Introduction
Tube-ovarian cancer (OC) ranks eighth among
malignant neoplasms affecting females overall; however, it is the
fourth leading cancer in terms of mortality in patients of
childbearing age of <44 years (1,2).
Moreover, the incidence markedly increases in patients carrying
BRCA1/2 mutations (BRCA1mut and BRCA2mut), in whom the lifetime
breast cancer risk is >60%, with an OC risk of 40–60 and 15–30%
for those with BRCA1mut and BRCA2mut, respectively (3–5).
Inflammation has been implicated in ovarian carcinogenesis and a
history of pelvic inflammatory disease or endometriosis is
associated with an increased risk of epithelial OC (6–8).
Fertility-sparing surgery (FSS) can be proposed only
for patients with selected histotypes of OC at initial stages
apparently confined to the ovary, consisting of unilateral
salpingo-oophorectomy and peritoneal and lymph-node surgical
staging according to the histopathology of the primary tumor, and
preferably with a minimally invasive surgical approach (9,10).
However, it is unclear what the ideal FSS treatment is in case of
primary fallopian tube carcinoma, as there are no data regarding
ovarian preservation following salpingectomy, to the best of our
knowledge. Furthermore, available data regarding fertility
preservation (FP) in OC with oocytes retrieval are associated with
borderline ovarian tumors (BOTs) and non-epithelial ovarian tumors,
whilst there are no data regarding the safety of FP with oocyte
retrieval in case of invasive tube-ovarian epithelial carcinoma
(11). Additionally, the FP options
available to patients with OC are dependent on staging,
histological type, location and spread; and the options consist of
oocytes or embryos cryopreservation. FSS should be an option in
patients with early-stage OC, followed by close follow-up (1).
There are few retrospective studies and case reports
regarding assisted reproductive techniques (ART) in young patients
with OC (12,13). Moreover, there is not enough data to
provide an accurate estimate on the incidence of relapse, the
actual chance of pregnancy and its outcome. There is also currently
no data regarding FP techniques for patients with Fallopian tubes
cancer, to the best of our knowledge.
The present report describes the first case, to the
best of our knowledge, of a pregnancy with a healthy newborn using
vitrified oocytes after FSS in a young patient with a BRCA-2
mutation and invasive Fallopian tube cancer [International
Federation of Gynecology and Obstetrics (FIGO) stage IC1] (14).
Case report
In September 2019, a 32-year-old female patient was
referred to Department of Obstetrics and Gynecology at the
Institute for Maternal and Child Health IRCCS ‘Burlo Garofolo’
(Trieste, Italy) by an attending gynecologist due to suspected
bilateral hydrosalpinx identified with vaginal ultrasound. The
patient was actively seeking pregnancy and waiting to start ART.
The patient was nulliparous and did not report a family history of
gynecological cancers or other pathologies. The patient had no
prior history of symptoms indicative of pelvic inflammatory disease
or sexually transmitted infections, and denied experiencing
dysmenorrhea, dyspareunia or chronic pelvic pain. The patient had a
body mass index (BMI) of 30 kg/m2 and was a smoker.
During pre-operative checks, a diagnostic work-up
was performed, in which a second level transvaginal ultrasound
confirmed the hypothesis of a left 22×47 mm hydrosalpinx. Ovarian
tumor markers CA125, carcinoembryonic antigen, CA 19.9 and
α-fetoprotein were negative. A total of 5 months later, the patient
underwent a laparoscopic bilateral salpingectomy, in which
laparoscopic exploration showed both tubes congested and enlarged,
especially on the left side, attached to the ipsilateral ovarian
fossa, the posterior wall of the uterus and the omentum. The right
ovary showed an unilocular neoformation of 2 cm, with a
tight-elastic consistency. The left ovary was regular. There were
no signs of peritoneal carcinomatosis or suspicious peritoneal
nodularity. There was no pelvic or aortic lymph node enlargement.
During mobilization, a surgical spillage of the left tube occurred,
with the release of purulent, thick and brownish liquid that was
promptly aspirated (data not shown).
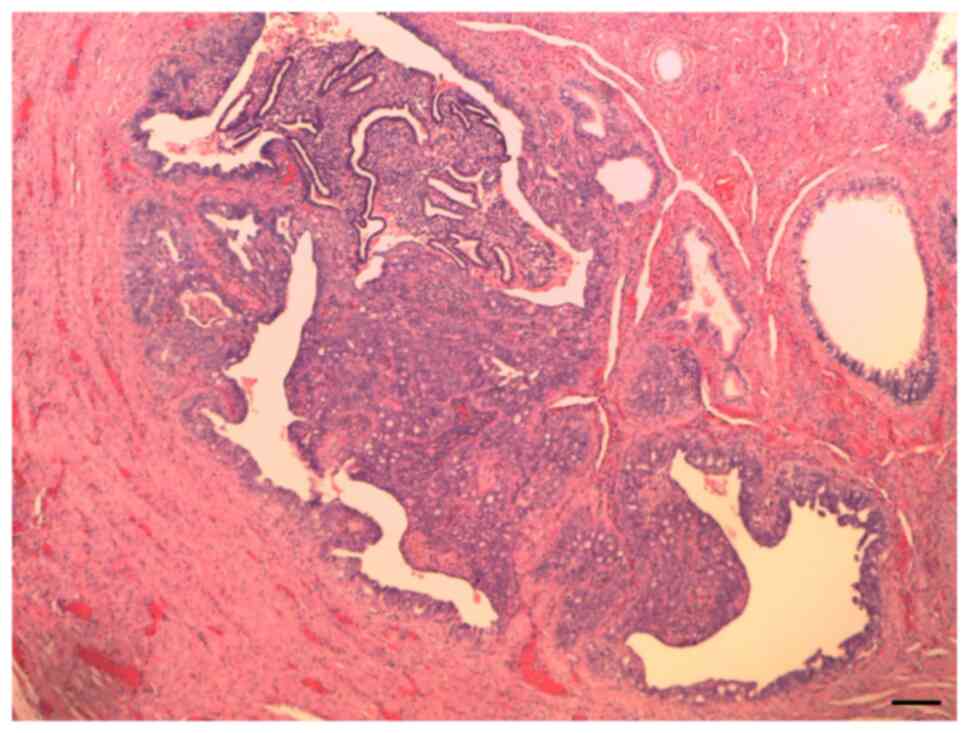
Histological examination revealed a primary
endometrioid carcinoma of the Fallopian tube, G2 (Fig. 1). Tissue samples were fixed in 4%
formaldehyde at room temperature for 24 h, embedded in paraffin,
sectioned at 4 µm, and stained with hematoxylin and eosin (H&E)
using the HistoCore SPECTRA ST automated stainer (Leica Biosystems)
at room temperature, for ~1 h. Light microscopic evaluation was
carried out with the Aperio AT2 system (Leica Biosystems).
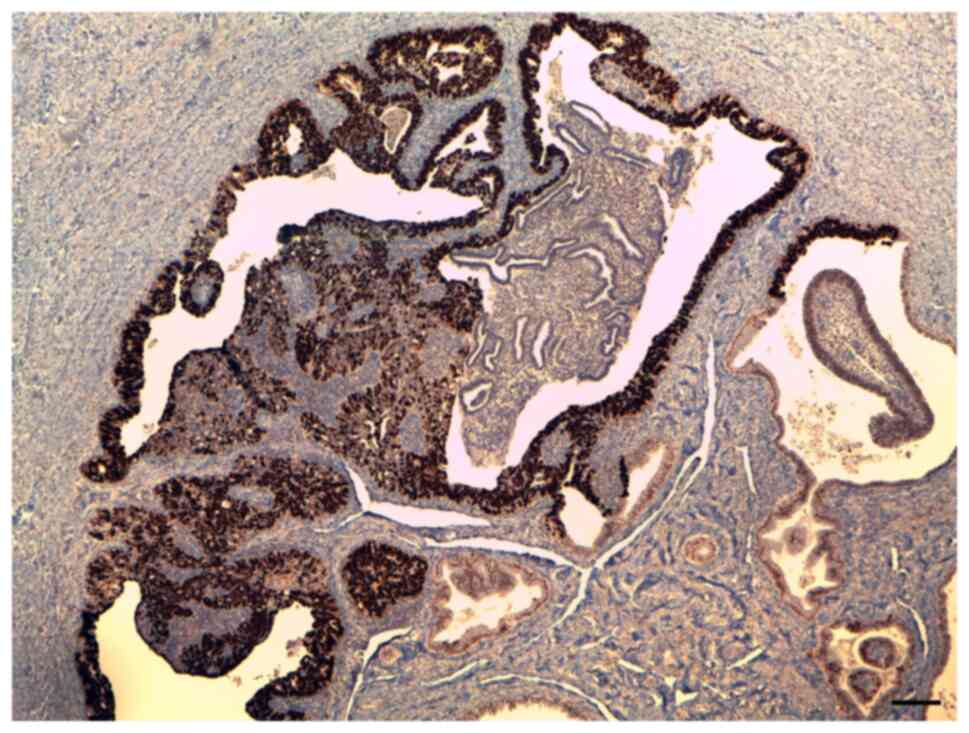
Immunohistochemistry (IHC) demonstrated p53
positivity (Fig. 2) as well as
positive staining for ER and PR receptors. Representative IHC
images are not shown (the official pathology report is available
upon request). Tissue preparation followed the same fixation and
embedding protocol as aforementioned. All IHC procedures were
performed using the BenchMark ULTRA system (Roche Tissue
Diagnostics), strictly following the manufacturer's instructions.
For p53 staining, the CONFIRM anti-p53 (DO-7) Primary Antibody (REF
800–2912, Ventana, Roche) was used together with the OptiView DAB
IHC Detection Kit (REF 760–700, Ventana, Roche). Estrogen receptor
(ER) expression was assessed using the CONFIRM anti-ER (SP1) Rabbit
Monoclonal Primary Antibody (REF 790–4324, Ventana, Roche), while
progesterone receptor (PR) staining was performed with the CONFIRM
anti-PR (1E2) Rabbit Monoclonal Primary Antibody (cat. no.
790-2223, Ventana, Roche). Detection of ER and PR was achieved
using the ultraView Universal DAB Detection Kit (REF 760–500,
Ventana, Roche).
(Immunohistochemistry images of positive estrogen
and progesterone are not presented; however, an official report is
available on request.)
After the diagnosis of Fallopian tube cancer, the
patient was counselled about surgical restaging. Preoperative
work-up included chest-abdomen-pelvis PET/CT that was normal, and a
hysteroscopic endometrial biopsy that was negative for
neoplasia.
Before surgical restaging, the patient was referred
to an oncofertility consultant, considering the young age and the
strong reproductive desire of the patient. The patient underwent
oocyte preservation in July 2020. Anti-Müllerian hormone levels
were 2.08 ng/ml and ovarian stimulation was performed with 5 mg/day
letrozole + recombinant follicle-stimulating hormone (rFSH) from
the second day of the menstrual cycle. On day 5 of rFSH
stimulation, a gonadotropin-releasing hormone (GnRH) antagonist was
administered to prevent a premature luteinizing hormone surge and
discontinued on the day of the trigger, which was achieved using a
GnRH agonist, triptorelin acetate (0.2 mg). After ovarian pick-up,
12 oocytes were cryopreserved.
In September 2020, the patient underwent
laparoscopic surgical restaging with a bilateral ovariectomy,
systematic pelvic lymphadenectomy, total omentectomy, multiple
peritoneal biopsies and peritoneal washing. Histological
examination of all the specimens was negative, and a cytological
examination of peritoneal washing did not show any cells suggestive
of malignancy. The final stage was IC1 (FIGO 2014). Furthermore,
post-surgery genetic counseling and next-generation sequencing
revealed a germinal BRCA-2 mutation in December 2020.
The present case was presented at a gynecological
oncology multidisciplinary meeting, and based on the
histopathological characteristics and tumor stage, two potential
management options were proposed: Adjuvant chemotherapy or close
surveillance. The patient expressed a strong preference against
chemotherapy and opted for a closely monitored gynecological and
breast follow-up approach. This included gynecological evaluation
with pelvic ultrasonography performed every 4 months, in addition
to an annual breast ultrasound examination.
After 3 years of negative clinical and radiological
follow-up, the patient decided to undergo embryo transfer. In July
2023, an oocyte intra cytoplasmatic sperm injection with partner
semen was performed, and a single blastocyst was transferred into
the uterus of the patient with success. Adequate endometrial
preparation was performed with oral administration of estradiol
valerate (4 mg administered on cycle days 1–3, then a dose increase
to 6 mg per day on days 10–13). After ultrasound revealed a regular
endometrium thickness, progesterone was orally administered for
adequate luteal phase support. A total of 11 days after embryo
transfer, a positive serum pregnancy test was achieved. Therefore,
the patient was administered oral estradiol and progesterone until
12 weeks.
Pregnancy was complicated by early fetal growth
restriction, for which the patient underwent amniocentesis. This
was negative with a normal karyotype 46XY at 18 weeks of gestation.
At 30 weeks of gestation, the patient developed preeclampsia and
was hospitalized for close monitoring with ultrasound and
cardiotocography. Due to a worsening of flowmetry, a caesarean
section was performed at 33 weeks and 5 days, with the birth of a
healthy male newborn of 1,152 g and Apgar of 5 and 8 at 1 and 5
min, respectively, an arterial pH of 7.33 and base excess, 5.4. The
baby was hospitalized for 1 month in neonatal intensive care due to
the gestational age and weight. The patient was discharged after 1
week with well-controlled blood pressure without puerperal
complications.
Following delivery, the patient regularly continued
gynecological and breast surveillance, consisting of clinical
evaluation and pelvic ultrasound every 6 months, and an annual
breast ultrasound. The most recent follow up was performed in
February 2025, in which the patient was asymptomatic, the
gynecological examination unremarkable and imaging studies were
within normal limits. The patient also underwent a recent breast
assessment, which was regular. The patient remains on a scheduled
follow up.
Literature review
A narrative review was performed by searching the
PubMed (pubmed.ncbi.nlm.nih.gov/), Scopus (scopus.com/), Web of
Science (https://www.webofscience.com/wos/) and Google Scholar
(https://scholar.google.com/) databases
for articles published between 2000 to November 2024 involving
patients with fallopian tubes cancer and BRCA mutations that
underwent a FP with vitrified oocytes and FSS. Articles not
relevant to the present review or not written in English were
excluded, as well as cases with multiple tumors and those with
patients who underwent ex vivo oocyte retrieval or other
types of preservation techniques.
The following keywords were used for the search:
‘Fallopian tubes Cancer’, ‘BRCA mutation’, ‘Fertility sparing
treatment’ and ‘Fertility preservation’. Different combinations of
the terms were used. Due to the rarity of this pathology and
procedure, numerous case reports or case series of patients with OC
that underwent a fertility sparing treatment with vitrified oocytes
were identified, but no cases of fallopian tube cancer. For this
reason, the data is presented in a descriptive manner with the aim
to delve deeper into the topic through parallels with OC.
A total of 44 articles were identified through the
search of databases and references of the retrieved articles.
However, only two articles reported data on pregnancy with
vitrified oocytes after FSS, and they included 3 cases (15,16).
These articles included a case report on a birth of a healthy
newborn using vitrified-warmed oocytes in a young patient with
invasive mucinous ovarian carcinoma (stage IC) and two cases in two
patients with BOTs. All the pregnancies reached full term with
healthy babies, without disease recurrence during pregnancy and in
the immediate postpartum period (Table
I).
 | Table I.Outcomes and characteristics of
patients in the literature. |
Table I.
Outcomes and characteristics of
patients in the literature.
| First author/s,
year | Age at diagnosis,
years | Site | Histo-type | FIGO 2014
stage | BRCA mutation | Surgical
treatment | Number of
oocytes | ART technique | Number of embryos
transferred | Age at pregnancy,
years | Type of
delivery | Gestational age at
delivery, weeks | Complications | (Refs.) |
|---|
| Alvarez et
al, 2014 | 28 | Ovaries | Invasive mucinous
ovarian carcinoma | IC | / | Bilateral
salpingo-oophorectomy, appendectomy, pelvic and aortic
lymphadenectomy, and-omentectomy | 14 | ICSI | 2 | 29 | Elective Caesarian
section | 38 | Cornual ectopic
pregnancy of 1 embryo after 18 days | (15) |
| Mayeur et
al, 2021 | 36 | Ovaries | Borderline ovarian
cancer | / | / | / | 15 | / | 2 | 37 | / | / | / | (16) |
|
| 26 | Ovaries | Borderline ovarian
cancer | / | / | / | 16 | / | 2 | 27 | / | / | / |
|
| Present case | 32 | Tubes | Endometrioid
G2 | IC1 | Yes | Bilateral
salpingo-oophorectomy, omentectomy, bilateral pelvic
linphade-nectomy, peritoneal biopsy and peritoneal washing | 12 | ICSI | 1 | 34 | Urgent Caesarian
section | 33 + 5 | Early FGR,
preecla-mpsia and flowmetry anomalies | - |
In case 1, the 28-year-old patient underwent two
cycles of ovarian stimulation, and 14 oocytes were vitrified before
FSS. A total of 1 year later, a transfer of two embryos was
performed, and 18 days after the transfer, the patient underwent a
laparotomy as a right cornual ectopic pregnancy in the uterus was
diagnosed. A wedge resection was performed, and an elective
Caesarean section was performed at week 38 of gestation, resulting
in the birth of a healthy male newborn, weighing 2,650 g.
In case 2, the two patients were 36 and 26 years
old, respectively, with BOTs. A total of 15 and 16 oocytes were
retrieved, respectively, and after 14 and 12 months, respectively,
embryo transfer was performed with two embryos. However, in this
article, the course of the pregnancies, the mode of delivery, and
the sex and birth weight of the newborns were not reported.
Discussion
Pregnancy following gynecological cancer is an
option that has only been considered and offered to patients in
recent years (17). For this
reason, very little data is available regarding ART after
gynecological cancer, particularly after FSS for OC.
According to international guidelines on ovarian and
fallopian tube cancer, conservative surgery can be proposed in
selected patients who are strongly motivated to preserve fertility,
and according to the histotype and stage disease (18,19).
FSS is feasible in patients with BOTs, non-epithelial tumors and
low-grade epithelial tumors (serous, endometrioid or mucinous
expansile sub-type) stage IA and selected IC1 stages (20). Moreover, FSS in OC presents an
acceptable overall recurrence rate of ~11%, comparable with
patients treated with radical surgery at initial stages (21). Following surgery, spontaneous
pregnancy seeking is recommended, although there is no established
timing. The spontaneous pregnancy rate following FSS for OC is high
at ~84% (22).
Conversely, data regarding ART in patients with OC
are extremely limited. Adequate evidence does not currently exist
to support the oncologic safety or harmfulness of ovarian
stimulation by pituitary gonadotropins after FSS for OC (23). In particular, an increased rate of
BOTs recurrence after stimulation is reported, ranging from
19.4–27.7%, but without an impact on mortality (24). Nevertheless, ovarian stimulation
after FSS for BOTs could be contraindicated in certain high-risk
cases, such as the presence of peritoneal implants, a
micropapillary pattern, the presence of microinvasion and in cases
of recurrence (25). Moreover,
regarding ART after invasive epithelial OC, there is limited data
from case reports and case series, which provide conflicting
information (26,27).
In patients with cancer, options for preservation of
fertility before gonadotoxic treatments include the following:
Cryopreservation (oocytes or embryos), ovarian transposition (if
pelvic irradiation is planned), pharmacological protection with
GnRH analogous and ovarian tissue cryopreservation (OTC), and
transplantation. OTC or ovarian tissue transposition (OTT) are
options for patients with certain type of cancers (such as
hematological cancers); however, OTT cannot be considered for
patients with fallopian tube cancer (28–30).
Moreover, oocyte/embryo cryopreservation is recommended in
oncological patients if the initiation of chemotherapy can be
delayed. Due to the fewer number of oocytes expected to be
retrieved and limitations associated with preimplantation genetic
diagnosis (31,32), two consecutive stimulations should
be considered for those patients when feasible (31). However, for patients with cancer who
require radical surgery and have limited time available for FP,
controlled ovarian stimulation (COS) is not feasible. In these
cases, immature oocyte collection without COS, followed by in
vitro maturation (IVM) and oocyte freezing, represents an
alternative option. This technique has been used in conjunction
with ovarian tissue freezing. IVM is proposed as an additional
strategy to enhance the effectiveness of ovarian tissue freezing.
As antral follicles are devoid of cancer cell infiltration, oocytes
retrieved from these follicles provide a reliable option for
patients with cancer with a high risk of ovarian involvement or
in situ OC. This method offers a potential strategy for FP
in patients with recurrent disease, without the risk of cancer cell
spillage associated with standard transvaginal oocyte retrieval
(33).
Furthermore, there are issues associated with the
fertility of patients with a BRCA2 mutation. According to certain
studies, BRCA2 mutation-positive patients have lower levels of
anti-Müllerian hormone compared with patients who are BRCA-negative
and BRCA1 mutation-positive (34–37).
This may reflect a diminished oocyte reserve in these patients
(34–37). Among the patients who tried to
conceive, infertility was observed in 30.8% of those who were BRCA2
mutation-positive. Moreover, BRCA mutations result in defective DNA
repair, leading to oocyte damage with apoptotic cell death
(35,36). According to previous research, even
if patients with a BRCA2 mutation have lower levels of
anti-Müllerian hormone, this does not appear to have a negative
impact on reproductive outcome (34). Therefore, this should be considered
during onco-fertility counseling of these patients.
For the patient in the present case, after
multidisciplinary consultation with the oncologist and the
reproductive medicine consultant, adequate oncofertility counseling
was performed. Considering the risk-benefit ratio of FSS, the
low-grade histology of the tumor and the desire of the patient, it
was decided in the present case to perform in vivo oocyte
cryopreservation before restaging surgery. As there were no data
regarding the safety of ovarian preservation in early-stage primary
Fallopian tube carcinoma, the patient underwent bilateral
oophorectomy, preserving the uterus exclusively.
In vitro fertilization for oocyte
cryopreservation requires ovarian stimulation, which results in an
increased estrogen environment. For other hormone-sensitive tumors,
such as breast cancer, the safety of FP has now been established,
and indeed, controlled hormonal stimulation does not impact
progression free survival or and overall survival (38).
COS protocols have been enhanced by the
incorporation of aromatase inhibitors (39,40).
In the present case, letrozole was administered during the
stimulation phase to suppress the aromatization of androgens into
estrogens, thereby reducing circulating estrogen levels. Beyond its
role in limiting estrogen synthesis, letrozole also promotes an
increase in androgen levels, which has been associated with
improved ovarian responsiveness to stimulation (41).
Moreover, in the present case, all ultrasound
examinations performed during ovarian stimulation appeared normal,
with no evidence of cysts or suspicious areas. Before
cryopreservation, a hysteroscopy was performed in consideration of
the endometrial histotype of cancer. Rienzi et al (28) reported that, to achieve a successful
live birth through cryopreserving oocytes, ≥8 oocytes are required
for patients aged <38 years. In the present case, 12 oocytes
were cryopreserved. Additionally, the laparoscopic approach has
been described as a feasible technique by several authors (42–46).
Considering the negative thoracic-abdominal PET-CT scans in the
present case, complete laparoscopic surgical restaging with
bilateral ovariectomy was performed.
Embryo transfer was performed following hormonal
replacement therapy, initiated with oral estradiol (E2) on days 1–3
of the cycle to prepare the endometrium and inhibit spontaneous
follicular development. Estradiol was administered either at a
fixed daily dose (6 mg) or via a step-up regimen. Although no
randomized controlled trials have directly compared these
approaches, a large retrospective analysis of 8,254 oocyte donation
cycles reported similar live birth rates after a fixed daily dose
or after a step-up regimen (33.0 vs. 32.5%, respectively) (47). Furthermore, in the present case,
vaginal micronized progesterone was administrated (200 mg, ×3/day)
for adequate luteal phase support.
Regarding the fetal growth restriction and the
development of early preeclampsia in the present case, these were
complications associated with the BMI of the patient (30
kg/m2), nulliparity and smoking (48), as well as because it was >2 years
since the cancer diagnosis and the patient had not undergone
chemotherapy.
Pregnancies after gynecological cancer need to be
managed by different medical specialties; therefore, it is
desirable to have a close collaboration between consultants with an
expertise in oncology, reproductive medicine and high-risk
pregnancy in referral oncological centers.
The present case is the first case, to the best of
our knowledge, of ART after FSS for a primary Fallopian tube cancer
in a patient with BRCA2 mutation. However, there are certain
reflections to be made: On the one hand, the present report
presents the first case of the feasibility of FP with COS in case
of diagnosis of low-grade primary Fallopian tube cancer at initial
stage; whilst on the other hand, there was a fertility issue
regarding a germline pathogenic variant in the BRCA1/2 gene. In
fact, certain research suggests that the ovarian reserve of
patients with the BRCA1/2 mutation may be reduced (34). Therefore, we hypothesize a
practice-changing strategy: FP could be systematically offered to
healthy patients carrying a BRCA mutation who have not yet
completed the reproductive cycle, and then perform a risk-reducing
bilateral salpingo-oophorectomy after 35 years.
In conclusion, due to the limited reproductive
research in patients with Fallopian tube cancer, evaluating the
safety, efficacy and feasibility of FP, and post-diagnosis
pregnancy, should be considered a research priority. Reporting the
first case in the literature of pregnancy after epithelial primary
Fallopian tube cancer using conservative surgery and oocyte
freezing, to the best of our knowledge, the present report aims to
give impetus to further research in this field. Oocyte
cryopreservation appears to be a feasible method to preserve
fertility in selected cases of OC. FP by oocyte/embryo freezing
employing specific ovarian stimulation protocols could represent a
therapeutic option in young patients with BRCA1/BRCA 2 mutations
who have not completed their reproductive cycle within the 35
years, even if further evidence is needed for elucidation of the
role of BRCA mutations on fertility.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Ministry of Health (Rome,
Italy), in collaboration with the Institute for Maternal and Child
Health IRCCS ‘Burlo Garofolo’ (Trieste, Italy).
Availability of data and materials
The next-generation sequencing data are not publicly
available as they contain information that could compromise the
privacy of research participants; however, they may be requested
from the corresponding author. All other data generated in the
present study may be requested from the corresponding author.
Authors' contributions
GS, SC, AV, GZ and GR conceived and designed the
study; AR and AM performed the experiments. GR, LN and AM performed
the analysis and interpretation of data. GS, AV, AR, GZ, SC and AM
wrote the manuscript. GS, AV, AR, AM, GZ and LN revised the
manuscript. All authors read and approved the final manuscript. GS
and AV confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All procedures performed in the present study were
in accordance with the ethical standards of the institutional
research committee and with the 1964 Helsinki Declaration and its
later amendments or comparable ethical standards. The present study
was approved by the Institutional Review Board of the Institute for
Maternal and Child Heath IRCCS ‘Burlo Garofolo’ (approval no. RC
08/2022; April 15, 2022).
Patient consent for publication
Written informed consent was obtained from the
patient to publish the present paper.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Santos ML, Pais AS and Almeida Santos T:
Fertility preservation in ovarian cancer patients. Gynecol
Endocrinol. 37:483–489. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Agency for Reaserch on
Cancer, Global Cancer Observatory, Cancer Today Globocan 2022, .
Ovary factsheet. https://gco.iarc.who.int/media/globocan/factsheets/cancers/25-ovary-fact-sheet.pdfFebruary
8–2024
|
|
3
|
Taylan E and Oktay K: Fertility
preservation in gynecologic cancers. Gynecol Oncol. 155:522–529.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Comprehensive Cancer Network, .
Ovarian Cancer (Version1.2017). https://www.nccn.org/professionals/phys-ician_gls/pdf/ovarian.pdfApril
15–2024
|
|
5
|
Arcieri M, Tius V, Andreetta C, Restaino
S, Biasioli A, Poletto E, Damante G, Ercoli A, Driul L, Fagotti A,
et al: How BRCA and homologous recombination deficiency change
therapeutic strategies in ovarian cancer: A review of literature.
Front Oncol. 14:13351962024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasmussen CB, Kjaer SK, Albieri V, Bandera
EV, Doherty JA, Høgdall E, Webb PM, Jordan SJ, Rossing MA, Wicklund
KG, et al: Pelvic inflammatory disease and the risk of ovarian
cancer and borderline ovarian tumors: A pooled analysis of 13
Case-control studies. Am J Epidemiol. 185:8–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Seta F, Banco R, Turrisi A, Airoud M,
De Leo R, Stabile G, Ceccarello M, Restaino S and De Santo D:
Pelvic inflammatory disease (PID) from Chlamydia trachomatis versus
PID from Neisseria gonorrhea: From clinical suspicion to therapy. G
Ital Dermatol Venereol. 147:423–430. 2012.PubMed/NCBI
|
|
8
|
Barnard ME, Farland LV, Yan B, Wang J,
Trabert B, Doherty JA, Meeks HD, Madsen M, Guinto E, Collin LJ, et
al: Endometriosis typology and ovarian cancer risk. JAMA.
332:482–489. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SY and Lee JR: Fertility preservation
option in young women with ovarian cancer. Future Oncol.
12:1695–1698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavoue V, Huchon C, Akladios C, Alfonsi P,
Bakrin N, Ballester M, Bendifallah S, Bolze PA, Bonnet F, Bourgin
C, et al: Management of epithelial cancer of the ovary, fallopian
tube, primary peritoneum. Long text of the joint French clinical
practice guidelines issued by FRANCOGYN, CNGOF, SFOG,
GINECO-ARCAGY, endorsed by INCa. (Part 2: Systemic, intraperitoneal
treatment, elderly patients, fertility preservation, follow-up). J
Gynecol Obstet Hum Reprod. 48:379–386. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mangili G, Somigliana E, Giorgione V,
Martinelli F, Filippi F, Petrella MC, Candiani M and Peccatori F:
Fertility preservation in women with borderline ovarian tumours.
Cancer Treat Rev. 49:13–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsonis O and Kopeika J: Fertility
preservation in patients with gynaecologic malignancy: Response to
ovarian stimulation and long-term outcomes. Eur J Obstet Gynecol
Reprod Biol. 290:93–100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akel RA, Guo XM, Moravek MB, Confino R,
Smith KN, Lawson AK, Klock SC, Tanner Iii EJ and Pavone ME: Ovarian
stimulation is safe and effective for patients with gynecologic
cancer. J Adolesc Young Adult Oncol. 9:367–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
15
|
Alvarez M, Solé M, Devesa M, Fábregas R,
Boada M, Tur R, Coroleu B, Veiga A and Barri PN: Live birth using
vitrified-warmed oocytes in invasive ovarian cancer: Case report
and literature review. Reprod Biomed Online. 28:663–668. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mayeur A, Puy V, Windal V, Hesters L,
Gallot V, Benoit A, Grynberg M, Sonigo C and Frydman N: Live birth
rate after use of cryopreserved oocytes or embryos at the time of
cancer diagnosis in female survivors: A retrospective study of ten
years of experience. J Assist Reprod Genet. 38:1767–1775. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitecki R, Clapp MA, Fu S, Lamiman K,
Melamed A, Brady PC, Kaimal A, Del Carmen MG, Woodard TL, Meyer LA,
et al: Outcomes of the first pregnancy after Fertility-sparing
surgery for Early-stage ovarian cancer. Obstet Gynecol.
137:1109–1118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ledermann JA, Matias-Guiu X, Amant F,
Concin N, Davidson B, Fotopoulou C, González-Martin A, Gourley C,
Leary A, Lorusso D, et al: ESGO-ESMO-ESP consensus conference
recommendations on ovarian cancer: Pathology and molecular biology
and early, advanced and recurrent disease. Ann Oncol. 35:248–266.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
NCCN Guidelines Version 1.2024, . Ovarian
Cancer/Fallopian Tube Cancer/Primary Perit. PubMed/NCBI
|
|
20
|
Armstrong DK, Alvarez RD, Backes FJ,
Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM,
Chitiyo VC, Cristea M, et al: NCCN Guidelines® Insights:
Ovarian cancer, version 3.2022. J Natl Compr Canc Netw. 20:972–980.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bentivegna E, Gouy S, Maulard A, Pautier
P, Leary A, Colombo N and Morice P: Fertility-sparing surgery in
epithelial ovarian cancer: A systematic review of oncological
issues. Ann Oncol. 27:1994–2004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zapardiel I, Cruz M, Diestro MD, Requena A
and Garcia-Velasco JA: Assisted reproductive techniques after
fertility-sparing treatments in gynaecological cancers. Hum Reprod
Update. 22:281–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomao F, Di Pinto A, Sassu CM, Bardhi E,
Di Donato V, Muzii L, Petrella MC, Peccatori FA and Panici PB:
Fertility preservation in ovarian tumours. Ecancermedicalscience.
12:88520185PubMed/NCBI
|
|
24
|
Daraï E, Fauvet R, Uzan C, Gouy S,
Duvillard P and Morice P: Fertility and borderline ovarian tumor: A
systematic review of conservative management, risk of recurrence
and alternative options. Hum Reprod Update. 19:151–166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poulain M, Vandame J, Tran C, Koutchinsky
S, Pirtea P and Ayoubi JM: Fertility preservation in borderline
ovarian tumor patients and survivors. Horm Mol Biol Clin Investig.
43:179–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fortin A, Hazout A, Thoury A, Alvès K,
Bats AS, Dhainaut C and Madelenat P: Assistance médicale à la
procréation après traitement conservateur d'une tumeur de l'ovaire
invasive ou à la limite de la malignité Assisted reproductive
technologies after conservative management of borderline or
invasive ovarian tumours. Gynecol Obstet Fertil. 33:488–4897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zaami S, Melcarne R, Patrone R, Gullo G,
Negro F, Napoletano G, Monti M, Aceti V, Panarese A, Borcea MC, et
al: Oncofertility and reproductive counseling in patients with
breast cancer: A retrospective study. J Clin Med. 11:13112022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rienzi L, Cobo A, Paffoni A, Scarduelli C,
Capalbo A, Vajta G, Remohí J, Ragni G and Ubaldi FM: Consistent and
predictable delivery rates after oocyte vitrification: An
observational longitudinal cohort multicentric study. Hum Reprod.
27:1606–1612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
SHRE Guideline Group on Female Fertility
Preservation, . Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de
Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, et al:
ESHRE guideline: Female fertility preservation. Hum Reprod Open.
2020:hoaa0522020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gullo G, Perino A and Cucinella G: Open vs
closed vitrification system: Which one is safer? Eur Rev Med
Pharmacol Sci. 26:1065–1067. 2022.PubMed/NCBI
|
|
31
|
Peccatori FA, Mangili G, Bergamini A,
Filippi F, Martinelli F, Ferrari F, Noli S, Rabaiotti E, Candiani M
and Somigliana E: Fertility preservation in women harboring
deleterious BRCA mutations: Ready for prime time? Hum Reprod.
33:181–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gullo G, Basile G, Cucinella G, Greco ME,
Perino A, Chiantera V and Marinelli S: Fresh vs. frozen embryo
transfer in assisted reproductive techniques: A single center
retrospective cohort study and Ethical-legal implications. Eur Rev
Med Pharmacol Sci. 27:6809–6823. 2023.PubMed/NCBI
|
|
33
|
Park CW, Lee SH, Yang KM, Lee IH, Lim KT,
Lee KH and Kim TJ: Cryopreservation of in vitro matured oocytes
after ex vivo oocyte retrieval from gynecologic cancer patients
undergoing radical surgery. Clin Exp Reprod Med. 43:119–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponce J, Fernandez-Gonzalez S, Calvo I,
Climent M, Peñafiel J, Feliubadaló L, Teulé A, Lázaro C, Brunet JM,
Candás-Estébanez B and Durán Retamal M: Assessment of ovarian
reserve and reproductive outcomes in BRCA1 or BRCA2 mutation
carriers. Int J Gynecol Cancer. 30:83–88. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oktay K, Kim JY, Barad D and Babayev SN:
Association of BRCA1 mutations with occult primary ovarian
insufficiency: A possible explanation for the link between
infertility and breast/ovarian cancer risks. J Clin Oncol.
28:240–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oktay K, Turan V, Titus S, Stobezki R and
Liu L: BRCA mutations, DNA repair deficiency, and ovarian aging.
Biol Reprod. 93:672015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang ET, Pisarska MD, Bresee C, Chen YD,
Lester J, Afshar Y, Alexander C and Karlan BY: BRCA1 germline
mutations may be associated with reduced ovarian reserve. Fertil
Steril. 102:1723–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kufel-Grabowska J, Podolak A, Maliszewski
D, Bartoszkiewicz M, Ramlau R and Lukaszuk K: Fertility counseling
in BRCA1/2-mutated women with breast cancer and healthy
individuals. J Clin Med. 11:39962022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marklund A, Lekberg T, Hedayati E,
Liljegren A, Bergh J, Lundberg FE and Rodriguez-Wallberg KA:
Relapse rates and Disease-specific mortality following procedures
for fertility preservation at time of breast cancer diagnosis. JAMA
Oncol. 8:1438–1446. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim J, Turan V and Oktay K: Long-term
safety of letrozole and gonadotropin stimulation for fertility
preservation in women with breast cancer. J Clin Endocrinol Metab.
101:1364–1371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oktay K, Buyuk E, Libertella N, Akar M and
Rosenwaks Z: Fertility preservation in breast cancer patients: A
prospective controlled comparison of ovarian stimulation with
tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol.
23:4347–4353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dias Nunes J, Demeestere I and Devos M:
BRCA mutations and fertility preservation. Int J Mol Sci.
25:2042023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tomao F, Peccatori F, Del Pup L, Franchi
D, Zanagnolo V, Panici PB and Colombo N: Special issues in
fertility preservation for gynecologic malignancies. Crit Rev Oncol
Hematol. 97:206–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martinez A, Poilblanc M, Ferron G, De
Cuypere M, Jouve E and Querleu D: Fertility-preserving surgical
procedures, techniques. Best Pract Res Clin Obstet Gynaecol.
26:407–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bruno M, Ludovisi M, Ronsini C, Capanna G,
Stabile G and Guido M: Tertiary cytoreduction for isolated
lymphnode recurrence (ILNR) ovarian cancer in a BRCA2 mutated
patient: Our experience and prevalence of BRCA 1 or 2 genes
mutational status in ILNR. Medicina (Kaunas). 59:6062023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Restaino S, Finelli A, Pellecchia G,
Biasioli A, Mauro J, Ronsini C, Martina MD, Arcieri M, Della Corte
L, Sorrentino F, et al: Scar-free laparoscopy in BRCA-mutated
women. Medicina (Kaunas). 58:9432022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Madero S, Rodriguez A, Vassena R and
Vernaeve V: Endometrial preparation: Effect of estrogen dose and
administration route on reproductive outcomes in oocyte donation
cycles with fresh embryo transfer. Hum Reprod. 31:1755–1764. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bartsch E, Medcalf KE, Park AL and Ray JG:
Clinical risk factors for pre-eclampsia determined in early
pregnancy: Systematic review and Meta-analysis of large cohort
studies. BMJ. 353:i17532016. View Article : Google Scholar : PubMed/NCBI
|