Introduction
Gastrointestinal malignancies account for >25% of
the global incidence of all tumors and 35% of tumor-related
mortality, which poses a notable threat to the health of
individuals worldwide (1,2). Based on histological features,
gastrointestinal malignancies can be classified into
adenocarcinoma, squamous cell carcinoma, sarcomatoid carcinoma and
undifferentiated carcinoma (3,4). Among
them, switch/sucrose non-fermentable (SWI/SNF) related matrix
associated actin-dependent regulator of chromatin sub-family A
member 4 (SMARCA4)-deficient undifferentiated carcinoma (DUC) of
the gastrointestinal tract is a subtype of gastrointestinal
undifferentiated carcinoma (4,5).
SMARCA4 deficiency is more commonly observed in
non-small cell lung cancer, with an incidence of 5–10%. However, it
is relatively rare in gastrointestinal cancer types, with limited
reports available (6). The absence
of SMARCA4 in primary tumors is associated with higher tumor
invasiveness and a poorer prognosis. Compared with common tumors,
there may be differences in clinical features, imaging
manifestations, pathological characteristics and treatment
responses (7,8). A previous study validated that in 436
cancer cell lines, tumors with low SMARCA4 expression demonstrated
markedly higher IC50 values for multiple
chemotherapeutic drugs compared with those with high SMARCA4
expression, which implied that SMARCA4 deficiency may be a key
reason for patient drug resistance (9). Another previous study indicated that
SMARCA4 deficiency induces chemoresistance in tumor cells and also
activates the EMT process, which thereby enhances tumor invasion
(10). Therefore, additional
studies and reports of cases of SMARCA4-deficient gastrointestinal
undifferentiated carcinoma may enhance the current understanding of
its biological behavior and potentially provide key insights for
clinical diagnosis, the formulation of treatment strategies and
prognosis assessment in the future.
The present study aimed to report a rare case of
small intestinal SMARCA4-DUC. The patient presented with intestinal
intussusception and progression to metastasis in the lungs, liver,
spleen and skin. The condition of the patient deteriorated rapidly,
with death occurring 42 days following the initial detection of the
lesion. The present study aimed to provide a detailed description
of the clinical features, imaging manifestations, pathological
characteristics and molecular biological features of the present
case, to aid in the diagnosis and treatment of SMARCA4-deficient
gastrointestinal undifferentiated carcinoma.
Case report
A 69-year-old man with gastrointestinal discomfort
was admitted to Weifang No. 2 People's Hospital (Weifang, China) on
April, 2024 (day 1). The patient reported that they had suffered
from chronic gastritis and gastric ulcers for >10 years. The
mental health of the patient was poor at the time of admission,
with dyspnea and no other abnormalities.
After the patient was admitted to the hospital, a
biochemical examination was conducted (Table I). Results of the examination
demonstrated carcinoembryonic antigen levels of 22.81 ng/ml
(reference value, 0–4.5 ng/ml), indicative of a malignant
neoplastic lesion. The medical team conducted a chest CT (Optima
CT660, GE HealthCare; 5-mm-layer-thickness; helical scanning;
Table II) with plain scanning and
enhancement examination. Results of the CT examination revealed
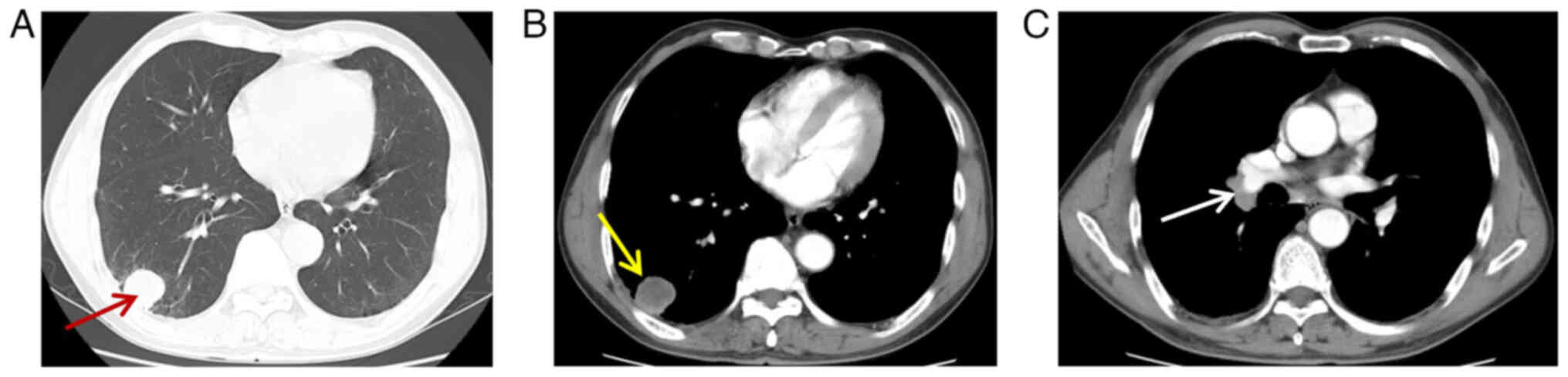
multiple nodules and masses in both lungs, with the largest mass
located in the right lower lobe of the lung in the outer basal
segment. The mass exhibited a diameter of ~31 mm, with an average
CT value of 19 Hounsfiled unit (HU), clear borders and smooth edges
(Fig. 1A). There was no notable
enhancement in the central area of the lesion on enhanced scanning
(double-phase enhancement CT values of 20 HU and 21 HU,
respectively), with circumferential enhancement around the edges
(Fig. 1B). Furthermore, the lymph
node in the right hilar region of the right lung was enlarged
(Fig. 1C), with a maximal short
diameter of ~15 mm. An abdominal CT scan (Optima CT660, Cytiva;
5-mm layer thickness; spiral scan) and enhanced examination
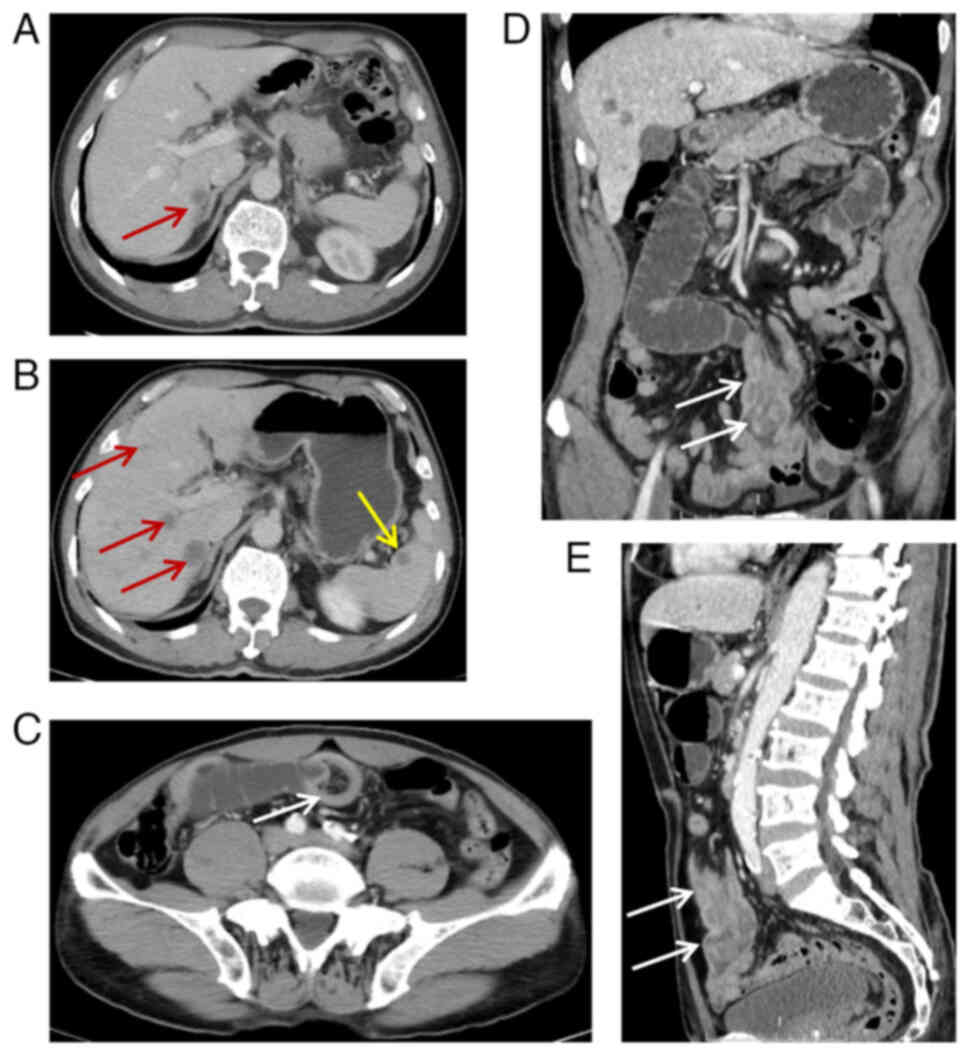
revealed multiple hypodense nodules in the liver, with the largest
nodule observed in the hepatic segment VIII (Fig. 2A). This nodule exhibited a diameter
of ~12 mm, a CT value of 20 HU on scanning and enhanced scans of 30
HU, 35 HU and 39 HU in hepatic arterial, portal and delayed phases,
respectively. A brain CT was also performed and indicated no
evidence of metastasis, hemorrhage or infarction.
 | Table I.General examination and laboratory
test results on admission. |
Table I.
General examination and laboratory
test results on admission.
| A, General clinical
examination |
|---|
|
|---|
| Parameter | Value | Reference
range |
|---|
| Body temperature,
°C | 36.6 | 36.3-37.2 |
| Heart rate, beats
per min | 82 | 60-100 |
| Respiratory rate,
breaths per min | 18 | 15-20 |
| Blood pressure,
mmHg | 130/82 | 90/60-120/80 |
|
| B, ABG
analysis |
|
|
Parameter | Value | Reference
range |
|
| pCO2,
mmHg | 36.00 | 35-45 |
| pO2,
mmHg | 86.00 | 80-100 |
| HCO3,
mmol/l | 24.50 | 18-23 |
| TCO2,
mmol/l | 25.60 | 22-28 |
| SO2C,
% | 97.00 | 95-98 |
| A-aDO2,
mmHg | 20.00 | 15 |
| BE, mmol/l | 0.30 | −2-3 |
|
| C, Tumor marker
assays |
|
|
Parameter | Value | Reference
range |
|
| CEA, µg/l | 22.81 | 0-10 |
| CA125, U/ml | 28.62 | 0-35 |
| CA19-9, U/ml | 26.88 | 0-27 |
| CA72-4, U/ml | 2.97 | 0-6.9 |
| AFP, ng/ml | 1.85 | 0-20 |
|
| D, Pathogen
detection |
|
| G test,
pg/ml | 32.53 | <60 |
|
| CP-DNA test | Negative | Negative |
| MP-DNA test | Negative | Negative |
| GM ratio | 0.21 | <0.5 |
| Fungal smear
test | Negative | Negative |
 | Table II.Summary of patient radiological
examination results. |
Table II.
Summary of patient radiological
examination results.
| Day | Radiological
examination | Results |
|---|
| Day 1 | Chest CT | Multiple nodules
and masses found in both lungs, enlargement of right hilar lymph
node in right lung |
|
| Abdominal CT | Multiple
low-density nodules in the liver, with larger nodules located in
segment VIII of the liver |
| Day 18 | Abdominal CT | Enlarged liver
metastases, new metastases in the spleen, intussusception in the
jejunal segment of the small intestine, intestinal obstruction |
| Day 31 | Chest CT | Enlarged lesions in
both lungs with new ground-glass shadows in the dorsal aspect of
both lungs |
The clinical history of the patient, imaging and
laboratory findings were suggestive of a malignant neoplastic
lesion. On day 6, the patient underwent a puncture biopsy of a mass
in the extramedullary segment of the lower lobe of the right lung.
The puncture specimen was a greyish-white tissue of two strips with
a length of 0.8-1.0 cm and a diameter of 0.1 cm. The puncture
specimen was further examined using H&E staining. The tissue
sections were fixed with 4% paraformaldehyde at room temperature
for 30 min. The thickness of each section was 5 µm. Hematoxylin
staining was performed at room temperature for 7 min, followed by
eosin staining at room temperature for 1 min. The sections were
then observed under an light microscope. The fresh specimen was
dehydrated using alcohol in a room-temperature gradient, soaked in
xylene at room temperature and embedded in paraffin at 65°C.
Subsequently, the pathological tissue was cut into 5-µm slices and
soaked in room temperature xylene, anhydrous ethanol, 95% ethanol,
80% ethanol and H2O. The tissue sample was stained using
hematoxylin for 5 min and eosin for 1 min, followed by gradient
alcohol dehydration and neutral resin sealing. Tissue samples were
observed using a light microscope (scale bar, 200 µm). The tumor
cells in the punctured tissue of the patient were diffusely
distributed in the form of sheet nests. Furthermore, the tumor
cells exhibited unclear borders, the cytoplasm was weakly
basophilic or translucent, the nuclei were ovoid or short
spindle-shaped, the frequent mitotic figures was irregular and
small nucleoli were partially visible. Mitotic figures and a high
amount of necrosis were observed (Fig.
3A). Histopathological staining of the patient tissue sample
revealed negative expression of tumor markers for colorectal or
lung cancer, though there is focal expression of squamous cell
carcinoma markers. The tissue samples were fixed with 4%
paraformaldehyde at room temperature for 30 min, and serial
sections were cut at a thickness of 5 µm. After blocking, the
sections were incubated with the primary antibody at 37°C for 30
min, followed by incubation with the secondary antibody at room
temperature for 8 min. To visualize the nuclei, the sections were
treated with 3,3′-diaminobenzidine chromogenic solution for 8 min
at room temperature and then counterstained with hematoxylin for 7
min at room temperature. Finally, the stained sections were
examined under a light microscope. However, the specific origin of
the tumor cannot be determined based solely on these findings.
Therefore, clinical manifestations must be taken into account. The
patient was admitted due to hematochezia and was found to have a
positive fecal occult blood test. No pulmonary symptoms were
observed. Imaging studies demonstrated tumors in the lungs and
liver, which were peripherally located and well-defined, which
suggested a higher possibility of metastatic tumors. Additionally,
the mass causing intussusception had already invaded the muscular
layer of the small intestine. Combining these clinical findings, it
was concluded that the systemic tumors in the patient most likely
originated from the small intestine.
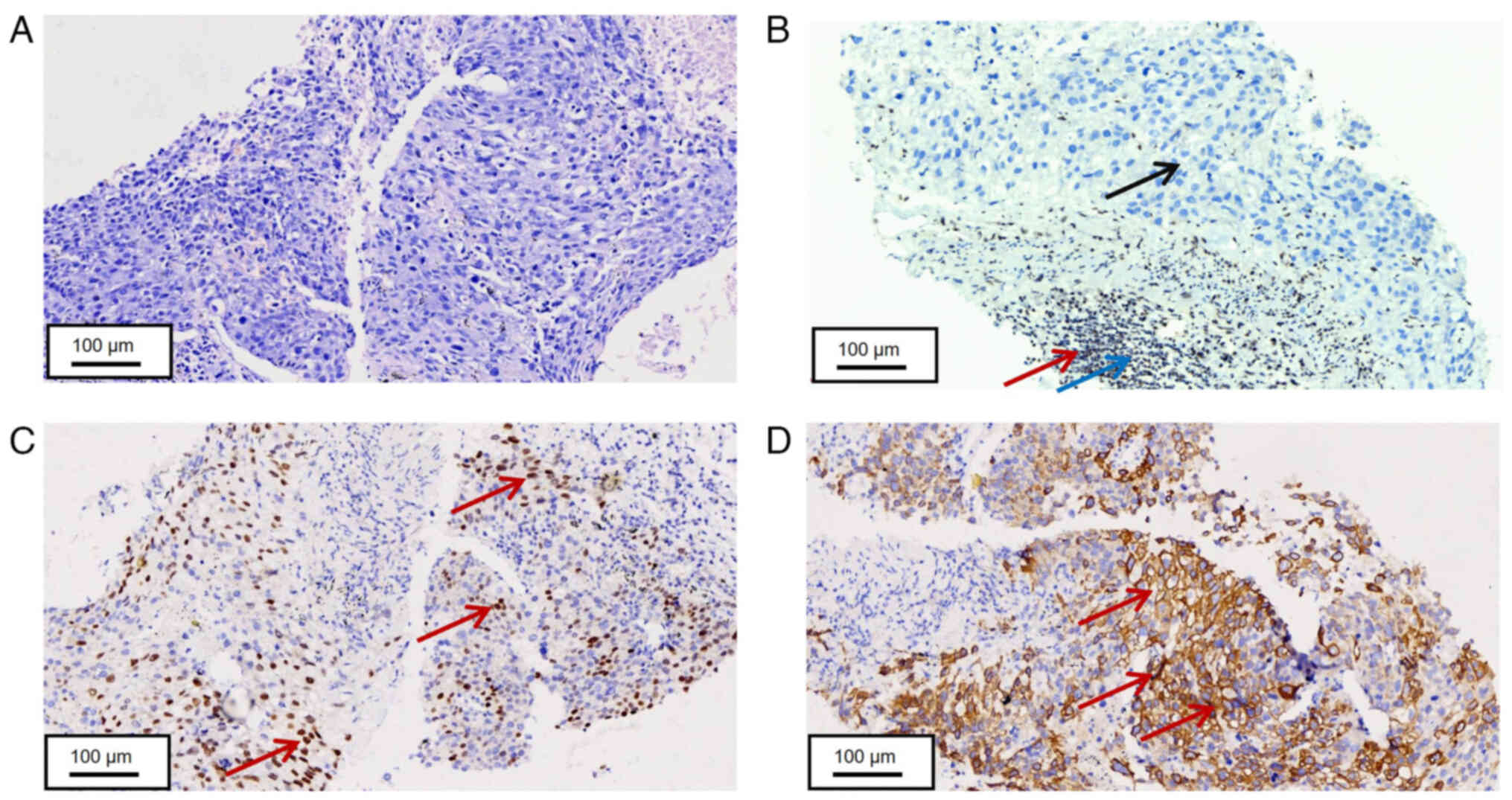 | Figure 3.Histopathological staining of lung
biopsy tissue. (A) Tumor cells in lung biopsy tissue were arranged
in solid sheets, with some cells exhibiting visible nucleoli
following H&E staining (scale bar, 100 µm). (B) Tumor cells
obtained from the lung biopsy tissue revealed negative expression
of SMARCA4 (Red arrows indicate SMARCA4-positive sites, blue arrows
indicate lymphoid regions, black arrows indicate tumor regions,
scale bar, 100 µm). (C) Partial positive expression of p40 in tumor
cells obtained from the lung biopsy tissue (Red arrows indicate
p40-positive sites, scale bar, 100 µm). (D) Positive expression of
CK7 in tumor cells obtained from the lung biopsy tissue (red arrows
indicate CK7-positive sites, scale bar, 100 µm). SMARCA4,
switch/sucrose non-fermentable (SWI/SNF) related matrix associated
actin-dependent regulator of chromatin sub-family A member 4; CK,
cytokeratin. |
Immunohistochemical staining was performed using
Benchmark GX (Roche Diagnostics GmbH). The tissue samples were
fixed with 4% paraformaldehyde at room temperature for 30 min,
after which they were embedded in paraffin wax. Permeabilization
was performed by treating the sections with 0.1% Triton X-100 at
room temperature for 15 min. Each section was cut to a thickness of
5 µm. EDTA (pH, 8) was used for antigen repair and repair
parameters were set at 100°C for 30 min, according to the
manufacturer's protocol. Endogenous catalase was blocked using 3%
hydrogen peroxide for 5 min at room temperature. Tumor tissues were
incubated with monoclonal antibodies against thyroid transcription
factor-1 (TTF-1), NapsinA, cytokeratin (CK)5/6, synaptophysin
(Syn), CK7, p40 and SMARCA4 at 37°C for 30 min. Following primary
incubation, samples were incubated with the HRP-labeled secondary
antibodies (Roche Diagnostics GmbH) for 8 min at room temperature.
Subsequently, the nuclei were visualized using
3,3′-diaminobenzidine chromogenic solution for 8 min at room
temperature and stained using hematoxylin for 7 min at room
temperature (Table III).
 | Table III.Sources of reagents for patient
testing. |
Table III.
Sources of reagents for patient
testing.
| Reagent or
medicine | Manufacturer | Cat. no. | Dilution |
|---|
| EGFR-KRAS-ALK gene
mutation combination test kit | Shenzhen Huada Gene
Technology Co Ltd | RM0914 | Not applicable |
| Anti-Rabbit IgG
H&L (HRP) | Roche Diagnostics
GmbH | 760-500 | 1:1 |
| Anti-human NUT | Guangzhou Anbiping
Medical Technology | IR429 | 1:1 |
| Anti-human
TTF-1 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0677 | 1:1 |
| Anti-human
CK5/6 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0774 | 1:1 |
| Anti-human Syn | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0742 | 1:1 |
| Anti-human
Villin | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0540 | 1:1 |
| Anti-human
CK20 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0834 | 1:1 |
| Anti-human
CDX2 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1056 | 1:1 |
| Anti-human
CD34 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1076 | 1:1 |
| Anti-human CgA | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0707 | 1:1 |
| Anti-human
INSMI | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1017 | 1:1 |
| Anti-human
MelanA | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1033 | 1:1 |
| Anti-human
S-100 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | Kit-0007 | 1:1 |
| Anti-human
Myogenin | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0362 | 1:1 |
| Anti-human
CD30 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0023 | 1:1 |
| Anti-human CK7 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0828 | 1:1 |
| Anti-human CK8 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1002 | 1:1 |
| Anti-human p40 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | RMA-0815 | 1:1 |
| Anti-human
CD56 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0743 | 1:1 |
| Anti-human
SMARCA4 | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-1016 | 1:1 |
| Anti-human
NapsinA | Fuzhou Maixin
Biotechnology Development Co., Ltd. | MAB-0704 | 1:1 |
Results of the present study demonstrated that the
tumor tissues obtained from the patient were negative for TTF-1,
NapsinA, CK5/6 and Syn, positive for CK7, partially positive for
p40 and negative for SMARCA4 (Fig.
3B-D; Table IV). The specimen
was pathologically examined and used the 7th edition American Joint
Committee on Cancer staging system for small bowel cancer (11). The tumor of the patient was stage
IV, T2NXM1. The NX staging was due to insufficient peritumoral
lymph nodes sampled during surgery. However, with distant
metastasis (M1), all T and N categories default to stage IV
(12). Results of the pathological
analysis demonstrated a SMARCA4-DUC with localized squamous
carcinoma expression (Table IV).
Given the low incidence of small intestine cancer and the lack of
specific clinical guidelines, the guidelines for gastrointestinal
tumors were referred to, which indicated that programmed cell death
protein-1 (PD-1) therapy as the first-line treatment for advanced
patients with SMARCA4-DUC (13).
Additionally, previous studies have reported that SMARCA4
deficiency can lead to resistance to platinum-based and other
traditional chemotherapeutic drugs (9,10,14).
Therefore, PD-1 therapy was chosen as the primary treatment without
employing a combination therapy regimen. Tislelizumab (200 mg,
intravenous; cat. no. BGB-A317; BeOne Medicines) was intravenously
administered to the patient in the clinic on day 11. Paclitaxel and
nedaplatin were suggested for the patient on day 13 but not
administered due to bleeding that occurred in the patient. The
patient was passing tarry stools; thus, a follow-up blood test was
performed. The results demonstrated hemoglobin levels of 57 g/l
(healthy range, 130–175 g/l). Gastrointestinal bleeding was
considered, chemotherapy was stopped and fasting, blood transfusion
(4 units) and nutritional support were advised. However, the
patient did not agree to the test because the results of the
immunotherapy did not meet the expectations of the patient and they
were not willing to continue with the immunotherapy.
 | Table IV.Summary of patient IHC results. |
Table IV.
Summary of patient IHC results.
| Name | pH | Incubation time,
min | IHC result |
|---|
| NUT | 8 | 32 | - |
| TTF-1 | 8 | 32 | - |
| CD5/6 | 8 | 32 | - |
| Syn | 8 | 32 | - |
| Villin | 8 | 32 | - |
| CDX2 | 8 | 32 | - |
| CD34 | 8 | 32 | - |
| CgA | 8 | 32 | - |
| INSM1 | 8 | 32 | - |
| Myogenin | 8 | 32 | - |
| CD30 | 8 | 32 | - |
| CK7 | 8 | 32 | + |
| p40 | 8 | 32 | + |
| CD56 | 8 | 32 | + |
| SMARCA4 | 8 | 32 | - |
| NapsinA | 8 | 32 | - |
| MelanA | 8 | 28 | - |
| S-100 | 8 | 24 | - |
| CK8 | 8 | 24 | + |
| CD20 | 8 | 24 | - |
On day 18, the patient had not passed gas for 1 day.
A complete abdominal CT examination and enhanced examination
(Optima CT660, Cytiva; 5-mm layer thickness; helical scan) revealed
increased and enlarged liver metastases, novel metastases in the
spleen, intestinal intussusception in the jejunal segment of the
small intestine and intestinal obstruction (Fig. 2B-E). On day 20, a laparoscopic
partial resection of the small intestine was performed and observed
a resected small intestinal specimen of 8 cm in length in the lumen
of the lassoed area. A red elevated area was observed in the
intestinal lumen of the intestinal trocar, measuring 3.5×2.5×1.5
cm. This area exhibited an ulcerated vesiculated surface and the
cut surface was grayish-white and grayish-red with a moderate solid
texture. H&E staining was performed using the previously
described protocol and light microscopy revealed that the tumor
cells were diffusely distributed in nests of sheets. In addition,
the tumor cells were large with unclear borders, weakly basophilic
or translucent cytoplasm and large, rounded or oval nuclei with
pronounced nuclear membranes. Multiple small nucleoli were observed
and nuclear schizophrenia was readily visible. There were numerous
hemorrhagic necroses within the lesion and small blood vessels in
the tumor interstitium were hyperplastic and partially dilated with
bruising (Fig. 4A).
Immunohistochemistry demonstrated lack of expression for TTF-1,
CK5/6, Syn, PSA, villin, CK20, caudal type homeobox 2 (CDX-2),
CD34, Syn, CgA, insulinoma-associated protein 1 (INSM1), melanoma
antigen (MelanA), S100, myogenin, leukocyte common antigen and
CD30, and positive expression for CK7 and CK8. There were small
foci of p40 positive expression, partial positive expression of
glypican-3 and CD56, and lack of expression of SMARCA4 and nuclear
protein in the testis (Fig. 4B-D;
Table V). Small intestinal tumor
samples obtained from the patient were investigated for 50 genes
associated with lung cancer and 23 genes associated with intestinal
cancer using next-generation sequencing (NGS). High-throughput
sequencing was conducted on the MGISEQ-2000 platform. DNA libraries
were constructed with the EGFR-KRAS-ALK Gene Mutation Combination
Test kit (Cat. No. RM0914, Shenzhen Huada Gene Technology Co.,
Ltd.) to generate 150-bp paired-end reads. Library integrity was
validated using the Qsep1™ capillary electrophoresis
system (Product No. 100001, BiOptic Inc.) with the Standard
Cartridge kit (Cat. No. C105201, BiOptic Inc.). Final library
concentrations were quantified with the Equalbit 1× dsDNA HS Assay
Kit (Cat. No. EQ121-02, Vazyme) and adjusted to 10 pM for cluster
generation. Raw sequencing data were processed and analyzed using
HALOS software (v4.1.3, Shenzhen Huada Gene Technology Co., Ltd.),
and mutations in PTEN, TP53, ataxia telangiectasia mutated (ATM),
ephrin type-A receptor 5 (EPHA5) and EGFR p.F997L were observed
(Table SI). The raw sequence data
reported in the present case report have been deposited in the
Genome Sequence Archive (GSA) in the National Genomics Data Center,
China National Center for Bioinformation/Beijing Institute of
Genomics, Chinese Academy of Sciences (GSA-Human database;
accession no. HRA011751) that are publicly accessible at GSA for
Human (ngdc.cncb.ac.cn/gsa-human/browse/HRA011751) (15,16).
The genes tested include both those recommended for testing by Food
and Drug Administration, National Medical Products Administration
and National Comprehensive Cancer Network authoritative guidelines
and key genes associated with drug targets during clinical trials
and the development of diseases (17–27).
Pan-cancer studies have linked SMARCA4/PTEN loss to DNA repair
defects that may underlie the TP53, ATM, EPHA5 and EGFR mutations,
as unrepaired DNA damage can trigger oncogenic mutations (28–30).
Thus, the clinical features, imaging manifestations and
pathological features were indicative of undifferentiated carcinoma
with localized squamous carcinoma expression and intussusception in
SMARCA4-deficient small intestine, with metastasis to the lung,
liver and spleen. Although these mutations were detected, the
treatment plan could not be adjusted and optimized based on them
due to the rapid progression of the condition of the patient.
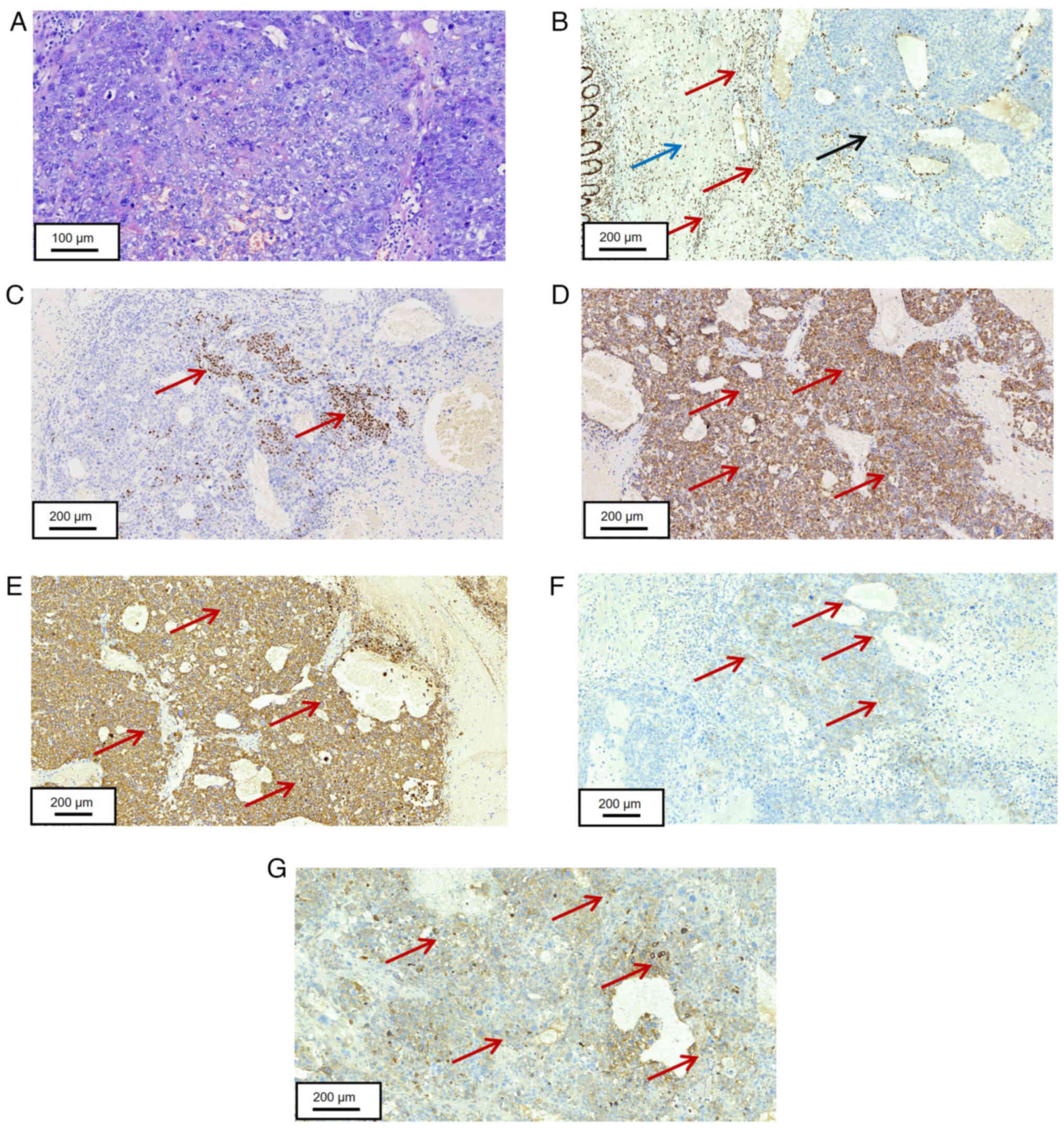 | Figure 4.Histopathological staining of tumor
tissue in the small intestine. (A) Tumor cells in the small
intestine were arranged in solid nests, with large, lightly stained
nuclei and one or more visible small nucleoli (scale bar, 100 µm).
(B) Negative SMARCA4 expression in tumor cells of the small
intestine (red arrows indicate SMARCA4-positive sites, blue arrows
indicate lymphoid regions, black arrows indicate tumor regions,
scale bar, 200 µm). (C) Partial positive expression of p40 in tumor
cells of the small intestine (red arrows indicate p40-positive
sites, scale bar, 200 µm). (D) Positive expression of CK7 in tumor
cells of the small intestine (Red arrows indicate CK7-positive
sites, scale bar, 200 µm). (E) Positive expression of CK8 in tumor
cells of lung biopsy tissue (Red arrows indicate CK8-positive
sites, scale bar, 200 µm). (F) Positive expression of CD56 in tumor
cells of lung biopsy tissue (red arrows indicate CD56-positive
sites, scale bar, 200 µm). (G) Positive expression of GPC-3 in
tumor cells of lung biopsy tissue (red arrows indicate
GPC-3-positive sites, scale bar, 200 µm). SMARCA4, switch/sucrose
non-fermentable (SWI/SNF) related matrix associated actin-dependent
regulator of chromatin sub-family A member 4; CK, cytokeratin;
GPC-3, glypican-3. |
 | Table V.Immunohistochemistry of tumor tissues
from different sites in patients. |
Table V.
Immunohistochemistry of tumor tissues
from different sites in patients.
|
| Lung tumor | Small bowel
tumor |
|---|
| CK5/6 | - | - |
| p40 | + | + |
| CK7 | + | + |
| TTF-1 | - | - |
| NapsinA | - | Untested |
| Syn | - | Untested |
| CK8 | Untested | + |
| Villin | Untested | - |
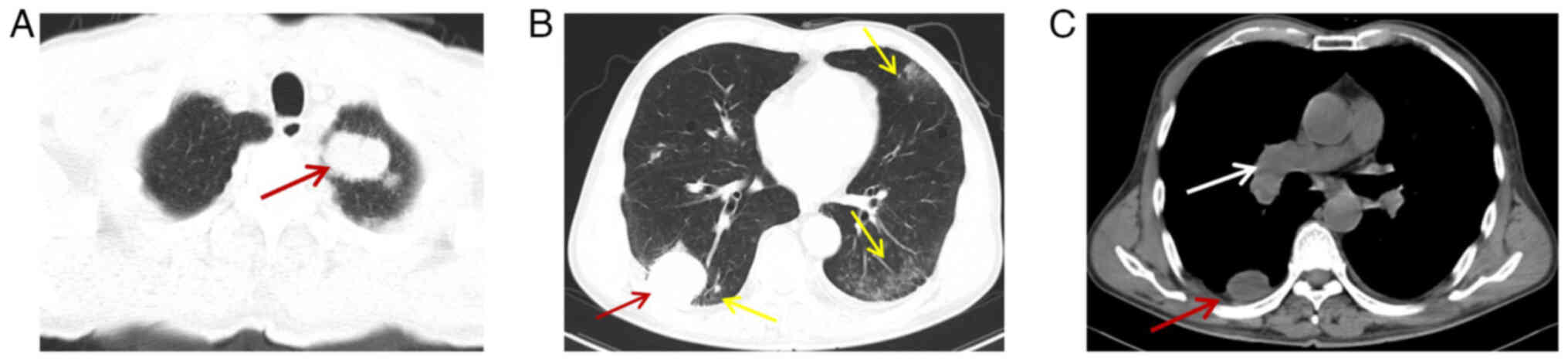
On day 31, a CT examination of the chest was
performed. The patient exhibited enlarged and increased lesions in
both lungs (Fig. 5A-C) and new
ground-glass shadows in the dorsal aspect of both lungs, with no
evidence of infection, the Response Evaluation Criteria in Solid
Tumors (RECIST) score was disease progression (31). Furthermore, immune-associated
pneumonia-non-specific interstitial pneumonitis was considered
(Fig. 5B) (32). Multiple new lesions were observed on
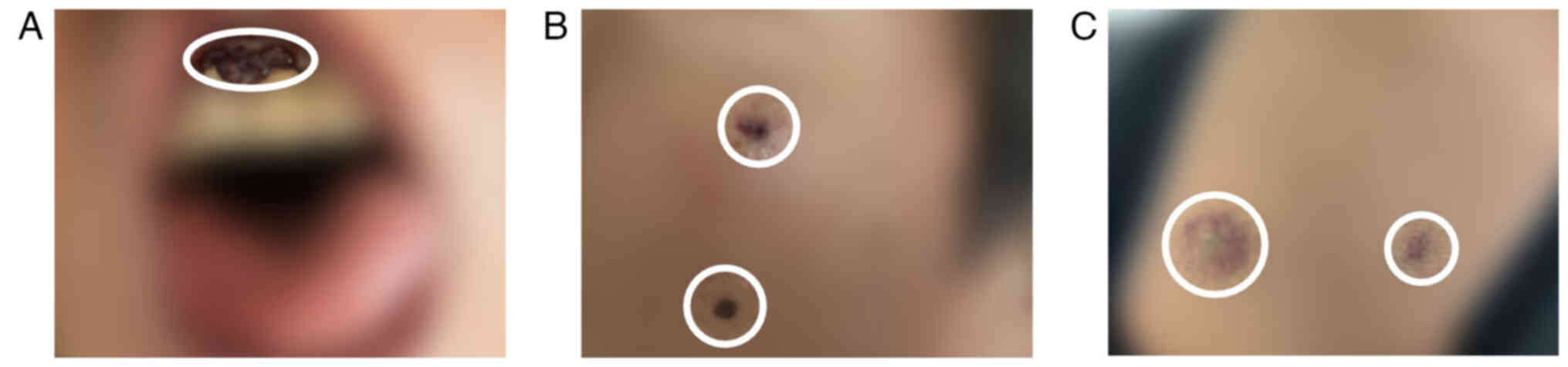
the skin and gingiva, indicative of metastasis (Fig. 6A-C). The patient presented with
persistent symptoms of gastrointestinal bleeding and a decrease in
hemoglobin levels (69 g/l) and was therefore administered 9 units
of erythrocyte transfusions and posterior pituitary hormones (a
dilute acetic acid sterilized aqueous solution of posterior
pituitary powder containing posterior pituitary hormone) for
hemostasis (2 ml intravenously once daily), and was provided with
nutritional support. The family of the patient requested that the
patient be discharged from the hospital on day 42 and the patient
was discharged on the same day. Within 24 h of discharge, the
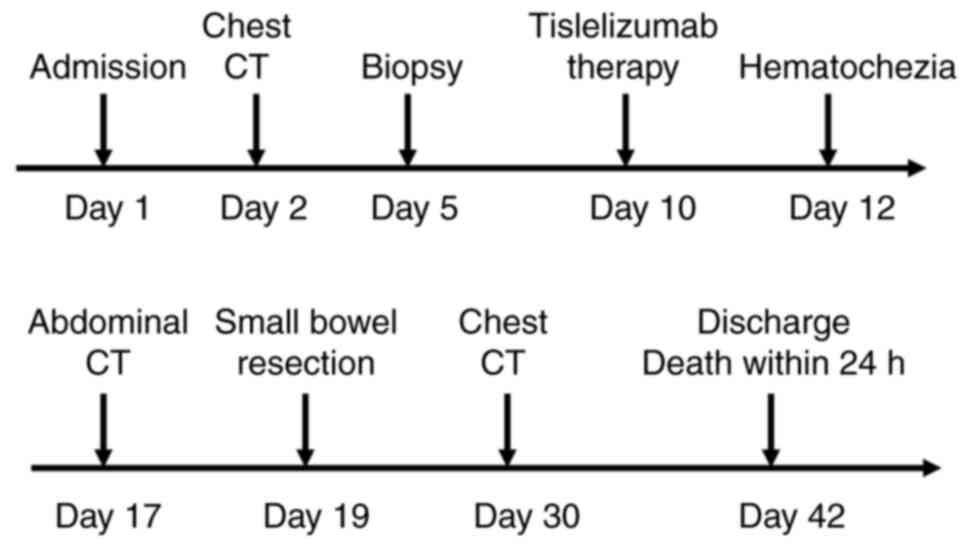
patient succumbed to the disease. The complete timeline is shown in
Fig. 7.
Discussion
The SMARCA4 gene subgroup belongs to the SWI/SNF
family, which serve a key role in chromatin remodeling and repair.
Results from previous studies demonstrated that SMARCA4 serves a
key role in developmental processes, transcriptional regulation,
DNA repair, cell cycle regulation, tumorigenesis and tumor
development. Furthermore, inactivating mutations in SMARCA4 lead to
a loss of protein expression in the nucleus, which result in
malignancies associated with SMARCA defects (33,34).
In non-small cell lung cancer, mutations in the SMARCA4 gene occur
in ~10% of cases. Notably, these mutations have been reported in
malignant tumors in alternative sites, including the ovaries,
esophagus, stomach, uterus and liver; however, the majority of
previous studies were case reports (35–39).
SMARCA4-DUC is a rare yet highly malignant tumor
that is aggressive and associated with poor prognosis (Table VI). Cases of SMARCA4-DUC in the
gastrointestinal tract predominantly affects elderly men and the
common sites of development include the stomach, colon, small
intestine and lower esophageal cardia, with few cases affecting the
ileum, rectum and pancreas (40,41).
In a study by Wang et al (42), it was noted that lung cancer cells
undergo SMARCA4 loss, which results in a reduced response to
anti-PD-1 immunotherapy, which may be associated with a notable
reduction in the infiltration of dendritic cells and
CD4+ T cells into the tumor microenvironment (TME);
however, this study focused on cellular and animal experiments. The
results of previous studies examining the impact of SMARCA4 loss on
the response to PD-1 immunotherapy in lung cancer are conflicting
and these discrepancies may be associated with differences in study
models. As noted in a study of advanced undifferentiated carcinoma
of the duodenum, PD-1 therapy is effective in some patients with
SMARCA4 deficiency, which potentially provides an option for the
treatment of these highly malignant tumors (43). A clinical study reported that
partial SMARCA4 deletion may make patients with lung cancer more
sensitive to PD-1 monoclonal antibody drugs (27). However, this study primarily
included an animal model for its experiments and the underlying
mechanisms may be more complex in humans. Tumors are often located
within the muscular wall of the digestive tract and involve the
mucosa and may also form masses outside of the wall of the
digestive tract (38,44–47).
Few characteristic imaging features of SMARCA4-DUC lead to
complexities in diagnosis; however, the majority of these tumors
exhibit notable signs of malignancy, such as large foci that are
lobulated, which encircle blood vessels, invade adjacent tissues
and metastasis to mediastinal lymph nodes and distant organs
(5). In the present case, a chest
CT examination demonstrated multiple nodules and masses with clear
borders and smooth edges and enhanced CT examination demonstrated
that the majority of areas in the lesions did not exhibit notable
enhancement. Furthermore, the edges of the lesions were notably
enhanced, which was consistent with the manifestation of
bloodstream metastasis in both lungs. Enhanced abdominal CT
examination revealed localized condensation of the jejunal segment
of the small intestine, with edema and thickening of the tube wall.
In addition, multiple nodules in the liver and spleen were
observed, with moderate bulls-eye enhancement observed through
enhanced examination. These findings were consistent with the
manifestation of hematogenous metastasis in the liver and spleen.
The imaging manifestations of bilateral lung, liver and spleen
metastases were consistent with those reported in previous
literature (48–50); however, to the best of our
knowledge, the present study is the first to report the case of
intestinal intussusception caused by SMARCA4-DUC in the small
intestine.
 | Table VI.Summary of key findings on SMARCA4
loss in various malignancies. |
Table VI.
Summary of key findings on SMARCA4
loss in various malignancies.
| First author,
year | Type of cancer | Key finding | (Refs.) |
|---|
| Witkowski et
al, 2023 | Ovarian | Associated with
tumor aggressiveness and poor prognosis. | (35) |
| Yu et al,
2024 | Esophageal | Led to reduced
sensitivity to chemotherapeutic drugs. | (55) |
| Yu et al,
2024 | Gastric | Associated with
tumor aggressiveness and poor prognosis. | (55) |
| Affandi et
al, 2018 | Endometrial | Associated with
tumor aggressiveness and poor prognosis. | (57) |
| Garcia-Porrero
et al, 2024 | Liver | Associated with
tumor aggressiveness and poor prognosis. | (58) |
| Xue et al,
2021; | Non-small cell | Reduced sensitivity
to chemotherapeutic drugs; activated | (9) |
| Kim et al,
2021 | lung cancer | EMT process and
enhanced tumor invasiveness | (10) |
The histology of SMARCA4-DUC is characterized by
rounded cells and epithelioid cell structures arranged in sheets,
nests or beams within the tumor tissue, which may be accompanied by
a loss of adherent growth. Furthermore, rhabdoid morphology, which
is defined as the presence of cells with large, eosinophilic
cytoplasm, prominent nucleoli and a vesicular chromatin pattern is
seen in focal areas in ~2/3 of the cases (34). Results from previous studies
revealed that tumor cells are often uniform. However, tumor cells
that were pleomorphic, contained spindle cell components or
exhibited pseudoadenoids or pseudoglandular structures were
observed in some cases (5,51). A previous study demonstrated that
undifferentiated carcinomas of the gastrointestinal tract often
possess multifarious rhabdomyolysis features, accompanied by
deletion of SMARCB1, SMARCA2, SMARCA4 and AT-rich interaction
domain-containing protein 1A (ARID1A) (41). Notably, a deletion in SMARCA4 is
often present in conjunction with a deletion in SMARCA2, which
affects ~39.9% of cases. These deletions are often accompanied by
negative or reduced expression (<10% positivity) of epithelial
markers, including CKpan, CK7, CK20 and CDX2. In addition to
epithelial markers, tumor cell markers, such as Sox2, spalt-like
protein 4, p53, ARID1A and neuroendocrine markers, such as CgA,
Syn, CD56 and INSM1, may also be expressed to varying degrees in
SMARCA4-DUC (46,52–54).
In a previous study (36), it was
noted that SMARCA4 deletion and TP53 have similar mutation rates in
gastrointestinal tumors and that this mutation seems to occur more
often in the esophagus compared with the stomach or elsewhere,
which suggests a possible location of origin for gastrointestinal
tumors. The patient from the present case also demonstrated focal
expression of p40; however, SMARCA4 deletion was detected in small
bowel cancer in a study on SMARCA4 deletion in lung cancer, which
indicated that tumors with deletion of SMARCA4 were mostly
non-squamous cell carcinomas and the expression of p40 was mostly
negative or weakly positive at the focal level (34). This is similar to the expression of
p40 detected in the patient in the present case. Considering the
DNA repair function of SMARCA4, SMARCA4 deletion may lead to a
variety of morphological and immunophenotypic features of tumor
cells during differentiation and the focal expression of p40 may
reflect the partial squamous differentiation tendency of the
associated tumor cells (55).
According to previous studies, diffuse strong positivity of p40
expression in tumor tissue can be used to diagnose squamous cell
carcinoma. However, the present tumor tissue showed focal p40
expression, which did not meet the diagnostic criteria. Therefore,
the patient's treatment plan was based on the criteria for
undifferentiated carcinoma (56,57).
During clinical diagnosis, a combination of
histological and molecular pathological analyses is required to
distinguish SMARCA4-DUC from large-cell neuroendocrine carcinomas
with rhabdomyosiform morphology, often accompanied by diffuse or
strong positive expression of neuroendocrine markers (58,59).
Notably, melanomas with rhabdomyosiform morphology often express
melanocyte markers, such as S100, MelanA and human melanoma
black-45 (60,61). Rhabdomyosarcoma with distinctive
rhabdomyosarcoma-like features often express muscle-specific
markers, such as myogenic differentiation antigen 1 and myogenin
(62,63). Cases of primary interstitial
large-cell lymphoma of the gastrointestinal tract often include
large cells with a pronounced nucleolus and these may exhibit high
levels of expression of lymphocyte markers, such as CD30 and ALK
(64,65). Furthermore, proximal-type
epithelioid sarcoma with epithelioid morphology often express
epithelioid markers, such as CK and epithelial membrane antigen
(66,67), which aid in identification and
differentiation.
Genomic analysis of SMARCA4-DUC demonstrated that
common co-mutations included TP53, KRAS, serine/threonine kinase 11
(STK11) and Kelch-like ECH-associated protein 1 (KEAP1), with less
common co-mutations with common genes, such as EGFR, ROS
proto-oncogene 1, receptor tyrosine kinase and ALK (68,69).
The Dana-Farber Cancer Institute (Boston, USA) reported that the
KRAS gene is the most commonly co-mutated gene in SMARCA4-DUC,
which accounts for 5–11% of gene mutations (70). Patients with SMARCA4-DUC often
present with a poor prognosis, with markedly shorter median
progression-free survival (4.1 vs. 1.4 months) and median overall
survival (15.1 vs. 3.0 months) compared with patients exhibiting
the SMARCA4/KRAS wild-type (71,72).
Treatment of SMARCA4-DUC is complex, due to its
unique physiological characteristics and low prevalence rates. In
addition, there are no unified treatment guidelines to follow, with
a lack of effective therapies used in clinical practice (73). Partial SMARCA4 deletion may make
patients with lung cancer more sensitive to PD-1 monoclonal
antibody drugs, which was noted in a clinical study (44). A previous case report demonstrated
that surgical resection may be an effective initial treatment
option for patients with SMARCA4-DUC with clinical staging
(74). However, the present case
report also demonstrated that 4 months following surgery, patients
with SMARCA4-DUC developed tumor recurrence with invasion to the
adjacent ribs. Results from a previous study revealed that patients
with SMARCA4-DUC may benefit from platinum-based chemotherapy and
the systemic effects of chemotherapy may control distal tumor
metastasis to some extent, due to the high aggressiveness and
metastatic nature of the disease (75). Furthermore, targeted drug therapies,
including anilotinib, sindilizumab and bevacizumab, may aid in
controlling the progression of SMARCA4-DUC (54,76).
In a previous case report, a patient with SMARCA4-DUC who developed
genetic mutations in STK11/KEAP1, KEAP1, PBRM1, SMARCA4 and STK11
received tirilizumab immunotherapy (77). However, rapid disease progression
was observed, with hepatic and pulmonary metastases within 1 month,
which ultimately led to the death of the patient. Findings from
previous studies revealed that STK11/KEAP1, KEAP1, PBRM1 and STK11
gene mutations may affect the tumor microenvironment of patients,
which weakens the tumor-killing effect of immune cells and
increases the risk of disease progression following immunotherapy
(78–80). Due to the high degree of
heterogeneity of tumor cells in patients with SMARCA4-DUC, NGS
technology is required for the development of personalized
treatment plans (81). The rapid
disease progression of the present patient after immunotherapy
suggested that there may be hyperprogression, although there is no
direct evidence that SMARCA4 may be an independent influence on
hyperprogression of immunotherapy in gastrointestinal patients. It
was noted in Han et al (82)
that environmental factors, especially the gut microbiome, can have
an impact on immunotherapy and it is possible that the
intussusception and gastrointestinal bleeding that the patient
suffered at a later stage would have led to an imbalance in the gut
microbiome, which could be an important cause of
hyperprogression.
In addition, the present patient tumor exhibited
partial squamous carcinoma and in a case report by Misra et
al (83), it was noted that
patients with SMARCA4 in the gastrointestinal tract exhibiting
focal squamous carcinoma differentiation can be resistant to a
variety of chemotherapeutic agents (cisplatin and docetaxel) and
lead to an increase in their invasiveness, which may also explain
the rapid progression of the patient in the present case (84,85).
Due to the highly heterogeneous nature of tumor cells in
SMARCA4-DUC patients, deep gene sequencing of SMARCA4-DUC patients
using NGS technology is of great importance for the development of
personalized treatment protocols, taking into account the impact of
different gene mutations on patient prognosis and the toxicity and
side effects of traditional chemotherapeutic agents (81,86).
In addition, some non-pharmacological interventions, such as
magnetic fields, exosomes, may also provide options for the
treatment of SMARCA4-DUC (87–89).
In the present case, the patient was administered tislelizumab once
following diagnosis and the disease was reassessed at 22-day
intervals. After 1 month of treatment, the symptoms of the patient
had worsened, with an increase in the number of metastatic lesions
in the lungs and liver, enlargement, novel metastases in the
spleen, the development of a CT pattern indicative of
immune-associated pneumonia-nonspecific interstitial pneumonitis in
both lungs and a Response Evaluation Criteria in Solid Tumors score
of progressive disease (31).
Following intussusception surgery, gastrointestinal bleeding and
treatment for aggressive symptoms, the death of the patient
occurred after 12 days. Although the specific cause of death was
not determined via autopsy, it was considered that the highly
aggressive nature of SMARCA4-DUC, which led to multiple organ
metastasis was the main cause of death and the gastrointestinal
hemorrhage and immune-associated pneumonia caused by the
intussusception may have accelerated the deterioration of the
disease.
The present study exhibits numerous limitations. For
example, the present study included a retrospective analysis of
rare cases, with a lack of guideline support and inexperience. In
the present case, diagnosis and treatment were delayed, as it
remained unclear whether immunotherapy caused hyperprogression of
the disease. In addition, the specific cause of death in the
patient was not determined due to the absence of autopsy
confirmation.
In conclusion, the present study reported a rare
case of SMARCA4-DUC in the gastrointestinal tract, which provides a
theoretical basis for the clinical management of this disease.
SMARCA4-DUC often affects elderly male patients and imaging often
reveals large lesions, invasion of surrounding tissues and
metastasis to lymph nodes and distant organs. Definitive diagnosis
of the disease relies on pathological examination,
immunohistochemistry and molecular testing. A diagnosis of
SMARCA4-DUC should be considered following the observation of solid
high-grade undifferentiated morphology of cells and negative
expression of genes used for conventional staging. Thus,
appropriate immunohistochemical and molecular tests should be
carried out to aid in early diagnosis and effective treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Weifang Municipal Health
Commission Scientific Research Program (grant nos. WFWSJK-2022-090,
WFWSJK-2022-150 and WFWSJK-2024-200).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The raw sequence data
generated in the present study may be found in the Genome Sequence
Archive (GSA) (14) in National
Genomics Data Center (15), China
National Center for Bioinformation/Beijing Institute of Genomics,
Chinese Academy of Sciences, under accession number (accession no.
HRA011751) or at the following URL:
ngdc.cncb.ac.cn/gsa-human/browse/HRA011751.
Authors' contributions
MQ, LL, BY, and YZ analyzed data. BY and XY designed
the study protocol. XC and SL were the primary care physicians of
the patient and developed and implemented the treatment plan. YW,
QG and ZC performed the literature review and analyzed and
interpreted the data in the paper. XY drafted the work and
critically revised intellectual content. BY and XY confirm the
authenticity of all the raw data. All authors commented on the
manuscript and agreed with the conclusions of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Ethics
Committee of the Weifang No. 2 People's Hospital (approval no.
KY2023-055-01; Weifang, China) and was performed in accordance with
the Declaration of Helsinki.
Patient consent for publication
The patient's family member provided written
informed consent for the publication of the manuscript including
any identifying images or data.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
|
|
2
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar
|
|
3
|
Zhan T, Betge J, Schulte N, Dreikhausen L,
Hirth M, Li M, Weidner P, Leipertz A, Teufel A and Ebert MP:
Digestive cancers: Mechanisms, therapeutics and management. Signal
Transduct Target Ther. 10:242025. View Article : Google Scholar
|
|
4
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar
|
|
5
|
Zhu PP, Li XX, Liu JH, Du XL, Su HY and
Wang J: SMARCA4-deficient undifferentiated carcinoma of the
gastrointestinal tract: a clinicopathological and
immunohistochemical study of nine cases. Zhonghua Bing Li Xue Za
Zhi. 51:868–874. 2022.(In Chinese).
|
|
6
|
Tian Y, Xu L, Li X, Li H and Zhao M:
SMARCA4: Current status and future perspectives in non-small-cell
lung cancer. Cancer Lett. 554:2160222023. View Article : Google Scholar
|
|
7
|
Lombardi M, D'Ercole M, Midolo De Luca V,
Chiari M, Spaggiari L and Bertolaccini L: The impact of SMARCA4
loss in non-small cell lung cancer therapy. Heliyon. 11:e411282024.
View Article : Google Scholar
|
|
8
|
Shi M, Pang L, Zhou H, Mo S, Sheng J,
Zhang Y, Liu J, Sun D, Gong L, Wang J, et al: Rare
SMARCA4-deficient thoracic tumor: Insights into molecular
characterization and optimal therapeutics methods. Lung Cancer.
192:1078182024. View Article : Google Scholar
|
|
9
|
Xue Y, Morris JL, Yang K, Fu Z, Zhu X,
Johnson F, Meehan B, Witkowski L, Yasmeen A, Golenar T, et al:
SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by
restricting IP3R3-mediated Ca2+ flux to mitochondria.
Nat Commun. 12:54042021. View Article : Google Scholar
|
|
10
|
Kim J, Jang G, Sim SH, Park IH, Kim K and
Park C: SMARCA4 depletion induces cisplatin resistance by
activating YAP1-mediated epithelial-to-mesenchymal transition in
triple-negative breast cancer. Cancers (Basel). 13:54742021.
View Article : Google Scholar
|
|
11
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar
|
|
12
|
Locher C, Batumona B, Afchain P, Carrère
N, Samalin E, Cellier C, Aparicio T, Becouarn Y, Bedenne L, Michel
P, et al: Small bowel adenocarcinoma: French intergroup clinical
practice guidelines for diagnosis, treatments and follow-up (SNFGE,
FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis.
50:15–19. 2018. View Article : Google Scholar
|
|
13
|
Kelly RJ, Bever K, Chao J, Ciombor KK, Eng
C, Fakih M, Goyal L, Hubbard J, Iyer R, Kemberling HT, et al:
Society for Immunotherapy of Cancer (SITC) clinical practice
guideline on immunotherapy for the treatment of gastrointestinal
cancer. J Immunother Cancer. 11:e0066582023. View Article : Google Scholar
|
|
14
|
Liu H, Hong Q, Zheng S, Zhang M and Cai L:
Effective treatment strategies and key factors influencing
therapeutic efficacy in advanced SMARCA4-deficient non-small cell
lung cancer. Lung Cancer. 198:1080222024. View Article : Google Scholar
|
|
15
|
Chen T, Chen X, Zhang S, Zhu J, Tang B,
Wang A, Dong L, Zhang Z, Yu C, Sun Y, et al: The genome sequence
archive family: Toward explosive data growth and diverse data
types. Genomics Proteomics Bioinformatics. 19:578–583. 2021.
View Article : Google Scholar
|
|
16
|
CNCB-NGDC Members and Partners, . Database
resources of the national genomics data center, china national
center for bioinformation in 2022. Nucleic Acids Res. 50(D1):
D27–D38. 2022. View Article : Google Scholar
|
|
17
|
Saura C, Roda D, Roselló S, Oliveira M,
Macarulla T, Pérez-Fidalgo JA, Morales-Barrera R, Sanchis-García
JM, Musib L, Budha N, et al: A First-in-human phase I study of the
ATP-competitive AKT inhibitor ipatasertib demonstrates robust and
safe targeting of AKT in patients with solid tumors. Cancer Discov.
7:102–113. 2017. View Article : Google Scholar
|
|
18
|
Therkildsen C, Bergmann TK,
Henrichsen-Schnack T, Ladelund S and Nilbert M: The predictive
value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment
in metastatic colorectal cancer: A systematic review and
meta-analysis. Acta Oncol. 53:852–864. 2014. View Article : Google Scholar
|
|
19
|
Wang ZH, Gao QY and Fang JY: Loss of PTEN
expression as a predictor of resistance to anti-EGFR monoclonal
therapy in metastatic colorectal cancer: Evidence from
retrospective studies. Cancer Chemother Pharmacol. 69:1647–1655.
2012. View Article : Google Scholar
|
|
20
|
Forster MD, Dedes KJ, Sandhu S, Frentzas
S, Kristeleit R, Ashworth A, Poole CJ, Weigelt B, Kaye SB and
Molife LR: Treatment with olaparib in a patient with PTEN-deficient
endometrioid endometrial cancer. Nat Rev Clin Oncol. 8:302–306.
2011. View Article : Google Scholar
|
|
21
|
Koehler K, Liebner D and Chen JL: TP53
mutational status is predictive of pazopanib response in advanced
sarcomas. Ann Oncol. 27:539–543. 2016. View Article : Google Scholar
|
|
22
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar
|
|
23
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar
|
|
24
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar
|
|
25
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar
|
|
26
|
Bendell JC, Rodon J, Burris HA, de Jonge
M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, et
al: Phase I, dose-escalation study of BKM120, an oral pan-class I
PI3K inhibitor, in patients with advanced solid tumors. J Clin
Oncol. 30:282–290. 2012. View Article : Google Scholar
|
|
27
|
Weber J and McCormack PL: Panitumumab: In
metastatic colorectal cancer with wild-type KRAS. BioDrugs.
22:403–411. 2008. View Article : Google Scholar
|
|
28
|
Sørensen SG, Shrikhande A, Poulsgaard GA,
Christensen MH, Bertl J, Laursen BE, Hoffmann ER and Pedersen JS:
Pan-cancer association of DNA repair deficiencies with whole-genome
mutational patterns. Elife. 12:e812242023. View Article : Google Scholar
|
|
29
|
Kassem PH, Montasser IF, Mahmoud RM,
Ghorab RA, AbdelHakam DA, Fathi MESA, Wahed MAA, Mohey K, Ibrahim
M, Hadidi ME, et al: Genomic landscape of hepatocellular carcinoma
in Egyptian patients by whole exome sequencing. BMC Med Genomics.
17:2022024. View Article : Google Scholar
|
|
30
|
Watanabe T, Semba S and Yokozaki H:
Regulation of PTEN expression by the SWI/SNF chromatin-remodelling
protein BRG1 in human colorectal carcinoma cells. Br J Cancer.
104:146–154. 2011. View Article : Google Scholar
|
|
31
|
Gouda MA, Janku F, Wahida A, Buschhorn L,
Schneeweiss A, Abdel Karim N, De Miguel Perez D, Del Re M, Russo A,
Curigliano G, et al: Liquid biopsy response evaluation criteria in
solid tumors (LB-RECIST). Ann Oncol. 35:267–275. 2024. View Article : Google Scholar
|
|
32
|
Ebner L, Christodoulidis S, Stathopoulou
T, Geiser T, Stalder O, Limacher A, Heverhagen JT, Mougiakakou SG
and Christe A: Meta-analysis of the radiological and clinical
features of usual interstitial pneumonia (UIP) and nonspecific
interstitial pneumonia (NSIP). PLoS One. 15:e02260842020.
View Article : Google Scholar
|
|
33
|
Zeng X, Yao B, Liu J, Gong GW, Liu M, Li
J, Pan HF, Li Q, Yang D, Lu P, et al: The SMARCA4R1157W
mutation facilitates chromatin remodeling and confers PRMT1/SMARCA4
inhibitors sensitivity in colorectal cancer. NPJ Precis Oncol.
7:282023. View Article : Google Scholar
|
|
34
|
Liang X, Gao X, Wang F, Li S, Zhou Y, Guo
P, Meng Y and Lu T: Clinical characteristics and prognostic
analysis of SMARCA4-deficient non-small cell lung cancer. Cancer
Med. 12:14171–14182. 2023. View Article : Google Scholar
|
|
35
|
Witkowski L, Nichols KE, Jongmans M, van
Engelen N, de Krijger RR, Herrera-Mullar J, Tytgat L, Bahrami A,
Mar Fan H, Davidson AL, et al: Germline pathogenic SMARCA4 variants
in neuroblastoma. J Med Genet. 60:987–992. 2023. View Article : Google Scholar
|
|
36
|
Neil AJ, Zhao L, Isidro RA, Srivastava A,
Cleary JM and Dong F: SMARCA4 mutations in carcinomas of the
esophagus, esophagogastric junction, and stomach. Mod Pathol.
36:1001832023. View Article : Google Scholar
|
|
37
|
Pors J, Devereaux KA, Hildebrandt D and
Longacre TA: Primary uterine synovial sarcoma with SMARCA4 loss.
Histopathology. 80:1135–1137. 2022. View Article : Google Scholar
|
|
38
|
Koyasu S, Sugimoto A, Matsubara J, Muto M
and Nakamoto Y: SMARCA4-deficient poorly differentiated
adenocarcinoma of the gallbladder. Clin Nucl Med. 49:688–689. 2024.
View Article : Google Scholar
|
|
39
|
Akunuru S, James Zhai Q and Zheng Y:
Non-small cell lung cancer stem/progenitor cells are enriched in
multiple distinct phenotypic subpopulations and exhibit plasticity.
Cell Death Dis. 3:e3522012. View Article : Google Scholar
|
|
40
|
Xu J and Chi Z: Esophageal carcinoma with
SMARCA4 mutation: A narrative review for this rare entity. Transl
Gastroenterol Hepatol. 9:242024. View Article : Google Scholar
|
|
41
|
Chang B, Sheng W, Wang L, Zhu X, Tan C, Ni
S, Weng W, Huang D and Wang J: SWI/SNF complex-deficient
undifferentiated carcinoma of the gastrointestinal tract:
Clinicopathologic study of 30 cases with an emphasis on variable
morphology, immune features, and the prognostic significance of
different SMARCA4 and SMARCA2 subunit deficiencies. Am J Surg
Pathol. 46:889–906. 2022. View Article : Google Scholar
|
|
42
|
Wang Y, Meraz IM, Qudratullah M, Kotagiri
S, Han Y, Xi Y, Wang J and Lissanu Y: SMARCA4 mutation induces
tumor cell-intrinsic defects in enhancer landscape and resistance
to immunotherapy. bioRxiv. Jun 22–2024.(Epub ahead of print).
|
|
43
|
Shi YN, Zhang XR, Ma WY, Lian J, Liu YF,
Li YF and Yang WH: PD-1 antibody in combination with chemotherapy
for the treatment of SMARCA4-deficient advanced undifferentiated
carcinoma of the duodenum: Two case reports. World J Clin Oncol.
15:456–463. 2024. View Article : Google Scholar
|
|
44
|
Schoenfeld AJ, Bandlamudi C, Lavery JA,
Montecalvo J, Namakydoust A, Rizvi H, Egger J, Concepcion CP, Paul
S, Arcila ME, et al: The genomic landscape of SMARCA4 alterations
and associations with outcomes in patients with lung cancer. Clin
Cancer. 26:5701–5708. 2020. View Article : Google Scholar
|
|
45
|
Agaimy A, Daum O, Märkl B, Lichtmannegger
I, Michal M and Hartmann A: SWI/SNF complex-deficient
undifferentiated/rhabdoid carcinomas of the gastrointestinal tract:
A series of 13 cases highlighting mutually exclusive loss of
SMARCA4 and SMARCA2 and frequent co-inactivation of SMARCB1 and
SMARCA2. Am J Surg Pathol. 40:544–553. 2016. View Article : Google Scholar
|
|
46
|
Liu J, Song L and Wang J: Primary
SMARCA4-deficient undifferentiated sarcomatoid tumor of the
gastroesophageal junction. Hum Pathol Case Rep. 22:2004322020.
View Article : Google Scholar
|
|
47
|
Wong AK, Shanahan F, Chen Y, Lian L, Ha P,
Hendricks K, Ghaffari S, Iliev D, Penn B, Woodland AM, et al: BRG1,
a component of the SWI-SNF complex, is mutated in multiple human
tumor cell lines. Cancer Res. 60:6171–6177. 2000.
|
|
48
|
Ankoh K, Shinji S, Yamada T, Matsuda A,
Ohta R, Sonoda H, Hotta M, Takahashi G, Kaneya Y, Iwai T, et al: A
rapidly growing small-intestinal metastasis from lung cancer. J
Nippon Med Sch. 89:540–545. 2022. View Article : Google Scholar
|
|
49
|
Xu Y, Zhang B and Wang J: Gastrointestinal
stromal tumour with liver metastasis presenting as gastric cancer.
Diagnostics (Basel). 13:3762023. View Article : Google Scholar
|
|
50
|
Altaf K, Mckernan G, Skaife P and Slawik
S: Splenic metastasis in colorectal cancer. Tech Coloproctol.
20:795–796. 2016. View Article : Google Scholar
|
|
51
|
Li X, Tian S, Shi H, Ta N, Ni X, Bai C,
Zhu Z, Chen Y, Shi D, Huang H, et al: The golden key to open
mystery boxes of SMARCA4-deficient undifferentiated thoracic tumor:
Focusing immunotherapy, tumor microenvironment and epigenetic
regulation. Cancer Gene Ther. 31:687–697. 2024. View Article : Google Scholar
|
|
52
|
Kakkar A, Ashraf SF, Rathor A, Adhya AK,
Mani S, Sikka K and Jain D: SMARCA4/BRG1-deficient sinonasal
carcinoma. Arch Pathol Lab Med. 146:1122–1130. 2022. View Article : Google Scholar
|
|
53
|
Nambirajan A, Singh V, Bhardwaj N, Mittal
S, Kumar S and Jain D: SMARCA4/BRG1-deficient non-small cell lung
carcinomas: A case series and review of the literature. Arch Pathol
Lab Med. 145:90–98. 2021. View Article : Google Scholar
|
|
54
|
Zhou P, Fu Y and Wang W: Case report:
Gastrointestinal neuroendocrine carcinoma with SMARCA4 deficiency:
A clinicopathological report of two rare cases. Front Oncol.
13:12907172023. View Article : Google Scholar
|
|
55
|
Yu L and Wu D: SMARCA2 and SMARCA4
participate in DNA damage repair. Front Biosci (Landmark Ed).
29:2622024. View Article : Google Scholar
|
|
56
|
Berezowska S, Maillard M, Keyter M and
Bisig B: Pulmonary squamous cell carcinoma and lymphoepithelial
carcinoma-morphology, molecular characteristics and differential
diagnosis. Histopathology. 84:32–49. 2024. View Article : Google Scholar
|
|
57
|
Affandi KA, Tizen NMS, Mustangin M and Zin
RRMRM: p40 immunohistochemistry is an excellent marker in primary
lung squamous cell carcinoma. J Pathol Transl Med. 52:283–289.
2018. View Article : Google Scholar
|
|
58
|
Garcia-Porrero G, Wood F, Faria S, Kelly
PJ and McCluggage WG: ‘Aberrant’ expression of skeletal muscle
markers in neuroendocrine carcinomas: A significant diagnostic
pitfall. Virchows Arch. 485:625–629. 2024. View Article : Google Scholar
|
|
59
|
Vocino Trucco G, Righi L, Volante M and
Papotti M: Updates on lung neuroendocrine neoplasm classification.
Histopathology. 84:67–85. 2024. View Article : Google Scholar
|
|
60
|
Watter H, Milkins R, Chambers C and
O'Brien B: Melanoma with rhabdomyosarcomatous features: A potential
diagnostic pitfall. BMJ Case Rep. 16:e2564272023. View Article : Google Scholar
|
|
61
|
Tran TAN, Linos K, de Abreu FB and Carlson
JA: Undifferentiated sarcoma as intermediate step in the
progression of malignant melanoma to rhabdomyosarcoma: Histologic,
histologic, immunohistochemical, and molecular studies of a new
case of malignant melanoma with rhabdomyosarcomatous
differentiation. Am J Dermatopathol. 41:221–229. 2019. View Article : Google Scholar
|
|
62
|
Tuohy JL, Byer BJ, Royer S, Keller C,
Nagai-Singer MA, Regan DP and Seguin B: Evaluation of myogenin and
MyoD1 as immunohistochemical markers of canine rhabdomyosarcoma.
Vet Pathol. 58:516–526. 2021. View Article : Google Scholar
|
|
63
|
Agard H, Clark C, Massanyi E, Steele M and
McMahon D: Chemoradiotherapy-induced cytodifferentiation in
bladder/prostate rhabdomyosarcoma with genetic downregulation of
myogenin and MyoD1 gene expression: A case study and review of the
literature. Urology. 137:173–177. 2020. View Article : Google Scholar
|
|
64
|
Mishra P, Patra S, Srinivasan A, Padhi S,
Sable MN, Samal SC and Mohapatra S: Primary gastrointestinal
anaplastic large cell lymphoma: A critical reappraisal with a
systematic review of the world literature. J Cancer Res Ther.
17:1307–1313. 2021. View Article : Google Scholar
|
|
65
|
Radha S, Afroz T and Reddy R: Primary
anaplastic large cell lymphoma of caecum and ascending colon.
Indian J Pathol Microbiol. 64:168–170. 2021. View Article : Google Scholar
|
|
66
|
Hasegawa T, Matsuno Y, Shimoda T, Umeda T,
Yokoyama R and Hirohashi S: Proximal-type epithelioid sarcoma: A
clinicopathologic study of 20 cases. Mod Pathol. 14:655–663. 2001.
View Article : Google Scholar
|
|
67
|
Echchaoui A, Sadrati Y, Elbir Y, Elktaibi
A, Benyachou M, Mazouz SE, Gharib NE and Abbassi A: Proximal-type
epithelioid sarcoma: A new case report and literature review. Pan
Afr Med J. 24:2382016. View Article : Google Scholar
|
|
68
|
Quintanal-Villalonga Á, Costa E,
Wohlheiter C, Tischfield S, Redin E, Sridhar H, Funnel A, Poirier
JT, Hao Y, Kinyua D, et al: 228P SMARCA4 inactivation drives
aggressiveness in STK11/KEAP1 co-mutant lung adenocarcinomas
through the induction of TGFβ signaling. ESMO Open. 9 (Suppl
3):S1028782024. View Article : Google Scholar
|
|
69
|
An HR, Kim HD, Ryu MH and Park YS:
SMARCA4-deficient undifferentiated gastric carcinoma: A case series
and literature review. Gastric Cancer. 27:1147–1152. 2024.
View Article : Google Scholar
|
|
70
|
Wilson BG and Roberts CWM: SWI/SNF
nucleosome remodellers and cancer. Nat Rev Cancer. 11:481–492.
2011. View Article : Google Scholar
|
|
71
|
Liu L, Ahmed T, Petty WJ, Grant S, Ruiz J,
Lycan TW, Topaloglu U, Chou PC, Miller LD, Hawkins GA, et al:
SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: A
multi-cohort analysis. Mol Oncol. 15:462–472. 2021. View Article : Google Scholar
|
|
72
|
Alessi JV, Ricciuti B, Spurr LF, Gupta H,
Li YY, Glass C, Nishino M, Cherniack AD, Lindsay J, Sharma B, et
al: SMARCA4 and other SWItch/sucrose nonfermentable family genomic
alterations in NSCLC: Clinicopathologic characteristics and
outcomes to immune checkpoint inhibition. J Thorac Oncol.
16:1176–1187. 2021. View Article : Google Scholar
|
|
73
|
Cui M, Lemmon K, Jin Z and Uboha NV:
Esophageal carcinoma with SMARCA4 mutation: Unique diagnostic
challenges. Pathol Res Pract. 248:1546922023. View Article : Google Scholar
|
|
74
|
Yin C, Liu ZJ, He C and Yu HX: A case of
surgically treated non-metastatic SMARCA4-deficient
undifferentiated thoracic tumor: A case report and literature
review. Front Oncol. 14:13998682024. View Article : Google Scholar
|
|
75
|
Bell EH, Chakraborty AR, Mo X, Liu Z,
Shilo K, Kirste S, Stegmaier P, McNulty M, Karachaliou N, Rosell R,
et al: SMARCA4/BRG1 is a novel prognostic biomarker predictive of
cisplatin-based chemotherapy outcomes in resected non-small cell
lung cancer. Clin Cancer Res. 22:2396–2404. 2016. View Article : Google Scholar
|
|
76
|
Dagogo-Jack I, Schrock AB, Kem M, Jessop
N, Lee J, Ali SM, Ross JS, Lennerz JK, Shaw AT and Mino-Kenudson M:
Clinicopathologic characteristics of BRG1-deficient NSCLC. J Thorac
Oncol. 15:766–776. 2020. View Article : Google Scholar
|
|
77
|
Naito T, Umemura S, Nakamura H, Zenke Y,
Udagawa H, Kirita K, Matsumoto S, Yoh K, Niho S, Motoi N, et al:
Successful treatment with nivolumab for SMARCA4-deficient non-small
cell lung carcinoma with a high tumor mutation burden: A case
report. Thorac Cancer. 10:1285–1288. 2019. View Article : Google Scholar
|
|
78
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar
|
|
79
|
Leinonen HM, Kansanen E, Pölönen P,
Heinäniemi M and Levonen AL: Dysregulation of the keap1-Nrf2
pathway in cancer. Biochem Soc Trans. 43:645–649. 2015. View Article : Google Scholar
|
|
80
|
Gatalica Z, Xiu J, Swensen J and Vranic S:
Comprehensive analysis of cancers of unknown primary for the
biomarkers of response to immune checkpoint blockade therapy. Eur J
Cancer. 94:179–186. 2018. View Article : Google Scholar
|
|
81
|
Concepcion CP, Ma S, LaFave LM, Bhutkar A,
Liu M, DeAngelo LP, Kim JY, Del Priore I, Schoenfeld AJ, Miller M,
et al: Smarca4 inactivation promotes lineage-specific
transformation and early metastatic features in the lung. Cancer
Discov. 12:562–585. 2022. View Article : Google Scholar
|
|
82
|
Han XJ, Alu A, Xiao YN, Wei YQ and Wei XW:
Hyperprogression: A novel response pattern under immunotherapy.
Clin Transl Med. 10:e1672020. View Article : Google Scholar
|
|
83
|
Misra S, Szeto W, Centeno L, Castillo D,
Rucker A, Rahmanuddin S and Mannan R: SMARCA4 deficient gastric
carcinoma with squamous differentiation in a young patient with
aggressive clinical course. Int J Surg Pathol. 33:1016–1021. 2025.
View Article : Google Scholar
|
|
84
|
Bhat V, Koneru M, Knapp K, Joneja U,
Morrison J and Hong YK: Identification and treatment of SMARCA4
deficient poorly differentiated gastric carcinoma. Am Surg.
89:4987–4989. 2023. View Article : Google Scholar
|
|
85
|
Kido K, Nojima S, Motooka D, Nomura Y,
Kohara M, Sato K, Ohshima K, Tahara S, Kurashige M, Umeda D, et al:
Ovarian high-grade serous carcinoma cells with low SMARCA4
expression and high SMARCA2 expression contribute to platinum
resistance. J Pathol. 260:56–70. 2023. View Article : Google Scholar
|
|
86
|
Mokashi A and Bhatia NM: Integrated
network ethnopharmacology, molecular docking, and ADMET analysis
strategy for exploring the anti-breast cancer activity of ayurvedic
botanicals targeting the progesterone receptor. BIO Integr. 5:1–17.
2024. View Article : Google Scholar
|
|
87
|
Ji X, Tian X, Feng S, Zhang L, Wang J, Guo
R, Zhu Y, Yu X, Zhang Y, Du H, et al: Intermittent F-actin
perturbations by magnetic fields inhibit breast cancer metastasis.
Research (Wash DC). 6:00802023.
|
|
88
|
Guo XF, Gu SS, Wang J, Sun H, Zhang YJ, Yu
PF, Zhang JS and Jiang L: Protective effect of mesenchymal stem
cell-derived exosomal treatment of hippocampal neurons against
oxygen-glucose deprivation/reperfusion-induced injury. World J
Emerg Med. 13:46–53. 2022. View Article : Google Scholar
|
|
89
|
Luo Z, Mei J, Wang X, Wang R, He Z, Geffen
Y, Sun X, Zhang X, Xu J, Wan R, et al: Voluntary exercise
sensitizes cancer immunotherapy via the collagen
inhibition-orchestrated inflammatory tumor immune microenvironment.
Cell Rep. 43:1146972024. View Article : Google Scholar
|