Introduction
Breast fat necrosis is a relatively uncommon benign
breast condition, with an overall incidence of ~0.6%, accounting
for 2.75% of all benign breast lesions (1). The pathogenesis of fat necrosis
includes a non-suppurative inflammatory process of adipose tissue,
fundamentally caused by aseptic fat saponification. Common causes
of fat necrosis include trauma, surgery, radiotherapy,
anticoagulant therapy or idiopathic factors (2). It is key to diagnose fat necrosis
because it often mimics breast cancer, which can lead to
unnecessary biopsies of breast lesions. Breast cancer is a
heterogeneous disease influenced by both genetic and environmental
factors. Key genetic factors involve high-penetrance mutations
(e.g., BRCA1/2) and family history, the latter conferring a
2–3-fold risk increase with an affected first-degree relative.
Modifiable risks encompass prolonged estrogen exposure, lifestyle
factors like postmenopausal obesity and alcohol use, and
reproductive history such as nulliparity and late first birth
(3). Globally, it is one of the
most common female cancers, representing 25% of new cases and
accounting for 2.1 million diagnoses in 2018 (3). Invasive breast cancer is the most
frequent subtype, with the main pathological types being invasive
ductal carcinoma (70–80%) and invasive lobular carcinoma (5–15%)
(3). The occurrence of massive
breast fat necrosis associated with a small focus of invasive
breast cancer is relatively uncommon, with, to the best of our
knowledge, only 5 cases reported in the literature, all including a
history of seatbelt trauma from road traffic accidents (4). The imaging differentiation between
breast fat necrosis and breast cancer presents notable diagnostic
challenges. Breast fat necrosis can mimic several imaging features
of breast cancer, such as spiculated margins due to fibrosis or
inflammatory reactions, calcification foci resulting from
saponification of necrotic cells and irregular enhancement patterns
on contrast-enhanced scans similar to those seen in malignant
tumors (5–7). This notable overlap in imaging
manifestations has led to breast fat necrosis being termed the
‘great mimicker’ (8,9). While current studies primarily focus
on differentiating between these two entities, the imaging
characteristics of their coexistence remain insufficiently explored
(10–12). The present study reported a case
primarily characterized by the relatively uncommon coexistence of
massive breast fat necrosis with invasive breast cancer,
emphasizing the key role of imaging findings in diagnosis to
prevent overlooking malignant foci hidden within benign
lesions.
Case report
A 74-year-old female patient was admitted to The
Third Affiliated Hospital of Zunyi Medical University (The First
People's Hospital of Zunyi) (Zunyi, China) in March 2023 with a
2-month history of a palpable nodule in the left breast,
accompanied by intermittent swelling and self-perceived distending
pain. On physical examination, a mass measuring ~2.0×1.0 cm was
identified at the 2 o'clock position of the left breast, 4 cm from
the nipple. The mass was hard, had an irregular surface, poorly
defined margins and an irregular shape, with limited mobility and
no tenderness. When the patient and their family were queried
regarding a relevant medical history, the patient and their family
denied having a history of trauma, surgery, radiotherapy,
anticoagulant therapy or other relevant medical histories. No
notable abnormalities were identified in other examinations.
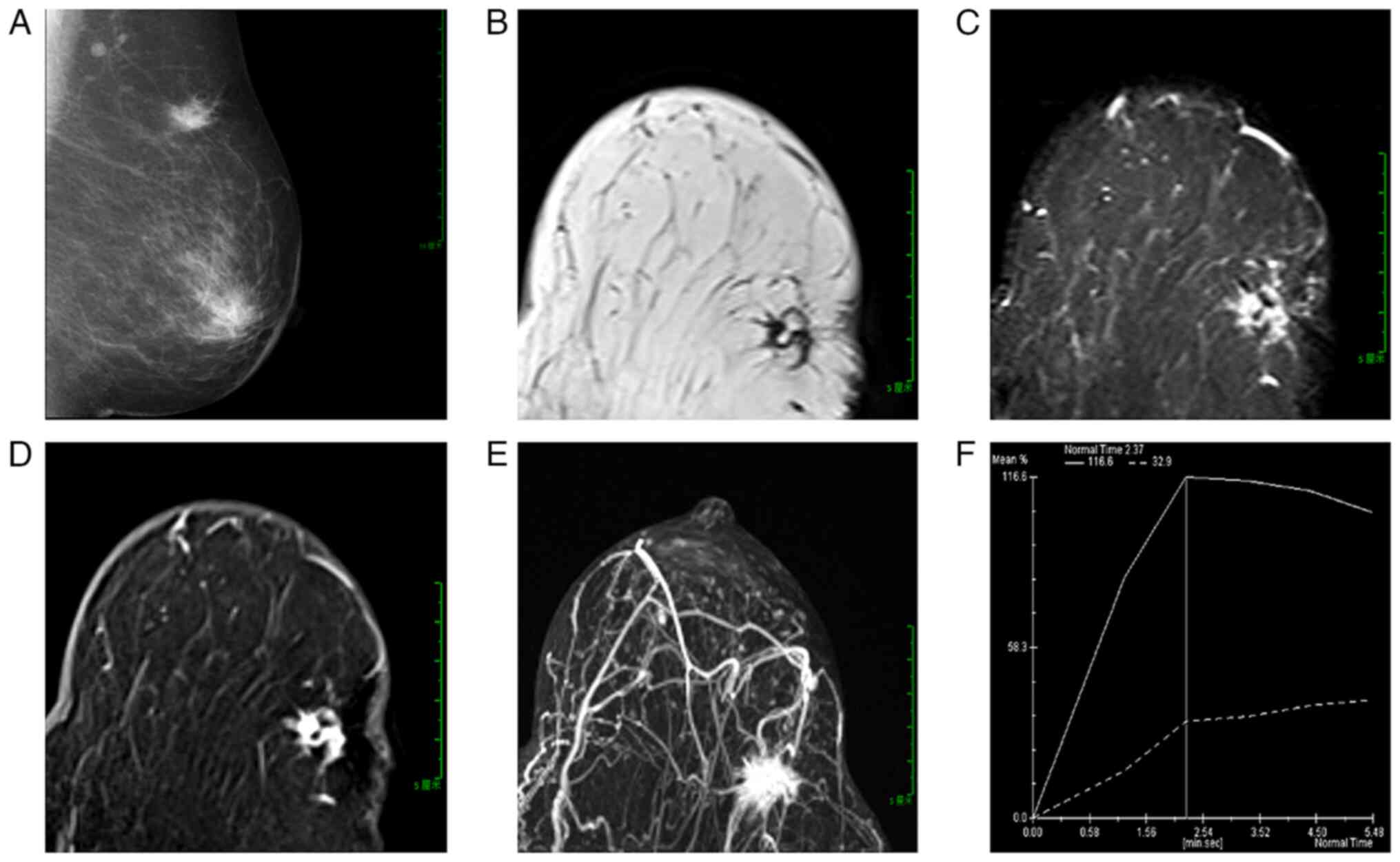
In March 2023, mammography revealed an irregular,
high-density mass in the upper outer quadrant of the left breast,
located 8.6 cm from the nipple, with a diameter of ~2.1 cm. The
lesion exhibited indistinct margins and spiculated edges (Fig. 1A). The mammographic findings
suggested a mass in the upper outer quadrant of the left breast,
highly suspicious for breast cancer.
In March 2023, MRI revealed an irregular lesion in
the upper outer quadrant of the left breast, 7.0 cm from the
nipple, measuring ~2.2×1.2×1.2 cm. The lesion had poorly defined
margins and spiculated edges. On T1-weighted imaging (T1WI), it
appeared hypointense with small focal areas of hyperintensity
(Fig. 1B). On fat-suppressed T1WI,
the focal areas appeared hypointense. On fat-suppressed T2WI, the
lesion exhibited slightly hyperintense signals with focal
hypointense areas that were lower in intensity compared with
surrounding fat, forming a ‘black hole sign’ (Fig. 1C). Diffusion-weighted imaging (DWI)
revealed heterogeneous hyperintensity, while apparent diffusion
coefficient (ADC) mapping revealed hypointensity. A
contrast-enhanced MRI demonstrated marked heterogeneous enhancement
in the early phase, with patchy non-enhancing areas within the
lesion (Fig. 1E). The
time-intensity curve (TIC) displayed a washout pattern (Fig. 1D). Maximum intensity projection
images indicated a rich blood supply to the mass (Fig. 1F). Based on MRI findings, the lesion
in the upper outer quadrant of the left breast was considered
consistent with fat necrosis.
In March 2023, surgical excision of the mass and
surrounding normal glandular tissue (weighing 50 g) was performed.
A gross examination of the specimen revealed extensive gray-yellow
necrotic fat interspersed with white, speckled cancerous foci
(Fig. 2A). The histopathological
analysis performed in March 2023 using hematoxylin and eosin
(H&E) staining (Fig. 2B)
confirmed invasive breast carcinoma with focal fat necrosis in the
surrounding tissue. For H&E staining, surgical specimens were
fixed in formalin at room temperature for 12 h, followed by
paraffin embedding and sectioning into 4-µm slices. The sections
were baked at 70°C for 1 h, deparaffinized in xylene and rehydrated
through a graded alcohol series at room temperature. The sections
were stained with hematoxylin for 5 min and eosin for 1 min at room
temperature. Subsequently, the sections were dehydrated through a
graded alcohol series, cleared in xylene and mounted with neutral
balsam for examination under a DM750 light microscope (Leica
Microsystems). Immunohistochemical analysis was conducted manually
on 4-µm formalin-fixed, paraffin-embedded tissue sections. After
baking at 70°C for 1 h, sections were deparaffinized in xylene and
rehydrated through a graded alcohol series. Antigen retrieval was
performed under high pressure at 100°C using either EDTA (pH 9.0)
or citrate-based buffer (pH 6.0) according to the specific
requirements of each primary antibody (7 min at full pressure
followed by 5 min standing). Following cooling and PBS rinses,
endogenous peroxidase activity was blocked with 3%
H2O2 for 10 min at room temperature. Sections
were then incubated with primary antibodies from Tongling
Biotechnology (50–100 µl/section) at 37°C for 1 h, all primary
antibodies were used at ready-to-use concentration including Ki-67
(cat. no. AM0241; EDTA pretreatment), erythroblastic oncogene B-2
receptor (c-erbB-2) (cat. no. AM0037; EDTA), non-metastatic protein
23 (nm 23) (cat. no. AM0382; EDTA), estrogen receptor (ER) (cat.
no. AR0710; CBS), progesterone receptor (PR) (cat. no. AR0711;
CBS), E-cadherin (cat. no. AR0240; EDTA), epidermal growth factor
receptor (EGFR) (cat. no. AR0251; EDTA), cytokeratin 5/6 (CK5/6)
(cat. no. AM0101; EDTA), p63 (cat. no. AM0186; EDTA), and p120
(cat. no. AM0268; EDTA). After PBS washes, sections were incubated
with HRP-conjugated secondary antibody (cat. no. DD13; Tongling
Biotechnology) at 37°C for 30 min, followed by DAB development
(cat. no. KS-005A/B; Tongling Biotechnology) for 5 min at room
temperature. Counterstaining was performed with hematoxylin for 1
min at room temperature, followed by differentiation in 1%
hydrochloric acid-alcohol solution (1% HCl in 70% ethanol).
Finally, sections were dehydrated through graded alcohols,
air-dried and mounted for examination under a DM750 light
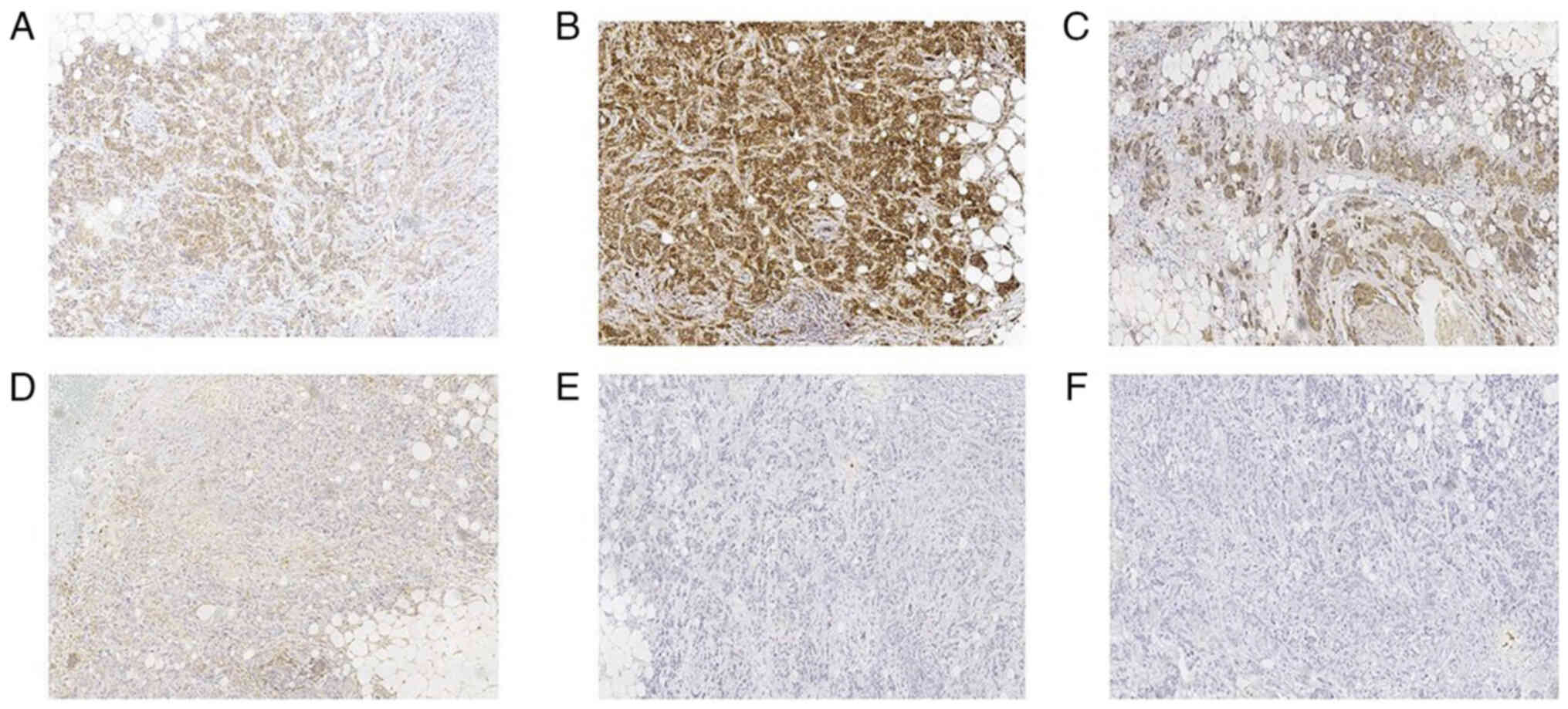
microscope (Leica Microsystems). In March 2023, immunohistochemical
staining revealed positive expression of Ki-67 (+; ~45%) (Fig. 2C), E-cadherin (+) (Fig. 2D), ER (3+; ~90%) (Fig. 2E), PR (+; ~50%) (Fig. 2F), c-erbB-2 (+) (Fig. 3A), p120 (on the tumor cell membrane
+) (Fig. 3B) and nm23 (+) (Fig. 3C), while EGFR (Fig. 3D), CK5/6 (Fig. 3E) and p63 (Fig. 3F) were negative. The date of last
follow-up was September 2025, with annual telephone follow-ups
conducted.
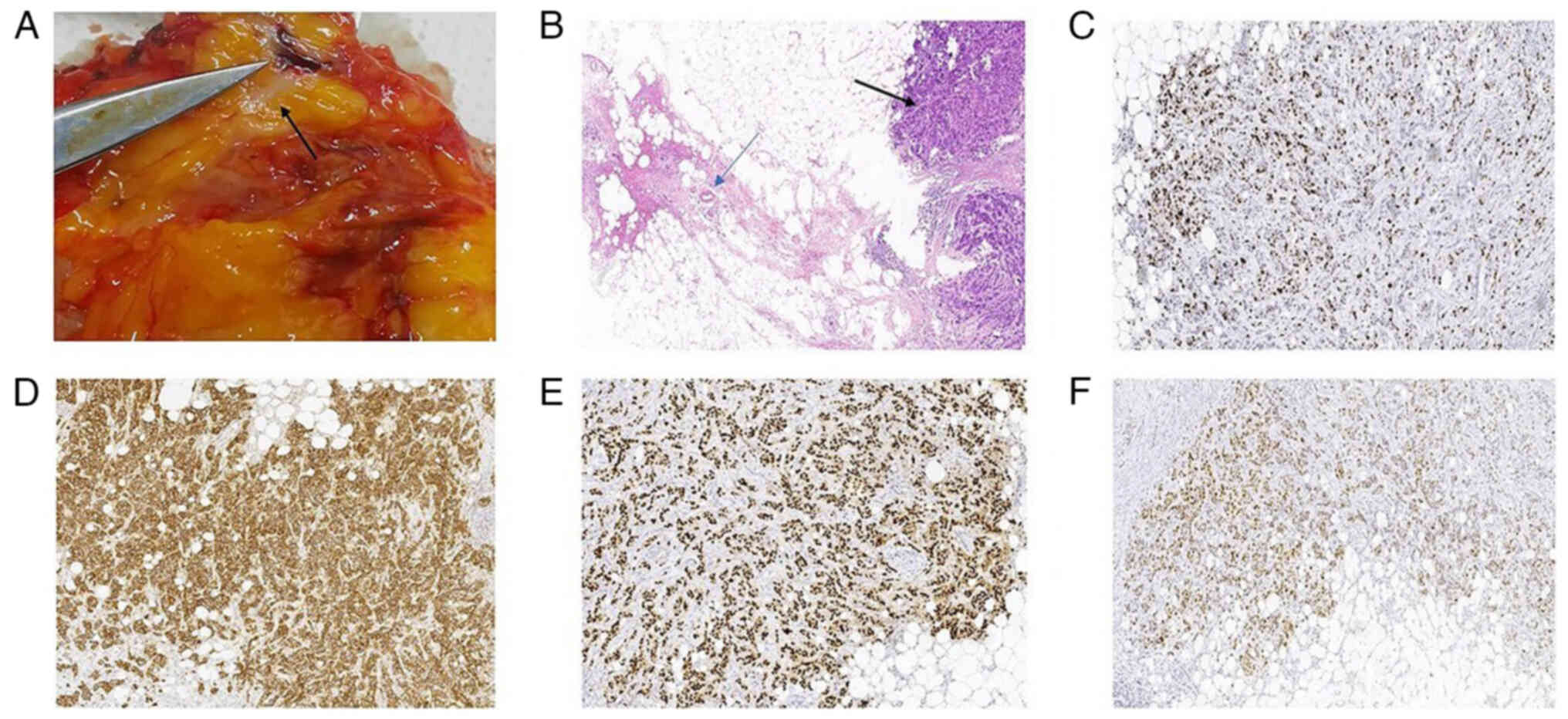 | Figure 2.(A) The gross specimen exhibits
extensive gray-yellow necrotic fat with white spot-like cancer foci
(indicated by the black arrow). (B) Fat necrosis and invasive
breast cancer (H&E stain): The area indicated by the blue arrow
displays ruptured or disrupted adipocytes, with their normal round
structure destroyed. Macrophages are visible. The area indicated by
the black arrow displays highly heterogeneous cancer cell
morphology and size, with an increased nucleus-to-cytoplasm ratio.
The cancer cell cytoplasm stains light pink, and the cancer cells
infiltrate the surrounding fibrous stroma in a cord-like pattern
(magnification, ×5). (C) Invasive breast cancer (Ki-67):
Ki-67+ cells have brownish-yellow-stained nuclei. The
positive staining is confined to the cell nucleus, with no staining
in the cytoplasm. (D) Invasive breast cancer (E-cadherin):
E-cadherin(+) displays brownish-yellow positive staining on the
cancer cell membranes, with staining primarily localized to the
cell membrane, suggesting that the cancer cells still retain some
adhesive function. (E) Invasive breast cancer (ER): ER+
cells have brownish-yellow-stained nuclei. (F) Invasive breast
cancer (PR): PR+ cells have brownish-yellow-stained
nuclei (magnification, ×10). ER, estrogen receptor; PR,
progesterone receptor. |
Discussion
Breast fat necrosis is a common benign breast
condition with imaging features that often mimic breast cancer,
such as spiculated margins due to fibrosis or inflammatory
reactions, calcification foci resulting from saponification of
necrotic cells and irregular enhancement patterns on
contrast-enhanced scans similar to those seen in malignant tumors
(5–7). Breast fat necrosis is characterized by
granulomatous inflammation resulting from the enzymatic
liquefaction of necrotic tissue. The process of fat necrosis has
four stages: i) The hyperacute stage; ii) the acute inflammatory
stage; iii) the oil cyst formation stage; and iv) the chronic
granulomatous reaction stage. Each stage exhibits distinct imaging
characteristics. When the imaging findings resemble those of
malignancy, a pathological examination is required for a definitive
diagnosis (5,8). Invasive breast cancer is defined by
the penetration of cancer cells through the basement membrane into
the breast stroma, resulting in a higher degree of malignancy and
an increased risk of metastasis compared with non-invasive breast
cancer (13,14).
The patient was admitted to The Third Affiliated
Hospital of Zunyi Medical University (The First People's Hospital
of Zunyi); Zunyi, China) in March 2023 due to a palpable left
breast nodule for >2 months, accompanied by self-perceived
distending pain that occurred intermittently. Although the patient
reported no history of breast trauma or surgery, imaging
examinations are of notable necessity (15). This is because imaging examinations
can make up for the limitations of palpation, clearly display the
detailed characteristics of the nodule such as its size, shape,
boundary, internal echo, density or signal and whether it is
accompanied by calcification, and can even detect ‘occult or tiny
nodules’ that cannot be identified by palpation (16).
Breast fat necrosis and breast cancer can have
similar imaging appearances, which makes them difficult to
distinguish. On mammography, fat necrosis often presents as round
or oval low-density cysts with fat-fluid levels caused by the
separation of oil and serous fluid (17). As the condition progresses,
calcifications may develop (5). By
comparison, breast cancer appears on mammography as an irregular,
high-density mass with spiculated margins. The calcifications
associated with breast cancer are often described as clustered
microcalcifications or fine, irregular microcalcifications
(18). In the present case,
mammography revealed an irregular, high-density mass with unclear
borders and spiculated edges, features highly suggestive of breast
cancer. However, mammography has limited tissue-specific resolution
and previous reports have indicated that fat necrosis can mimic
malignant tumors (8,9,12).
Therefore, breast MRI is necessary, as it provides more detailed
information using multiparametric imaging techniques.
In breast MRI, fat necrosis often appears as a
hyperintense lesion on T1WI and T2WI, becoming hypointense on
fat-suppressed images, with a typically clear border. However,
fibrosis or inflammation may cause slight spiculation. On enhanced
scanning, it often exhibits no enhancement or only rim enhancement.
However, enhancing fat necrosis mimicking malignancy can exhibit
irregular mass enhancement (9),
with a varied TIC to a certain extent similar to breast cancer,
making differentiation difficult (5). Notably, on inversion recovery
sequences, most intracellular lipids, such as those in lipid-rich
mammary lesions (19) and the
triglyceride droplets of breast hamartomas (20) indicate marked signal reduction.
However, fat necrosis has a lower signal intensity compared with
surrounding normal fat, termed the ‘black hole’ sign, considered
specific for fat necrosis. This is because the necrotic fat is
replaced by fibrosis, calcification or fluid, with a narrower
distribution of T1 relaxation times around the inversion time (TI),
the specific time delay used in an MRI inversion recovery pulse
sequence, leading to more complete fat suppression at specific TI
times. The ‘black hole sign’ often presents in the oil cyst
formation stage (where the necrotic region undergoes cystic change
and liquefied fat is confined within the cyst cavity) and the
chronic granulomatous reaction stage (where the central necrotic
area is completely fibrotic, with or without calcification) of fat
necrosis. By contrast, breast cancer often lacks this sign
(19,21,22).
The ‘black hole’ sign seen in the breast MRI of the present case
suggested fat necrosis. However, apart from the characteristic
‘black hole’ sign of fat necrosis on MRI, other imaging features
were similar to those of breast cancer, making differentiation
difficult and prompting clinical vigilance and biopsy. The
histopathological analysis of the surgical specimen suggested that
the coexistence of massive mammary fat necrosis with invasive
breast cancer, which is a relatively uncommon clinical presentation
with only a few documented cases in the literature (4).
Previous studies have predominantly focused on
differentiating fat necrosis from breast cancer, yet they have
overlooked their imaging manifestations when they coexist (10–12).
In the present case, performing MRI after mammography was necessary
for accurate preoperative assessment of the lesion's size and
delineating the extent of the lesion. Firstly, mammography has
relatively weak tissue resolutions due to the interference of
breast glands (23,24), while the multi-parametric imaging
technology of MRI provides more detailed information, thus serving
a complementary role (25).
Furthermore, MRI can evaluate the condition of the contralateral
(healthy side) breast. It is worth noting that when mammography
suggests a malignant lesion, MRI becomes particularly key, as it
serves a key role in diagnosis and treatment (26). The present case highlighted that
even with the characteristic ‘black hole’ sign of fat necrosis on
MRI, if other imaging features resemble breast cancer and are
indistinguishable, the possibility of coexisting breast cancer
within fat necrosis should be considered.
Under the microscope, fat necrosis is characterized
by ruptured adipocytes, infiltration of inflammatory cells,
saponification of fat, formation of foam cells and fibrosis
(2,21,27,28).
The pathological features of invasive ductal carcinoma of the
breast include cellular atypia, loss of normal glandular
architecture (14,29), and immunohistochemical staining
results demonstrated negative expression for myoepithelial markers
CK5/6 and p63 and positive expression of p120 and E-cadherin. Fat
necrosis is a proliferative condition often induced by hypoxia
(27). Following ischemic injury,
adipocyte rupture alters the tumor microenvironment, creating
conditions that may promote cancer development (30).
The differential diagnosis between breast fat
necrosis and invasive breast cancer requires distinguishing it from
conditions such as breast sarcoma, inflammatory breast cancer (IBC)
and simple invasive breast cancer. Breast sarcoma often includes
the entire breast, with rare calcification and axillary
lymphadenopathy. On T1WI, it reveals isointensity or
hyperintensity, except in necrotic or cystic areas. The TIC of
breast sarcoma demonstrates washout, indicating slow enhancement
and heterogeneous signal intensity (SI) distribution (31). IBC presents with specific
mammography and MRI features, such as breast enlargement and
diffuse or nodular skin thickening. Lesions are often multifocal
and widespread, making accurate tumor size measurement challenging
(32). Pure infiltrating ductal
carcinoma generally appears on mammography as an irregular,
spiculated, dense mass with malignant calcification. On MRI, it
exhibits mixed T2WI signals, with SI dependent on internal
components. Enhancement can be homogeneous, heterogeneous or
rim-like, and TIC demonstrates washout. On DWI, it demonstrates
hyperintense and hypointense signals on ADC, indicating restricted
diffusion (33,34). Pure breast fat necrosis has distinct
imaging characteristics. The imaging presentation depends on the
stage of fat necrosis, the degree of inflammatory reaction, the
amount of liquefied fat, fibrosis and calcifications, as well as
the etiology of the fat necrosis. Therefore, knowledge of imaging
appearances of fat necrosis at different stages is essential for a
radiologist to make accurate interpretation and avoid unnecessary
invasive workup (35). Mammography
of fat necrosis often reveals well-defined, low-density oil cysts,
which may have coarse, ring-like or ‘eggshell’ calcifications. MRI
of fat necrosis often reveals hyperintensity on both T1WI and T2WI,
with clear margins. However, fibrosis or inflammation can cause
slight spiculation. The ‘black hole’ sign on T2WI fat-saturated
sequences is a specific marker for fat necrosis. Enhancement is
often absent or rim-like, with low restricted diffusion. In
summary, analyzing these imaging features can effectively
distinguish fat necrosis from invasive breast cancer and other
breast diseases, providing a reliable clinical diagnosis.
In conclusion, the present study reported a
relatively uncommon case of massive breast fat necrosis coexisting
with invasive breast cancer. Imaging findings reveal several
similarities between fat necrosis and breast cancer, often making
them difficult to differentiate. Although previous literature has
largely focused on the differential diagnosis between the two, the
present case highlighted the possibility of their concurrent
occurrence, with a minor focus on invasive breast cancer, which is
key for the improvement of diagnostic accuracy. Furthermore, the
present case provided additional evidence supporting the close
association between inflammation and cancer development, which
aligns with findings from previous related studies.
Acknowledgements
Not applicable.
Funding
The present case report was funded by Guizhou Provincial Natural
Science Foundation [grant no. Qian Ke He Jichu-ZK (2021) Yiban
479], Natural Science Foundation of Zunyi [grant no. Zun Shi Ke He
HZ Zi(2020) 143] and the First People's Hospital of Zunyi Yanjiu Yu
Shiyan Fazhan R&D [grant no. Yuan Ke Zi (2020) 9].
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
AZ designed the present case report and wrote the
manuscript. LS, BT, YL, XY and XC performed all the experiments. GJ
provided data and preliminarily interpretated them. LJ performed
data analysis, conceptualization and critical review. GJ and LJ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fat necrosis as a differential diagnosis
for recurrence in a treated case of breast cancer, . A case report
| Semantic Scholar.
|
|
2
|
Vasei N, Shishegar A, Ghalkhani F and
Darvishi M: Fat necrosis in the Breast: A systematic review of
clinical. Lipids Health Dis. 18:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Houghton SC and Hankinson SE: Cancer
progress and priorities: Breast Cancer. Cancer Epidemiol Biomarkers
Prev. 30:822–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song CT, Teo I and Song C: Systematic
review of seat-belt trauma to the female breast: A new diagnosis
and management classification. J Plast Reconstr Aesthet Surg.
68:382–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tayyab SJ, Adrada BE, Rauch GM and Yang
WT: A pictorial review: Multimodality imaging of benign and
suspicious features of fat necrosis in the breast. Br J Radiol.
91:201802132018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taboada JL, Stephens TW, Krishnamurthy S,
Brandt KR and Whitman GJ: The many faces of fat necrosis in the
breast. AJR Am J Roentgenol. 192:815–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daly CP, Jaeger B and Sill DS: Variable
appearances of fat necrosis on breast MRI. AJR Am J Roentgenol.
191:1374–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganau S, Tortajada L, Escribano F, Andreu
X and Sentís M: The great mimicker: Fat necrosis of the
breast-magnetic resonance mammography approach. Curr Probl Diagn
Radiol. 38:189–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guirguis MS, Adrada B, Santiago L,
Candelaria R and Arribas E: Mimickers of breast malignancy: Imaging
findings, pathologic concordance and clinical management. Insights
Imaging. 12:532021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen QD and Kharsa A: Magnetic resonance
imaging enhancement two years post lumpectomy: An unusual timing
for fat necrosis. Cureus. 13:e147942021.PubMed/NCBI
|
|
11
|
Parikh RP, Doren EL, Mooney B, Sun WV,
Laronga C and Smith PD: Differentiating fat necrosis from recurrent
malignancy in fat-grafted breasts: An imaging classification system
to guide management. Plast Reconstr Surg. 130:761–772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adejolu M, Huo L, Rohren E, Santiago L and
Yang WT: False-positive lesions mimicking breast cancer on FDG PET
and PET/CT. AJR Am J Roentgenol. 198:W304–W314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L: Mammography with deep learning for
breast cancer detection. Front Oncol. 14:12819222024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen W, Wang G and Zhang G: Insights into
the transition of ductal carcinoma in situ to invasive ductal
carcinoma: Morphology, molecular portraits, and the tumor
microenvironment. Cancer Biol Med. 19:1487–1495. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Genova R and Garza RF: Breast Fat
Necrosis. StatPearls. StatPearls Publishing; Treasure Island (FL):
2025
|
|
16
|
Kolb TM, Lichy J and Newhouse JH:
Comparison of the performance of screening mammography, physical
examination, and breast US and evaluation of factors that influence
them: An analysis of 27,825 patient evaluations. Radiology.
225:165–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Upadhyaya VS, Uppoor R and Shetty L:
Mammographic and sonographic features of fat necrosis of the
breast. Indian J Radiol Imaging. 23:366–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pulappadi VP, Dhamija E, Baby A, Mathur S,
Pandey S, Gogia A and Deo SVS: Imaging features of breast cancer
subtypes on mammography and ultrasonography: An analysis of 479
patients. Indian J Surg Oncol. 13:931–938. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trimboli RM, Carbonaro LA, Cartia F, Di
Leo G and Sardanelli F: MRI of fat necrosis of the breast: The
‘black hole’ sign at short tau inversion recovery. Eur J Radiol.
81:e573–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohamed A: Breast hamartoma: Unusual
radiological presentation. Radiol Case Rep. 15:2714–2717. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kerridge WD, Kryvenko ON, Thompson A and
Shah BA: Fat necrosis of the breast: A pictorial review of the
mammographic, ultrasound, CT, and MRI findings with histopathologic
correlation. Radiol Res Pract. 2015:6131392015.PubMed/NCBI
|
|
22
|
Hassan HHM, El Abd AM, Abdel Bary A and
Naguib NNN: Fat necrosis of the Breast: Magnetic resonance imaging
characteristics and pathologic correlation. Acad Radiol.
25:985–992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melnikow J, Fenton JJ, Whitlock EP,
Miglioretti DL, Weyrich MS, Thompson JH and Shah K: Supplemental
Screening for breast cancer in women with dense breasts: A
Systematic Review for the U.S. Preventive Service Task Force.
Agency for Healthcare Research and Quality (US); Rockville (MD):
2016
|
|
24
|
Nakamura N, Okafuji Y, Adachi S, Takahashi
K, Nakakuma T and Ueno S: Effect of different breast densities and
average glandular dose on contrast to noise ratios in Full-field
digital mammography: Simulation and phantom study. Radiol Res
Pract. 2018:61925942018.PubMed/NCBI
|
|
25
|
Raikhlin A, Curpen B, Warner E, Betel C,
Wright B and Jong R: Breast MRI as an adjunct to mammography for
breast cancer screening in high-risk patients: Retrospective
review. AJR Am J Roentgenol. 204:889–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Debruhl ND, Lee SJ, Mahoney MC, Hanna L,
Tuite C, Gatsonis CA and Lehman C: MRI Evaluation of the
contralateral breast in women with recently diagnosed breast
cancer: 2-Year Follow-up. J Breast Imaging. 2:50–55. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Majithia J, Haria P, Popat P, Katdare A,
Chouhan S, Gala KB, Kulkarni S and Thakur M: Fat necrosis: A
consultant's conundrum. Front Oncol. 12:9263962022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan PH, Lai LM, Carrington EV, Opaluwa AS,
Ravikumar KH, Chetty N, Kaplan V, Kelley CJ and Babu ED: Fat
necrosis of the breast-a review. Breast. 15:313–318. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nasrazadani A, Li Y, Fang Y, Shah O,
Atkinson JM, Lee JS, McAuliffe PF, Bhargava R, Tseng G, Lee AV, et
al: Mixed invasive ductal lobular carcinoma is clinically and
pathologically more similar to invasive lobular than ductal
carcinoma. Br J Cancer. 128:1030–1039. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu WH, Ji QL, Li ZZ, Wang QN, Liu SY and
Yu JF: Mammography and MRI manifestations of breast angiosarcoma.
BMC Womens Health. 19:732019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papalouka V and Gilbert FJ: Inflammatory
breast cancer-importance of breast imaging. Eur J Surg Oncol.
44:1135–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langman EL, Kuzmiak CM, Brader R, Thomas
SM, Alexander SL, Lee SS and Jordan SG: Breast cancer in young
women: Imaging and clinical course. Breast J. 27:657–663. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen R, Hu B, Zhang Y, Liu C, Zhao L,
Jiang Y and Xu Y: Differential diagnosis of plasma cell mastitis
and invasive ductal carcinoma using multiparametric MRI. Gland
Surg. 9:278–290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan PYL, Wong T, Chau CM, Fung WY, Lai
KB, Chan RLS, Wong WCW, Yung WT and Ma JKF: Fat necrosis in the
breast: A multimodality imaging review of its natural course with
different aetiologies. Clin Radiol. 78:323–332. 2023. View Article : Google Scholar : PubMed/NCBI
|