Introduction
Esophageal neuroendocrine carcinoma (ENEC) is a rare
and highly aggressive extrapulmonary neuroendocrine malignancy,
characterized by low incidence and notable challenges in clinical
diagnosis and treatment. It is considered one of the most lethal
forms of extrapulmonary neuroendocrine carcinoma, with globally
reported median survival times ranging from 14 to 28 months
(1–3). Even in patients diagnosed at a
localized stage and treated with comprehensive multimodal therapy,
the global 5-year overall survival (OS) rate remains as low as 8.2%
(4). Owing to its rarity,
large-scale, multicenter studies are lacking, and treatment
strategies for ENEC remain controversial.
The RAS-RAF-MEK-ERK signaling cascade is one of the
most critical oncogenic pathways involved in tumorigenesis
(5). BRAF, as a key protein in this
pathway, serves an essential role in the regulation of cellular
proliferation, differentiation and apoptosis (6,7).
Previous research has shown that dysregulation of this pathway is
closely associated with the development and progression of
neuroendocrine tumors, suggesting that BRAF inhibitors or RAF-1
activators may represent viable therapeutic targets (8). In colorectal neuroendocrine
carcinomas, the prevalence of BRAF V600E mutations has been
reported to range from 28 to 46.7% (9–11).
However, the expression status and clinical implications of BRAF
V600E mutations in ENEC remain unclear due to limited available
data.
Programmed cell death protein 1 (PD-1) and
programmed death ligand 1 (PD-L1) are critical immune checkpoints
that regulate antitumor immunity and immune tolerance (12). In previous years, PD-1/PD-L1
inhibitors have demonstrated notable therapeutic efficacy across
various malignancies, including esophageal and lung cancers
(13–16). Several clinical trials have
demonstrated that the combination of chemotherapy with PD-1/PD-L1
inhibitors markedly improves OS and progression-free survival (PFS)
in patients with small cell lung cancer (SCLC) (17–19).
However, the suitability of immunotherapy for ENEC remains
uncertain.
Emerging evidence suggests that the BRAF V600E
mutation may contribute to immune evasion by upregulating PD-L1
expression, a phenomenon observed in several tumor types, including
melanoma, colorectal cancer and non-small cell lung cancer
(20). If this mutation is also
present in ENEC, it may influence tumor immunogenicity and modulate
the response to immunotherapy.
In the present study, a comprehensive analysis of
the clinical characteristics and histopathological features of ENEC
was performed. For the first time, the expression status of both
BRAF V600E mutation and PD-L1 were simultaneously assessed in this
rare carcinoma, contributing novel insights into the molecular
landscape of ENEC. Additionally, potential clinical and
pathological factors associated with patient prognosis were
investigated, aiming to provide a theoretical and practical
foundation for future therapeutic strategies.
Materials and methods
Patients
In the present study, clinical data from 3,816
patients with esophageal cancer who were admitted and treated at
the Department of Thoracic Surgery, Affiliated Hospital of North
Sichuan Medical College (Nanchong, China) between January 2019 and
September 2024 were reviewed. The study was reviewed and approved
by The Ethics Committee of Affiliated Hospital of North Sichuan
Medical College (approval no. 2024ER682-1).
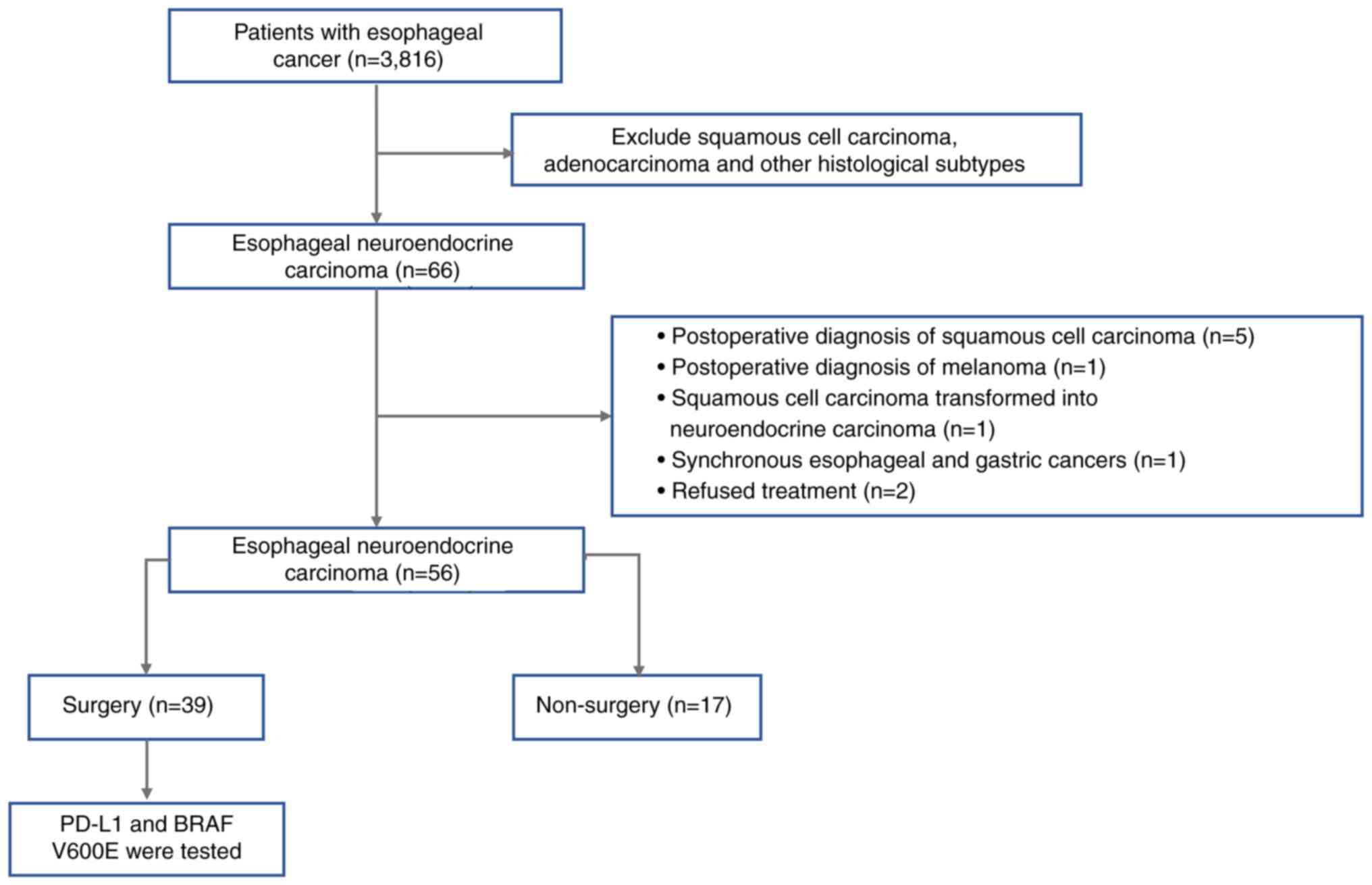
The patient selection process is illustrated in
Fig. 1. A total of 66 patients with
a pathological diagnosis of ENEC were identified based on the
diagnostic criteria outlined in the 2019 World Health Organization
Classification of Tumors of the Digestive System (21). Among these, 6 patients were
initially suspected of having neuroendocrine carcinoma based on
biopsy findings but were later confirmed to have different
pathological types after surgical resection: A total of 5 cases
were diagnosed with squamous cell carcinoma and 1 case with
melanoma and 1 patient was found to have synchronous primary
tumors, with squamous cell carcinoma in the middle esophagus and
neuroendocrine carcinoma at the esophagogastric junction. Another
patient had previously been diagnosed with esophageal squamous cell
carcinoma and received definitive radiotherapy. Subsequently, 3
years later, post-surgical pathology revealed a mixed-type ENEC
consisting of 30% squamous cell carcinoma and 70% large-cell
neuroendocrine carcinoma components. Additionally, 2 patients with
confirmed ENEC refused treatment. After excluding the
aforementioned cases, a total of 56 patients were included in the
final analysis, of whom 39 underwent surgical treatment and 17
received non-surgical management.
Follow-up and definitions
Patient follow-up data were collected through
regular outpatient visits and telephone interviews, including
information on tumor progression and survival status. All patients
were followed up at 3-month intervals for a minimum of 6 months
from the initiation of treatment. PFS was defined as the time from
the start of treatment to the first confirmed disease progression
or mortality from any cause. OS was defined as the time from the
start of treatment to mortality from any cause. R0 resection was
defined as a complete tumor resection with no microscopic residual
tumor at the surgical margins.
Immunohistochemical staining
A total of 39 patients who underwent surgical
resection and were confirmed to have ENEC based on postoperative
pathological diagnosis were included in the immunohistochemical
analysis with PD-L1 and BRAF V600E. Postoperative tumor specimens
were retrieved from the Pathology Specimen Bank of the Affiliated
Hospital of North Sichuan Medical College. The primary antibodies
were anti-PD-L1 (cat. no. #13684; 1:100; Cell Signaling Technology,
Inc.) and anti-BRAF V600E (cat. no. ab228461; 1:100; Abcam).
Antibodies used for immunohistochemical identification of
neuroendocrine differentiation and proliferative activity were as
follows: Cytokeratin (CK; cat. no. HA721455; 1:200), chromogranin A
(CgA; cat. no. ET1703-08; 1:1,000), synaptophysin (Syn; cat. no.
HA723196; 1:200), CD56 (cat. no. HA722755; 1:1,000) and Ki-67 (cat.
no. HA721115; 1:5,000; all Hangzhou Huaan Biotechnology Co.,
Ltd.).
Immunohistochemical staining was performed using the
UltraSensitive™ S-P IHC kit (mouse/rabbit; Fuzhou Maixin
Biotechnology Development Co., Ltd.) according to the
manufacturer's instructions. Briefly, specimens were fixed in 10%
neutral buffered formalin, embedded in paraffin, and sectioned at a
thickness of 4 µm. Primary antibody incubation was performed at
room temperature, followed by secondary antibody and detection
reagent application using the UltraSensitive™ S-P system with DAB
as the chromogen. The secondary antibodies were included in the
detection kit. Microscopic examination and image acquisition were
performed using a light microscope with Olympus cellSens Entry
software (version 4.2; Olympus Corporation). Human tonsil tissue
was used as the positive control for PD-L1 staining, while thyroid
tissue known to harbor the BRAF V600E mutation served as the
positive control for BRAF V600E staining. For negative controls,
phosphate buffered saline was substituted for the primary
antibodies.
All slides were independently evaluated by two
experienced pathologists with intermediate or senior professional
titles. In cases of discordant interpretation, a joint review was
performed, and final decisions were made by consensus. PD-L1
expression was evaluated based on membranous staining of tumor
cells. Following criteria established in previous studies (22,23), a
tumor was considered PD-L1 positive when ≥1% of tumor cells (tumor
proportion score, TPS) demonstrated membranous staining with an
intensity clearly above background levels. BRAF V600E expression
was assessed based on cytoplasmic staining. Tumor cells were
considered positive if distinct cytoplasmic staining was
observed.
Statistical analysis
All statistical analyses were performed using IBM
SPSS software (IBM Corp.; version 27.0). Continuous variables with
normal distribution are expressed as mean ± standard deviation and
compared between groups using an independent samples t-test.
Categorical variables were presented as frequency and percentage,
and differences between groups were analyzed using a χ2
test or Fisher's exact test, as appropriate. For ordinal
categorical variables and continuous variables not conforming to
normal distribution, non-parametric comparisons were performed
using a rank sum test.
Survival outcomes, including PFS and OS, were
estimated using the Kaplan-Meier method with corresponding 95%
confidence intervals (CI). Differences in survival between groups
were assessed using the log-rank test. Kaplan-Meier survival curves
were generated using MedCalc software (MedCalc Software Ltd.;
version 22.018). To further identify independent prognostic factors
in patients with ENEC, univariate and multivariate Cox proportional
hazards regression models were constructed. All statistical tests
were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 56 patients were included in the
analysis. Baseline characteristics are summarized in Table I. The mean age of the patients was
67.1±8.3 years. The majority of patients were male (69.6%). Tumors
were predominantly located in the middle (50.0%) and lower (41.1%)
esophagus. The most common presenting symptom was dysphagia
(60.7%), followed by subxiphoid pain (21.4%). In most cases
(69.6%), the disease duration ranged from 1 to 6 months at the time
of diagnosis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Total (n=56) | Non-surgery
(n=17) | Surgery (n=39) | P-value |
|---|
| Age, mean ± SD | 67.1±8.3 | 67.4±7.8 | 67.0±8.6 | 0.893 |
| BMI, mean ± SD | 22.3±3.3 | 21.5±2.4 | 22.7±3.5 | 0.197 |
| Sex |
|
|
| 0.463 |
|
Female | 17 (30.4) | 4 (23.5) | 13 (33.3) |
|
|
Male | 39 (69.6) | 13 (76.5) | 26 (66.7) |
|
| Smoking |
|
|
| 0.270 |
| No | 26 (46.4) | 6 (35.3) | 20 (51.3) |
|
|
Yes | 30 (53.6) | 11 (64.7) | 19 (48.7) |
|
| Drinking |
|
|
| 0.075 |
| No | 33 (58.9) | 7 (41.2) | 26 (66.7) |
|
|
Yes | 23 (41.1) | 10 (58.8) | 13 (33.3) |
|
| Hypertension |
|
|
| 0.800 |
| No | 44 (78.6) | 13 (76.5) | 31 (79.5) |
|
|
Yes | 12 (21.4) | 4 (23.5) | 8 (20.5) |
|
| Diabetes |
|
|
| 0.908 |
| No | 53 (94.6) | 16 (94.1) | 37 (94.9) |
|
|
Yes | 3 (5.4) | 1 (5.9) | 2 (5.1) |
|
| Tumor location |
|
|
| 0.302 |
|
Upper | 3 (5.4) | 1 (5.9) | 2 (5.1) |
|
|
Middle | 28 (50.0) | 11 (64.7) | 17 (43.6) |
|
|
Lower | 23 (41.1) | 5 (29.4) | 20 (51.3) |
|
| Length of
tumor |
|
|
| 0.023 |
| ≤3
cm | 15 (26.8) | 1 (5.9) | 14 (35.9) |
|
| >3
cm | 41 (73.2) | 16 (94.1) | 25 (64.1) |
|
| Thickness of
tumor |
|
|
| 0.003 |
| ≤10
mm | 23 (41.1) | 2 (11.8) | 21 (53.8) |
|
| >10
mm | 33 (58.9) | 15 (88.2) | 18 (46.2) |
|
| Symptom |
|
|
| 0.179 |
|
Dysphagia | 34 (60.7) | 12 (70.6) | 22 (56.4) |
|
|
Subxiphoid pain | 12 (21.4) | 1 (5.9) | 11 (28.2) |
|
|
Dysphagia with pain | 6 (10.7) | 3 (17.6) | 3 (7.7) |
|
| Medical
screening | 4 (7.1) | 1 (5.9) | 3 (7.7) |
|
| Disease
duration |
|
|
| 0.308 |
| <1
month | 14 (25.0) | 4 (23.5) | 10 (25.6) |
|
| 1–6
months | 39 (69.6) | 13 (76.5) | 26 (66.7) |
|
| >6
months | 3 (5.4) | 0 (0.0) | 3 (7.7) |
|
| Clinical T
stage |
|
|
| 0.005 |
|
T1/T2 | 22 (39.3) | 2 (11.8) | 20 (51.3) |
|
|
T3/T4 | 34 (60.7) | 15 (88.2) | 19 (48.7) |
|
| Clinical N
stage |
|
|
| <0.001 |
| N0 | 30 (53.6) | 1 (5.9) | 29 (74.4) |
|
| N+ | 26 (46.4) | 16 (94.1) | 10 (25.6) |
|
| Clinical M
stage |
|
|
| 0.088 |
| M0 | 54 (96.4) | 15 (88.2) | 39 (100.0) |
|
| M1 | 2 (3.6) | 2 (11.8) | 0 (0.0) |
|
Based on upper gastrointestinal imaging and
contrast-enhanced chest computed tomography, tumor length was
stratified into >3 and ≤3 cm, while tumor thickness was
categorized as >1 and ≤1 cm. Among all patients, 73.2% had
tumors >3 cm in length, and 58.9% had tumors >1 cm in
thickness. Clinical staging revealed that 60.7% of patients were at
tumor (T)3/T4 stage, and 46.4% were at lymph node (N)1-N3 stage.
Distant metastasis was observed in 2 patients (3.6%) at initial
diagnosis.
Patients were divided into surgical and non-surgical
groups based on treatment strategy. Significant differences were
observed between the two groups in tumor length and thickness and
clinical T and clinical N stage. No statistically significant
differences were noted for other baseline characteristics, and the
groups were otherwise comparable (Table
I).
Surgical data
In the present study cohort, a total of 39 patients
received surgery. As shown in Table
SI, the R0 resection rate was 97.4% (n=38), and minimally
invasive surgery was performed in 79.5% (n=31) of cases. The number
of lymph nodes that were intraoperatively excised was 15 (range,
4–50). The mean operative time was 210 min (range, 133–365 min),
and the mean intraoperative blood loss was 80 ml (range, 10–500
ml). The median postoperative hospital stay was 10 days (range,
7–42 days). Postoperative complications were most common with
pulmonary infection with an incidence of 28.2% (n=11), followed by
anastomotic fistula (5.1%, n=2). A total of 79.4% (n=31) of
patients did not receive preoperative neoadjuvant therapy and 25
(64.1%) did not receive postoperative adjuvant therapy.
As presented in Table
II, postoperative pathological analysis revealed that the most
common macroscopic tumor type was ulcer type (53.8%; n=21), while
the narrowing type was the least frequent (2.6%; n=1; Table II). Immunohistochemical staining
showed positive expression of cytokeratin in 51.3% of cases,
chromogranin A in 33.3%, synaptophysin in 89.7% and CD56 in 84.6%.
A high proliferative index, defined as Ki-67 >55%, was observed
in 74.4% of patients. The incidence of perineural invasion was
7.7%, and vascular invasion was identified in 20.5% of cases.
 | Table II.Pathological results. |
Table II.
Pathological results.
| Pathological
result | Surgical group
(n=39) |
|---|
| Morphological
subtype |
|
|
Mushroom type | 6 (15.4) |
| Ulcer
type | 21 (53.8) |
|
Medullary type | 6 (15.4) |
|
Narrowing type | 1 (2.6) |
| Mucosal
abnormality | 5 (12.8) |
| CK |
|
|
(−) | 4 (10.3) |
|
(+) | 20 (51.3) |
|
Absent | 15 (38.5) |
| CgA |
|
|
(−) | 23 (59.0) |
|
(+) | 13 (33.3) |
|
Absent | 3 (7.7) |
| Syn |
|
|
(−) | 4 (10.3) |
|
(+) | 35 (89.7) |
| CD56 |
|
|
(−) | 6 (15.4) |
|
(+) | 33 (84.6) |
| Ki-67, % |
|
|
≤55 | 10 (25.6) |
|
>55 | 29 (74.4) |
| PD-L1 (+) | 6 (15.4) |
| BRAF V600E (+) | 5 (12.8) |
| Pathological T
stage |
|
|
T1a | 2 (5.1) |
|
T1b | 13 (33.3) |
| T2 | 10 (25.6) |
| T3 | 14 (35.9) |
| Pathological N
stage |
|
| N0 | 18 (46.2) |
| N1 | 11 (28.2) |
| N2 | 7 (17.9) |
| N3 | 3 (7.7) |
| Vascular
invasion | 8 (20.5) |
| Perineural
invasion | 3 (7.7) |
| Preoperative
diagnosis |
|
|
Squamous cell carcinoma | 13 (33.3) |
|
Neuroendocrine carcinoma | 15 (38.5) |
|
Diagnosis uncertain | 11 (28.2) |
| Postoperative
diagnosis |
|
| Small
cell neuroendocrine carcinoma | 28 (71.7) |
| Large
cell neuroendocrine carcinoma | 1 (2.6) |
|
MiNEC-SCC | 9 (23.1) |
|
MiNEC-AC | 1 (2.6) |
According to the pathological T, N, metastasis (M)
staging, 64.1% of patients were classified as T1-T2, while 35.9%
were staged as T3. Lymph node metastasis was observed in 53.8% of
patients. A, 74.3% of tumors were classified as pure neuroendocrine
carcinoma, with small cell neuroendocrine carcinoma (SCNEC) being
the predominant subtype (71.7%). Additionally, 23.1% of tumors were
identified as mixed neuroendocrine carcinoma and squamous cell
carcinoma (MiNEC-SCC), and 2.6% were classified as mixed
neuroendocrine carcinoma and adenocarcinoma.
Expression and clinical
characterization of PD-L1 and BRAF V600E
Immunohistochemical staining for PD-L1 and BRAF
V600E was performed in patients who received surgery to evaluate
their expression profiles in ENEC and to explore the clinical
characteristics associated with biomarker positivity. As shown in
Fig. S1, PD-L1 was normally
expressed on the cell membrane, while BRAF V600E is normally
expressed in the cytoplasm (Fig.
S1). As shown in Table II, the
BRAF V600E mutation was detected in 12.8% of cases (5/39), while
PD-L1 positivity was observed in 15.4% (6/39).
To further investigate potential clinical
associations, patients were stratified into biomarker-positive and
-negative groups. As shown in Table
SII, a significant difference in presenting symptoms was
identified between the PD-L1 positive and negative groups. In the
PD-L1 positive group, subxiphoid pain was the most common initial
symptom (50%), and 83.3% of patients reported pain-related
symptoms. By contrast, the PD-L1 negative group predominantly
presented with dysphagia (63.6%). Tumor length also differed
significantly between the BRAF V600E positive and negative groups.
In the positive group, 80% of tumors were ≤3 cm in length, whereas
70.6% of tumors in the negative group measured >3 cm.
As summarized in Table
SIII, a pooled analysis of patients with positive PD-L1 and
BRAF expression was performed. Among the 9 patients with positive
biomarker expression, 88.9% (8/9) were >60 years of age. The
most common pathological subtype in this group was MiNEC-SCC,
accounting for 50.0% (3/6) of PD-L1-positive cases and 60.0% (3/5)
of BRAF V600E-positive cases. This was followed by SCNEC, observed
in 33.3% (2/6) of PD-L1-positive cases and 40.0% (2/5) of BRAF
V600E-positive cases. Notably, two patients with MiNEC-SCC were
found to co-express both PD-L1 and BRAF V600E.
Survival outcomes
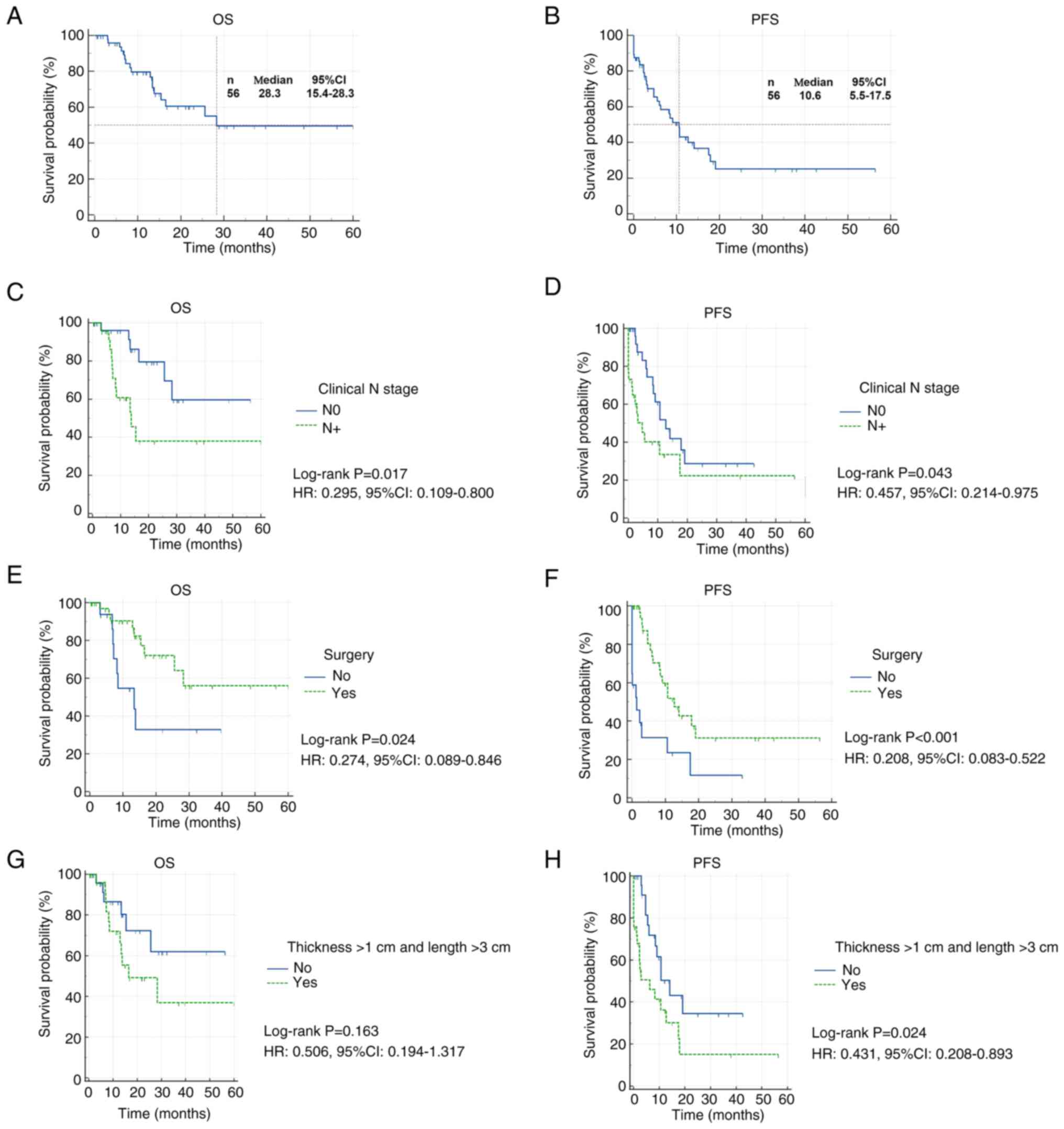
As illustrated in Fig.
2, the median follow-up duration for the entire cohort was 16.8
months. In the overall population, the median OS was 28.3 months
and the median PFS was 10.6 months. The 1-year OS and PFS rates
were 79.7 and 43.1%, respectively, while the 3-year OS and PFS
rates were 49.5 and 25.1%, respectively.
Survival analysis by clinical N stage showed that
patients with N0 disease had significantly improved outcomes
compared with those with N1-N3 disease. Specifically, N0 patients
exhibited longer OS [hazard ratio (HR), 0.295, 95% CI, 0.109–0.800]
and PFS (HR, 0.450; 95% CI, 0.214–0.975). Patients who received
surgery demonstrated significantly improved survival compared with
those who did not. OS was significantly longer in the surgical
group (HR, 0.274; 95% CI, 0.089–0.846) and PFS was also
significantly prolonged (HR, 0.208; 95% CI, 0.083–0.522). Tumors
with a thickness >1 cm and length >3 cm were defined as more
aggressive. Patients with less aggressive tumors showed improved
PFS compared with those with more aggressive disease (HR, 0.431;
95% CI, 0.208–0.893), while no statistically significant difference
in OS was observed.
As shown in Table
III, univariate Cox regression analysis identified clinical N
stage and surgery as prognostic factors for OS; however, these were
not retained as independent predictors in the multivariate model.
For PFS, univariate analysis revealed that clinical N stage, tumor
aggressiveness and surgery were significantly associated with
prognosis. Multivariate analysis demonstrated that surgery was an
independent predictor of improved PFS (HR, 0.345; 95% CI,
0.146–0.816).
 | Table III.Univariate and multivariate Cox
regression analysis in the overall population. |
Table III.
Univariate and multivariate Cox
regression analysis in the overall population.
|
| OS | PFS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical N
stage |
|
|
|
|
|
|
|
|
| N0 |
|
|
|
|
|
|
|
|
|
N1-3 | 3.208 | 0.023 | 2.469 | 0.200 | 2.040 | 0.050 | 0.916 | 0.860 |
|
| (1.173, 8.775) |
| (0.619, 9.853) |
| (0.999, 4.163) |
| (0.344, 2.435) |
|
| Tumor size and
length |
|
|
|
|
|
|
|
|
|
Thickness ≤1 cm or |
|
|
|
|
|
|
|
|
| length
≤3 cm |
|
|
|
|
|
|
|
|
|
Thickness >1 cm and | 2.005 | 0.172 | 0.958 | 0.948 | 2.244 | 0.030 | 1.956 | 0.133 |
| length
>3 cm | (0.739, 5.441) |
| (0.265, 3.462) |
| (1.084, 4.648) |
| (0.816, 4.689) |
|
| Surgical
treatment |
|
|
|
|
|
|
|
|
| No |
|
|
|
|
|
|
|
|
|
Yes | 0.346 | 0.031 | 0.557 | 0.315 | 0.314 | 0.002 | 0.345 | 0.015 |
|
| (0.132, 0.910) |
| (0.178, 1.743) |
| (0.152, 0.650) |
| (0.146, 0.816) |
|
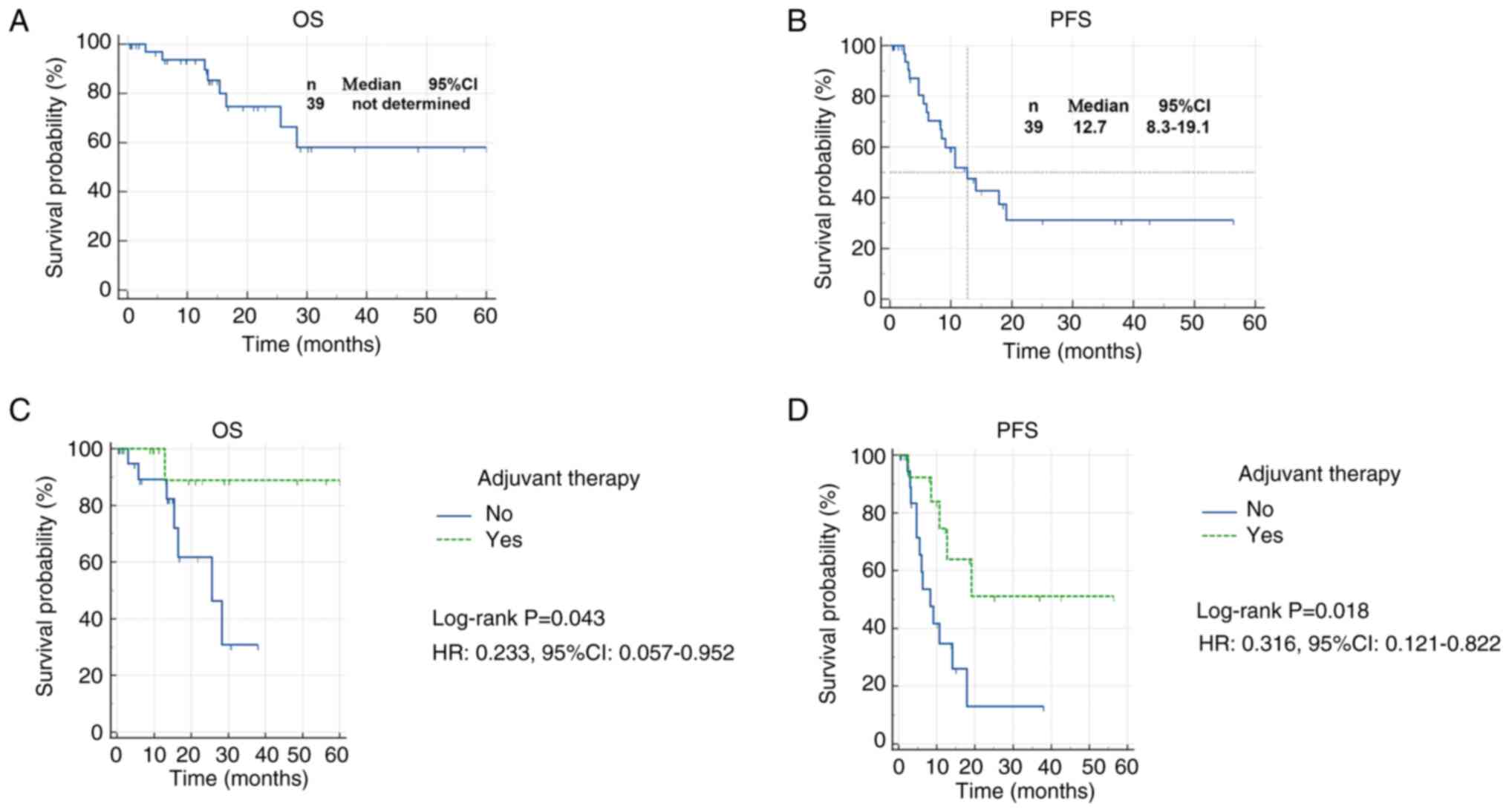
In the surgical cohort, survival analysis was
performed with a median follow-up duration of 15 months. As shown
in Fig. 3, the median OS was not
reached, while the median PFS was 12.7 months. The 1-year OS and
PFS rates were 93.6 and 51.8%, respectively, while the 3-year OS
and PFS rates were 58.1 and 31.2%, respectively.
Patients who received postoperative adjuvant therapy
demonstrated significantly improved survival outcomes compared with
those who did not. Specifically, adjuvant therapy was associated
with prolonged OS (HR, 0.233; 95% CI, 0.057–0.952) and improved PFS
(HR, 0.316; 95% CI, 0.121–0.822). As shown in Table SIV, Cox regression analysis further
demonstrated that postoperative adjuvant therapy was an independent
prognostic factor for PFS in the surgical cohort (HR, 0.252; 95%
CI,0.081–0.781).
Discussion
ENEC most commonly exhibits small cell morphology,
resembling SCLC, rather than the large-cell morphology typically
seen in other gastrointestinal neuroendocrine carcinomas (GINECs).
In the present study, 71.7% (28/39) of patients had tumors
classified as SCNEC. Molecular analyses have revealed notable
overlap between ENEC and SCLC in terms of genomic alterations and
molecular subtypes (24,25). Due to the lack of disease-specific
treatment guidelines, ENEC is currently managed using protocols
extrapolated from either SCLC or other gastrointestinal
neuroendocrine tumors.
PD-L1 expression has been reported in >70% of
SCLC cases and is considered a favorable prognostic biomarker
(26). This success has prompted
interest in the potential role of immunotherapy for ENEC. However,
data on PD-L1 expression in ENEC are limited and inconsistent.
Yamashita et al (27)
reported a PD-L1 positivity rate of 33% in a small ENEC cohort. In
another study, Huang et al (28) similarly found a PD-L1 positivity
rate of 33% (3/9). By contrast, Xing et al (29) reported a notably lower PD-L1
positivity rate of 9.1% in ENEC, compared with 29% in GINEC,
raising concerns about the applicability of PD-1/PD-L1 monotherapy
in ENEC. These discrepancies highlight the potential need for
combination therapy strategies in this patient population.
The BRAF V600E mutation may upregulate PD-L1
expression, thereby promoting immune evasion (20). Preclinical studies have shown that
combining immune checkpoint inhibitors with BRAF-targeted therapy
may enhance treatment efficacy in BRAF-mutated tumors (30,31).
Tian et al (20) reported
that the combination of PD-1, BRAF and MEK inhibitors results in a
notably higher objective response rate (25.0%; 95% CI, 10.7–44.9%)
compared with BRAF and MEK inhibition alone (7.0%; 95% CI,
1.5–19.1%), highlighting the potential synergistic benefit of
incorporating immunotherapy into targeted therapy regimens.
In the present study, the expression profiles of
PD-L1 and BRAF V600E mutations were analyzed in ENEC to explore
potential targets for such combination strategies. The results
revealed a relatively low prevalence of BRAF V600E mutations
(12.8%; 5/39) and PD-L1 positivity (15.4%; 6/39), suggesting that
ENEC may lack the features of a highly immune-infiltrated tumor
microenvironment and instead represents an ‘immune cold’ phenotype
distinct from SCLC. Consistent with the present findings, Zhang
et al (32) previously
demonstrated that ENEC tumors exhibited sparse immune cell
infiltration, which may contribute to reduced antitumor immune
responses and limited immunotherapeutic efficacy. However, the
expression of PD-L1 in ENEC needs to be further clarified in
studies with larger samples.
Furthermore, the present results corroborate
previous studies showing that the BRAF mutation rate in ENEC is
markedly lower compared with in other GINECs (9). These observations imply that BRAF
V600E is unlikely to be a principal oncogenic driver in ENEC, and
its clinical phenotype may not be associated with mutation status.
The present study observed that clinical symptoms differed between
the PD-L1 positive and negative groups, while tumor length showed a
significant difference between the BRAF V600E-positive and negative
groups. However, due to the limited number of positive cases,
potential bias cannot be ruled out, and these associations should
be interpreted with caution.
Notably, however, PD-L1 and BRAF V600E expression
was notably more frequent in MiNEC-SCC subtypes, with a positivity
rate of 33.3% (3/9) for both markers. This raises the possibility
that BRAF V600E mutation and PD-L1 expression may be involved in
the phenotypic plasticity or lineage conversion between
neuroendocrine and squamous histology. Therefore, screening for
relevant markers in patients with MiNEC-SCC is recommended, who are
more likely to benefit from immunotherapy or targeted therapy.
ENEC is characterized by its high aggressiveness and
early propensity for metastasis (33). In the present cohort, two patients
presented with distant metastases at initial diagnosis, and 46.4%
were clinically staged as N1 or higher. Among the surgical
population, 74.4% of tumors exhibited a Ki-67 proliferation index
>55%, indicating a high proliferative potential. The incidences
of perineural invasion and vascular invasion were 7.7 and 20.5%,
respectively. Although 64.1% of patients were pathologically staged
as T1 or T2, the lymph node positivity rate reached 53.8%,
suggesting a strong tendency for early lymphatic spread and
vascular invasion even in tumors classified as early-stage.
Patients with ENEC have an extremely poor prognosis
(34,35). A large systematic review including
1,176 patients with esophageal SCNEC reported a median OS of only
11.1 months (36). In the present
study cohort, the median OS reached 28.3 months, and the median PFS
was 10.6 months. The 1-year OS and PFS rates were 79.7 and 43.1%,
respectively, while the 3-year OS and PFS rates were 49.5 and
25.1%. Subgroup analysis revealed that advanced N stage and more
aggressive tumor characteristics (thickness >1 cm and length
>3 cm) were associated with worse PFS outcomes, consistent with
the findings of Zou et al (37). Surgery significantly improved
survival outcomes. Patients who underwent surgery exhibited longer
OS and PFS compared with those who did not receive surgery.
Moreover, multivariate Cox regression analysis identified surgery
as an independent prognostic factor for PFS. Although potential
selection bias cannot be excluded-due to certain patients being
ineligible for surgery due to advanced disease-the survival
advantage associated with surgical intervention remains evident.
Similar findings have been reported in previous studies. Erdem
et al (38) found that the
2-year OS was significantly higher in the surgical group (57.3%)
compared with the non-surgical group (35.2%). Xu et al
(4) demonstrated that patients with
stage I or IIA esophageal SCNEC who underwent surgery alone had
improved survival compared with those who did not (median OS, 29.0
vs. 17.4 months; P=0.031). Chen et al (34) also reported a survival benefit
associated with surgery (P=0.003).
In the present surgical cohort, the median PFS was
12.7 months. The 1-year OS and PFS rates were 93.6 and 51.8%,
respectively, while the 3-year OS and PFS were 58.1 and 31.2%.
Although limited by a relatively short follow-up period and small
sample size, the modest 1-year PFS highlights the aggressive nature
of ENEC and its tendency toward early progression. Postoperative
adjuvant therapy emerged as another key factor influencing
prognosis. Patients who received adjuvant therapy after surgery had
a significantly reduced risk of disease progression, by nearly 70%.
Although the OS benefit was not statistically significant in
multivariate Cox analysis, Kaplan-Meier curves revealed a trend
toward prolonged OS in the adjuvant therapy group. Several previous
studies have demonstrated that surgery combined with adjuvant
therapy markedly improves outcomes in ENEC (37,39–41).
However, some reports suggest that definitive chemoradiotherapy may
provide superior survival benefits in patients with esophageal
SCNEC compared with surgery combined with chemotherapy (42). Zhu et al (43) further indicated that patients with
tumors located in the lower third of the esophagus or those with
tumors >5 cm derived greater OS benefit from surgery plus
chemotherapy, while chemoradiotherapy was more effective in tumors
located in the middle third or ≤5 cm in length. More large-sample
studies are needed in the future to determine the best treatment
for patients with ENEC.
A notable limitation of the present study is the
relatively small sample size, with only 39 patients available for
analysis. No formal power analysis was conducted, as the rarity of
ENEC inherently restricted case accrual, which may have limited the
statistical power. In addition, immunohistochemical images for CK,
Syn, CgA, and Ki-67 were not available, as these data were
retrieved from electronic medical records rather than original
pathological slides. Nevertheless, to the best of our knowledge,
this represents one of the largest single-institution cohorts to
date that systematically evaluated PD-L1 expression and BRAF V600E
mutation in ENEC. While the findings should be interpreted with
caution, they provide valuable preliminary evidence and may serve
as a reference point for future multi-center studies with larger
cohorts to validate these results.
In conclusion, PD-L1 expression and BRAF V600E
mutations are rare in ENEC but more common in MiNEC-SCC. Due to the
overall poor prognosis of ENEC, surgery combined with adjuvant
therapy may offer clinical benefit for selected patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Nanchong City University
Science and Technology Strategic Cooperation Special Fund (grant
no. 22SXQT0095) and the scientific research foundation for advanced
talents, Affiliated hospital of North Sichuan Medical College
(grant no. 2023GC006).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MF performed the study design, CZ performed the
statistical analysis and was a major contributor in writing the
manuscript. LZ and BX performed experiments. GS interpreted data.
CZ and MF confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by The
Ethics Committee of Affiliated Hospital of North Sichuan Medical
College (approval no. 2024ER682-1). According to national
legislation and institutional requirements, written informed
consent was not required for participation in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ENEC
|
esophageal neuroendocrine
carcinoma
|
|
SCLC
|
small cell lung cancer
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PD-1
|
programmed cell death protein 1
|
|
PD-L1
|
programmed death ligand 1
|
|
SCNEC
|
small cell neuroendocrine
carcinoma
|
|
MiNEC-SCC
|
mixed neuroendocrine carcinoma and
squamous cell carcinoma
|
|
CI
|
confidence interval
|
|
GINEC
|
gastrointestinal neuroendocrine
carcinoma
|
References
|
1
|
Li Z, Hu J, Chen P and Zeng Z: Incidence,
treatment, and survival analysis in esophageal neuroendocrine
carcinoma population. Transl Cancer Res. 9:4317–4329. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong AT, Shao M, Rineer J, Osborn V,
Schwartz D and Schreiber D: Treatment and survival outcomes of
small cell carcinoma of the esophagus: An analysis of the National
Cancer Data Base. Dis Esophagus. 30:1–5. 2017.
|
|
3
|
Ji A, Jin R, Zhang R and Li H: Primary
small cell carcinoma of the esophagus: Progression in the last
decade. Ann Transl Med. 8:5022020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu L, Li Y, Liu X, Sun H, Zhang R, Zhang
J, Zheng Y, Wang Z, Liu S and Chen X: Treatment strategies and
prognostic factors of Limited-stage primary small cell carcinoma of
the esophagus. J Thorac Oncol. 12:1834–1844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roskoski R: Targeting oncogenic Raf
protein-serine/threonine kinases in human cancers. Pharmacol Res.
135:239–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roskoski R: RAF protein-serine/threonine
kinases: Structure and regulation. Biochem Biophys Res Commun.
399:313–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beeram M, Patnaik A and Rowinsky EK: Raf:
A strategic target for therapeutic development against cancer. J
Clin Oncol. 23:6771–6790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fazio N, Abdel-Rahman O, Spada F, Galdy S,
De Dosso S, Capdevila J and Scarpa A: RAF signaling in
neuroendocrine neoplasms: From bench to bedside. Cancer Treat Rev.
40:974–979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dizdar L, Werner TA, Drusenheimer JC,
Möhlendick B, Raba K, Boeck I, Anlauf M, Schott M, Göring W,
Esposito I, et al: BRAFV600E mutation: A promising target in
colorectal neuroendocrine carcinoma. Int J Cancer. 144:1379–1390.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Idrees K, Padmanabhan C, Liu E, Guo Y,
Gonzalez RS, Berlin J, Dahlman KB, Beauchamp RD and Shi C: Frequent
BRAF mutations suggest a novel oncogenic driver in colonic
neuroendocrine carcinoma. J Surg Oncol. 117:284–289. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capdevila J, Arqués O, Hernández Mora JR,
Matito J, Caratù G, Mancuso FM, Landolfi S, Barriuso J,
Jimenez-Fonseca P, Lopez Lopez C, et al: Epigenetic EGFR gene
repression confers sensitivity to therapeutic BRAFV600E blockade in
colon neuroendocrine carcinomas. Clin Cancer Res. 26:902–909. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pedoeem A, Azoulay-Alfaguter I, Strazza M,
Silverman GJ and Mor A: Programmed death-1 pathway in cancer and
autoimmunity. Clin Immunol. 153:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Xu X, Wang D, Liu J, Sun J, Lu M,
Wang R, Hui B, Li X, Zhou C, et al: Neoadjuvant sintilimab and
chemotherapy in patients with potentially resectable esophageal
squamous cell carcinoma (KEEP-G 03): An Open-label, single-arm,
phase 2 trial. J Immunother Cancer. 11:e0058302023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H,
Zhu L, Shen Y, Zhang H, Sun Y, et al: Original research:
Multicenter, single-arm, phase II trial of camrelizumab and
chemotherapy as neoadjuvant treatment for locally advanced
esophageal squamous cell carcinoma. J Immunother Cancer.
10:e0042912022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H,
Gong L, Liu H, Tian F, Lu Q, et al: Tislelizumab combined with
chemotherapy as neoadjuvant therapy for surgically resectable
esophageal cancer: A prospective, single-arm, phase II study
(TD-NICE). Int J Surg. 103:1066802022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horn L, Mansfield AS, Szczęsna A, Havel L,
Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio
M, et al: First-line atezolizumab plus chemotherapy in
extensive-stage small-cell lung cancer. N Engl J Med.
379:2220–2229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng Y, Han L, Wu L, Chen J, Sun H, Wen
G, Ji Y, Dvorkin M, Shi J, Pan Z, et al: Effect of First-line
serplulimab vs placebo added to chemotherapy on survival in
patients with Extensive-stage small cell lung cancer: The
ASTRUM-005 randomized clinical trial. JAMA. 328:1223–1232. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian J, Chen JH, Chao SX, Pelka K,
Giannakis M, Hess J, Burke K, Jorgji V, Sindurakar P, Braverman J,
et al: Combined PD-1, BRAF and MEK inhibition in BRAFV600E
colorectal cancer: A phase 2 trial. Nat Med. 29:458–466. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guleria P, Kumar S, Malik PS and Jain D:
PD-L1 expression in small cell and large cell neuroendocrine
carcinomas of lung: An immunohistochemical study with review of
literature. Pathol Oncol Res. 26:2363–2370. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lantuejoul S, Sound-Tsao M, Cooper WA,
Girard N, Hirsch FR, Roden AC, Lopez-Rios F, Jain D, Chou TY, Motoi
N, et al: PD-L1 testing for lung cancer in 2019: Perspective from
the IASLC pathology committee. J Thorac Oncol. 15:499–519. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li R, Yang Z, Shao F, Cheng H, Wen Y, Sun
S, Guo W, Li Z, Zhang F, Xue L, et al: Multi-omics profiling of
primary small cell carcinoma of the esophagus reveals RB1
disruption and additional molecular subtypes. Nat Commun.
12:37852021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ooki A, Osumi H, Fukuda K and Yamaguchi K:
Potent molecular-targeted therapies for gastro-entero-pancreatic
neuroendocrine carcinoma. Cancer Metastasis Rev. 42:1021–1054.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishii H, Azuma K, Kawahara A, Yamada K,
Imamura Y, Tokito T, Kinoshita T, Kage M and Hoshino T:
Significance of programmed cell Death-ligand 1 expression and its
association with survival in patients with small cell lung cancer.
J Thorac Oncol. 10:426–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamashita S, Abe H, Yamashita H, Yagi K,
Seto Y and Ushiku T: PD-L1 and HLA-class I expression status and
their therapeutic implication in oesophageal small-cell carcinoma.
Histopathology. 83:264–275. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Z, Jin Y, Cai X, Chen L, Shen X, Li
B, Chen H and Li Y: Association of the programmed death ligand-1
combined positive score in tumors and clinicopathological features
in esophageal cancer. Thorac Cancer. 13:523–532. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xing J, Ying H, Li J, Gao Y, Sun Z, Li J,
Bai C, Cheng Y and Wu H: Immune checkpoint markers in
neuroendocrine carcinoma of the digestive system. Front Oncol.
10:1322020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebert PJR, Cheung J, Yang Y, McNamara E,
Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, et
al: MAP kinase inhibition promotes T cell and anti-tumor activity
in combination with PD-L1 checkpoint blockade. Immunity.
44:609–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Mayes PA, Eastman S, Shi H,
Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson
C, et al: The BRAF and MEK inhibitors dabrafenib and trametinib:
Effects on immune function and in combination with immunomodulatory
antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res.
21:1639–1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Yu B, Liu Z, Wei J, Pan J, Jiang
C and Li Z: Analysis of the clinicopathological characteristics,
prognosis, and lymphocyte infiltration of esophageal neuroendocrine
neoplasms: A Surgery-based cohort and Propensity-score matching
study. Cancers (Basel). 15:17322023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giannetta E, Guarnotta V, Rota F, de Cicco
F, Grillo F, Colao A and Faggiano A; NIKE: A rare rarity:
Neuroendocrine tumor of the esophagus. Crit Rev Oncol Hematol.
137:92–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen C, Hu H, Zheng Z, Yang Y, Chen W,
Qiao X, Li P and Zhang S: Clinical characteristics, prognostic
factors, and survival trends in esophageal neuroendocrine
carcinomas: A population-based study. Cancer Med. 11:4935–4945.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gu YM, Yang YS, Shi GD, Yan CY, Shang QX,
Zhang HL, Wang WP, Yuan Y and Chen LQ: Limited-stage small cell
carcinoma of the esophagus treated with curative esophagectomy: A
multicenter retrospective cohort study. J Surg Oncol.
126:1396–1402. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu X, Luo J, Ling Y, Kong YZ, Feng LL,
Zhou J and Wang F: Management of small cell carcinoma of esophagus
in China. J Gastrointest Surg. 17:1181–1187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou B, Li T, Zhou Q, Ma D, Chen Y, Huang
M, Peng F, Xu Y, Zhu J, Ding Z, et al: Adjuvant therapeutic
modalities in primary small cell carcinoma of esophagus patients: A
retrospective cohort study of multicenter clinical outcomes.
Medicine (Baltimore). 95:e35072016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Erdem S, Troxler E, Warschkow R, Tsai C,
Yerokun B, Schmied B, Stettler C, Blazer DG III, Hartwig M, Worni M
and Gloor B: Is There a role for surgery in patients with
neuroendocrine tumors of the esophagus? A Contemporary View from
the NCDB. Ann Surg Oncol. 27:671–680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng HY, Ni PZ, Wang YC, Wang WP and Chen
LQ: Neuroendocrine carcinoma of the esophagus: Clinical
characteristics and prognostic evaluation of 49 cases with surgical
resection. J Thorac Dis. 8:1250–1256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Hou P, Zha KJ, Wang F, Zhou K, He
W and Gao JB: Prognostic value of pretreatment contrast-enhanced
computed tomography in esophageal neuroendocrine carcinoma: A
multi-center follow-up study. World J Gastroenterol. 26:4680–4693.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu S, Ge X, Gao Z, Zhou Q, Shi Y, Jiang
W, Yang M and Sun X: Clinicopathological analysis of 67 cases of
esophageal neuroendocrine carcinoma and the effect of postoperative
adjuvant therapy on prognosis. Medicine (Baltimore).
100:e273022021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng MB, Zaorsky NG, Jiang C, Tian LJ,
Wang HH, Liu CL, Wang J, Tao Z, Sun Y, Wang J, et al: Radiotherapy
and chemotherapy are associated with improved outcomes over surgery
and chemotherapy in the management of limited-stage small cell
esophageal carcinoma. Radiother Oncol. 106:317–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu J, Wang Y, Sun H, Zhang Y, Zhang W,
Shen W, Yang N, Tan B, Su X, Li L, et al: Surgery versus
radiotherapy for limited-stage small cell esophageal carcinoma: A
multicenter, retrospective, cohort study in China (ChiSCEC). Int J
Surg. 110:956–964. 2024. View Article : Google Scholar : PubMed/NCBI
|