Introduction
Castleman disease (CD) is a rare lymphoproliferative
disorder. It was first described by Benjamin Castleman in 1954
(1), with an estimated incidence of
~2 per 100,000 individuals in the US (2). CD is classified into unicentric CD
(UCD) and multicentric CD (MCD) types based on the extent of lymph
node involvement. A 2024 systematic review of articles published in
1995–2021 on ≥5 cases of CD reported that UCD was slightly more
frequent in women (53.7%), whereas idiopathic MCD (iMCD; 59.1%) and
human herpesvirus 8 (HHV-8)+ MCD (88.5%) were predominant in men
(3). The diagnosis of CD primarily
relies on complete excisional biopsy of the affected lymph nodes
and subsequent histological examination, with pathological features
such as hyaline-vascular, plasma-cell or mixed type serving as the
diagnostic gold standard. Simultaneously, it is key to exclude
other diseases that can produce CD-like changes in lymph nodes
(e.g., lymphoma, tuberculosis and immunological disorders)
(4,5). Paraneoplastic pemphigus (PNP) and
follicular dendritic cell sarcoma (FDCS) are closely associated
with CD. It has been reported that 10–32% of patients with CD
develop PNP (6–8), while 7–10% of FDCS cases are
associated with hyaline-vascular CD (9). However, the coexistence of CD with
both PNP and FDCS remains exceedingly uncommon. Herein, we describe
a rare case of pelvic unicentric CD complicated by concurrent PNP
and low- to intermediate-grade FDCS, emphasizing the diagnostic
challenges and clinical implications of this unique
association.
Case report
A 67-year-old woman was admitted to HuiYa Hospital
of The First Affiliated Hospital of Sun Yat-sen University
(Huizhou, China) in July 2024 with an oral ulcer and systemic
purple skin spots that had persisted for 1 month and a pelvic mass
that had persisted for 5 days. The patient had presented with
bilateral conjunctival hyperemia 1 month earlier, which gradually
progressed to systemic purple skin spots and oral ulcers. The
condition of the patient worsened: Ulcers developed on the lips and
external genitalia, prompting the patient to seek medical
attention. The patient had a 10-year history of type 2 diabetes.
The latest hypoglycemic regimen is: miglitol (50 mg once daily) and
liraglutide. The starting date for liraglutide treatment is July
2024 and the treatment dose is 0.6 mg once daily, and it will be
gradually increased to 1.2 mg once daily. Marriage and childbearing
history were unremarkable and there were no patients with similar
diseases in the family.
Physical examination (July 2024)
A general physical examination on admission revealed
no abnormalities plus a pulse oxygen saturation of 98% on room air.
Palpation revealed no superficial lymphadenopathy. Cardiovascular,
pulmonary and abdominal examination results were normal. A
dermatological evaluation revealed that the skin lesions were
predominantly located in the mucosal areas. The oral mucosa
exhibited erosions with white exudates, while the perioral region
exhibited erosions, ulcerations and crusting with a noticeable
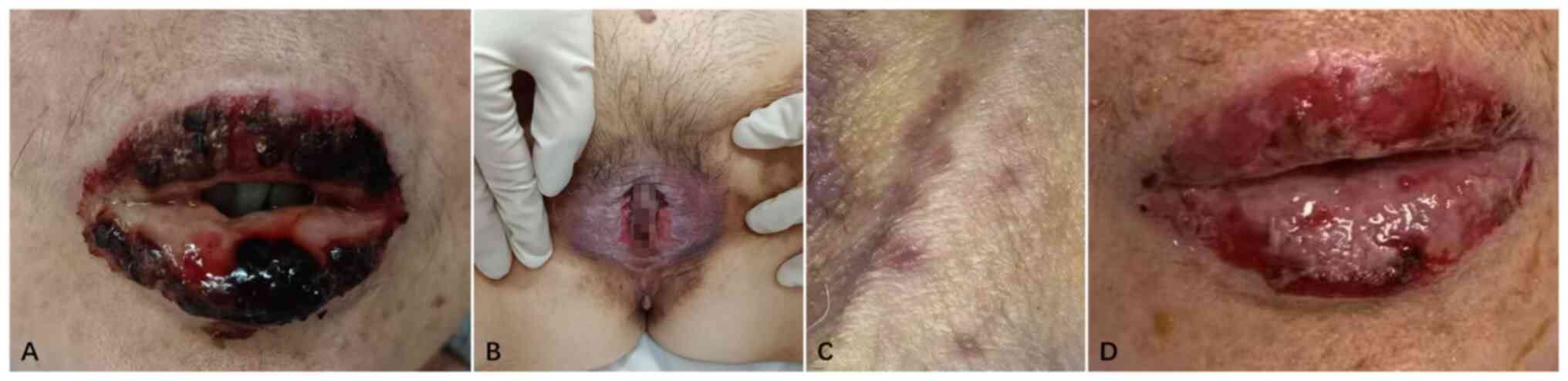
mouth-opening limitation (Fig. 1A).
An examination of the external genitalia revealed erosive and
ulcerative lesions (Fig. 1B).
Furthermore, multiple purple skin spots with variable morphologies
and well-defined borders were noted throughout the body (Fig. 1C).
Laboratory and imaging findings
Laboratory findings (July 2024) were as follows:
Blood glucose, 10.49 mmol/l (normal range, 3.9-6.1 mmol/l);
fibrinogen, 4.05 g/l (normal range, 2.0-4.0 g/l); serum albumin,
36.0 g/l (normal range, 35–50 g/l); neutrophils,
6.65×109/l (normal range, 1.8-6.3×109/l);
normal white blood cells (normal range, 3.5-9.5×109/l),
red blood cells (normal range, 3.8-5.1×109/l); platelet
counts (normal range, 125–350×109/l); hemoglobin, 137
g/l (normal range, 115–150 g/l); and C-reactive protein, 25.96 mg/l
(normal range, 0.0-10.0 mg/l). The erythrocyte sedimentation rate
was normal (normal range, 0–20 mm/h). The creatinine level (normal
range, 53–115 µmol/l) and estimated glomerular filtration rate were
normal (normal range, 90–120 ml/min/1.73 m2) and the
urine protein levels were negative. The human chorionic
gonadotropin (normal range, 0–6.00 mIU/ml) and estradiol levels
were normal (normal range, ≤138 pg/ml). Human papillomavirus
analysis (July 2024; PCR) was positive for the high-risk PV56
genotype. HIV, hepatitis virus antibodies and syphilis-specific
antibodies were negative. The tumor marker levels were within
normal limits [carcinoembryonic antigen, <5.00 ng/ml;
α-fetoprotein, <20.00 ng/ml; carbohydrate antigen (CA)15-3,
<35.00 KU/l; CA199, 0–35.00 U/ml; CA125, 0–35.00 U/ml; CA72-4,
0–6.9 U/ml; cytokeratin 19 fragment, 0–3.30 ng/ml; neuron-specific
enolase, 0–13.00 ng/ml]. Screening for pemphigus-related
autoantibodies, including anti-bullous pemphigoid antigen (BP)180,
BP230, desmoglein (Dsg)1 and Dsg3, exhibited anti-desmoplakin 3,
anti-desmoplakin 1 and anti-BP230 antibody positivity. Antinuclear
antibodies and specific autoantibodies (ANA, anti-double-stranded
DNA antibody, anti-nRNP/Sm, anti-Sm, anti-SSA, anti-Ro-52,
anti-SSB, anti-Scl-70, anti-J0-1) associated with connective tissue
diseases were absent. IL-10 levels were 27.81 pg/ml (normal range,
0–10 pg/ml) and parathyroid hormone levels were 87.41 pg/ml (normal
range, 15–60 pg/ml), while normal levels were obtained for IL-6
(normal range, 0–7 pg/ml), C3 (normal range, 0.5-2.8 g/l)/C4
(normal range, 0.1-0.4 g/l)/IgM (normal range, 0.3-2.2 g/l)/IgG
(normal range, 8.6-17.4 g/l)/IgA (normal range, 1–4.2 g/l) and
anti-Müllerian hormone (normal range, 0–0.39 ng/ml).
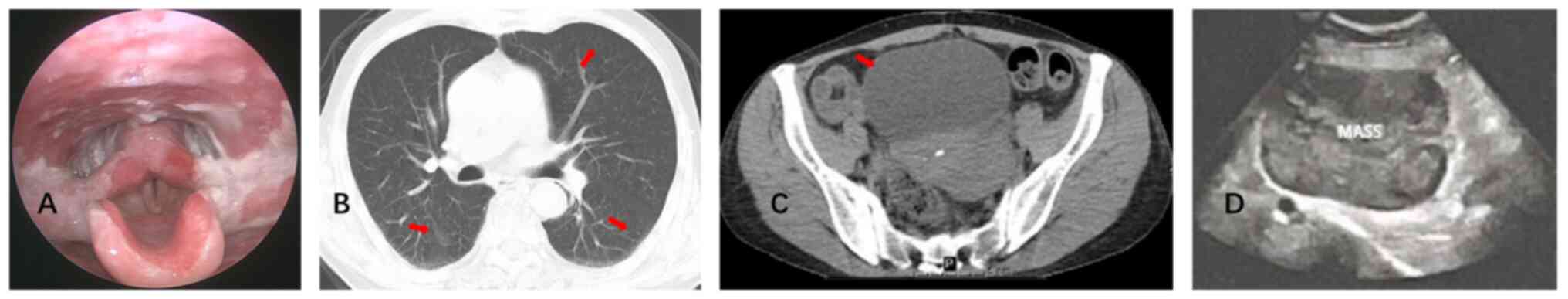
Laryngoscopy (July 2024) revealed erosions and white
pseudomembranous exudates (Fig.
2A). Chest CT (July 2024) revealed bronchitis and bilateral
mild bronchial dilation (Fig. 2B).
No enlarged mediastinal lymph nodes were observed. Pelvic CT (July
2024) (plain and contrast-enhanced) revealed a solitary mass
measuring ~114×96×116 mm in the left pelvic region. The lesion had
iso- to low-density characteristics with heterogeneous enhancement
and contained multiple patchy and non-enhancing necrotic areas
(Fig. 2C). The venous drainage of
the lesion converged into the left ovarian vein without enlarged
lymph nodes. The liver and spleen were normal in size. Transvaginal
ultrasonography (July 2024) revealed a hypoechoic mass measuring
~114×86×118 mm in the anterosuperior aspect of the left side of the
uterus (Fig. 2D).
Pathology and immunohistochemistry
(pelvic mass)
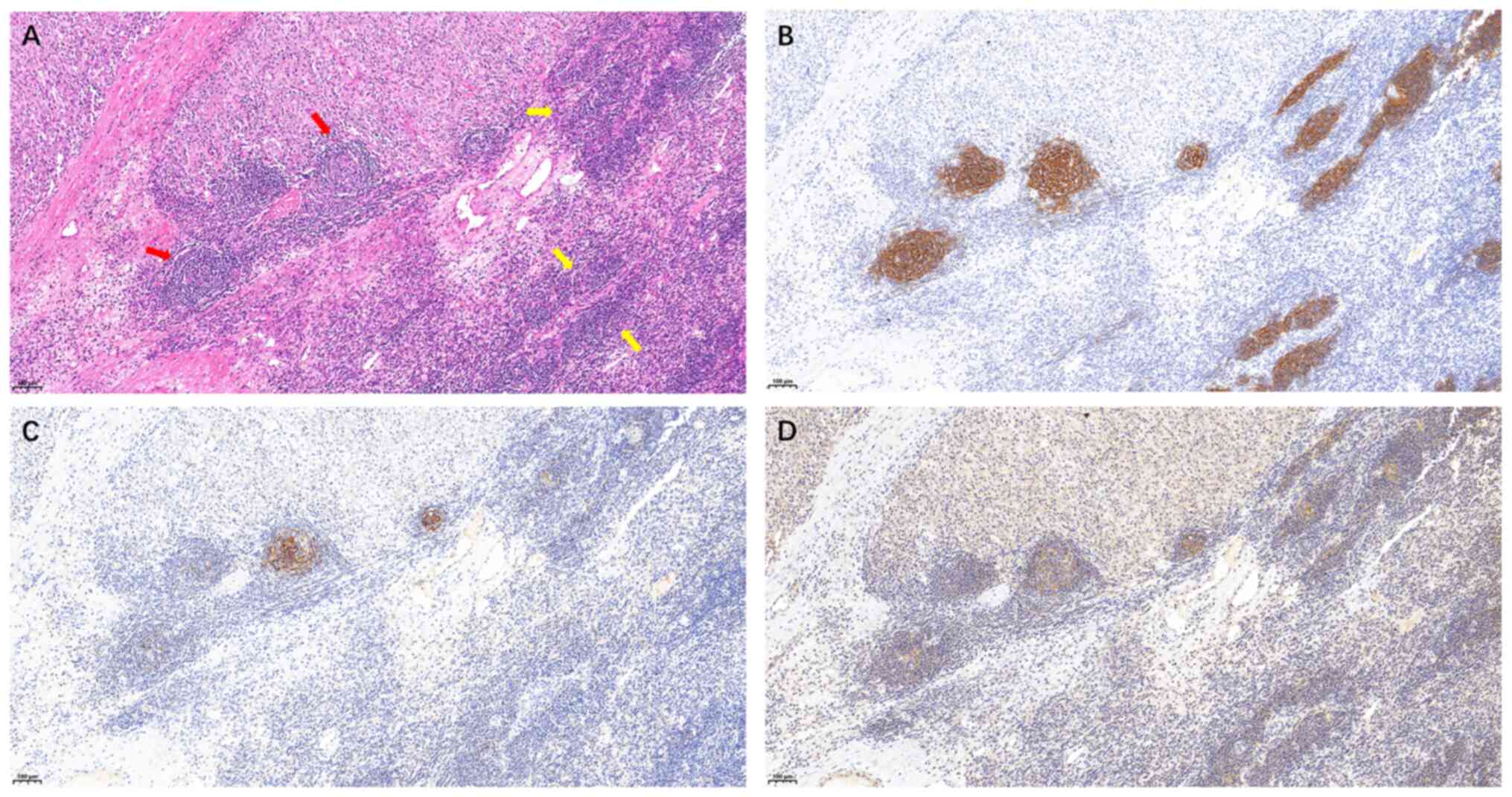
The findings of a histopathological examination of
the pelvic mass were consistent with CD with focal progression to
low- to intermediate-grade FDCS. H&E staining revealed the left
side exhibited a concentric (‘onion-skin’) configuration with
attenuation of lymphoid follicles, whereas the right side revealed
effacement of the normal follicular architecture with replacement
by a compressive mass consistent with progression to an FDC tumor
(Fig. 3A). Immunohistochemical
staining revealed the following (August 2024): Positive (focal or
scattered) for CD21, CD23, CD35, C-X-C motif chemokine ligand 13
(data not shown) and CD68 (scattered; data not shown); negative for
podoplanin, somatostatin receptor 2, CD20, CD3, CD1a, anaplastic
lymphoma kinase, CD30, S-100, smooth muscle actin, CD117, melanin
A, human melanoma black 45, CD34, cytokeratin, desmin and
Epstein-Barr virus-encoded RNAs; and a Ki-67 proliferation index of
~10% (data not shown). The results described in this paper are
based on the pathological reports. Some immunohistochemical results
are non-accessible image files, such as CXCL13, CD68 and Ki-67,
etc. The immunohistochemical results suggested the focal
progression to low- to intermediate-grade FDCS (Fig. 3B-D). The specific experimental
materials and procedures can be found in the supplementary methods
and Tables SI and SII..
Diagnosis
The patient was diagnosed in August 2024 with
unicentric CD (UCD; hyaline-vascular) with PNP and FDCS (7). CT evaluation of the patient revealed a
solitary large mass within the pelvic region, with no evidence of
additional masses or lymphadenopathy elsewhere. Histopathological
examination was consistent with CD, fulfilling diagnostic criteria
for involvement confined to a single lymph node station. Based on
H&E staining features, the lesion was classified as the
hyaline-vascular variant. In the diagnostic workup for PNP,
infectious, metabolic, and primary neurological disorders were
initially excluded. The detection of pemphigus-associated
autoantibodies served as a key diagnostic indicator, and in
conjunction with the identified tumor, confirmed the diagnosis. The
diagnosis of FDCS was established primarily based on
histopathological characteristics observed on H&E staining,
supported by positive immunohistochemical staining for CD21, CD25
and CD35.
Treatment
After admission, the patient was treated with
intravenous methylprednisolone succinate (40 mg/dose once daily,
intravenous drip), Human immunoglobulin (pH4) [20 g/dose once daily
for a 4-day treatment course, intravenous drip (July 2024)] and
thalidomide tablets (50 mg/evening, orally administered). Following
the aforementioned treatments, a slight improvement was noted in
the mucosal ulceration with reduced trismus severity. In July 2024,
the patient underwent an exploratory laparotomy, pelvic tumor
resection, adhesiolysis of the abdominal cavity and pelvic
adhesiolysis. Postoperatively, the patient received antibiotic
therapy (cefuroxime sodium for injection 1.5 g, twice a day,
lasting for 2 days) and continued corticosteroid treatment
(intravenous methylprednisolone succinate, 40 mg/dose once daily,
lasting for 18 days) along with supportive care. A tacrolimus
ointment (0.1%, twice a day) was applied to the lips. Postoperative
complications included hypoalbuminemia (albumin level, 22.4 g/l),
which was managed with an intravenous human albumin infusion (10
g/dose twice daily for 6 days). Upon the confirmation of CD through
a pathological examination of the pelvic tumor, the treatment was
as follows: Thalidomide 100 mg orally once nightly,
cyclophosphamide 300 mg/m2 orally once a week and
prednisone 1 mg/kg orally twice a week.
Outcomes
After treatment (August 13th, 2024), the neutrophil
count of the patient (5.12×109/l), and C-reactive
protein (9.15 mg/l) and albumin levels (39.6 g/l) normalized. The
generalized purple skin spots markedly subsided compared with that
at admission and the oral and vulvar ulcers improved, although the
erosions and pain persisted (Fig.
1D). The surgical incision healed well with normotension,
reduced facial edema and decreased lip crusting. Despite the
initial postoperative improvements, subsequent telephone follow-up
(January 20th, 2025) revealed that the patient had ultimately
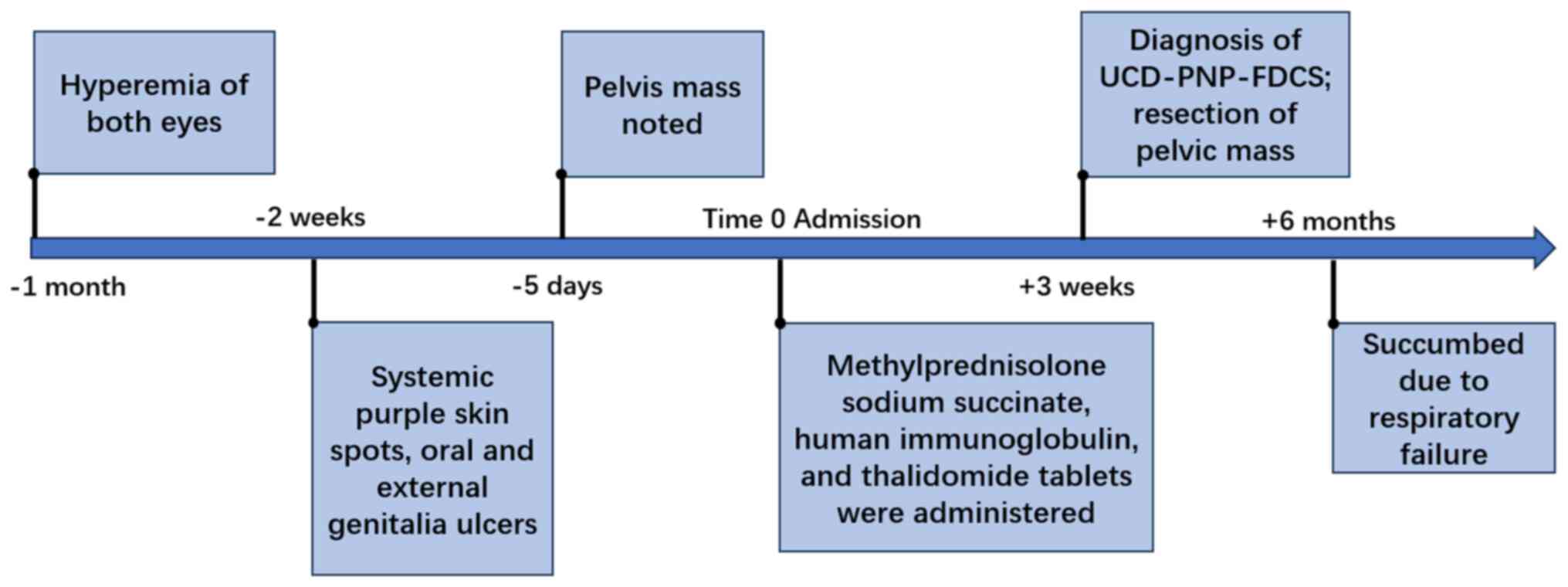
succumbed due to respiratory failure. The timeline of the condition
of the patient is shown in Fig.
4.
Discussion
UCD typically involves a single lymph node and often
presents without systemic symptoms. UCD most commonly occurs in the
mediastinum, accounting for ~29% of all cases, followed by single
lymph nodes or nodal regions in the cervical, abdominal and
retroperitoneal areas (10). UCD
also arises in rare sites such as the adrenal gland, liver,
paravertebral region and breast (11–14).
UCD is generally asymptomatic; however, when lesions are large or
located in sensitive regions, patients may develop mass-effect
symptoms such as dysphagia or localized pain and a minority may
exhibit systemic manifestations including fever, night sweats,
weight loss or anemia (15). MCD is
characterized by lymphadenopathy in multiple regions and is further
subdivided into HHV-8+ and HHV-8−
(idiopathic) types. Unlike those with UCD, patients with MCD
exhibit lymphadenopathy and commonly present with systemic
manifestations such as fever, night sweats, fatigue, weight loss,
anemia, hepatic dysfunction, renal impairment and volume overload
(for example, generalized edema, pleural effusion and ascites)
(16). Both UCD and MCD can be
complicated by PNP and bronchiolitis obliterans (BO), which are
associated with a poor prognosis (7,10,17).
PNP is a severe autoimmune blistering disease that
primarily involves the oral mucosa and skin, and often initially
presents as persistent oral ulcers or erosions. Other mucosal
sites, such as the conjunctiva and nasal passages, may also be
affected along with the skin, where polymorphic bullae and erythema
are common (18). The respiratory
system is frequently involved, with ~54% of patients exhibiting
respiratory symptoms, including pulmonary dysfunction and, in
certain cases, respiratory failure (18). FDCS is a rare low- to
intermediate-grade sarcoma that typically presents as a
slow-growing, painless and localized mass (9). Most patients are asymptomatic and
local symptoms usually arise only when the tumor compresses the
adjacent structures. Respiratory system involvement is rare. On
H&E staining, the tumor cells of FDCS displayed abundant pale
to eosinophilic cytoplasm, oval to elongated spindle-shaped nuclei,
occasional cytoplasmic vacuolation and small nucleoli.
Immunohistochemically, FDCS typically expresses follicular
dendritic cell markers including CD21, CD23 and CD35; the diagnosis
is established by associating these characteristic morphologic
features with a supportive immunophenotype (9,19,20).
The present study further reviewed case reports and
meta-analyses of UCD coexisting with FDCS and PNP. These findings
suggested that adults with UCD as well as FDCS or PNP generally do
not present with notable laboratory abnormalities and that CD
complicated by PNP may not exhibit positive pemphigus-related
autoantibodies. Due to the complexity and difficulty of diagnosing
CD, international evidence-based consensus diagnostic and treatment
guidelines were promulgated in previous years (7,10,16).
The diagnosis of CD and its associated complications depends
primarily on a biopsy and histopathological examination (3,21).
Furthermore, these guidelines advocate comprehensive laboratory and
imaging evaluations to exclude conditions that may produce CD-like
changes such as infections, lymphomas and polyneuropathy,
organomegaly, endocrinopathy, monoclonal protein and skin changes
syndrome (10).
Surgical resection, the treatment of choice for UCD,
is often curative. Surgical intervention not only enables complete
excision of the CD lesion, but it also mitigates the associated
hyperinflammatory state (10,22).
In patients with UCD and marked inflammation whose lesions are
initially unresectable, treatment regimens extrapolated from iMCD,
such as siltuximab in combination with glucocorticoids or the
thalidomide-cyclophosphamide-prednisone protocol, may be employed
(22). Surgical excision should be
performed if medical therapy induces sufficient lesion regression
for complete removal (10).
However, patients with UCD and concomitant PNP and BO tend to have
a worse prognosis. In cases of UCD associated with PNP,
international consensus and guidelines recommend surgical resection
(7,10). Nonetheless, even when the primary
lesion is controlled, PNP frequently leads to respiratory
complications (for example, BO), which may result in fatal
respiratory failure in certain patients (7,23).
Timely intervention is necessary and lung transplantation is an
option. Complete surgical resection is the preferred treatment
option for patients with FDCS. In cases that cannot be cured or
show recurrence, adjuvant radiotherapy, chemotherapy and targeted
immunotherapy may be considered; however, large-scale evidence to
support these treatment methods is currently lacking (9).
Due to the rarity of CD coexisting with FDCS,
survival outcomes are referenced from FDCS cases, which report a
median progression-free survival of ~21 months and median overall
survival (OS) of ~50 months (24).
CD with concomitant PNP is associated with a poor prognosis;
patients with PNP have 1- and 3-year OS rates of 76.9 and 57.6%,
respectively, compared with 98.6 and 88.3% in those without PNP
(P<0.001) (8). The development
of BO is the key adverse prognostic factor in PNP (8,25).
According to the Consensus Statements of
Deployment-Related Respiratory Disease and Chinese Guidelines for
Paraneoplastic Pemphigus, patients with CD should undergo regular
pulmonary function testing (PFTs). A decline in diffusing capacity
warrants further evaluation using high-resolution chest CT and, if
indicated, a lung biopsy to confirm or exclude BO (26,27).
The European Guidelines further advise complete PFTs (including
diffusing capacity of the lungs for carbon monoxide),
inspiratory-expiratory high-resolution CT, 6-min pulmonary walk
test or cardiopulmonary exercise examination (when available) and
arterial blood gas analysis at diagnosis (27). For patients with active disease or
progressive respiratory symptoms, symptom review and spirometry
every 3 months are recommended, with repeat high-resolution CT or
additional examinations (for example, cardiopulmonary exercise
examination or surgical biopsy) if a rapid forced expiratory volume
in 1 sec decline or worsening symptoms occur. In clinically stable
patients, PFT intervals may be extended to every 6 months; in
long-term stable cases, it can be extended to 6–12 months (28). These observations underscore the
importance of guideline-directed dynamic surveillance for BO in
patients with confirmed CD and/or PNP.
The present case represents a rare instance of CD
associated with PNP and FDCS. Upon admission, the patient was
treated with methylprednisolone, thalidomide and immunoglobulin to
alleviate the inflammation along with mucosal care of the lips and
perineum. Surgical resection was performed after the oral mucosal
condition of the patient was deemed tolerable for intubation. Upon
the confirmation of CD through a pathological examination of the
pelvic tumor, the treatment was as follows: Thalidomide 100 mg
orally once nightly, cyclophosphamide 300 mg/m2 orally
once a week and prednisone 1 mg/kg orally twice a week. However,
during subsequent follow-up, the patient succumbed due to
respiratory failure and obstructive bronchiolitis was included in
the mortality diagnosis as reported by the family.
In conclusion, CD is a key cause of PNP and BO.
Complete surgical resection of the enlarged lymph nodes is the key
treatment for patients with UCD and PNP. Therefore, monitoring for
postoperative BO is necessary. If a patient is further diagnosed
with BO, a lung transplantation can restore lung function and
improve the prognosis. Several previous studies have demonstrated
that patients experienced pulmonary function normalizing following
surgery, with nearly complete resolution of oral mucosal lesions
and marked improvement in health-related quality of life (21,29).
In addition, patients who underwent a lung transplantation achieved
a notable survival benefit compared with non-transplant with a
5-year survival rate of ~62.5% and no recurrence of CD was observed
after transplantation. Although postoperative infection remained
the predominant complication, the overall findings support lung
transplantation as an effective therapeutic option for patients
with end-stage disease (30,31).
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the 2024 Huizhou Key Project of
Science and Technology (grant no. 2024CZ010004) in the field of
health care (self-financing).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL and FZ obtained and analyzed the information of
the patient and wrote the manuscript. ZW, GZ and QZ obtained and
analyzed the information of the patient and reviewed the discussion
part of the clinical diagnosis and treatment. QZ partially revised
the article and generated the figures, and confirmed the
authenticity of all the raw data. YL and FZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present case report was approved by The HuiYa
Hospital of The First Affiliated Hospital, Sun Yat-sen University
(Huizhou, China; approval no. 2025-0519-001).
Patient consent for publication
Written informed consent to publish the present case
information and accompanying images was obtained from the patient
and their family.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castleman B and Towne VW: Case records of
the Massachusetts general hospital: Case No. 40231. N Engl J Med.
250:1001–1005. 1954.
|
|
2
|
Simpson D: Epidemiology of castleman
disease. Hematol Oncol Clin North Am. 32:1–10. 2018. View Article : Google Scholar
|
|
3
|
Hoffmann C, Oksenhendler E, Littler S,
Grant L, Kanhai K and Fajgenbaum DC: The clinical picture of
Castleman disease: A systematic review and meta-analysis. Blood
Adv. 8:4924–4935. 2024. View Article : Google Scholar
|
|
4
|
Dispenzieri A and Fajgenbaum DC: Overview
of castleman disease. Blood. 135:1353–1364. 2020. View Article : Google Scholar
|
|
5
|
Carbone A, Borok M, Damania B, Gloghini A,
Polizzotto MN, Jayanthan RK, Fajgenbaum DC and Bower M: Castleman
disease. Nat Rev Dis Primers. 7:842021. View Article : Google Scholar
|
|
6
|
Nikolskaia OV, Nousari CH and Anhalt GJ:
Paraneoplastic pemphigus in association with Castleman's disease.
Br J Dermatol. 149:1143–1151. 2003. View Article : Google Scholar
|
|
7
|
Hematology Committee of Chinese Medical
Association; Hematological Oncology Committee of China Anti-Cancer
Association; China Castleman Disease Network (CCDN), . The
consensus of the diagnosis and treatment of Castleman disease in
China (2021). Zhonghua Xue Ye Xue Za Zhi. 42:529–534. 2021.(In
Chinese).
|
|
8
|
Dong Y, Wang M, Nong L, Wang L, Cen X, Liu
W, Zhu S, Sun Y, Liang Z, Li Y, et al: Clinical and laboratory
characterization of 114 cases of Castleman disease patients from a
single centre: Paraneoplastic pemphigus is an unfavourable
prognostic factor. Br J Haematol. 169:834–842. 2015. View Article : Google Scholar
|
|
9
|
Facchetti F, Simbeni M and Lorenzi L:
Follicular dendritic cell sarcoma. Pathologica. 113:316–329. 2021.
View Article : Google Scholar
|
|
10
|
van Rhee F, Oksenhendler E, Srkalovic G,
Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S,
Streetly M, et al: International evidence-based consensus
diagnostic and treatment guidelines for unicentric Castleman
disease. Blood Adv. 4:6039–6050. 2020. View Article : Google Scholar
|
|
11
|
Raja A, Malik K and Mahalingam S: Adrenal
castleman's disease: Case report and review of literature. J Indian
Assoc Pediatr Surg. 27:109–111. 2022. View Article : Google Scholar
|
|
12
|
Chen H, Pang X, Li J, Xu B and Liu Y: Case
Report: A rare case of primary hepatic Castleman's disease
mimicking a liver tumor. Front Oncol. 12:9742632022. View Article : Google Scholar
|
|
13
|
Chen HY, Wu SH, Chew FY and Lee SY:
Mediastinal castleman disease presenting as a paraspinal mass
causing back pain and shortness of breath in a young adult.
Heliyon. 10:e407922024. View Article : Google Scholar
|
|
14
|
Parra-Medina R, Guio JI and López-Correa
P: Localized castleman's disease in the breast in a young woman.
Case Rep Pathol. 2016:84139872016.
|
|
15
|
Boutboul D, Fadlallah J, Chawki S, Fieschi
C, Malphettes M, Dossier A, Gérard L, Mordant P, Meignin V,
Oksenhendler E and Galicier L: Treatment and outcome of unicentric
castleman disease: A retrospective analysis of 71 cases. Br J
Haematol. 186:269–273. 2019. View Article : Google Scholar
|
|
16
|
Fajgenbaum DC, Uldrick TS, Bagg A, Frank
D, Wu D, Srkalovic G, Simpson D, Liu AY, Menke D, Chandrakasan S,
et al: International, evidence-based consensus diagnostic criteria
for HHV-8-negative/idiopathic multicentric Castleman disease.
Blood. 129:1646–1657. 2017. View Article : Google Scholar
|
|
17
|
Liu AY, Nabel CS, Finkelman BS, Ruth JR,
Kurzrock R, van Rhee F, Krymskaya VP, Kelleher D, Rubenstein AH and
Fajgenbaum DC: Idiopathic multicentric Castleman's disease: A
systematic literature review. Lancet Haematol. 3:e163–e175. 2016.
View Article : Google Scholar
|
|
18
|
Zou M, Zhan K, Zhang Y, Li L, Li J, Gao J,
Liu X and Li W: A systematic review of paraneoplastic pemphigus and
paraneoplastic autoimmune multiorgan syndrome: Clinical features
and prognostic factors. J Am Acad Dermatol. 92:307–310. 2025.
View Article : Google Scholar
|
|
19
|
Chen T and Gopal P: Follicular dendritic
cell sarcoma. Arch Pathol Lab Med. 141:596–599. 2017. View Article : Google Scholar
|
|
20
|
Jiménez-Heffernan JA, Díaz Del Arco C and
Adrados M: A cytological review of follicular dendritic
cell-derived tumors with emphasis on follicular dendritic cell
sarcoma and unicentric castleman disease. Diagnostics (Basel).
12:4062022. View Article : Google Scholar
|
|
21
|
Chen R, Teng Y, Xiao Y, Zhang L, Han X,
Wang W, Lu Z and Tian X: Constrictive bronchiolitis and
paraneoplastic pemphigus caused by unicentric Castleman disease in
a young woman: A case report. Front Med (Lausanne). 11:14682512024.
View Article : Google Scholar
|
|
22
|
Zhang MY, Jia MN, Chen J, Feng J, Cao XX,
Zhou DB, Fajgenbaum DC, Zhang L and Li J: UCD with MCD-like
inflammatory state: Surgical excision is highly effective. Blood
Adv. 5:122–128. 2021. View Article : Google Scholar
|
|
23
|
Kaur S, Singh C, Bal A and Jain A:
Unicentric Castleman disease-associated paraneoplastic pemphigus
successfully managed with surgical resection and rituximab. BMJ
Case Rep. 18:e2632752025. View Article : Google Scholar
|
|
24
|
Jain P, Milgrom SA, Patel KP, Nastoupil L,
Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J, et al:
Characteristics, management, and outcomes of patients with
follicular dendritic cell sarcoma. Br J Haematol. 178:403–412.
2017. View Article : Google Scholar
|
|
25
|
Irrera M, Bozzola E, Cardoni A, DeVito R,
Diociaiuti A, El Hachem M, Girardi K, Marchesi A and Villani A:
Paraneoplastic pemphigus and Castleman's disease: A case report and
a revision of the literature. Ital J Pediatr. 49:332023. View Article : Google Scholar
|
|
26
|
Chinese Society of Dermatology CDA,
Dermatology Branch of China International Exchange, Promotive
Association for Medical and Health Care, Rare Skin Diseases
Committee, China Alliance for Rare Diseases, National Clinical
Research Center for Dermatologic and Immunologic Diseases, . Expert
consensus on the diagnosis and treatment of paraneoplastic
pemphigus in China (2025 version). Chin J Dermatol. 58:289–296.
2025.(In Chinese).
|
|
27
|
Falvo MJ, Sotolongo AM, Osterholzer JJ,
Robertson MW, Kazerooni EA, Amorosa JK, Garshick E, Jones KD,
Galvin JR, Kreiss K, et al: Consensus statements on
deployment-related respiratory disease, inclusive of constrictive
bronchiolitis. Chest. 163:599–609. 2023. View Article : Google Scholar
|
|
28
|
Antiga E, Bech R, Maglie R, Genovese G,
Borradori L, Bockle B, Caproni M, Caux F, Chandran NS, Corrà A, et
al: S2k guidelines on the management of paraneoplastic
pemphigus/paraneoplastic autoimmune multiorgan syndrome initiated
by the European academy of dermatology and venereology (EADV). J
Eur Acad Dermatol Venereol. 37:1118–1134. 2023. View Article : Google Scholar
|
|
29
|
Chen W, Zhao L, Guo L, Zhao L, Niu H, Lian
H, Dai H, Chen J and Wang C: Clinical and pathological features of
bronchiolitis obliterans requiring lung transplantation in
paraneoplastic pemphigus associated with Castleman disease. Clin
Respir J. 16:173–181. 2022. View Article : Google Scholar
|
|
30
|
Liu YT, Zhen JF, Gao YH, Li SY, Dang Y, Xu
HY, Zhang L and Li J: Unicentric Castleman disease complicated with
bronchiolitis obliterans: A single-centre retrospective study from
China. Br J Haematol. 206:1129–1135. 2025. View Article : Google Scholar
|
|
31
|
Yue B, Huang J, Jing L, Yu H, Wei D, Zhang
J, Chen W and Chen J: Bilateral lung transplantation for Castleman
disease with end-stage bronchiolitis obliterans. Clin Transplant.
36:e144962022. View Article : Google Scholar
|