Introduction
Lung cancer persists as the leading cause of
cancer-related mortality in men and ranks second in women worldwide
(1). Notably, ~70% of lung cancer
cases are diagnosed at advanced stages with metastatic
dissemination (2). The adrenal
glands constitute a frequent metastatic site in this population,
with reported incidence rates ranging from 18 to 42% across
studies. Notably, 2–4% of these patients present with isolated
adrenal metastases that may be amenable to curative interventions
(3–5). Emerging evidence has demonstrated that
aggressive local therapies, including surgical resection and
stereotactic ablative radiotherapy, can markedly improve survival
outcomes in patients with solitary adrenal metastases (6,7). These
findings underscore the critical need for accurate restaging of
solitary adrenal masses in lung cancer management.
Nevertheless, differentiating metastatic lesions
from benign adrenal lesions in patients with lung cancer remains
clinically challenging (8),
particularly for small lesions [long diameter (LD) ≤3 cm] with
hyperattenuating features [unenhanced computed tomography (CT)
values ≥10 HU] (9–11). Although conventional imaging
modalities offer diagnostic parameters such as CT attenuation
values, delayed contrast-enhanced CT patterns and magnetic
resonance imaging (MRI) chemical-shift characteristics (12–14),
their clinical application faces three major limitations. First,
lesion-specific diagnostic thresholds may lack generalizability due
to heterogeneous tumor biology. Second, concurrent primary lung
malignancies can complicate radiological interpretation. Third,
practical challenges exist, including increased radiation exposure
from multiphase CT protocols, time-consuming MRI acquisitions that
disrupt clinical workflows and contraindications that compromise
image quality (such as motion artifacts or metallic implants)
(15,16).
18F-Fluorodeoxyglucose (FDG) positron
emission tomography (PET)/CT is an imaging modality that provides
both anatomical and metabolic information by assessing glucose
uptake. This technique has emerged as a reliable non-invasive tool
for evaluating adrenal masses (17,18).
However, its diagnostic accuracy is limited by two key factors: i)
Some benign adrenal lesions (for example, functional adenomas)
exhibit increased FDG uptake, potentially leading to
Tumor-Node-Metastasis stage overestimation (19,20);
and ii) certain adrenal metastases in patients with lung cancer may
remain undetected due to low FDG avidity (21). Additionally, previous studies have
primarily focused on individual metabolic parameters for
differentiating adrenal tumors (22,23),
while comprehensive PET/CT-based analyses of solitary small
hyperattenuating adrenal lesions in patients with lung cancer
remain scarce.
Support vector machine (SVM), a supervised machine
learning algorithm, is particularly effective for classification
tasks with limited sample sizes. SVM constructs an optimal decision
boundary by maximizing the margin between two classes in the
training dataset, thereby improving model generalizability
(24). SVM has shown promise in
classifying solitary pulmonary nodules and lymph nodes (25,26);
however, its application to solitary small hyperattenuating adrenal
lesions in lung cancer remains unexplored.
To address these gaps in the knowledge, the present
study aimed to develop an interpretable SVM-based classification
model to enhance the diagnostic accuracy of 18F-FDG
PET/CT in distinguishing between metastatic and benign solitary
small hyperattenuating adrenal lesions in patients with lung
cancer.
Patients and methods
Patients
Patients treated in Tangshan People's Hospital
(Tangshan, China) between October 2022 and October 2024 were
retrospectively included in the present study if they met the
following criteria: i) Histopathologically confirmed lung cancer
prior to 18F-FDG PET/CT examination; and ii) the
presence of a solitary small (LD ≤3 cm) hyperattenuating
(unenhanced CT value ≥10 HU) adrenal lesion. The current study was
approved by the Institutional Ethics Committee of Tangshan People's
Hospital (approval no. RMYY-LLKS-2023202). The requirement for
written informed consent was waived due to the retrospective nature
of the study. The diagnostic criteria for adrenal metastases were
as follows: i) Histopathological confirmation; or ii) interval
development of an adrenal mass on follow-up CT (compared with a
prior scan showing normal adrenal glands); or iii) short-term
interval growth [defined as a ≥20% increase in total tumor burden
within 6 months (27)]. The
diagnostic criteria for benign lesions were as follows: i)
Histopathological confirmation; or ii) stability in size (no
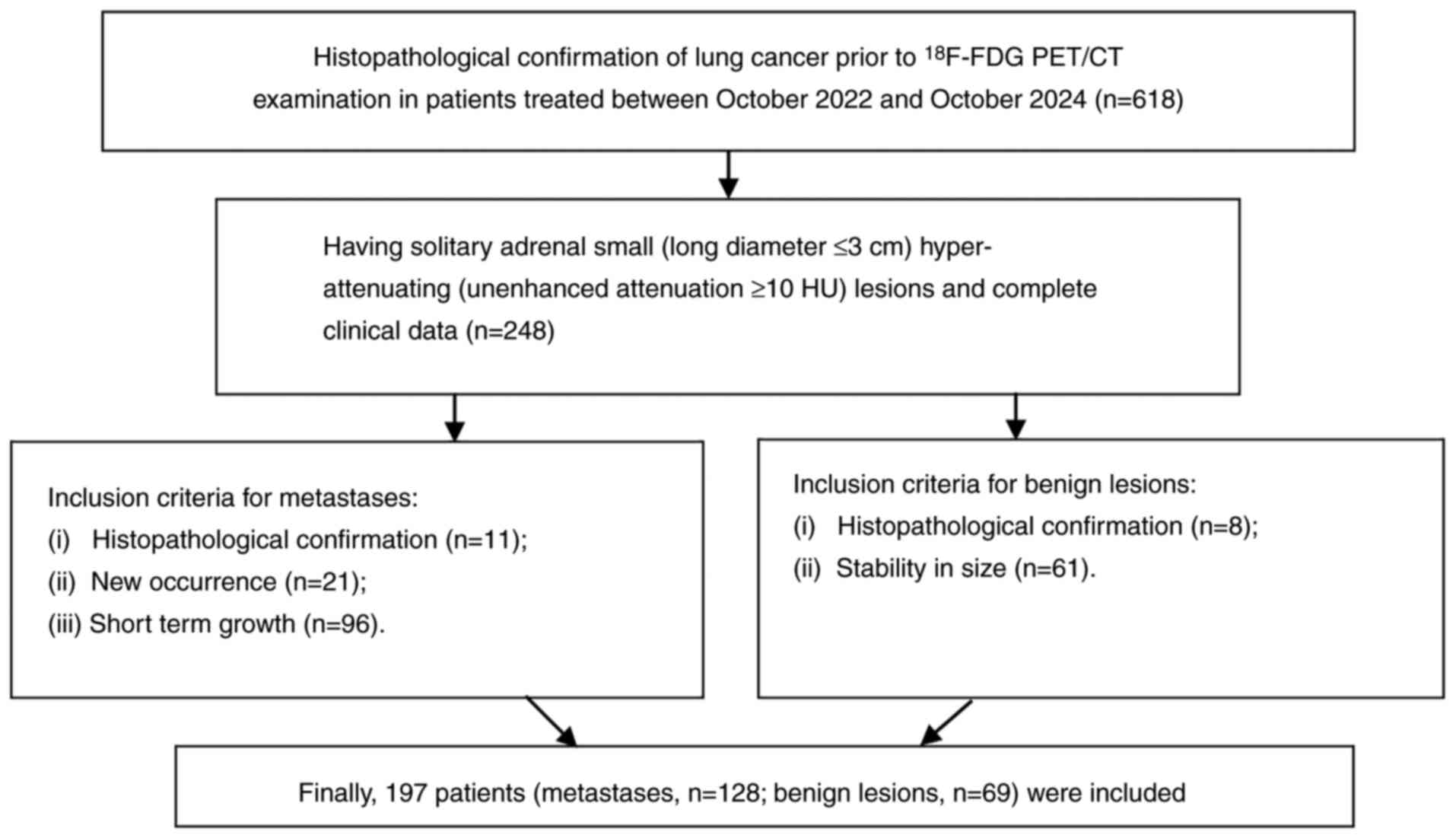
change) during ≥6 months of follow-up. A total of 197 patients (128
with metastases and 69 with benign lesions) were included to
develop and validate the models (Fig.
1).
18F-FDG PET/CT procedure. In the
present study, PET/CT imaging was performed using a Discovery MI
scanner (GE Healthcare). All patients received an intravenous
injection of 18F-FDG (4.2 MBq/kg), and imaging was
conducted ~60 min post-injection. Prior to the examination,
patients were required to fast for ≥6 h and to maintain blood
glucose levels at <11 mmol/l. Initially, unenhanced CT images
were acquired with the following parameters: 120 kV, 80 mAsec and a
slice thickness of 5 mm, covering the region from the skull vertex
to the mid-femur during tidal breathing. Subsequently, dedicated
full-ring PET images were obtained from the mid-thigh to the vertex
of the head during shallow breathing. Image reconstruction was
performed using an ordered-subset expectation maximization
algorithm with CT-based attenuation correction.
18F-FDG PET/CT image analysis. Two
nuclear medicine radiologists with 4 and 6 years of PET/CT
diagnostic experience, respectively, independently reviewed the
PET/CT images. Disagreements between the two radiologists were
settled by consensus. On the CT component, the following adrenal
nodule characteristics were assessed: i) Short diameter (SD) and
LD; ii) location (left or right); iii) homogeneity (homogeneous or
heterogeneous); iv) unenhanced CT value, measured by manually
placing a region of interest (ROI) covering two-thirds of the
largest transverse lesion section while avoiding adjacent fat
tissue. In addition, ROI measurements excluded areas with
calcification, hemorrhagic components, cystic degeneration or
necrosis. On the PET component, the maximum standardized uptake
value (SUVmax) was measured by drawing a circular-oval
ROI encompassing the entire adrenal nodule and primary lung cancer,
carefully avoiding adjacent FDG-avid structures. For reference, an
additional ROI was placed in the right posterior superior liver
segment. The following metabolic ratios were calculated:
Adrenal-to-liver SUVmax ratio
(SURadrenal/liver) and lung-to-liver SUVmax
ratio (SURlung/liver). Therefore, six key features were
analyzed for each case: i) Morphological characteristics: Size (SD
and LD), location, homogeneity and unenhanced CT value; and ii)
metabolic parameters: Adrenal nodules [SUVmax(adrenal
and SURadrenal/liver] and lung cancer
(SUVmax(lung) and SURlung/liver).
Statistical analysis and model
development
In the present study, the models were constructed
using an SVM with a linear kernel function and a regularization
parameter (C) set to 1. To enhance model interpretability, a single
feature from either the metabolic or size features of adrenal
lesions or the metabolic features of lung cancer was iteratively
selected, combining it with other feature types to build each
model. Using stratified random sampling, the study cohort was
divided into training (n=148; 96 metastases and 52 benign lesions)
and validation (n=49; 32 metastases and 17 benign lesions) subsets
maintaining a 3:1 allocation ratio. To mitigate sampling bias, this
sampling process was repeated 1,000 times. During this process, the
area under the receiver operating characteristic curve (AUC) values
and accuracies of the models in the validation subsets were used to
evaluate their performance. To assess whether the classification
accuracy exceeded chance level, a null distribution of accuracies
was generated by randomly shuffling the metastatic status labels of
adrenal lesions during classifier training, thereby eliminating any
predictive information. The P-value was calculated by comparing the
actual classification accuracy (derived from correctly labeled
data) against the null distribution, defined as the proportion of
null accuracies equal to or greater than the observed accuracy
(28). Permutation tests were
conducted for the best-performing SVM model (namely the models with
the highest accuracy). To further evaluate whether SVM was the
optimal method for metastasis prediction, its performance was
compared with random forest and k-nearest neighbor (KNN)
classifiers. Each algorithm was run 1,000 times under identical
conditions to ensure comparability, and the distributions of
accuracy and AUC were analyzed. For the random forest model, five
decision trees were used, with a minimum tree depth of 1 and the
number of features per tree set to the square root of the total
feature count. For KNN, k was set at 5. To enhance clinical
applicability, the model was simplified by scoring features based
on their weights and the distribution of continuous variables. The
final risk score was computed as the sum of each feature's score
multiplied by its mean weight. All analyses were performed using
Python (version 3.12; Python Software Foundation). The AUC and
accuracy of different models were compared using the Kruskal-Wallis
test with Dunn's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics and PET/CT
features
The clinical characteristics of patients in both
cohorts are summarized in Table I.
The retrospective dataset comprised 197 patients (age range, 28–85
years; males, 126; females, 71), including 128 metastatic lesions
and 69 benign adrenal lesions. Among the lung cancer cases,
adenocarcinoma represented the predominant histological subtype
(134/197, 68.0%), followed by squamous cell carcinoma (48/197,
24.4%). Other histological variants included adenosquamous
carcinoma (7/197, 3.6%), large cell carcinoma (4/197, 2.0%),
sarcomatoid carcinoma (3/197, 1.5%) and pleomorphic carcinoma
(1/197, 0.5%). Table II summarizes
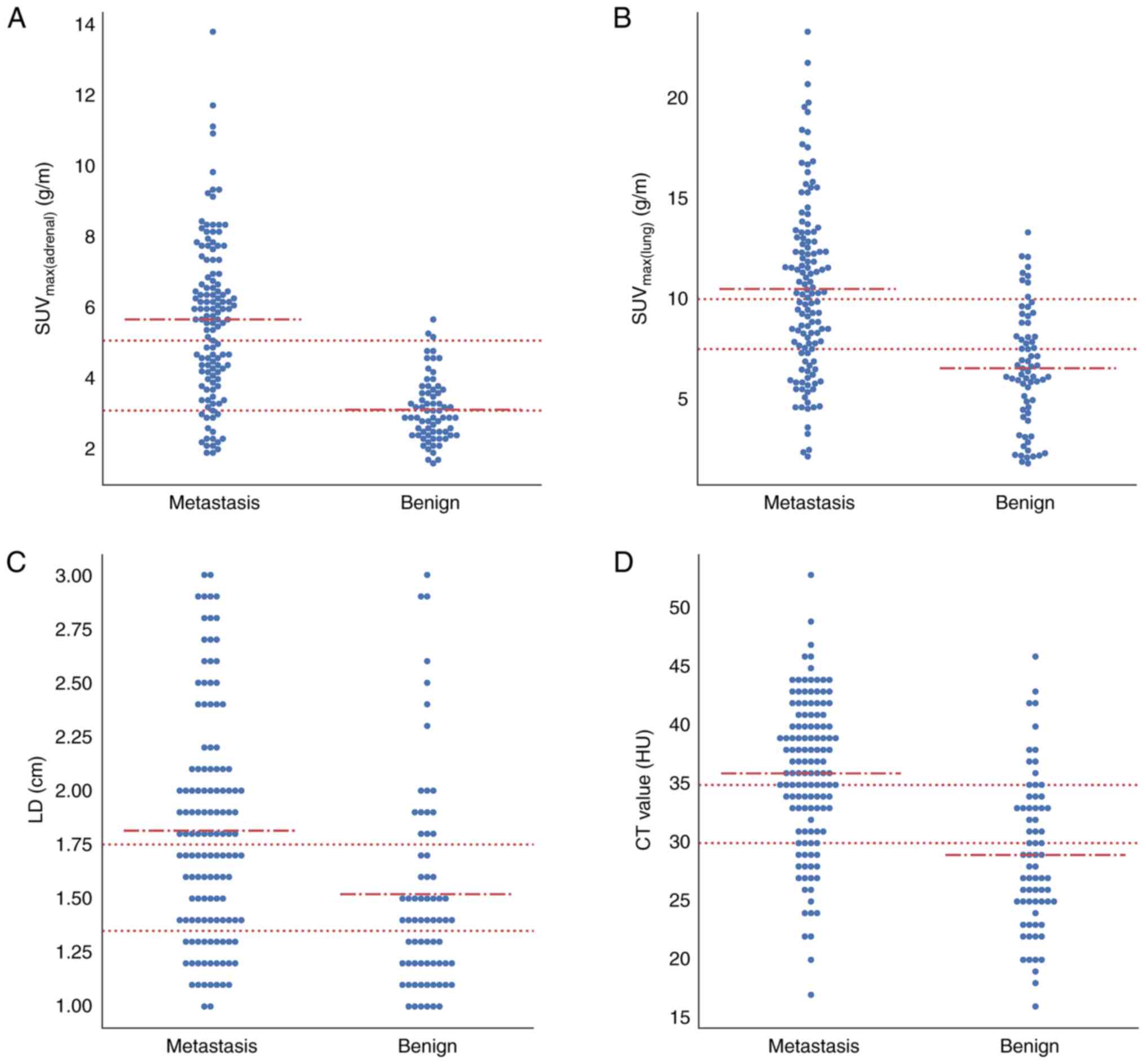
six key PET/CT features for both cohorts, including size (SD and
LD), metabolism (SUVmax(adrenal) and
SURadrenal/liver), unenhanced CT value, location and
homogeneity of adrenal nodules, and metabolism of lung cancer
(SUVmax(lung) and SURlung/liver).
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
|
Characteristics | Distribution |
|---|
| Sex, n |
|
|
Male | 126 |
|
Female | 71 |
| Age, years |
|
|
Range | 28-85 |
| Mean ±
standard deviation | 62.71±8.99 |
|
Median | 63 |
| Histological
subtype of lung cancer, n |
|
|
Adenocarcinoma | 134 |
|
Squamous cell carcinoma | 48 |
| Other
types | 15 |
|
Adenosquamous
carcinoma | 7 |
|
Large cell
carcinoma | 4 |
|
Sarcomatoid
carcinoma | 3 |
|
Pleomorphic
carcinoma | 1 |
| Group, n |
|
|
Metastases | 128 |
| Benign
lesions | 69 |
 | Table II.Positron emission tomography/CT
features of patients. |
Table II.
Positron emission tomography/CT
features of patients.
| Features | Metastases
(n=128) | Benign lesions
(n=69) |
|---|
| Size of adrenal
lesions, cma |
|
|
| SD | 1.39±0.41 | 1.23±0.33 |
| LD | 1.81±0.51 | 1.52±0.48 |
| Metabolism of
adrenal lesionsa |
|
|
|
SUVmax(adrenal),
g/m | 5.60±2.24 | 3.03±0.91 |
|
SURadrenal/liver | 1.74±0.69 | 1.02±0.26 |
|
Unenhanced CT value of adrenal
lesionsa | 35.97±6.40 | 28.99±6.48 |
| Location of adrenal
lesions, n |
|
|
|
Left | 83 | 48 |
|
Right | 45 | 21 |
| Homogeneity of
adrenal lesions, n |
|
|
|
Homogeneous | 108 | 54 |
|
Heterogeneous | 20 | 15 |
| Metabolism of lung
cancera |
|
|
|
SUVmax(lung),
g/m | 10.50±4.33 | 6.55±2.92 |
|
SURlung/liver | 3.27±1.35 | 2.25±1.06 |
Comparison of different models
A total of eight distinct SVM models were developed
in the present study, with their respective feature compositions
detailed in Table III. For each
model, 1,000 iterations of sampling were performed to ensure robust
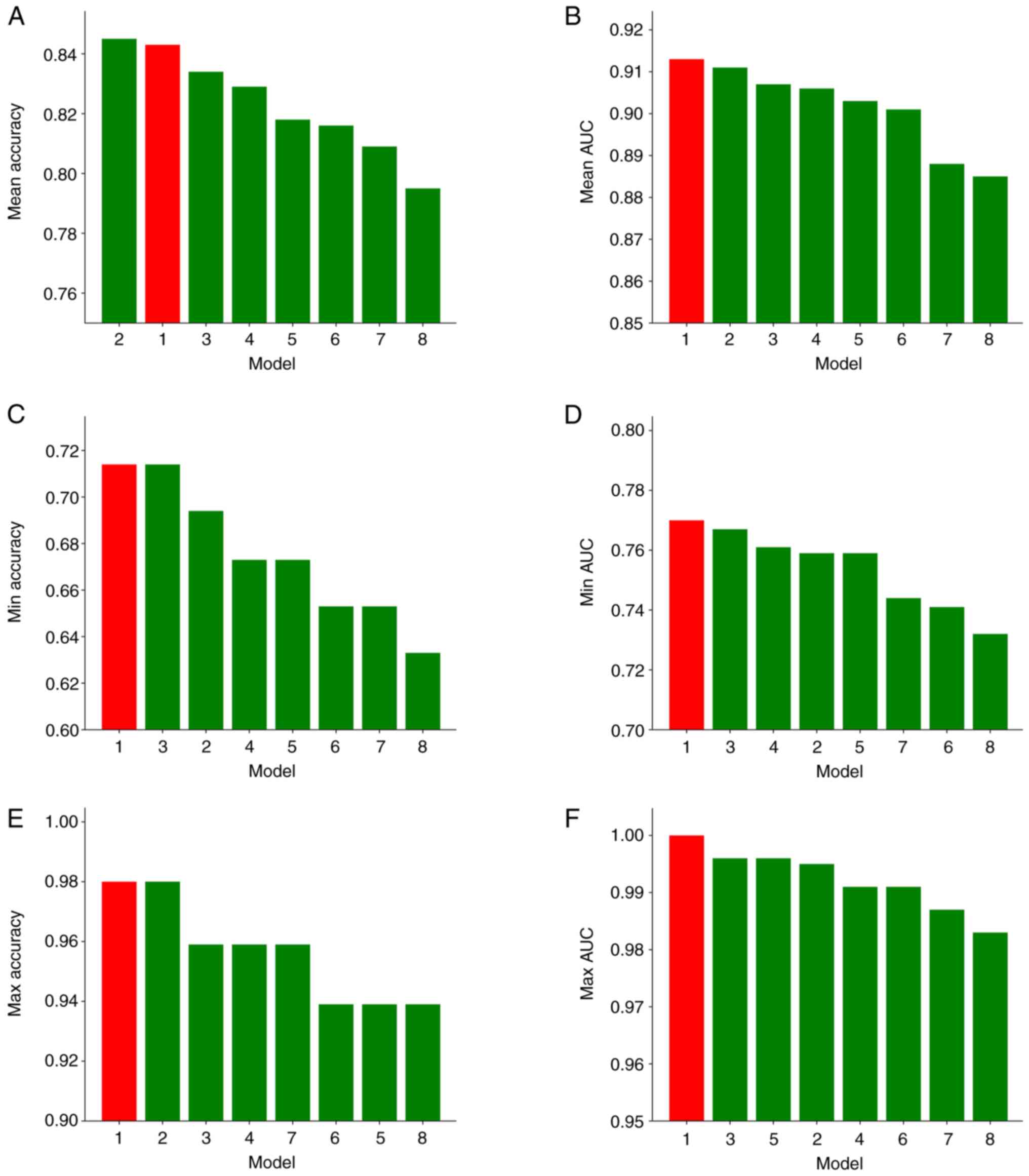
statistical evaluation. Model 1 emerged as the top-performing
model, demonstrating superior performance across multiple metrics,
including i) AUC: Maximum, 1.000; mean, 0.913; and minimum, 0.770;
and ii) accuracy: Maximum, 98.0% (95% CI, 93.9–100%); mean, 84.3%
(95% CI, 69.4–91.8%); and minimum, 71.4% (95% CI, 57.1–83.7%)
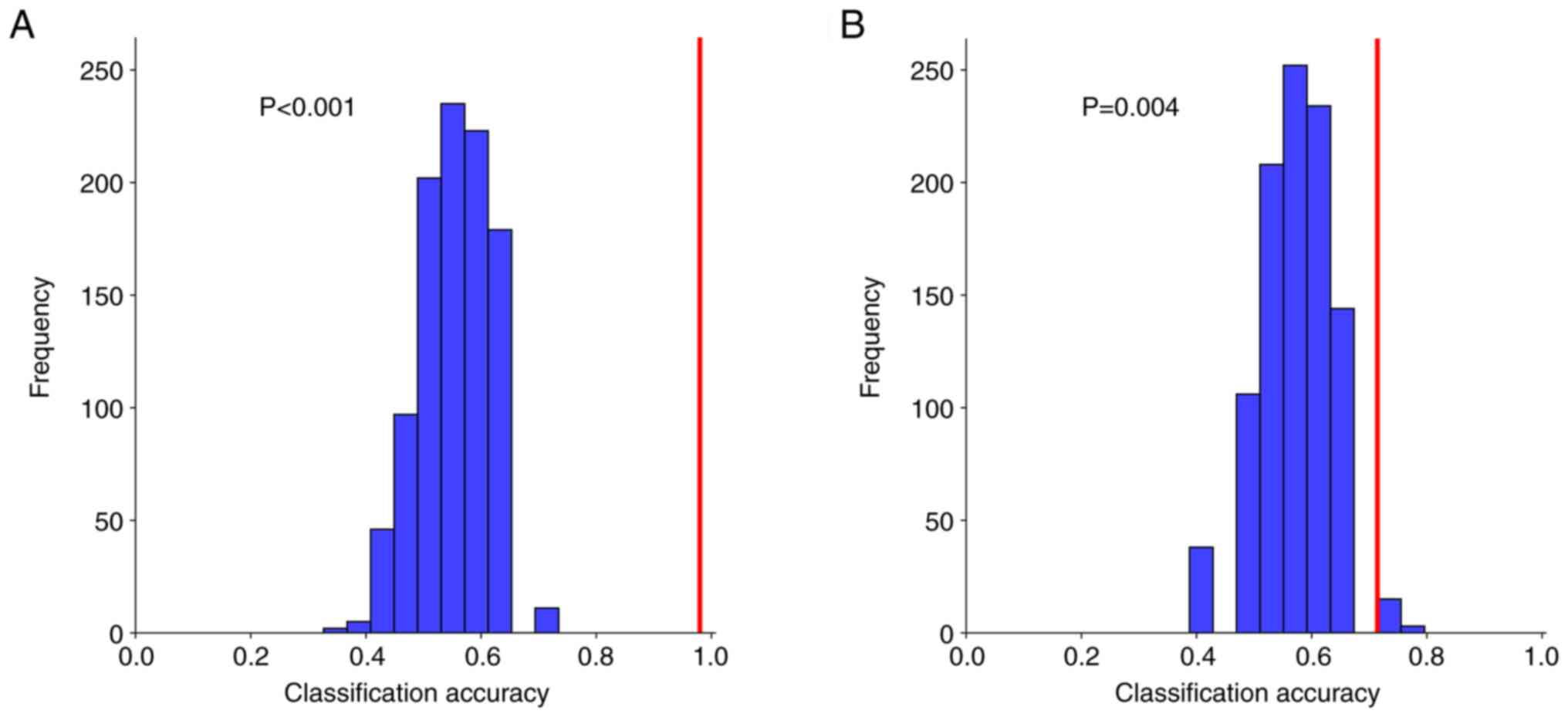
(Fig. 2). Permutation tests for
Model 1 yielded statistically significant results (P<0.001 for
the highest-accuracy iteration; P=0.004 for the lowest-accuracy
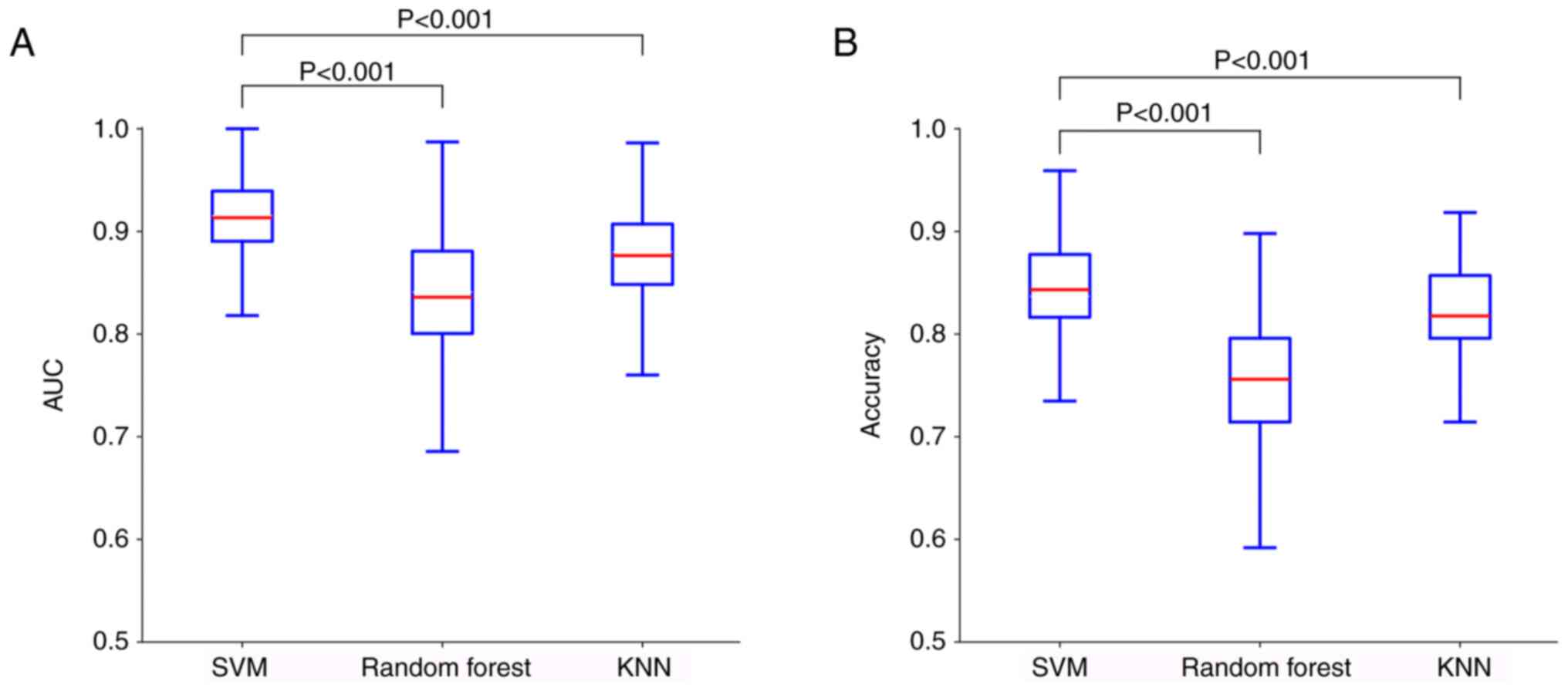
iteration) (Fig. 3). To validate
the predictive superiority of SVM, its performance was compared
against identically configured random forest and KNN models. SVM
significantly outperformed both alternatives in terms of accuracy
(both P<0.001) and AUC (both P<0.001) (Fig. 4).
 | Table III.Combination of different types of
features. |
Table III.
Combination of different types of
features.
| Model number | Metabolism of
adrenal lesions | Size of adrenal
lesions | Metabolism of lung
cancers | Unenhanced CT value
of adrenal lesions | Location of adrenal
lesions | Homogeneity of
adrenal lesions |
|---|
| 1 |
SUVmax(adrenal) | LD |
SUVmax(lung) | Hounsfield
unit | Right/left | Yes/no |
| 2 | SUV
max(adrenal) | LD |
SURlung/liver | Hounsfield
unit | Right/left | Yes/no |
| 3 | SUV
max(adrenal) | SD | SUV
max(lung) | Hounsfield
unit | Right/left | Yes/no |
| 4 | SUV
max(adrenal) | SD |
SURlung/liver | Hounsfield
unit | Right/left | Yes/no |
| 5 |
SURadrenal/liver | LD | SUV
max(lung) | Hounsfield
unit | Right/left | Yes/no |
| 6 |
SURadrenal/liver | LD |
SURlung/liver | Hounsfield
unit | Right/left | Yes/no |
| 7 |
SURadrenal/liver | SD | SUV
max(lung) | Hounsfield
unit | Right/left | Yes/no |
| 8 |
SURadrenal/liver | SD |
SURlung/liver | Hounsfield
unit | Right/left | Yes/no |
Feature weights of the best model
Table IV presents
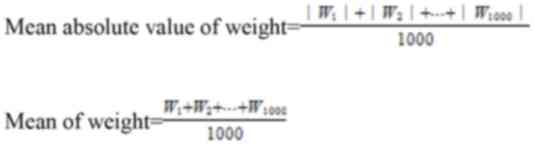
the 18F-FDG PET/CT feature weights of Model 1. The mean
absolute value of weights and the mean of weight were calculated as
follows:
 | Table IV.Weight of features for the best
model. |
Table IV.
Weight of features for the best
model.
|
Characteristics | Mean weight | Mean absolute value
of weight |
|---|
| Homogeneity of
adrenal lesions | −0.177±0.097 | 0.179±0.094 |
| Location of adrenal
lesions | −0.146±0.158 | 0.183±0.115 |
| Unenhanced CT value
of adrenal lesions | 0.702±0.114 | 0.702±0.114 |
| LD of adrenal
lesions | 0.443±0.112 | 0.443±0.112 |
|
SUVmax(adrenal) | 1.247±0.170 | 1.247±0.170 |
|
SUVmax(lung) | 0.709±0.137 | 0.709±0.137 |
The mean absolute weight reflects each feature's
relative importance in the model. A positive mean weight indicates
a positive association with metastases, whereas a negative value
suggests an inverse association. Key findings regarding feature
importance and association included: i) Homogeneity of adrenal
lesions had the lowest mean absolute value of weight; ii)
SUVmax(adrenal) had the highest mean absolute value of
weight; iii) homogeneity and location of adrenal lesions were
negatively correlated with metastases; and iv)
SUVmax(adrenal), SUVmax(lung), unenhanced CT
value and LD were positively correlated with metastases.
Adrenal metastasis scoring rule
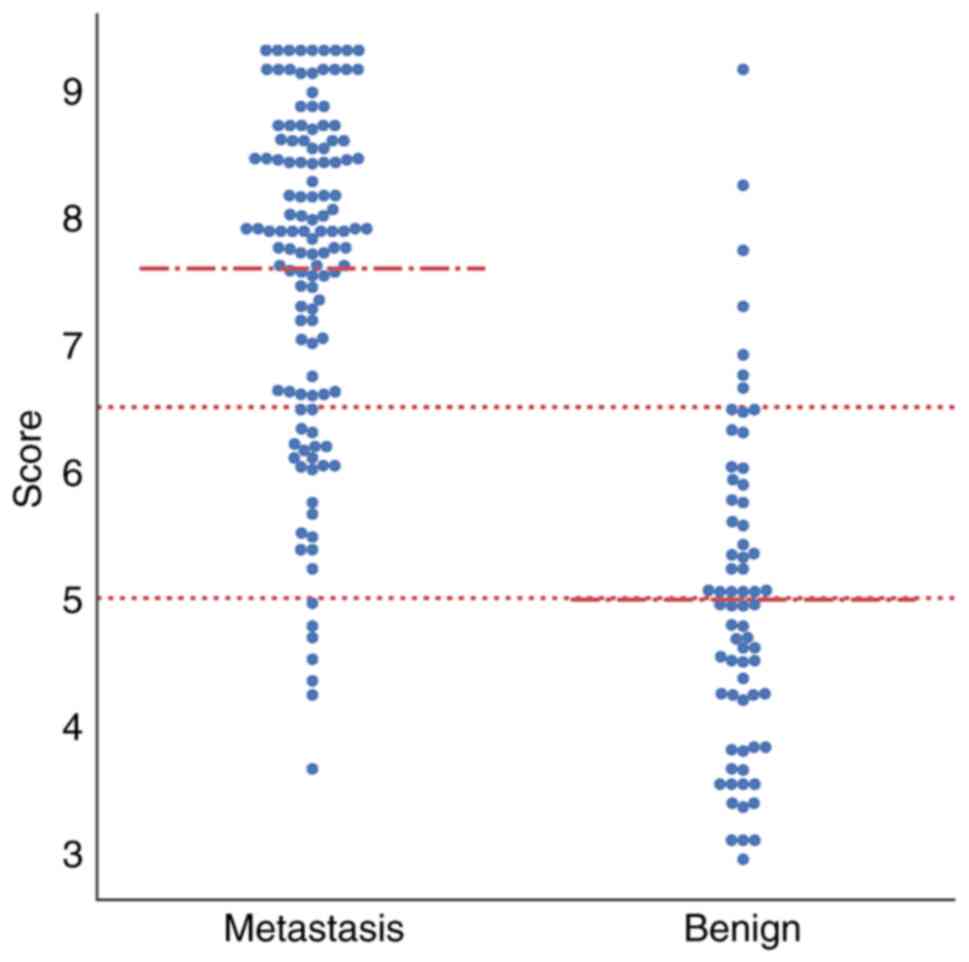
Fig. 5 displays the
distributions of SUVmax(adrenal),
SUVmax(lung), LD and unenhanced CT value. To facilitate
subsequent model simplification, each continuous variable was
categorized using thresholds derived from the approximate mean
values of the metastatic and benign groups. The variables were
categorized as follows: SUVmax(adrenal) was separated
into <3, 3–5 and ≥5 g/m; SUVmax(lung) was separated
into <7.5, 7.5–10 and ≥10 g/m; LD was separated into <1.35,
1.35–1.75 and ≥1.75 cm; and unenhanced CT value was separated into
<30, 30–35 and ≥35 HU. The mean weights were used as feature
coefficients for scoring. Table V
presents the detailed scoring system based on these distributions
and coefficients, whereby each factor was assigned a score
according to predetermined thresholds, and the final risk score was
calculated as the sum of each factor's score multiplied by its mean
weighting coefficient. Fig. 6
illustrates the final score distribution. The final scores were as
follows: 56.5% (39/69) of adrenal benign lesions scored <5,
whereas only 7.03% (9/128) of adrenal metastases scored <5.
Furthermore, only 5.80% (4/69) of the final scores of benign
lesions were >6.5, but 68.8% (88/128) of those for metastases
were >6.5. Finally, the following diagnostic criteria were
established: Adrenal nodules with scores <5 were benign; those
with scores >6.5 were metastatic; and those with scores 5–6.5
were suspicious for metastasis.
 | Table V.Final scoring rules for predicting
adrenal metastases. |
Table V.
Final scoring rules for predicting
adrenal metastases.
|
| SUVmax
(adrenal), g/m | SUVmax
(lung), g/m | LD, cm | Unenhanced CT
value, HU | Location | Homogeneity |
|---|
|
|
|
|
|
|
|
|
|---|
| Factor | <3 | 3-5 | ≥5 | <7.5 | 7.5–10 | ≥10 | <1.35 | 1.35–1.75 | ≥1.75 | <30 | 30-35 | ≥35 | L | R | Yes | No |
|---|
| Score | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 0 | 1 | 0 | 1 |
| Coefficient | 1.25 | 0.71 | 0.44 | 0.70 | −0.15 | −0.18 |
Discussion
The adrenal gland is a frequent site of metastasis,
particularly in patients with lung cancer (29). However, differentiating metastatic
from benign adrenal lesions in these patients remains clinically
challenging due to the overlapping prevalence of neoplastic and
non-neoplastic adrenal masses (30,31).
Compared with conventional CT or MRI, 18F-FDG PET/CT
offers distinct advantages by providing both functional metabolic
data and anatomical information. This synergistic capability has
verified the use of PET/CT as an invaluable tool in the evaluation
of primary lung cancer and its metastatic spread, particularly in
characterizing rare soft-tissue masses (32) and indeterminate solitary
hyperattenuating adrenal lesions (33). In the current study, an SVM model
was developed using 18F-FDG PET/CT parameters that
demonstrated superior performance to both KNN and random forest
algorithms, achieving high predictive accuracy (AUC=0.913) for
identifying adrenal metastases. Among the predictive features,
SUVmax(adrenal) emerged as the most significant
contributor, with a mean absolute weight of 1.25 in the model. To
enhance clinical applicability, the model was subsequently
simplified into a practical scoring system. This streamlined
approach maintains diagnostic accuracy while facilitating
implementation in routine clinical practice.
Recent studies have demonstrated the promising
diagnostic performance of 18F-FDG PET/CT in detecting
adrenal metastases in patients with lung cancer (23,34,35).
Evans et al (34) reported
that SUVmax exhibited marked diagnostic efficacy, with a
mean sensitivity of 91%, specificity of 81% and accuracy of 83% for
identifying adrenal metastases. Similarly, Orzechowski et al
(23) revealed that SUVs provided
reliable assessment of adrenal metastases, achieving an AUC of
0.83. Notably, Kim et al (35) demonstrated even higher diagnostic
accuracy using SURadrenal/liver (AUC, 0.933;
sensitivity, 87%; specificity, 100%) in patients with non-small
cell lung cancer. However, the small sample size of this previous
study (n=24 patients with suspicious adrenal masses) may limit the
generalizability of its findings. A critical limitation common to
these studies is their reliance on single metabolic parameters for
metastasis prediction. As established in the literature,
single-parameter approaches have inherent constraints: i) They
often demonstrate suboptimal diagnostic performance, and ii) they
cannot comprehensively characterize the multifaceted nature of
adrenal metastases. To enhance diagnostic accuracy, recent studies
have explored multi-parameter approaches for predicting adrenal
metastases (9,36,37).
Brady et al (9) demonstrated
that combining SUVmax >3.1 with mean attenuation
>10 HU achieved excellent diagnostic performance (sensitivity,
97.3%; specificity, 86.2%; accuracy, 90.5%). Similarly, Cho et
al (36) reported that an
adrenal-to-liver SUVmax ratio >1.3 combined with HU
>18 yielded a sensitivity of 97.7%, a specificity of 81.2% and
an accuracy of 93.4% in patients with lung cancer. Notably, Lu
et al (37) revealed that
integrating PET and CT features achieved optimal performance
(sensitivity, 100%; specificity, 98%; accuracy, 99%). These
findings collectively suggest that combining PET and CT parameters
may provide superior predictive value for adrenal metastases in
clinical practice. However, most previous studies included adrenal
masses of all sizes, limiting their applicability to solitary small
hyperattenuating lesions, a particularly challenging diagnostic
scenario where false-negative results frequently occur (1,19,34,35).
To address this limitation, the current study developed an SVM
model incorporating multiple clinically relevant PET and CT
features specifically for small adrenal lesions. The model
demonstrated robust performance, with a mean AUC of 0.913 and
accuracy of 84.3%. Notably, the clinical utility of the model was
enhanced by: i) Improving interpretability through feature
importance analysis, and ii) developing a simplified scoring system
to facilitate clinical staging decisions in patients with lung
cancer.
In the current linear SVM model, feature importance
was quantified by the mean absolute weight, where higher values
indicated stronger predictive contributions for adrenal metastases.
Among all features, SUVmax(adrenal) demonstrated the
weight of highest importance. This finding aligns with established
literature demonstrating the diagnostic value of metabolic
parameters such as SUVmax in detecting adrenal
metastases (18,20,38).
Koopman et al (38) reported
significantly higher SUVmax values in metastatic vs.
benign adrenal lesions, with optimal diagnostic performance (96%
sensitivity and specificity) at a cutoff of 3.7 for detecting
metastatic adrenal lesions in patients with lung cancer. The
biological basis for this observation relates to SUV being a
surrogate for Km (the absolute metabolic rate of glucose
consumption). Malignant lesions typically exhibit elevated Km
values due to their characteristically increased glucose metabolism
(39). The prominent weight of
SUVmax(adrenal) in the present model substantiates its
critical diagnostic role. Additionally, SUVmax(lung)
showed considerable predictive weight, suggesting that higher
metabolic activity in primary lung tumors may be associated with
greater metastatic potential. This association has been previously
documented (40,41): Zhang et al (40) identified primary tumor
SUVmax as a significant predictor of lymph node
metastasis and Zhu et al (41) similarly reported that elevated
SUVmax in non-small cell lung cancer is associated with
increased metastatic risk.
Multiple studies have established that while PET
features may yield false-negative results in cases of
micro-metastases, integrated PET/CT demonstrates superior
diagnostic accuracy for detecting adrenal metastases compared with
PET or CT alone (1,36,37).
This underscores the importance of incorporating CT features to
enhance diagnostic performance. The present analysis revealed that
unenhanced CT value served a corrective function in the model. This
finding aligns with the existing literature demonstrating that
adrenal metastases typically exhibit higher attenuation values than
benign lesions (36,42). For example, Cho et al
(36) reported significant
differences in unenhanced CT values between metastatic (29±8 HU)
and non-metastatic (9±13 HU) adrenal lesions, with a HU threshold
of >18 achieving an AUC of 0.925 (sensitivity, 86.7%;
specificity, 81.2%; accuracy, 85.2%). Chen et al (43) subsequently identified a pre-contrast
CT value of >30 HU as an independent predictor of metastases
(AUC, 0.766), a potentially more robust criterion. The underlying
pathophysiology relates to adipose tissue content, which serves as
a key diagnostic marker for adenomas (42). The present results corroborate these
established attenuation patterns. Furthermore, lesion size emerged
as another significant CT parameter, with metastases typically
demonstrating larger dimensions than benign lesions, which was
consistent with previous research (23,34).
Evans et al (34) documented
markedly larger metastatic lesions (mean, 3.0 cm; range, 1.0–9.2
cm) vs. benign lesions (mean, 1.9 cm; range, 0.7–5.3 cm). In
addition, Orzechowski et al (23) reported that a size threshold of
>25 mm yielded 83.33% sensitivity, 83.64% specificity and 83.49%
accuracy for metastasis detection.
The location and homogeneity of adrenal lesions also
contributed to the present predictive model. It was observed that
adrenal metastases demonstrated a left-sided predominance and more
heterogeneous appearance, consistent with prior studies (22,43).
However, the mean absolute weights for these features were markedly
lower than those of the four primary parameters
[SUVmax(adrenal), SUVmax(lung), unenhanced CT
value and size].
The current study has several limitations that
should be acknowledged. First, the subjective interpretation of
certain imaging features (particularly lesion homogeneity) may vary
between radiologists, with more experienced practitioners likely
demonstrating greater diagnostic accuracy. Future incorporation of
computer-assisted feature evaluation could enhance measurement
reproducibility and model stability. Second, as a single-center
study with a relatively small sample size, the findings may be
specific to the particular PET system, acquisition protocol and
reconstruction algorithm employed. Multi-center validation studies
with larger cohorts are needed to establish the generalizability of
the coefficients across different imaging platforms. Third, in the
21 patients with interval development of adrenal metastases, the
SUVmax of the primary lung cancer may have changed due
to tumor progression or recurrence. Future studies with larger
cohorts are warranted to systematically assess whether the change
in SUVmax has diagnostic value in differentiating
adrenal metastases and whether it affects the performance of the
model. Fourth, it is notable that some of the patients with lung
cancer included in the present study also had comorbid fatty liver.
Hepatic steatosis in patients with cancer may alter metabolic
activity and influence 18F-FDG uptake (44). Although the SUR was not a parameter
in the final model, further corrections should be considered in
future studies when the liver is used as a comparator for PET-CT
scans in patients with lung cancer.
In conclusion, the present study describes the
development of an interpretable SVM model using 18F-FDG
PET/CT features to predict adrenal metastases in patients with lung
cancer. The model identified SUVmax(adrenal) as the most
significant predictor, followed by SUVmax(lung),
unenhanced CT value, size, homogeneity and location. For clinical
implementation, the SVM model was simplified into a practical
scoring system that may facilitate staging decisions for patients
with lung cancer.
Acknowledgements
Not applicable.
Funding
This study received funding from the Tangshan City Key Research
and Development Program (grant no. 24150216C).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ, HC, WZ, JL, YL and LC designed the study. ZL, CH
and ZW collected the patient images, performed the statistical
analysis and wrote the manuscript. CL, LJ and LY critically
reviewed the manuscript. All authors contributed to the article and
have read and approved the final manuscript. CL and ZL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Tangshan People's Hospital (Tangshan, China). The
requirement for informed consent was waived due to the
retrospective nature of the study, and the study was performed in
accordance with The Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the receiver operating
characteristic curve
|
|
FDG
|
fluorodeoxyglucose
|
|
KNN
|
k-nearest neighbor
|
|
LD
|
long diameter
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
ROI
|
region of interest
|
|
SVM
|
support vector machine
|
|
SD
|
short diameter
|
|
SUVmax
|
maximum standardized uptake value
|
|
SURadrenal/liver
|
adrenal-to-liver SUVmax
ratio
|
|
SURlung/liver
|
lung-to-liver SUVmax
ratio
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Besse B, Adjei A, Baas P, Meldgaard P,
Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, et
al: 2nd ESMO consensus conference on lung cancer: Non-small-cell
lung cancer first-line/second and further lines of treatment in
advanced disease. Ann Oncol. 25:1475–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burt M, Heelan RT, Coit D, McCormack PM,
Bains MS, Martini N, Rusch V and Ginsberg RJ: Prospective
evaluation of unilateral adrenal masses in patients with operable
non-small-cell lung cancer. Impact of magnetic resonance imaging. J
Thorac Cardiovasc Surg. 107:584–599. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawai N, Tozawa K, Yasui T, Moritoki Y,
Sasaki H, Yano M, Fujii Y and Kohri K: Laparoscopic adrenalectomy
for solitary adrenal metastasis from lung cancer. JSLS.
18:e2014.00062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porte HL, Roumilhac D, Graziana JP, Eraldi
L, Cordonier C, Puech P and Wurtz AJ: Adrenalectomy for a solitary
adrenal metastasis from lung cancer. Ann Thorac Surg. 65:331–335.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao XL, Zhang KW, Tang MB, Zhang KJ, Fang
LN and Liu W: Pooled analysis for surgical treatment for isolated
adrenal metastasis and non-small cell lung cancer. Interact
Cardiovasc Thorac Surg. 24:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holy R, Piroth M, Pinkawa M and Eble MJ:
Stereotactic body radiation therapy (SBRT) for treatment of adrenal
gland metastases from non-small cell lung cancer. Strahlenther
Onkol. 187:245–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao L, Yang H, Yao D, Cai H, Wu H, Yu Y,
Zhu L, Xu W, Liu Y and Li J: Clinical-imaging-radiomic nomogram
based on unenhanced CT effectively predicts adrenal metastases in
patients with lung cancer with small hyperattenuating adrenal
incidentalomas. Oncol Lett. 28:3402024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brady MJ, Thomas J, Wong TZ, Franklin KM,
Ho LM and Paulson EK: Adrenal nodules at FDG PET/CT in patients
known to have or suspected of having lung cancer: A proposal for an
efficient diagnostic algorithm. Radiology. 250:523–530. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeiger MA, Siegelman SS and Hamrahian AH:
Medical and surgical evaluation and treatment of adrenal
incidentalomas. J Clin Endocrinol Metab. 96:2004–2015. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao L, Yang H, Wu H, Zhong H, Cai H, Yu Y,
Zhu L, Liu Y and Li J: Adrenal indeterminate nodules: CT-based
radiomics analysis of different machine learning models for
predicting adrenal metastases in lung cancer patients. Front Oncol.
14:14112142024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caoili EM, Korobkin M, Francis IR, Cohan
RH, Platt JF, Dunnick NR and Raghupathi KI: Adrenal masses:
Characterization with combined unenhanced and delayed enhanced CT.
Radiology. 222:629–633. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujiyoshi F, Nakajo M, Fukukura Y and
Tsuchimochi S: Characterization of adrenal tumors by chemical shift
fast low-angle shot MR imaging: Comparison of four methods of
quantitative evaluation. AJR Am J Roentgenol. 180:1649–1657. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayo-Smith WW, Song JH, Boland GL, Francis
IR, Israel GM, Mazzaglia PJ, Berland LL and Pandharipande PV:
Management of incidental adrenal masses: A white paper of the ACR
incidental findings committee. J Am Coll Radiol. 14:1038–1044.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haider MA, Ghai S, Jhaveri K and Lockwood
G: Chemical shift MR imaging of hyperattenuating (>10 HU)
adrenal masses: Does it still have a role? Radiology. 231:711–716.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koo HJ, Choi HJ, Kim HJ, Kim SO and Cho
KS: The value of 15-minute delayed contrast-enhanced CT to
differentiate hyperattenuating adrenal masses compared with
chemical shift MR imaging. Eur Radiol. 24:1410–1420. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsiao R, Chow A, Kluijfhout WP, Bongers
PJ, Verzijl R, Metser U, Veit-Haibach P and Pasternak JD: The
clinical consequences of functional adrenal uptake in the absence
of cross-sectional mass on FDG-PET/CT in oncology patients.
Langenbecks Arch Surg. 407:1677–1684. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arikan AE, Makay O, Teksoz S, Vatansever
S, Alptekin H, Albeniz G, Demir A, Ozpek A and Tunca F: Efficacy of
PET-CT in the prediction of metastatic adrenal masses that are
detected on follow-up of the patients with prior nonadrenal
malignancy: A nationwide multicenter case-control study. Medicine
(Baltimore). 101:e302142022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lang BH, Cowling BJ, Li JY, Wong KP and
Wan KY: High false positivity in positron emission tomography is a
potential diagnostic pitfall in patients with suspected adrenal
metastasis. World J Surg. 39:1902–1908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Detterbeck FC, Woodard GA, Bader AS, Dacic
S, Grant MJ, Park HS and Tanoue LT: The proposed ninth edition TNM
classification of lung cancer. Chest. 166:882–895. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vos EL, Grewal RK, Russo AE, Reidy-Lagunes
D, Untch BR, Gavane SC, Boucai L, Geer E, Gopalan A, Chou JF, et
al: Predicting malignancy in patients with adrenal tumors using
18F-FDG-PET/CT SUVmax. J Surg Oncol. 122:1821–1826.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu B, Gao J, Cui L, Wang H, Guan Z, Yao S,
Shen Z and Tian J: Characterization of adrenal metastatic cancer
using FDG PET/CT. Neoplasma. 59:92–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orzechowski S, Gnass M, Czyżewski D,
Wojtacha J, Sudoł B, Pankowski J, Zajęcki W, Ćmiel A, Zieliński M
and Szlubowski A: Ultrasound predictors of left adrenal metastasis
in patients with lung cancer: A comparison of computed tomography,
positron emission tomography-computed tomography, and endoscopic
ultrasound using ultrasound bronchoscope. Pol Arch Intern Med.
132:161272022.PubMed/NCBI
|
|
24
|
Noble WS: What is a support vector
machine? Nat Biotechnol. 24:1565–1567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin G, Song Y, Li X, Zhu L, Su Q, Dai D
and Xu W: Prediction of mediastinal lymph node metastasis based on
18F-FDG PET/CT imaging using support vector machine in
non-small cell lung cancer. Eur Radiol. 31:3983–3992. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang R, Zhu L, Cai Z, Jiang W, Li J, Yang
C, Yu C, Jiang B, Wang W, Xu W, et al: Potential feature
exploration and model development based on 18F-FDG PET/CT images
for differentiating benign and malignant lung lesions. Eur J
Radiol. 121:1087352019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang M, Su Q, Mouraux A and Iannetti GD:
Spatial patterns of brain activity preferentially reflecting
transient pain and stimulus intensity. Cereb Cortex. 29:2211–2227.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mendez AM, Petre EN, Ziv E, Ridouani F,
Solomon SB, Sotirchos V, Zhao K and Alexander ES: Safety and
efficacy of thermal ablation of adrenal metastases secondary to
lung cancer. Surg Oncol. 55:1021022024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andersen MB, Bodtger U, Andersen IR,
Thorup KS, Ganeshan B and Rasmussen F: Metastases or benign adrenal
lesions in patients with histopathological verification of lung
cancer: Can CT texture analysis distinguish? Eur J Radiol.
138:1096642021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al Fares HK, Abdullaj S, Gokden N and
Menon LP: Incidental, solitary, and unilateral adrenal metastasis
as the initial manifestation of lung adenocarcinoma. Cureus.
14:e326282022.PubMed/NCBI
|
|
32
|
Hashimoto K, Nishimura S and Akagi M: Lung
adenocarcinoma presenting as a soft tissue metastasis to the
shoulder: A case report. Medicina (Kaunas). 57:1812021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lococo F, Patricelli G, Galeone C and
Rapicetta C: eComment. Surgery for isolated adrenal metastasis from
non-small-cell lung cancer: The role of preoperative PET/CT scan'.
Interact Cardiovasc Thorac Surg. 24:72017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Evans PD, Miller CM, Marin D, Stinnett SS,
Wong TZ, Paulson EK and Ho LM: FDG-PET/CT characterization of
adrenal nodules: Diagnostic accuracy and interreader agreement
using quantitative and qualitative methods. Acad Radiol.
20:923–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim BS, Lee JD and Kang WJ:
Differentiation of an adrenal mass in patients with non-small cell
lung cancer by means of a normal range of adrenal standardized
uptake values on FDG PET/CT. Ann Nucl Med. 29:276–283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cho AR, Lim I, Na II, Choe du H, Park JY,
Kim BI, Cheon GJ, Choi CW and Lim SM: Evaluation of adrenal masses
in lung cancer patients using F-18 FDG PET/CT. Nucl Med Mol
Imaging. 45:52–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Y, Xie D, Huang W, Gong H and Yu J:
18F-FDG PET/CT in the evaluation of adrenal masses in lung cancer
patients. Neoplasma. 57:129–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koopman D, van Dalen JA, Stigt JA, Slump
CH, Knollema S and Jager PL: Current generation time-of-flight
(18)F-FDG PET/CT provides higher SUVs for normal adrenal glands,
while maintaining an accurate characterization of benign and
malignant glands. Ann Nucl Med. 30:145–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith TA: Facilitative glucose transporter
expression in human cancer tissue. Br J Biomed Sci. 56:285–292.
1999.PubMed/NCBI
|
|
40
|
Zhang S, Li S, Pei Y, Huang M, Lu F, Zheng
Q, Li N and Yang Y: Impact of maximum standardized uptake value of
non-small cell lung cancer on detecting lymph node involvement in
potential stereotactic body radiotherapy candidates. J Thorac Dis.
9:1023–1031. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu SH, Zhang Y, Yu YH, Fu Z, Kong L, Han
DL, Fu L, Yu JM and Li J: FDG PET-CT in non-small cell lung cancer:
Relationship between primary tumor FDG uptake and extensional or
metastatic potential. Asian Pac J Cancer Prev. 14:2925–2929. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sherlock M, Scarsbrook A, Abbas A, Fraser
S, Limumpornpetch P, Dineen R and Stewart PM: Adrenal
incidentaloma. Endocr Rev. 41:775–820. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, He Y, Zeng X, Zhu S and Li F:
Distinguishing between metastatic and benign adrenal masses in
patients with extra-adrenal malignancies. Front Endocrinol
(Lausanne). 13:9787302022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ali MA, El-Abd E, Morsi M, El Safwany MM
and El-Sayed MZ: The effect of hepatic steatosis on 18F-FDG uptake
in PET-CT examinations of cancer Egyptian patients. Eur J Hybrid
Imaging. 7:192023. View Article : Google Scholar : PubMed/NCBI
|