inhibiting SRY-box transcription factor 10 signaling, acting on
cell cycle-related proteins and promoting apoptosis (96). Moreover, astragalin has been
reported to upregulate the expression of superoxide dismutase and
exhibit skin protection effects (97).
Astragalin has been reported to have notable
biological effects for the treatment of prostate cancer by
inhibiting cell proliferation, migration and invasion, and inducing
apoptosis (103). Astragaloside,
the most abundant component of Cyperus exaltatus var.
iwasakii (CE) secondary metabolites, modulates transcription
factors and inhibits the expression of MMP-9, thereby inhibiting
invasion, migration and proliferation of prostate cancer cells.
In vivo studies have also reported that in an allogeneic
model, CE inhibits tumor loading (104). Moreover, Jurinea
mesopotamica Hand.-Mazz. contains high levels of astragalin,
which can destroy cell organelles, promote the generation of
reactive oxygen species, increase apoptotic gene expression and
transcription, regulate energy metabolism, inhibit cell
proliferation and induce cell death (103).
Liver cancer is a common primary cancer and a
high-risk site for cancer metastasis (105). Emerging Yttrium 90 microsphere
therapy has been reported to have notable efficacy in patients with
advanced, non-surgical liver cancer (106); however, this method requires
strict pre-operative screening, is relatively expensive and
requires a high level of expertise to perform the surgery (107). By contrast, it is advantageous to
explore the development of Traditional Chinese Medicine herbal
active ingredients. For example, astragalin has been reported to
inhibit the activity of hepatocellular carcinoma cells, suppressing
their proliferation and inducing apoptosis through several
mechanisms (108–110).
With 58,903 deaths, China had the highest number of
leukemia deaths in the world in 2021 (114). Personalized treatment approaches
for leukemia have been developed; however, there are issues of
treatment resistance, long-term side effects and quality of life
(115). Several studies have
reported that Chinese herbal medicine has satisfactory therapeutic
effects on myelosuppression (116). For example, astragalin has been
reported to inhibit growth and induce apoptosis in leukemia cells
(117).
Certain researchers have isolated drug components
with anticancer activity and clarified that astragalin is its main
antileukemia component (118). The
flavonoid derivative astragalin heptaacetate (AHA) can induce cell
death through non-specific caspases. AHA has been reported to
concentrate and cleave cell chromatin under aerobic conditions,
block the cell cycle in the G0-G1 phase and affect the
mitochondrial membrane potential. It also has been reported to
regulate cytokine expression, activate MAPK, exhibit notable
cytotoxicity and induce apoptosis at the organelle level (117).
The development of NB may be associated with the
neural spine during embryonic development (119). The disease is highly malignant
(120), with >50% of patients
presenting with high-risk phenotypes (121) and a 5-year survival rate of
<50% (122). The immune
microenvironment of NB is complex, with low immunogenicity of
tumor-associated antigens and immunosuppressive factors (such as
TGF-β and IL-10), hindering the effectiveness of immunotherapy
(123). Moreover, the high
clinical behavioral heterogeneity of NB makes it more difficult to
develop standardized treatment protocols (124,125).
Previous research has reported that mutations
associated with the RAS/MAPK pathway are present in the majority of
recurrent NBs (126,127). Astragalin exhibits biological
effects on this pathway, inhibiting cell activity and inducing
apoptosis (128). Furthermore,
astragalin pretreatment reverses the cytoprotective effect, which
is associated with the inhibition of oxidative stress and
phosphorylation of MAPK, as shown by a reduction in the levels of
extracellular protein-regulated protein kinases, p38 and c-Jun
N-terminal kinase, exerting their cytotoxic effects and inducing
apoptosis in NB SK-N-SH cells (128).
Research has demonstrated that the Chinese herbal
formulation, FFBZL, composed of Scutellaria barbata D. Don,
Astragalus membranaceus and Ligusticum chuanxiong
Hort, may be used in the treatment of OSCC; therefore, its
molecular mechanisms warrant in-depth exploration. As a crucial
component, Astragalus membranaceus exhibits marked antitumor
activity in laryngeal squamous cell carcinoma through its extract,
total flavonoids of Astragalus, suggesting the potential of its
active ingredients (138).
Astragalin, one of the key active components of Astragalus
membranaceus, currently lacks direct data demonstrating its
inhibitory effect on OSCC; however, network pharmacology combined
with bioinformatics analysis has revealed that six key ingredients
of FFBZL (including components from Astragalus membranaceus)
may interact with 820 potential target genes. These targets
intersect with OSCC-related target genes, generating 151 common
targets (139).
DLBCL is a common aggressive non-Hodgkin's lymphoma
that accounts for 30–40% of all non-Hodgkin's lymphomas (140). The R-CHOP regimen (rituximab +
cyclophosphamide + adriamycin + vincristine + prednisone) is the
first-line standard of care for DLBCL. However, certain patients
still relapse after treatment or transition to refractory cases
(141).
Astragalin has been reported to induce apoptosis in
DLBCL cells by modulating cytokines (142). A previous study reported that
astragalin inhibits DLBCL OCI-LY8 cell viability and disrupts the
nucleus to promote the emergence of apoptotic vesicles by affecting
the JAK/STAT signaling pathway, upregulating the levels of p53,
caspase-3 and Bax, and inhibiting Bcl-2 expression (142).
Astragalin has notable anticancer effects in several
types of cancer. It also scavenges free radicals in the body
(143). Furthermore,
overexpression of GLUT5 has been reported to lead to marked changes
in glucose metabolism and enhance glycolysis, which is a prominent
manifestation of cancer cells. This protein is expected to be used
as a biomarker for the diagnosis of cancer and as a therapeutic
target (144). A derivative of
astragalin, astragalin-6-glucoside, has been reported to inhibit
GLUT5 (145). Certain studies
suggest that it is associated with glycolysis; however, the exact
mechanism of action is not clear (146).
Based on a review of the mechanisms of astragalin
and its derivatives against several malignant tumors, the
anticancer mechanisms of astragalin are summarized as follows: i)
Regulation of signaling pathways: Astragalin can regulate the
PI3K/AKT, MAPKs, JAK/STAT and NF-κB signaling pathways, miRNA
expression and the Notch1 pathway (27,147),
which inhibits tumor cell growth and survival (148); ii) induction of apoptosis:
Astragalin can cause loss of caspase activity, mutation of p53 gene
and imbalance in the regulation of Bcl2 protein, leading to reduced
activity and programmed death of cancer cells (149). It also affects mitochondrial
function, reduces mitochondrial membrane potential and increases
the Bax/Bcl-2 ratio (150).
Notably, a study reported that astragalin ameliorated testicular
germ cell apoptosis in rats with varicocele by reducing the
expression of caspase 3 and the Bax/Bcl-2 ratio (151). Moreover, astragalin reduced
intracellular oxidative stress, activated caspase 3, 7 and 9, and
promoted programmed cell death in cancer cells, whilst protecting
normal cells from oxidative damage, suggesting that it has a dual
effect on the human body (7); iii)
inhibition of cell proliferation and migration: Astragalin is
closely associated with several angiogenesis-related factors (such
as prostaglandin-endoperoxide synthase 2, kinase insert domain
receptor and MMP9), revealing potential biological effects with
angiogenesis and suggesting new ideas for the treatment of tumors
in terms of reducing the number of blood vessels (152). Astragalin downregulates the
expression of specific protein factors, regulates miRNAs and
induces cell cycle arrest (26);
and iv) enhancement of drug sensitivity: In a previous study,
astragalin was administered with carboplatin and cisplatin over a
range of concentrations, and the results demonstrated that
astragalin was associated with an improved combined effect in
OVCAR-8 and SKOV-3 cells compared with carboplatin or cisplatin
alone (39). This indicates that
astragalin could enhance the accumulation of chemotherapeutic drugs
in tumor cells and reverse resistance to chemotherapeutic
drugs.
Astragalin has a wide range of advantages in cancer
treatment, especially in the process of cooperating with
chemotherapeutic drugs to reduce toxicity and increase
efficiency.
For different cancer types, astragalin has varied
anticancer mechanisms of action, but there are also
cross-overlapping mechanisms of effect. In general, they include
reducing the growth activity of tumor cells, inhibiting tumor cell
angiogenesis, inducing apoptosis, blocking the cell growth cycle,
and reprogramming the metabolic process of tumor cells, affecting
the proliferation, migration and invasion of tumor cells (58). Moreover, several studies have
reported that astragalin does not cause harm to normal human cells
(8,153).
Astragalin has shown promising results in different
types of cancer. For example, radiotherapy may lead to
neurotoxicity (154),
cancer-caused fatigue (155) and
gastrointestinal impairment, and other therapeutic adverse effects
(156,157). However, previous research has
suggested that if astragalin is used in combination with
chemotherapeutic drugs, the neuroprotective, anti-inflammatory,
antioxidant (158,159) and antibacterial effects of
astragalin could assist the action of the chemotherapeutic drugs
(160). Moreover, research has
reported that astragalin is involved in targeting cellular
resistance factors (89): It can
enhance the accumulation of chemotherapeutic drugs in cells and
increase the sensitivity of cells to the drugs, thus reversing
cellular resistance (39). However,
at present, there is a lack of direct research data on the
application of astragalin and chemotherapeutic agents in other
cancers. Based on the collection of existing literature, we
hypothesize that astragalin can serve a direct inhibitory and
blocking role in the growth and metabolism of tumor cells,
hindering cell growth, proliferation, invasion and migration, and
inducing apoptosis through several mechanisms. At the same time, we
hypothesize that it can serve a unique toxicity-reducing and
synergistic effect in the standardized radiotherapy process. This
gives it the potential to be used as a complementary or alternative
treatment to first-line chemotherapeutic agents.
Absorption, distribution, metabolism, excretion and
toxicity model predictions have reported that astragalin is not
susceptible to acute hazards or systemic toxicity and, meeting drug
safety requirements, may reduce accumulation toxicity to normal
tissues through exocytosis mechanisms (153). In A549 lung cancer cells,
astragalin had an IC50 of 150 µM (after 72 h treatment)
and inhibited 85% of lung cancer cells; however, to the best of our
knowledge, at this dose, there is no evidence of toxicity to normal
lung bronchial epithelial cells and cell survival was markedly
higher compared with that of cancer cells (26). Moreover, the IC50 was
20–50 µM in A498 renal carcinoma cells and ≤110 µM in normal renal
cells, indicating that the toxicity to normal cells was notably
lower than that to cancer cells (89). Additionally, the IC50 of
astragalin-containing extract of Citrus × aurantium
was 23.26 µg/ml in colon cancer HCT-116 cells; however, the
toxicity in normal lung fibroblasts (WI-38) was low, with no marked
IC50 value reported. This suggests that astragalin may
have a selective effect on cancer and normal cells (161). Moreover, the aforementioned
findings indicate that astragalin has a wide therapeutic window and
exhibits notable anticancer activity with a high safety profile for
normal cells.
In summary, astragalin has potent antitumor
biological effects with little or no toxic effects on normal
tissues; however, this needs to be supported by further evidence
from numerous future clinical trials.
Previous research reported that the absolute oral
bioavailability of astragalin in rats was <5% (162). This is mainly due to its molecular
structure (higher molecular weight and worse water solubility)
(163) and its easy conversion by
metabolic enzymes [such as uridine
diphosphate-glucuronosyltransferases (UGTs)] in the liver and
intestine, which is characterized by rapid absorption and clearance
(8). However, improved processing
or structural optimization may enhance gastric absorption and delay
metabolism, thereby increasing bioavailability (164,165). UGT enzymes have been reported to
be key metabolizing enzymes of astragalin in vivo. They
include CtUGT3 (166) and
AtUGT78D2 (167). Moreover,
astragalin is distributed in several organs, with the highest
concentration in the gastrointestinal tract. It can also cross the
blood-brain barrier (168) and can
be detected in the liver, lungs and kidneys (169). However, although there are studies
that investigated the pharmacokinetics of astragalin (8,170,171), there is a lack of clinical
pharmacokinetic validation, and the pharmacological properties of
the metabolites, lower bioavailability and stability enhancement
need to be further optimized.
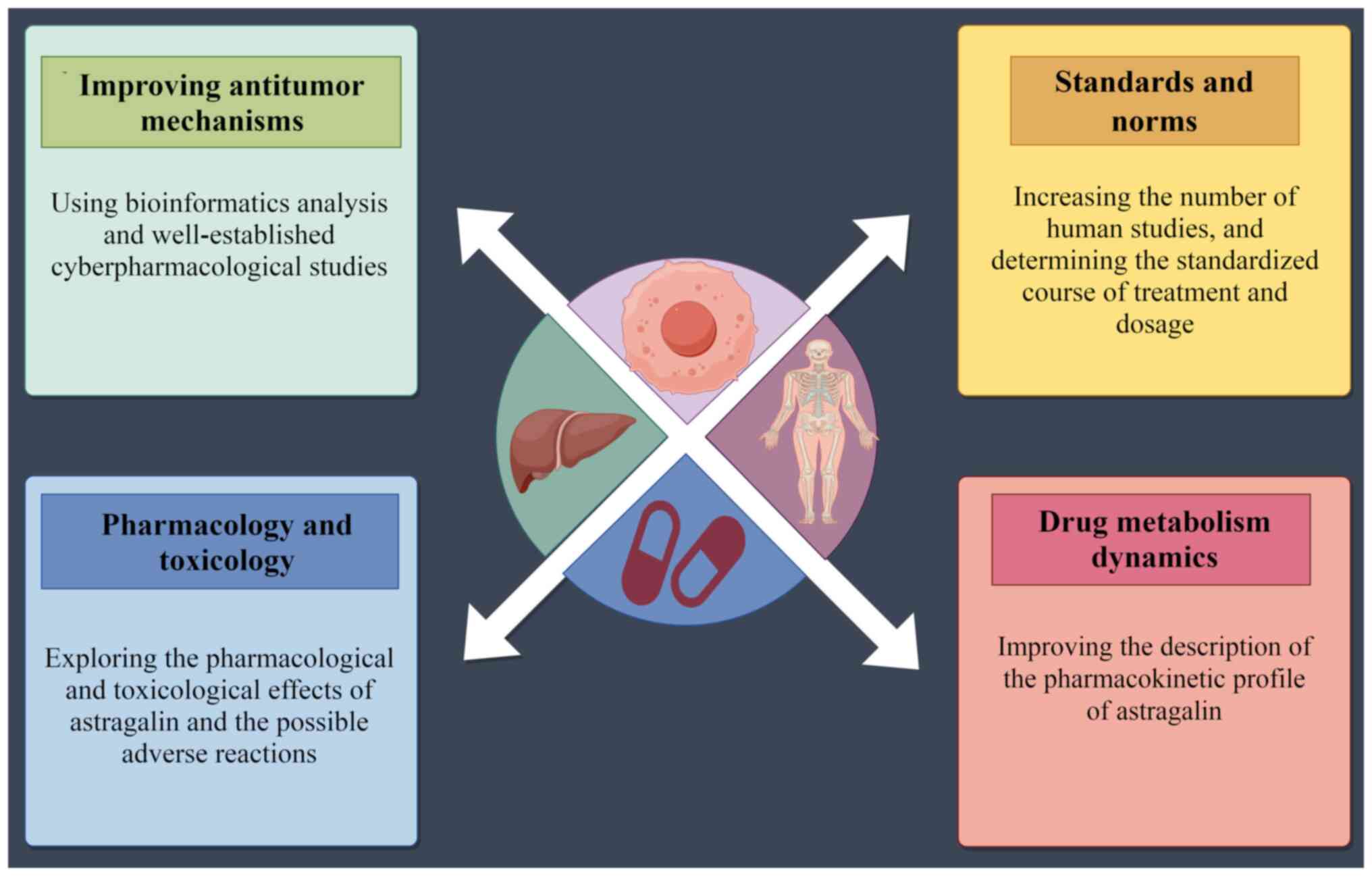
Therefore, future in-depth studies on astragalin
should focus on the advancement of data on the real drug
environment in the clinic, as well as data associated with drug
metabolism pathways and pharmacokinetics in a standardized manner,
with a view to providing an objective basis for the development of
new drugs and the update of therapeutic regimens.
In the research of malignant tumors, the
development and utilization of herbal medicines has become a major
topic. The results of the present review indicate that astragalin,
with its wide and natural sources, strong anticancer activity, high
safety value and low cost, has potential to be an alternative drug
with considerable efficacy in the prevention and treatment of
malignant tumors; however, further research is required. Although
astragalin has demonstrated promising antitumor effects, its
pharmacokinetic profile is incompletely defined, and its low oral
bioavailability limits its potential clinical application. Future
studies should investigate the bioavailability of astragalin
through systematic in vitro and animal experiments, and the
optimization of formulation processes for novel drug delivery
systems. With further research, astragalin has the potential to
become an alternative drug for cancer treatment in the future.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82274566).
Not applicable.
YFZ retrieved the data and participated in the
conceptualization and design of the article as well as the data
analysis; YQF wrote the original manuscript and made several
revisions with the assistance and editing of CL, DND and FYL. FJH
was involved in the design of the manuscript and the interpretation
of the data. Data authentication is not applicable. All authors
contributed to the article and read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Nussinov R, Tsai CJ and Jang H: Anticancer
drug resistance: An update and perspective. Drug Resist Updat.
59:1007962021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zilla MK, Nayak D, Amin H, Nalli Y, Rah B,
Chakraborty S, Kitchlu S, Goswami A and Ali A:
4′-Demethyl-deoxypodophyllotoxin glucoside isolated from
Podophyllum hexandrum exhibits potential anticancer activities by
altering Chk-2 signaling pathway in MCF-7 breast cancer cells. Chem
Biol Interact. 224:100–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martău GA, Bernadette-Emőke T, Odocheanu
R, Soporan DA, Bochiș M, Simon E and Vodnar DC: Vaccinium species
(Ericaceae): Phytochemistry and biological properties of medicinal
plants. Molecules. 28:15332023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruan J, Shi Z, Cao X, Dang Z, Zhang Q,
Zhang W, Wu L, Zhang Y and Wang T: Research progress on
anti-inflammatory effects and related mechanisms of astragalin. Int
J Mol Sci. 25:44762024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barge S, Deka B, Kashyap B, Bharadwaj S,
Kandimalla R, Ghosh A, Dutta PP, Samanta SK, Manna P, Borah JC and
Talukdar NC: Astragalin mediates the pharmacological effects of
Lysimachia candida Lindl on adipogenesis via downregulating PPARG
and FKBP51 signaling cascade. Phytother Res. 35:6990–7003. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mladenova SG, Vasileva LV, Savova MS,
Marchev AS, Tews D, Wabitsch M, Ferrante C, Orlando G and Georgiev
MI: Anti-Adipogenic effect of alchemilla monticola is mediated Via
PI3K/AKT signaling inhibition in human adipocytes. Front Pharmacol.
12:7075072021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ceramella J, Loizzo MR, Iacopetta D,
Bonesi M, Sicari V, Pellicanò TM, Saturnino C, Malzert-Fréon A,
Tundis R, Sinicropi MS, et al: Anchusa azurea Mill. (Boraginaceae)
aerial parts methanol extract interfering with cytoskeleton
organization induces programmed cancer cells death. Food Funct.
10:4280–4290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Zhong K, Qin S, Jing Y, Liu S, Li
D and Peng C: Astragalin: A food-origin flavonoid with therapeutic
effect for multiple diseases. Front Pharmacol. 14:12659602023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tao Y, Bao J, Zhu F, Pan M, Liu Q and Wang
P: Ethnopharmacology of Rubus idaeus Linnaeus: A critical review on
ethnobotany, processing methods, phytochemicals, pharmacology and
quality control. J Ethnopharmacol. 302:1158702023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao ZW, Zhang M, Wang G, Zou J, Gao JH,
Zhou L, Wan XJ, Zhang DW, Yu XH and Tang CK: Astragalin retards
atherosclerosis by promoting cholesterol efflux and inhibiting the
inflammatory response via upregulating ABCA1 and ABCG1 expression
in macrophages. J Cardiovasc Pharmacol. 77:217–227. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajani V, Umadevi S and Raju CN: A review
on exploring the phytochemical and pharmacological significance of
Indigofera astragalina. Pharmacogn Mag. 20:363–371. 2024.
View Article : Google Scholar
|
|
12
|
Wang N, Singh D and Wu Q: Astragalin
attenuates diabetic cataracts via inhibiting aldose reductase
activity in rats. Int J Ophthalmol. 16:1186–1195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bangar SP, Singh A, Chaudhary V, Sharma N
and Lorenzo JM: Beetroot as a novel ingredient for its versatile
food applications. Crit Rev Food Sci Nutr. 63:8403–8427. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohammed AE, Alghamdi SS, Shami A, Suliman
RS, Aabed K, Alotaibi MO and Rahman I: In silico prediction of
malvaviscus arboreus metabolites and green synthesis of silver
nanoparticles-opportunities for safer anti-bacterial and
anti-cancer precision medicine. Int J Nanomedicine. 18:2141–2162.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SC, Xiang XY, Han WC, Chen MY and Xie
BC: Research progress on pharmacological properties and mechanism
of astragalin. Chin J Tradit Chin Med. 40:118–123+287. 2022.(In
Chinese).
|
|
16
|
Yang M, Li WY, Xie J, Wang ZL, Wen YL,
Zhao CC, Tao L, Li LF, Tian Y and Sheng J: Astragalin inhibits the
proliferation and migration of human colon cancer HCT116 cells by
regulating the NF-κB signaling pathway. Front Pharmacol.
12:6392562021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Lv J, Li X and Lin Q: The
flavonoid Astragalin shows anti-tumor activity and inhibits
PI3K/AKT signaling in gastric cancer. Chem Biol Drug Des.
98:779–786. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian S, Wei Y, Hu H and Zhao H: Mixed
computational-experimental study to reveal the anti-metastasis and
anti-angiogenesis effects of Astragalin in human breast cancer.
Comput Biol Med. 150:1061312022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma R: Mapping of global, regional and
national incidence, mortality and mortality-to-incidence ratio of
lung cancer in 2020 and 2050. Int J Clin Oncol. 27:665–675. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rossi R, De Angelis ML, Xhelili E, Sette
G, Eramo A, De Maria R, Incani UC, Francescangeli F and Zeuner A:
Lung cancer organoids: The rough path to personalized medicine.
Cancers (Basel). 14:37032022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Prete M, Ballarin G, Porciello G, Arianna
A, Luongo A, Belli V, Scalfi L and Celentano E: Bioelectrical
impedance analysis-derived phase angle (PhA) in lung cancer
patients: A systematic review. BMC Cancer. 24:6082024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Wei W, Wang J and Chen T:
Theranostic applications of selenium nanomedicines against lung
cancer. J Nanobiotechnology. 21:962023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xi Chen and Jin Qi: Flavonoids and
Alkaloids in Lotus Leaves. Chin J Exp Tradit Med Formulae. 211–214.
2015.
|
|
24
|
Wang D, Yu J, Cheng L, Hu X, Xiang M, Zhou
Y, Tao B, Liu C and Xia Y: Research Progress on Main Constituents
and Biological Activities of Lotus Leaves. Agricultural Technology
& Equipment. 71–72. 742021.(In Chinese).
|
|
25
|
Jia XB, Zhang Q, Xu L, Yao WJ and Wei L:
Lotus leaf flavonoids induce apoptosis of human lung cancer A549
cells through the ROS/p38 MAPK pathway. Biol Res. 54:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu G, Yu B, Wang R, Jiang J, Wen F and Shi
X: Astragalin flavonoid inhibits proliferation in human lung
carcinoma cells mediated via induction of caspase-dependent
intrinsic pathway, ROS production, cell migration and invasion
inhibition and targeting JAK/STAT signalling pathway. Cell Mol Biol
(Noisy-le-grand). 67:44–49. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao D, Bai JC and Zhang B: Research
progress of signal pathways related to the anti-tumor effect of
Astragalin. J Pract Oncol. 38:62–66. 2024.
|
|
28
|
Liang Y and Chen W: Effect of Astragalin
on proliferation, migration, apoptosis and oxidative stress of lung
cancer A549 cells. Chin J Clin Pharmacol. 39:999–1003. 2023.(In
Chinese).
|
|
29
|
Chen M, Cai F, Zha D, Wang X, Zhang W, He
Y, Huang Q, Zhuang H and Hua ZC: Astragalin-induced cell death is
caspase-dependent and enhances the susceptibility of lung cancer
cells to tumor necrosis factor by inhibiting the NF-кB pathway.
Oncotarget. 8:26941–26958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan C, Pang X, Fan W, Dai W, Hu X, Mei Q
and Zeng C: Mechanism of leaves of Microcos paniculata
improving of dyspepsia based on network pharmacology and molecular
docking. Drugs & Clinic. 38:1335–1343. 2023.(In Chinese).
|
|
31
|
Li M, Zhao Z, Xuan J, Li Z and Ma T:
Advances in Studies on Chemical Constituents and Pharmacological
Effects of Lotus Leaves. Journal of Liaoning University of TCM.
22:135–138. 2020.(In Chinese).
|
|
32
|
Bai J, Chen Y, Zhao G and Gui R: In vitro
and vivo experiments revealing astragalin inhibited lung
adenocarcinoma development via LINC00582/miR-140-3P/PDPK1. J
Biochem Mol Toxicol. 38:e700422024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ribeiro V, Ferreres F, Macedo T,
Gil-Izquierdo Á, Oliveira AP, Gomes NGM, Araújo L, Pereira DM,
Andrade PB and Valentão P: Activation of caspase-3 in gastric
adenocarcinoma AGS cells by Xylopia aethiopica (Dunal) A. Rich.
fruit and characterization of its phenolic fingerprint by
HPLC-DAD-ESI(Ion Trap)-MSn and UPLC-ESI-QTOF-MS2. Food Res Int.
141:1101212021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
35
|
Webb PM and Jordan SJ: Global epidemiology
of epithelial ovarian cancer. Nat Rev Clin Oncol. 21:389–400. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G
and Jin WL: Immunotherapy for ovarian cancer: Adjuvant,
combination, and neoadjuvant. Front Immunol. 11:5778692020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gu L, Du N, Jin Q, Li S, Xie L, Mo J, Shen
Z, Mao D, Ji J, et al: Magnitude of benefit of the addition of poly
ADP-ribose polymerase (PARP) inhibitors to therapy for malignant
tumor: A meta-analysis. Crit Rev Oncol Hematol. 147:1028882020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song L and Fu Q: Study of the effect of
astragalin on proliferation of ovarian cancer cells by inhibiting
the glycolytic pathway induced Via HIF-1α. Pract Oncol J.
32:503–509. 2018.
|
|
39
|
Ling Song and Qiong Fu: Astragalin
inhibits hypoxia-induced proliferation and invasion of ovarian
cancer cells by up-regulating PHD2. J Pract Oncol. 33:407–414.
2019.
|
|
40
|
Mazzone M, Dettori D, de Oliveira RL,
Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, de
Almodovar CR, et al: Heterozygous deficiency of PHD2 restores tumor
oxygenation and inhibits metastasis via endothelial normalization.
Cell. 136:839–851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guentsch A, Beneke A, Swain L, Farhat K,
Nagarajan S, Wielockx B, Raithatha K, Dudek J, Rehling P and
Zieseniss A: PHD2 is a regulator for glycolytic reprogramming in
macrophages. Mol Cell Biol. 37:e00236–e00216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mehla K and Singh PK: MUC1: A novel
metabolic master regulator. Biochim Biophys Acta. 1845:126–135.
2014.PubMed/NCBI
|
|
43
|
Katayama Y, Uchino J, Chihara Y, Tamiya N,
Kaneko Y, Yamada T and Takayama K: Tumor neovascularization and
developments in therapeutics. Cancers (Basel). 11:3162019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li RL, He LY, Zhang Q, Liu J, Lu F, Duan
HX, Fan LH, Peng W, Huang YL and Wu CJ: HIF-1α is a potential
molecular target for herbal medicine to treat diseases. Drug Des
Devel Ther. 14:4915–4949. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang CZ, Wang SH, Zhang RH, Lin JH, Tian
YH, Yang YQ, Liu J and Ma YX: Neuroprotective effect of astragalin
via activating PI3K/Akt-mTOR-mediated autophagy on APP/PS1 mice.
Cell Death Discov. 9:152023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shu-ping Qu, Chun-jun Liu and Ju-hua
Liang: Correlation between serum miR-410,miR-875 expression with
HPV infection in cervical cancer patients. J Path Biol.
19:1404–1407. 2024.
|
|
48
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN Guidelines® insights: Cervical cancer,
version 1.2024. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raj S, Prasad RR and Ranjan A: Incidence
of vaginal toxicities following definitive chemoradiation in intact
cervical cancer: A meta-analysis. J Contemp Brachytherapy.
16:241–256. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zeng W and Chen L: Astragalin inhibits the
proliferation of high-risk HPV-positive cervical epithelial cells
and attenuates malignant cervical lesions. Cytotechnology.
77:802025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He M, Yasin K, Yu S, Li J and Xia L: Total
flavonoids in Artemisia absinthium L. and evaluation of its
anticancer activity. Int J Mol Sci. 24:163482023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zerrouki S, Mezhoud S, Yilmaz MA,
Yaglioglu AS, Bakir D, Demirtas I and Mekkiou R: LC/MS-MS analyses
and in vitro anticancer activity of Tourneuxia variifolia
extracts. Nat Prod Res. 36:4506–4510. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu X, Zhou N, Lan H, Yang F, Dong B and
Zhang X: The ferroptosis molecular subtype reveals characteristics
of the tumor microenvironment, immunotherapeutic response, and
prognosis in gastric cancer. Int J Mol Sci. 23:97672022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mamun TI, Younus S and Rahman MH: Gastric
cancer-Epidemiology, modifiable and non-modifiable risk factors,
challenges and opportunities: An updated review. Cancer Treat Res
Commun. 41:1008452024.PubMed/NCBI
|
|
56
|
Wang J, Du L and Chen X: Adenosine
signaling: Optimal target for gastric cancer immunotherapy. Front
Immunol. 13:10278382022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu Y, Huang T, Wang L, Wang Y, Liu Y, Bai
J, Wen X, Li Y, Long K and Zhang H: Traditional Chinese medicine in
the treatment of chronic atrophic gastritis, precancerous lesions
and gastric cancer. J Ethnopharmacol. 337:1188122025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Radziejewska I, Supruniuk K, Tomczyk M,
Izdebska W, Borzym-Kluczyk M, Bielawska A, Bielawski K and Galicka
A: p-Coumaric acid, Kaempferol, Astragalin and Tiliroside influence
the expression of glycoforms in AGS gastric cancer cells. Int J Mol
Sci. 23:86022022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chu ZH, He SY, Wang Y, Zhu RT, Gu YL and
Chen JY: Effects of Astragalin on apoptosis of undifferentiated
gastric cancer cells. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
38:520–524. 2022.(In Chinese). PubMed/NCBI
|
|
60
|
Sharafutdinov I, Tegtmeyer N, Linz B,
Rohde M, Vieth M, Tay AC, Lamichhane B, Tuan VP, Fauzia KA, Sticht
H, et al: A single-nucleotide polymorphism in Helicobacter pylori
promotes gastric cancer development. Cell Host Microbe.
31:1345–1358.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tan A, Scortecci KC, De Medeiros NM,
Kukula-Koch W, Butler TJ, Smith SM and Boylan F: Plukenetia
volubilis leaves as source of anti-Helicobacter pylori agents.
Front Pharmacol. 15:14614472024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Murphy CC and Zaki TA: Changing
epidemiology of colorectal cancer-birth cohort effects and emerging
risk factors. Nat Rev Gastroenterol Hepatol. 21:25–34. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ebnudesita FR, Dienanta SB, Jannah AR and
I'tishom R: Potency of soursop leaf extract and curcumin with
magnetic and mucus-penetrating nanoparticle as colorectal cancer
alternative therapY. J Vocational Health Studies. 5:186–191. 2022.
View Article : Google Scholar
|
|
64
|
Abedizadeh R, Majidi F, Khorasani HR,
Abedi H and Sabour D: Colorectal cancer: A comprehensive review of
carcinogenesis, diagnosis, and novel strategies for classified
treatments. Cancer Metastasis Rev. 43:729–753. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen L, Chen C, Wang P and Song T:
Mechanisms of cellular effects directly induced by magnetic
nanoparticles under magnetic fields. J Nanomaterials. 2017:1–13.
2017. View Article : Google Scholar
|
|
66
|
Eng C, Yoshino T, Ruíz-García E, Mostafa
N, Cann CG, O'Brian B, Benny A, Perez RO and Cremolini C:
Colorectal cancer. Lancet. 404:294–310. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zou Y, Wang S, Zhang H, Gu Y, Chen H,
Huang Z, Yang F, Li W, Chen C, Men L, et al: The triangular
relationship between traditional Chinese medicines, intestinal
flora, and colorectal cancer. Med Res Rev. 44:539–567. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Augustynowicz D, Lemieszek MK, Strawa JW,
Wiater A and Tomczyk M: Anticancer potential of acetone extracts
from selected Potentilla species against human colorectal cancer
cells. Front Pharmacol. 13:10273152022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhai Y, Sun J, Sun C, Zhao H, Li X, Yao J,
Su J, Xu X, Xu X, Hu J, et al: Total flavonoids from the dried root
of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer
growth through PI3K/AKT/mTOR signaling pathway. Phytother Res.
36:4263–4277. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tragulpakseerojn J, Yamaguchi N,
Pamonsinlapatham P, Wetwitayaklung P, Yoneyama T, Ishikawa N,
Ishibashi M and Apirakaramwong A: Anti-proliferative effect of
Moringa oleifera Lam (Moringaceae) leaf extract on human colon
cancer HCT116 cell line. Trop J Pharm Res. 16:371–378. 2017.
View Article : Google Scholar
|
|
71
|
Chidambaram A, Prabhakaran R, Sivasamy S,
Kanagasabai T, Thekkumalai M, Singh A, Tyagi MS and Dhandayuthapani
S: Male breast cancer: Current scenario and future perspectives.
Technol Cancer Res Treat. 23:153303382412618362024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Palmer BV, Walsh GA, McKinna JA and
Greening WP: Adjuvant chemotherapy for breast cancer: Side effects
and quality of life. BMJ. 281:1594–1597. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Di Nardo P, Lisanti C, Garutti M, Buriolla
S, Alberti M, Mazzeo R and Puglisi F: Chemotherapy in patients with
early breast cancer: Clinical overview and management of long-term
side effects. Expert Opin Drug Saf. 21:1341–1355. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim SD, Kwag EB, Yang MX and Yoo HS:
Efficacy and safety of ginger on the side effects of chemotherapy
in breast cancer patients: systematic review and meta-analysis. Int
J Mol Sci. 23:112672022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peeters NJMC, Kaplan ZLR, Clarijs ME,
Mureau MAM, Verhoef C, van Dalen T, Husson O and Koppert LB:
Health-related quality of life (HRQoL) after different axillary
treatments in women with breast cancer: A 1-year longitudinal
cohort study. Qual Life Res. 33:467–479. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Meng X, Zhang A, Wang X and Sun H: A
kaempferol-3-O-β-D-glucoside, intervention effect of astragalin on
estradiol metabolism. Steroids. 149:1084132019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zeb A, Khan W, Ul Islam W, Khan F, Khan A,
Khan H, Khalid A, Rehman NU, Ullah A and Al-Harrasi A: Exploring
the anticancer potential of astragalin in triple negative breast
cancer cells by attenuating glycolytic pathway through AMPK/mTOR.
Curr Med Chem. Jul 25–2024.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nunnery SE and Mayer IA: Targeting the
PI3K/AKT/mTOR pathway in hormone-positive breast cancer. Drugs.
80:1685–1697. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Song X, Shen L, Contreras JM, Liu Z, Ma K,
Ma B, Liu X and Wang DO: New potential selective estrogen receptor
modulators in traditional Chinese medicine for treating menopausal
syndrome. Phytother Res. 38:4736–4756. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Huq AM, Stanslas J, Nizhum N, Uddin MN,
Maulidiani M, Roney M, Abas F and Jamal JA: Estrogenic
post-menopausal anti-osteoporotic mechanism of Achyranthes aspera
L.: Phytochemicals and network pharmacology approaches. Heliyon.
10:e387922024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bellai A, Deka S, Tag H, Bhattacharya K
and Hui PK: Integrated pharmacognostic and computational analysis
of Hydrocotyle javanica thunb. Phytochemicals as a potential
HER2 tyrosine kinase inhibitor in breast cancer. Pept Sci.
119:e243722024. View Article : Google Scholar
|
|
82
|
Ahn S, Jung H, Jung Y, Lee J, Shin SY, Lim
Y and Lee Y: Identification of the active components inhibiting the
expression of matrix metallopeptidase-9 by TNFα in ethyl acetate
extract of Euphorbia humifusa Willd. J Appl Biol Chem. 62:367–374.
2019. View Article : Google Scholar
|
|
83
|
Shin SY, Kim CG, Jung YJ, Jung Y, Jung H,
Im J, Lim Y and Lee YH: Euphorbia humifusa Willd exerts inhibition
of breast cancer cell invasion and metastasis through inhibition of
TNFα-induced MMP-9 expression. BMC Complement Altern Med.
16:4132016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cirillo L, Innocenti S and Becherucci F:
Global epidemiology of kidney cancer. Nephrol Dial Transplant.
39:920–928. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Young M, Jackson-Spence F, Beltran L, Day
E, Suarez C, Bex A, Powles T and Szabados B: Renal cell carcinoma.
Lancet. 404:476–491. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Imamura R, Tanaka R, Taniguchi A, Nakazawa
S, Kato T, Yamanaka K, Namba-Hamano T, Kakuta Y, Abe T, Tsutahara
K, et al: Everolimus reduces cancer incidence and improves patient
and graft survival rates after kidney transplantation: A
multi-center study. J Clin Med. 11:2492022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen H: microRNA-Based cancer diagnosis
and therapy. Int J Mol Sci. 25:2302024. View Article : Google Scholar
|
|
88
|
Zhang W, Chan C, Zhang K, Qin H, Yu BY,
Xue Z, Zheng X and Tian J: Discovering a new drug against acute
kidney injury by using a tailored photoacoustic imaging probe. Adv
Mater. 36:e23113972024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhu L, Zhu L, Chen J, Cui T and Liao W:
Astragalin induced selective kidney cancer cell death and these
effects are mediated via mitochondrial mediated cell apoptosis,
cell cycle arrest, and modulation of key tumor-suppressive miRNAs.
J BUON. 24:1245–1251. 2019.PubMed/NCBI
|
|
90
|
Wu N, Li J and Tao J: Hot spots in
diagnosis of malignant melanoma. J Diagn Concepts Pract.
22:215–219. 2023.(In Chinese).
|
|
91
|
Arnold M, Singh D, Laversanne M, Vignat J,
Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC and Bray
F: Global burden of cutaneous melanoma in 2020 and projections to
2040. JAMA Dermatol. 158:495–503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Swetter SM, Johnson D, Albertini MR,
Barker CA, Bateni S, Baumgartner J, Bhatia S, Bichakjian C, Boland
G, Chandra S, et al: NCCN Guidelines® insights:
Melanoma: Cutaneous, version 2.2024: Featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 22:290–298. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ernst M and Giubellino A: The current
state of treatment and future directions in cutaneous malignant
melanoma. Biomedicines. 10:8222022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li J, Liu X, Wang W, Li C and Li X: MSK1
promotes cell proliferation and metastasis in uveal melanoma by
phosphorylating CREB. Arch Med Sci. 16:1176–1188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li N, Zhang K, Mu X, Tian Q, Liu W, Gao T,
Ma X and Zhang J: Astragalin attenuates UVB radiation-induced
actinic keratosis formation. Anticancer Agents Med Chem.
18:1001–1008. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
You OH, Shin EA, Lee H, Kim JH, Sim DY,
Kim JH, Kim Y, Khil JH, Baek NI and Kim SH: Apoptotic effect of
astragalin in melanoma skin cancers via activation of caspases and
inhibition of sry-related HMg-Box gene 10. Phytother Res.
31:1614–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wang J, Bian Y, Cheng Y, Sun R and Li G:
Effect of lemon peel flavonoids on UVB-induced skin damage in mice.
RSC Adv. 10:31470–31478. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Løvf M, Zhao S, Axcrona U, Johannessen B,
Bakken AC, Carm KT, Hoff AM, Myklebost O, Meza-Zepeda LA, Lie AK,
et al: multifocal primary prostate cancer exhibits high degree of
genomic heterogeneity. Eur Urol. 75:498–505. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lafontaine ML and Kokorovic A:
Cardiometabolic side effects of androgen deprivation therapy in
prostate cancer. Curr Opin Support Palliat Care. 16:216–222. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Moses KA, Sprenkle PC, Bahler C, Box G,
Carlsson SV, Catalona WJ, Dahl DM, Dall'Era M, Davis JW, Drake BF,
et al: NCCN Guidelines® insights: Prostate cancer early
detection, version 1.2023. J Natl Compr Canc Netw. 21:236–246.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Beer TM, Hotte SJ, Saad F, Alekseev B,
Matveev V, Fléchon A, Gravis G, Joly F, Chi KN, Malik Z, et al:
Custirsen (OGX-011) combined with cabazitaxel and prednisone versus
cabazitaxel and prednisone alone in patients with metastatic
castration-resistant prostate cancer previously treated with
docetaxel (AFFINITY): A randomised, open-label, international,
phase 3 trial. Lancet Oncol. 18:1532–1542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shahab MRF and Shahab N: Recent advances
and future directions in the management of metastatic
castration-resistant prostate cancer asing androgen receptor
signaling inhibitors and poly-adipose polymerase inhibitors: A
literature review. International J Multidisciplinary Research.
6:22024.
|
|
103
|
Koyuncu I, Temiz E, Seker F, Balos MM,
Akkafa F, Yuksekdag O, Yılmaz MA and Zengin G: A mixed-apoptotic
effect of Jurinea mesopotamica extract on prostate cancer cells: A
promising source for natural chemotherapeutics. Chem Biodivers.
21:e2023017472024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kim H, Hwang B, Cho S, Kim WJ, Myung SC,
Choi YH, Kim WJ, Lee S and Moon SK: The ethanol extract of Cyperus
exaltatus var. iwasakii exhibits cell cycle dysregulation,
ERK1/2/p38 MAPK/AKT phosphorylation, and reduced MMP-9-mediated
metastatic capacity in prostate cancer models in vitro and in vivo.
Phytomedicine. 114:1547942023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li X, Ramadori P, Pfister D, Seehawer M,
Zender L and Heikenwalder M: The immunological and metabolic
landscape in primary and metastatic liver cancer. Nat Rev Cancer.
21:541–557. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Chiang CL, Chiu KWH, Chan KSK, Lee FAS, Li
JCB, Wan CWS, Dai WC, Lam TC, Chen W, Wong NSM, et al: Sequential
transarterial chemoembolisation and stereotactic body radiotherapy
followed by immunotherapy as conversion therapy for patients with
locally advanced, unresectable hepatocellular carcinoma
(START-FIT): A single-arm, phase 2 trial. Lancet Gastroenterol
Hepatol. 8:169–178. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Pennington B, Akehurst R, Wasan H, Sangro
B, Kennedy AS, Sennfält K and Bester L: Cost-effectiveness of
selective internal radiation therapy using yttrium-90 resin
microspheres in treating patients with inoperable colorectal liver
metastases in the UK. J Med Econ. 18:797–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen C, Chen F, Gu L, Jiang Y, Cai Z, Zhao
Y, Chen L, Zhu Z and Liu X: Discovery and validation of COX2 as a
target of flavonoids in Apocyni Veneti Folium: Implications for the
treatment of liver injury. J Ethnopharmacol. 326:1179192024.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li W, Hao J, Zhang L, Cheng Z, Deng X and
Shu G: Astragalin reduces hexokinase 2 through increasing miR-125b
to inhibit the proliferation of hepatocellular carcinoma cells in
vitro and in vivo. J Agric Food Chem. 65:5961–5972. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hong Z, Lu Y, Ran C, Tang P, Huang J, Yang
Y, Duan X and Wu H: The bioactive ingredients in Actinidia
chinensis Planch. Inhibit liver cancer by inducing apoptosis. J
Ethnopharmacol. 281:1145532021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pirvu L, Stefaniu A, Neagu G, Albu B and
Pintilie L: In vitro cytotoxic and Antiproliferative Activity of
Cydonia oblonga flower petals, leaf and fruit pellet ethanolic
extracts. Docking simulation of the active flavonoids on
anti-apoptotic protein Bcl-2. Open Chemistry. 16:591–604. 2018.
View Article : Google Scholar
|
|
112
|
Kaidash OA, Kostikova VA, Udut EV, Shaykin
VV and Kashapov DR: Extracts of Spiraea hypericifolia L. and
Spiraea crenata L.: The phenolic profile and biological activities.
Plants. 11:27282022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhou K, Xiao S, Cao S, Zhao C, Zhang M and
Fu Y: Improvement of glucolipid metabolism and oxidative stress via
modulating PI3K/Akt pathway in insulin resistance HepG2 cells by
chickpea flavonoids. Food Chem X. 23:1016302024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Murray CJL: The global burden of disease
study at 30 years. Nat Med. 28:2019–2026. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nadiminti KVG, Sahasrabudhe KD and Liu H:
Menin inhibitors for the treatment of acute myeloid leukemia:
Challenges and opportunities ahead. J Hematol Oncol. 17:1132024.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lam CS, Peng LW, Yang LS, Chou HWJ, Li CK,
Zuo Z, Koon HK and Cheung YT: Examining patterns of traditional
Chinese medicine use in pediatric oncology: A systematic review,
meta-analysis and data-mining study. J Integr Med. 20:402–415.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Burmistrova O, Quintana J, Díaz JG and
Estévez F: Astragalin heptaacetate-induced cell death in human
leukemia cells is dependent on caspases and activates the MAPK
pathway. Cancer Lett. 309:71–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lee KH, Tagahara K, Suzuki H, Wu RY,
Haruna M, Hall IH, Huang HC, Ito K, Iida T and Lai JS: Antitumor
agents. 49 tricin, kaempferol-3-O-beta-D-glucopyranoside and
(+)-nortrachelogenin, antileukemic principles from Wikstroemia
indica. J Nat Prod. 44:530–535. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Delloye-Bourgeois C and Castellani V:
Hijacking of embryonic programs by neural crest-derived
neuroblastoma: From physiological migration to metastatic
dissemination. Front Mol Neurosci. 12:522019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gatta G, Botta L, Rossi S, Aareleid T,
Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour
B, et al: Childhood cancer survival in Europe 1999–2007: Results of
EUROCARE-5-a population-based study. Lancet Oncol. 15:35–47. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kholodenko IV, Kalinovsky DV, Doronin II,
Deyev SM and Kholodenko RV: Neuroblastoma origin and therapeutic
targets for immunotherapy. J Immunol Res. 2018:73942682018.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Berlanga P, Cañete A and Castel V:
Advances in emerging drugs for the treatment of neuroblastoma.
Expert Opin Emerg Drugs. 22:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Shekarian T and Valsesia-Wittmann S:
Considerations for the development of innovative therapies against
aggressive neuroblastoma: Immunotherapy and Twist1 targeting.
Neuroblastoma-Current State and Recent Updates. Gowda C: InTech;
2017, View Article : Google Scholar
|
|
124
|
Luksch R, Castellani MR, Collini P, De
Bernardi B, Conte M, Gambini C, Gandola L, Garaventa A, Biasoni D,
Podda M, et al: Neuroblastoma (Peripheral neuroblastic tumours).
Crit Rev Oncol Hematol. 107:163–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Lodrini M, Sprüssel A, Astrahantseff K,
Tiburtius D, Konschak R, Lode HN, Fischer M, Keilholz U, Eggert A
and Deubzer HE: Using droplet digital PCR to analyze MYCN and ALK
copy number in plasma from patients with neuroblastoma. Oncotarget.
8:85234–85251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Johnsen JI, Dyberg C, Fransson S and
Wickström M: Molecular mechanisms and therapeutic targets in
neuroblastoma. Pharmacol Res. 131:164–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zafar A, Wang W, Liu G, Wang X, Xian W,
McKeon F, Foster J, Zhou J and Zhang R: Molecular targeting
therapies for neuroblastoma: Progress and challenges. Med Res Rev.
41:961–1021. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chung MJ, Lee S, Park YI, Lee J and Kwon
KH: Neuroprotective effects of phytosterols and flavonoids from
Cirsium setidens and Aster scaber in human brain neuroblastoma
SK-N-SH cells. Life Sci. 148:173–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chamoli A, Gosavi AS, Shirwadkar UP,
Wangdale KV, Behera SK, Kurrey NK, Kalia K and Mandoli A: Overview
of oral cavity squamous cell carcinoma: Risk factors, mechanisms,
and diagnostics. Oral Oncol. 121:1054512021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S,
Nice EC, Tang J and Huang C: Oral squamous cell carcinomas: State
of the field and emerging directions. Int J Oral Sci. 15:442023.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Katirachi SK, Grønlund MP, Jakobsen KK,
Grønhøj C and von Buchwald C: The prevalence of HPV in oral cavity
squamous cell carcinoma. Viruses. 15:4512023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li H, Zhang Y, Xu M and Yang D: Current
trends of targeted therapy for oral squamous cell carcinoma. J
Cancer Res Clin Oncol. 148:2169–2186. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Arboleda LPA, de Carvalho GB, Santos-Silva
AR, Fernandes GA, Vartanian JG, Conway DI, Virani S, Brennan P,
Kowalski LP and Curado MP: Squamous cell carcinoma of the oral
cavity, oropharynx, and larynx: A scoping review of treatment
guidelines worldwide. Cancers (Basel). 15:44052023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ma YX, Li XJ, Jin Q, Ji L and Qiao Q:
Application of neoadjuvant immunotherapy in locally advanced
squamous cell carcinoma of head and neck. Chin J Cancer Prev Treat.
31:968–974. 2024.
|
|
135
|
Wang X, Li J, Chen R, Li T and Chen M:
Active ingredients from Chinese medicine for combination cancer
therapy. Int J Biol Sci. 19:3499–3525. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang X, Qiu H, Li C, Cai P and Qi F: The
positive role of traditional Chinese medicine as an adjunctive
therapy for cancer. Biosci Trends. 15:283–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Miao K, Liu W, Xu J, Qian Z and Zhang Q:
Harnessing the power of traditional Chinese medicine monomers and
compound prescriptions to boost cancer immunotherapy. Front
Immunol. 14:12772432023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhao S, Xiao S, Wang W, Dong X, Liu X,
Wang Q, Jiang Y and Wu W: Network pharmacology and transcriptomics
reveal the mechanisms of FFBZL in the treatment of oral squamous
cell carcinoma. Front Pharmacol. 15:14055962024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun Y, Cai J, Ding S and Bao S: Network
Pharmacology Was Used to Predict the Active Components and
Prospective Targets of Paeoniae Radix Alba for Treatment in
Endometriosis. Reprod Sci. 30:1103–1117. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Liu WL, Ji MW, Wang YY, Song LN, Jiang Y
and Shao YK: A case report of diffuse large B-cell lymphoma with
brachial plexus nerve injury as the first symptom. J Apoplexy Nerv
Dis. 37:842–844. 2020.
|
|
141
|
Qi RL, Qiu MH, Zhang T, Zhao XZ, He GP,
Mei HW and Wang HQ: Progress in treatment of recurrent/refractory
diffuse large B-cell lymphoma. Chin Clin Oncol. 27:758–763.
2022.
|
|
142
|
Wang Y, Zhu ML, He SY, Chen H and Chen JY:
Astragalin induces apoptosis of DLBCL cell line OCI-LY8. Chin J
Pathophysiol. 37:885–890. 2021.(In Chinese).
|
|
143
|
Lu Y, Wu N, Fang Y, Shaheen N and Wei Y:
An automatic on-line 2,2-diphenyl-1-picrylhydrazyl-high performance
liquid chromatography method for high-throughput screening of
antioxidants from natural products. J Chromatogr A. 1521:100–109.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hadzi-Petrushev N, Stojchevski R,
Jakimovska A, Stamenkovska M, Josifovska S, Stamatoski A, Sazdova
I, Sopi R, Kamkin A, Gagov H, et al: GLUT5-overexpression-related
tumorigenic implications. Mol Med. 30:1142024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Thompson AM, Iancu CV, Nguyen TTH, Kim D
and Choe J: Inhibition of human GLUT1 and GLUT5 by plant
carbohydrate products; insights into transport specificity. Sci
Rep. 5:128042015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ingram DK and Roth GS: Glycolytic
inhibition: An effective strategy for developing calorie
restriction mimetics. Geroscience. 43:1159–1169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Harikrishnan H, Jantan I, Alagan A and
Haque MA: Modulation of cell signaling pathways by Phyllanthus
amarus and its major constituents: Potential role in the prevention
and treatment of inflammation and cancer. Inflammopharmacology.
28:1–18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Devanaboyina M, Kaur J, Whiteley E, Lin L,
Einloth K, Morand S, Stanbery L, Hamouda D and Nemunaitis J: NF-κB
signaling in tumor pathways focusing on breast and ovarian cancer.
Oncol Rev. 16:105682022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Adebayo IA, Balogun WG and Arsad H:
Moringa oleifera: An apoptosis inducer in cancer cells. Trop J
Pharm Res. 16:2289–2296. 2017. View Article : Google Scholar
|
|
150
|
Yang F, Li AM, Zhang YW, Ma CM and Zhang
HR: Study on the effect of astragalin on apoptosis of human colon
cancer SW480 cells. Guo Ji Yi Yao Wei Sheng Dao Bao She.
26:3013–3017. 2020.(In Chinese).
|
|
151
|
Karna KK, Choi BR, You JH, Shin YS, Cui
WS, Lee SW, Kim JH, Kim CY, Kim HK and Park JK: The ameliorative
effect of monotropein, astragalin, and spiraeoside on oxidative
stress, endoplasmic reticulum stress, and mitochondrial signaling
pathway in varicocelized rats. BMC Complement Altern Med.
19:3332019. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yun W, Dan W, Liu J, Guo X, Li M and He Q:
Investigation of the mechanism of traditional Chinese medicines in
angiogenesis through network pharmacology and data mining. Evid
Based Complement Alternat Med. 2021:55399702021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Riaz A, Rasul A, Hussain G, Zahoor MK,
Jabeen F, Subhani Z, Younis T, Ali M, Sarfraz I and Selamoglu Z:
Astragalin: A bioactive phytochemical with potential therapeutic
activities. Adv Phar Sc. 2018:1–15. 2018.
|
|
154
|
Lee JH, Saxena A and Giaccone G:
Advancements in small cell lung cancer. Semin Cancer Biol.
93:123–128. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhou SB and Chen GZ: Research overview and
reflection on the treatment of cancer-related fatigue in lung
cancer by traditional Chinese medicine. Acta Chin Med Pharm.
52:1–4. 2024.(In Chinese).
|
|
156
|
Adelstein DJ, Moon J, Hanna E, Giri PGS,
Mills GM, Wolf GT and Urba SG: Docetaxel, cisplatin, and
fluorouracil induction chemotherapy followed by accelerated
fractionation/concomitant boost radiation and concurrent cisplatin
in patients with advanced squamous cell head and neck cancer: A
Southwest oncology group phase II trial (S0216). Head Neck.
32:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Andreyev HJN, Davidson SE, Gillespie C,
Allum WH and Swarbrick E; British Society of Gastroenterology;
Association of Colo-Proctology of Great Britain Ireland;
Association of Upper Gastrointestinal Surgeons; Faculty of Clinical
Oncology Section of the Royal College of Radiologists, : Practice
guidance on the management of acute and chronic gastrointestinal
problems arising as a result of treatment for cancer. Gut.
61:179–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Bower JE: The role of neuro-immune
interactions in cancer-related fatigue: Biobehavioral risk factors
and mechanisms. Cancer. 125:353–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
de Raaf PJ, Sleijfer S, Lamers CHJ, Jager
A, Gratama JW and van der Rijt CCD: Inflammation and fatigue
dimensions in advanced cancer patients and cancer survivors: an
explorative study. Cancer. 118:6005–6011. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wen YL, Li WY, Zhang N, Su RZ, Xia JR, Li
LF and Tian Y: The inhibitory effect of astragalin on escherichia
coli and its mechanism. J Yunnan Agricultural University.
37:471–477. 2022.
|
|
161
|
Oraibi AI, Dawood AH, Trabelsi G, Mahamat
OB, Chekir-Ghedira L and Kilani-Jaziri S: Antioxidant activity and
selective cytotoxicity in HCT-116 and WI-38 cell lines of LC-MS/MS
profiled extract from Capparis spinosa L. Front Chem.
13:15401742025. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yang X, Ma B, Zhang Q, Wu X, Gu G, Li J,
Sun J, Tang B, Zhu J, Qia H and Ying H: Comparative
pharmacokinetics with single substances and Semen Cuscutae extract
after oral administration and intravenous administration Semen
Cuscutae extract and single hyperoside and astragalin to rats. Anal
Methods. 6:72502014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Adamu UM, Renggasamy R, Stanslas J, Razis
AF, Fauzi F, Mulyani SW and Ramasamy R: In-silico prediction
analysis of polyphenolic contents of ethanolic extract of moringa
oleifera leaves. Malays J Med Health Sci. 19:9–15. 2023.
|
|
164
|
Liu J, Zou S, Liu W, Li J, Wang H, Hao J,
He J, Gao X, Liu E and Chang Y: An Established HPLC-MS/MS method
for evaluation of the influence of salt processing on
pharmacokinetics of six compounds in cuscutae semen. Molecules.
24:25022019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Choung WJ, Hwang SH, Ko DS, Kim SB, Kim
SH, Jeon SH, Choi HD, Lim SS and Shim JH: Enzymatic synthesis of a
novel Kaempferol-3-O-β-d-glucopyranosyl-(1→4)-O-α-d-glucopyranoside
using cyclodextrin glucanotransferase and its inhibitory effects on
aldose reductase, inflammation, and oxidative stress. J Agric Food
Chem. 65:2760–2767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Ren C, Xi Z, Xian B, Chen C, Huang X,
Jiang H, Chen J, Peng C and Pei J: Identification and
characterization of CtUGT3 as the key player of astragalin
biosynthesis in Carthamus Tinctorius L. J Agric Food Chem.
71:16221–16232. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Pei J, Dong P, Wu T, Zhao L, Fang X, Cao
F, Tang F and Yue Y: Metabolic engineering of Escherichia Coli for
astragalin biosynthesis. J Agric Food Chem. 64:7966–7972. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhang Y, Lei X, Xu H, Liu G, Wang Y, Sun
H, Geng F and Zhang N: Tissue distribution of total flavonoids
extracts of drynariae rhizoma in young and old rats by UPLC-MS/MS
determination. J Anal Methods Chem. 2022:1–30. 2022. View Article : Google Scholar
|
|
169
|
Li N, Lv T, Pan J, Liu C, Sun J, Lan Y,
Wang A, Li Y, Wang Y and Lu Y: Comparative tissue distribution of 6
major polyphenolic compounds in normal and myocardial ischemia
model rats after oral administration of the Polygonum
orientale L. Extract. Nat Prod Commun.
15:1934578X209294472020.
|
|
170
|
He B, Bai X, Tan Y, Xie W, Feng Y and Yang
GY: Glycosyltransferases: Mining, engineering and applications in
biosynthesis of glycosylated plant natural products. Synth Syst
Biotechnol. 7:602–620. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Han YM, Koh J, Kim JH, Lee J, Im JP and
Kim JS: Astragalin inhibits nuclear Factor-κB signaling in human
colonic epithelial cells and attenuates experimental colitis in
mice. Falk Symp. 15:100–108. 2021.
|