Introduction
Lymphomas can originate in almost all tissues and
organs, and may subsequently infiltrate peripheral tissues, distant
tissues and bone marrow (BM) (1,2).
Primary BM lymphoma (PBM) is a rare entity defined by lymphoma
confined to the BM without evidence of nodal or extranodal
involvement at diagnosis. Diffuse large B-cell lymphoma (DLBCL) is
the most common histological subtype of PBM, yet it accounts for
<1% of all DLBCL cases and is typically associated with an
aggressive clinical course and poor prognosis (3). Patients often present with
constitutional symptoms (fever, night sweats and weight loss) and
peripheral blood cytopenias, which can mimic a wide spectrum of
benign and malignant hematological diseases, frequently resulting
in diagnostic delay or error (4).
Diagnosis requires strict exclusion of systemic involvement (no
lymph node/organ enlargement confirmed by physical examination and
imaging) and is easily misdiagnosed as lymphoma of secondary BM
involvement. Peripheral blood lymphocyte counts and morphology are
usually unremarkable (5).
Myelofibrosis (MF) is a clonal myeloproliferative
neoplasm (MPN) of the BM caused by an abnormal clone of
hematopoietic stem cells (6). MF
can be classified into primary MF (PMF), a Philadelphia chromosome
(Ph)-negative MPN, and secondary MF, which refers to reactive MF
induced by a variety of underlying diseases or conditions. Accurate
differentiation between the two is critical for determining
appropriate therapeutic strategies and requires an integrated
assessment of clinical presentation, laboratory findings, BM
histopathology and molecular genetics (7). The pathophysiological association
between DLBCL and MF is considered to involve cytokine-mediated
activation of fibrotic pathways (for example, JAK/STAT and SMAD).
Profibrotic factors such as transforming growth factor-β (TGF-β)
and platelet-derived growth factor (PDGF), secreted by lymphoma
cells, lead to collagen deposition and subsequent extramedullary
hematopoiesis. Clinically, cytopenias, hepatosplenomegaly and
systemic symptoms predominate, with a median survival time of ~6
years (8).
The present study reports a rare and diagnostically
challenging case of primary BM-CLBCL (PBM-DLBCL) that was initially
misdiagnosed as a T-cell lymphoma with associated MF based on
initial BM histopathology and clinical presentation. The diagnosis
of PBM-DLBCL was established following two BM aspirates and
biopsies. The diagnosis and therapeutic timeline over the course of
the patient's two hospitalizations is summarized in Table I. The present case illustrates the
potential diagnostic pitfalls and emphasizes the necessity of
repeated and comprehensive BM histopathological assessment in
patients with persistent cytopenias.
 | Table I.Timeline of key clinical events,
interventions, and diagnostic findings. |
Table I.
Timeline of key clinical events,
interventions, and diagnostic findings.
| Date |
Event/intervention | Key diagnostic
findings/outcome |
|---|
| 2023-04 | Initial
admission | Presented with fever,
malaise. Found to have severe pancytopenia (hemoglobin, 52
g/l). |
|
| First bone marrow
biopsy | Flow cytometry:
79.54% T-cells (CD4:CD8=1.70), monoclonal TCR-γ rearrangement. |
|
|
| Biopsy: Collagen
fiber proliferation, multifocal lymphocytic infiltrates (30%
CD3+). |
|
|
| Suspected T-cell
lymphoma with MF. |
| 2023-04 to 06 | Cyclosporine
therapy | No significant
improvement in blood counts or symptoms. |
| 2023-06 | Methylprednisolone
therapy | Started 16 mg/day. No
response observed. |
| 2024-03 | Second admission | Worsening
pancytopenia (hemoglobin, 35 g/l) and fever. |
|
| Second bone marrow
biopsy | Flow cytometry: No
abnormal clone detected. |
|
|
| Biopsy and IHC:
CD20+, CD79a+ large B-cells (Ki-67
30%+). |
|
|
| Diagnosis: Primary
bone marrow diffuse large B-cell lymphoma with secondary MF. |
|
| Post-diagnosis | Immunochemotherapy
recommended but refused by the patient. and family |
| 2024-06 (Manuscript
preparation) | Outcome | Lost to follow-up.
Final outcome unknown. |
Case report
Patient presentation
A 70-year-old man with no significant past medical
history was admitted to the Department of Hematology of The First
Affiliated Hospital of Jishou University (Jishou, China) in April
2023, presenting with a 2-day history of fever and profound
malaise. Physical examination revealed marked pallor, without
evidence of lymphadenopathy, hepatosplenomegaly or hemorrhagic
manifestations.
Initial laboratory investigations demonstrated
severe pancytopenia, with a white blood cell count of
2.36×109/l (normal range, 4–10×109/l),
hemoglobin count of 52 g/l (normal range, 120–160 g/l) and
platelets count of 37×109/l (normal range,
100–400×109/l). C-reactive protein was markedly elevated
at 137.15 mg/l (normal range, 0–8 mg/l). Iron studies, vitamin B12,
folate levels and immunoglobulin profiles were within normal
limits. Lactate dehydrogenase (LDH) was elevated at 450 U/l,
exceeding the reference range of 120–250 U/l. Serological tests for
common viruses, including HBV, HCV, HIV and EBV, were negative.
Contrast-enhanced computed tomography (CT) of the chest and abdomen
revealed no lymphadenopathy or organomegaly. A positron emission
tomography (PET)-CT scan was not performed due to the patient's
clinical status and logistical constraints.
BM aspirate and biopsy were performed. The aspirate
demonstrated an elevated lymphocyte ratio (40%). Flow cytometric
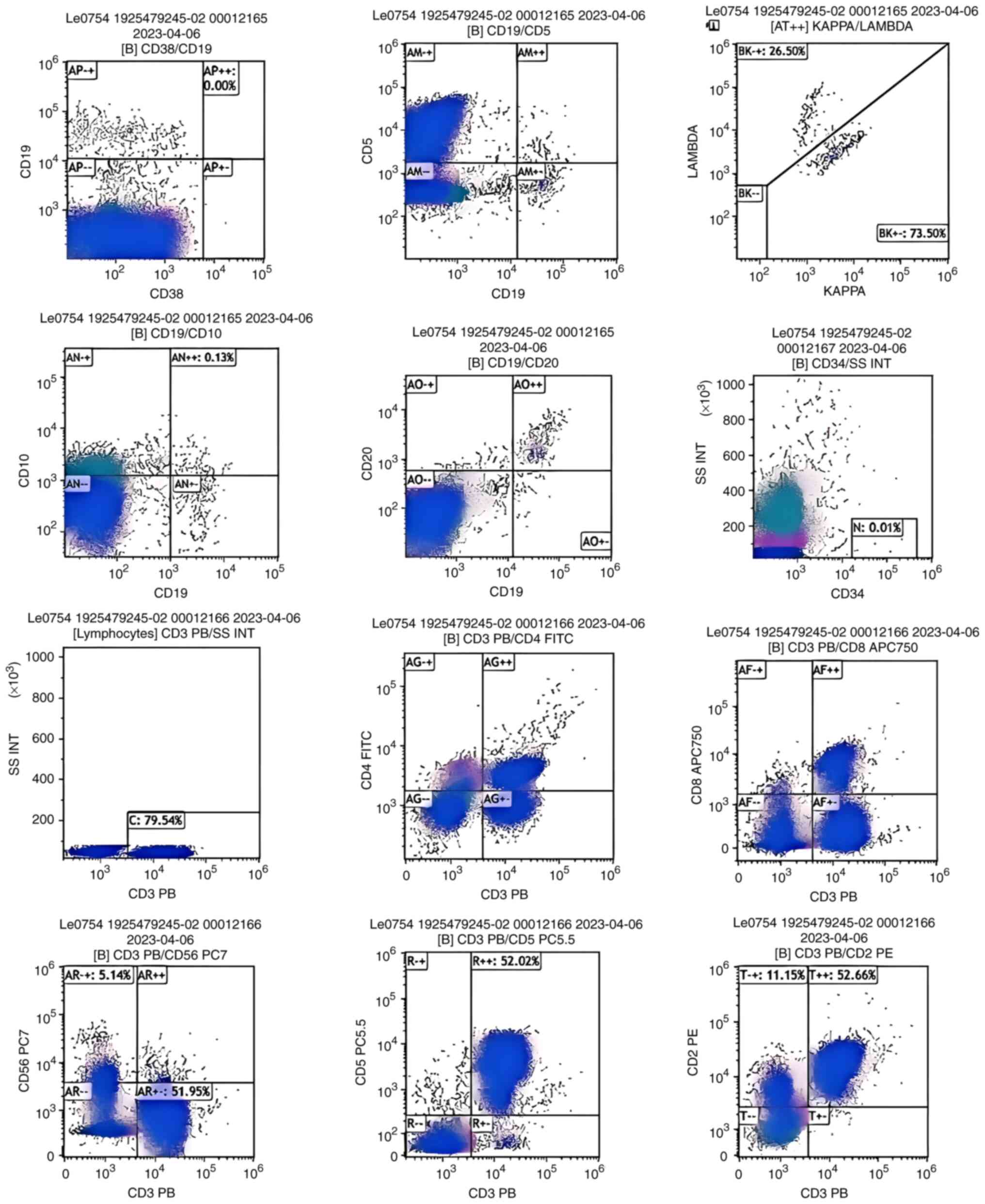
analysis (Fig. 1) revealed a
predominant T-cell population (79.54%; CD4:CD8 ratio of 1.70) with
monoclonal T-cell receptor (TCR)-γ gene rearrangement.
Immunoglobulin heavy chain gene rearrangement was not assessed in
the initial sample. No aberrant B-cell or NK-cell populations were
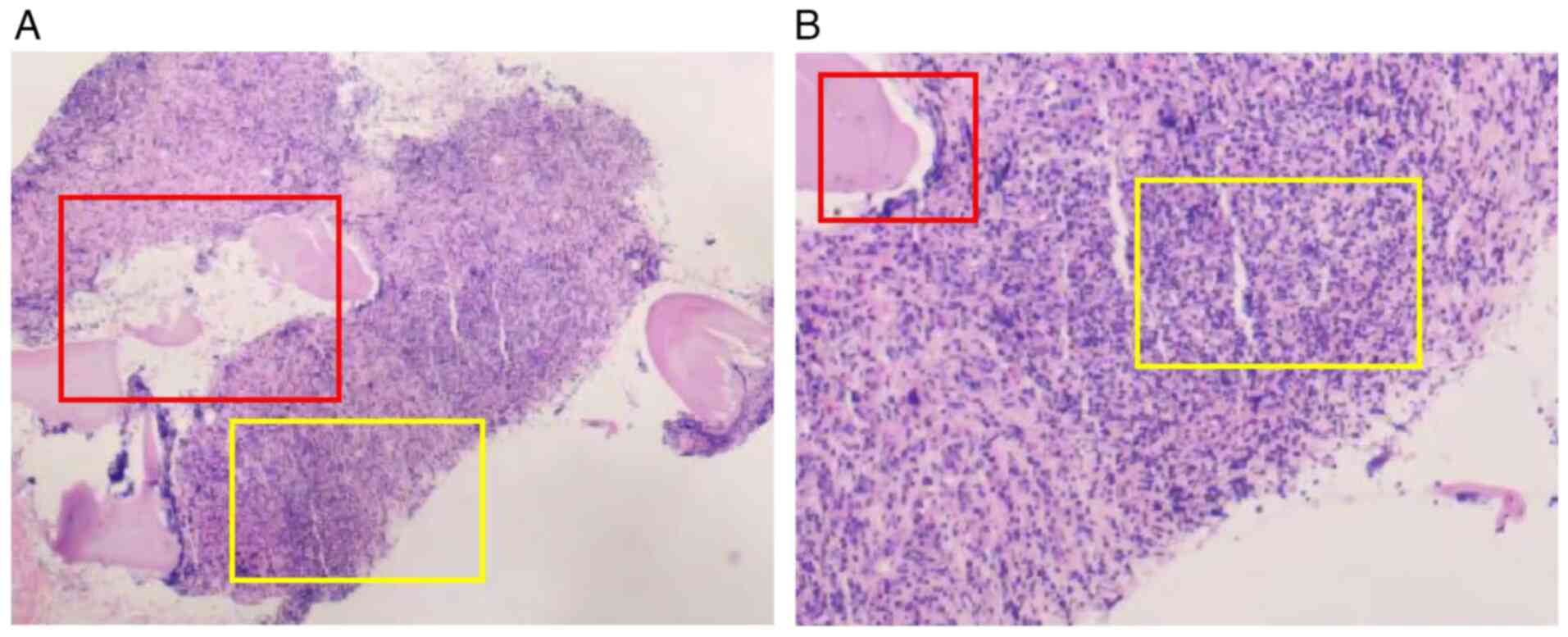
detected. The core biopsy (Fig. 2)
revealed a hypercellular marrow with marked collagen fibers
proliferation, multifocal lymphocytic aggregates (reticulin
staining confirmed persistent grade 2 MF), and megakaryocytes
without significant atypia. Immunohistochemistry (IHC) revealed
focal CD3 positivity (~30% of nucleated cells). The initial
integrated interpretation favored a T-cell lymphoproliferative
disorder complicated by secondary MF.
The initial administration of cyclosporine (50 mg
twice daily) represented an empirical therapeutic trial, aligned
with the Chinese Society of Clinical Oncology (CSCO) diagnosis and
treatment guidelines for malignant lymphoma 2021 (English version)
(9). This strategy aimed to
suppress a potential aberrant T-cell clone or immune-mediated
marrow suppression prior to establishing a definitive diagnosis.
Immunosuppressive therapy is a considered option under such
circumstances per guidelines including those from CSCO. This was
not a bypass of standard lymphoma treatment regimens but rather a
supportive care attempt aimed at the most life-threatening issue,
pancytopenia, at a time when a definitive lymphoma diagnosis had
not been established. However, after several months of treatments,
the patient's cytopenias and clinical symptoms showed no
improvement. A subsequent empirical course of methylprednisolone
(16 mg daily) initiated in June 2023, was also ineffective.
In March 2024, the patient was readmitted presenting
with worsening pancytopenia (hemoglobin, 35 g/l) accompanied by
recurrent fever. Repeat imaging with CT and ultrasonography again
revealed no evidence of lymphadenopathy or splenomegaly. A second
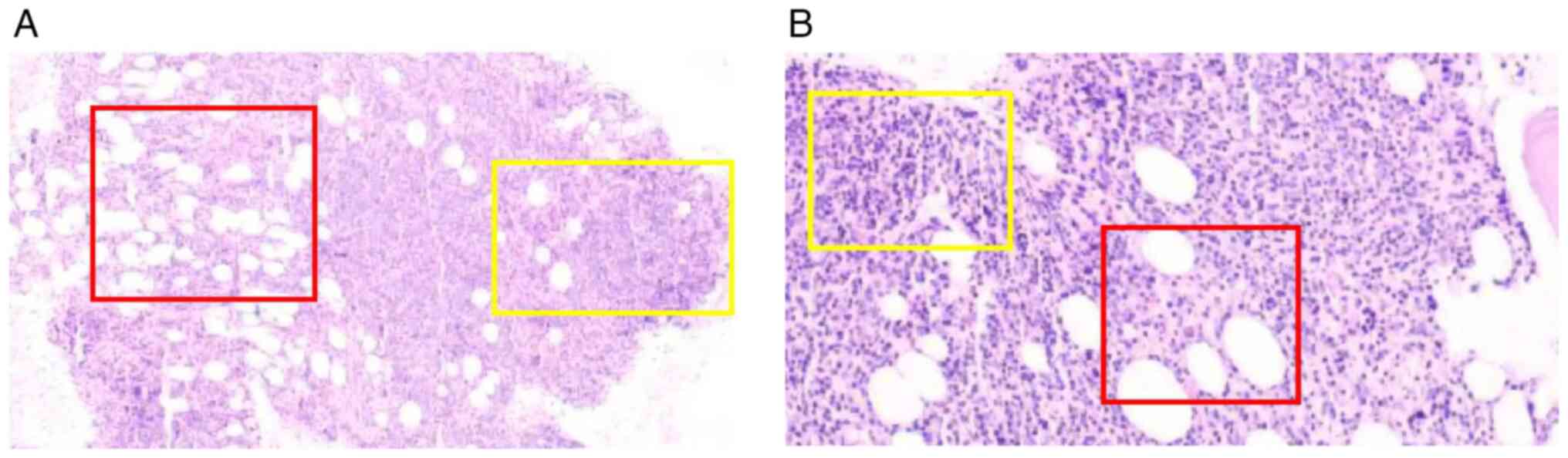
BM biopsy proved decisive. The aspirate (Fig. 3) demonstrated a marked lymphocytosis
(74% total lymphocytes; 30.02% T-cells; and 43.96% NK-cells). Flow
cytometry failed to detect the previously identified clonal T-cell
population, or any aberrant B-cell clone. However, the core biopsy
revealed a diffuse infiltrate of large lymphoid cells. IHC
confirmed B-cell lineage, with strong CD20 and CD79a expression,
and a Ki-67 proliferation index of ~30%. CD3 highlighted a residual
background population of T-cells. Reticulin staining confirmed
persistent grade 2 MF. Collectively, these findings established a
diagnosis of DLBCL. A definitive diagnosis of PBM-DLBCL with
secondary MF was established, in accordance with the World Health
Organization classification of hematolymphoid tumors (2022)
(10), owing to the absence of
extramedullary involvement.
The patient and their family declined the
recommended immunochemotherapy (R-CHOP regimen) and further
diagnostic tests, including molecular testing for JAK2, CALR and
MPL mutations or FISH for MYC/BCL2 rearrangements. The patient was
subsequently lost to follow-up, and the ultimate outcome remains
unknown.
Methods
Flow cytometric analysis
Immunophenotyping of bone marrow samples was
performed using multicolor flow cytometry. A comprehensive antibody
panel targeting lineage-associated antigens for lymphoid and
myeloid leukemias/lymphomas was employed for surface and/or
intracellular staining. Briefly, the staining protocol was as
follows: In total, ~1×106 cells were incubated with
pre-titrated antibody cocktails at room temperature for 15–20 min
in the dark. Subsequently, red blood cells were lysed using a
lysing solution, followed by two washes with phosphate-buffered
saline. The cells were then resuspended in fixation buffer prior to
acquisition.
Data acquisition was conducted on a BD FACSymphony
A5 flow cytometer (BD Biosciences). The acquired data were analyzed
using FlowJo™ software (version 10.8; BD Biosciences).
For data analysis, target lymphocyte populations and
other nucleated cell subsets were precisely gated based on forward
scatter and side scatter properties, combined with CD45 expression.
To eliminate spectral overlap in multicolor detection, fluorescence
compensation was applied using single-stained compensation beads.
Positive thresholds for specific antibody expression were
determined using isotype controls and/or fluorescence-minus-one
controls.
The antigens investigated, along with their
corresponding fluorochromes, catalog numbers and suppliers, are
detailed in Table II. All
antibodies were purchased from BD Biosciences and used according to
the manufacturer's recommended concentrations.
 | Table II.Flow cytometric analytes and
fluorochromes. |
Table II.
Flow cytometric analytes and
fluorochromes.
| Antigen | Fluorochrome | Clone | Catalog number | Supplier |
|---|
| CD45 | V500 | HI30 | 560777 | BD Biosciences |
| CD45 | PerCP-Cy5.5 | HI30 | 560777 | BD Biosciences |
| CD45 | APC-H7 | 2D1 | 560178 | BD Biosciences |
| CD3 | FITC | UCHT1 | 561806 | BD Biosciences |
| CD3 | PerCP-Cy5.5 | SK7 | 340916 | BD Biosciences |
| CD4 | FITC | SK3 | 340133 | BD Biosciences |
| CD8 | APC | SK1 | 561953 | BD Biosciences |
| CD19 | PE | HIB19 | 561741 | BD Biosciences |
| CD19 | V450 | HIB19 | 561297 | BD Biosciences |
| CD20 | PE-Cy7 | L27 | 560734 | BD Biosciences |
| CD5 | APC | L17F12 | 561896 | BD Biosciences |
| CD5 | PE-Cy7 | UCHT2 | 561899 | BD Biosciences |
| CD7 | PE | M-T701 | 561603 | BD Biosciences |
| CD2 | APC | RPA-2.10 | 561765 | BD Biosciences |
| CD10 | APC | HI10a | 561003 | BD Biosciences |
| CD38 | Brilliant Violet
711 | HB-7 | 563438 | BD Biosciences |
| CD56 | PE | NCAM16.2 | 562751 | BD Biosciences |
| Kappa | FITC | TB28-2 | 556867 | BD Biosciences |
| Lambda | PE | 1-155-2 | 556874 | BD Biosciences |
| CD34 | V450 | 581 | 561204 | BD Biosciences |
| CD117 | PE | 104D2 | 561199 | BD Biosciences |
| HLA-DR | PerCP-Cy5.5 | L243 | 552764 | BD Biosciences |
| CD33 | PE | P67.6 | 561816 | BD Biosciences |
| CD71 | APC | M-A712 | 561775 | BD Biosciences |
| CD41 | PE | HIP8 | 561433 | BD Biosciences |
Histological staining protocol
Bone marrow biopsy specimens were fixed in 10%
neutral buffered formalin at room temperature (22–25°C) for 12–24
h. Following fixation, tissues were processed through standard
dehydration, clearing and paraffin embedding procedures.
Consecutive sections were cut at a 3-µm thickness using a rotary
microtome and mounted on charged glass slides.
For hematoxylin and eosin staining, deparaffinized
and rehydrated sections were stained with hematoxylin solution
(room temperature, 8 min), blued in running tap water and
counterstained with eosin ethanol solution (room temperature, 3
min). Sections were then dehydrated through graded ethanol, cleared
in xylene and mounted with neutral balsam.
Reticular fiber staining was performed using
Gomori's methenamine silver method. The procedure included
treatment of deparaffinized sections with 0.5% periodic acid (room
temperature, 10 min) for oxidation, followed by impregnation in
freshly prepared methenamine silver working solution (room
temperature, 20–30 min). Subsequent steps involved toning with 0.2%
gold chloride and nuclear counterstaining with nuclear fast
red.
All staining procedures were conducted at room
temperature following standard protocols, with detailed information
on staining reagents provided in Table III. All sections were examined,
evaluated and imaged using an Olympus BX43 light microscope
(Olympus Corporation).
 | Table III.Staining reagents and conditions for
bone marrow biopsy. |
Table III.
Staining reagents and conditions for
bone marrow biopsy.
| A,
Immunohistochemistry |
|---|
|
|---|
| Target/stain | Supplier | Catalog no. | Dilution | Incubation
temperature, °C | Incubation
duration, min | Conjugate |
|---|
| Primary
antibodies |
|
|
|
|
|
|
|
CD10 | Jiangsu Calt
Biotechnology | CTB062V6 | 1:50 | 37 | 30 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
CD20 | Fuzhou Maixin
Biotechnology | Kit0001 | 1:200 | 37 | 30 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
CD23 | Fuzhou Maixin
Biotechnology | RMA0504 | 1:100 | 37 | 30 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
CD3 | Fuzhou Maixin
Biotechnology | MAB0740 | 1:100 | 37 | 30 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
CD34 | Zhongshan
Aoquan | NCL-L-END | 1:150 | 37 | 30 | / |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD5 | Zhongshan
Aoquan | RTU-CD5- | 1:150 | 37 | 30 | / |
|
| Biotechnology
Development | 4C7-QH |
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD56 | Zhongshan
Medical | ZM0057 | 1:150 | 37 | 30 | / |
|
| Technology
Development |
|
|
|
|
|
|
| (Guangzhou) Co.,
Ltd. |
|
|
|
|
|
|
CD61 | Zhongshan
Aoquan | NCL-L-CD61 | 1:100 | 37 | 30 | / |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD79a | Zhongshan
Medical | ZA0293 | 1:150 | 37 | 30 | / |
|
| Technology
Development |
|
|
|
|
|
|
| (Guangzhou) Co.,
Ltd. |
|
|
|
|
|
|
E-cad | Zhongshan
Aoquan | RTU-ECAD- | 1:100 | 37 | 30 | / |
|
| Biotechnology
Development | QH |
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
Ki-67 | Jiangsu Calt
Biotechnology | CTB004V6 | 1:150 | 37 | 30 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
Cyclin-D1 | Jiangsu Calt
Biotechnology | CTB026V6 | 1:100 | 32 | 35 | / |
|
| Development Co.,
Ltd. |
|
|
|
|
|
| Secondary
antibodies |
|
|
|
|
|
|
|
CD10 | Zhongshan
Aoquan | 7600 | 1:200 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD20 | Zhongshan
Aoquan | 7600 | 1:1,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD23 | Zhongshan
Aoquan | 7600 | 1:500 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD3 | Zhongshan
Aoquan | 7600 | 1:500 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD34 | Zhongshan
Aoquan | 7600 | 1:1,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD5 | Zhongshan
Aoquan | 7600 | 1:1,500 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD56 | Zhongshan
Aoquan | 7600 | 1:8,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD61 | Zhongshan
Aoquan | 7600 | 1:1,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
CD79a | Zhongshan
Aoquan | 7600 | 1:1,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
E-cad | Zhongshan
Aoquan | 7600 | 1:5,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
Ki-67 | Zhongshan
Aoquan | 7600 | 1:4,000 | 37 | 15 | DAB |
|
| Biotechnology
Development |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
|
Cyclin-D1 | Jiangsu Calt
Biotechnology | CTBK11-1 | 1:750 | 32 | 12 | DAB |
|
| Development Co.,
Ltd. |
|
|
|
|
|
|
| B, Hematoxylin
and eosin staining |
|
|
Target/stain |
Supplier | Catalog
no. |
Dilution | Incubation
temperature, °C | Incubation
duration, min |
Conjugate |
|
| Hematoxylin | Zhuhai Besso
Biotechnology | BA-4097 | RTU | 22-25 | 8-10 | / |
|
| Co., Ltd |
|
|
|
|
|
| Eosin | Zhuhai Besso
Biotechnology | BA-4098 | RTU | 22-25 | 1-3 | / |
|
| Co., Ltd |
|
|
|
|
|
|
| C, Reticular
fiber staining |
|
|
Target/stain |
Supplier | Catalog
no. |
Dilution | Incubation
temperature, °C | Incubation
duration, min |
Conjugate |
|
| Periodic acid | Sinopharm Chemical
Reagent | 10024118 | 0.5% (aq) | 22-25 | 10 | / |
|
| Co., Ltd. |
|
|
|
|
|
| GMS | Shenzhen
DAKEWE | D02013 | RTU | 22-25 | 20-30 | MS |
|
| Bio-engineering
Co., Ltd. |
|
|
|
|
|
| Gold chloride | Shanghai
Aladdin | G112803 | 0.2% (aq) | 22-25 | 22-25 | / |
|
| Biochemical
Technology |
|
|
|
|
|
|
| Co., Ltd. |
|
|
|
|
|
| Nuclear fast
red | Shanghai
Macklin | R817461 | 0.1% (aq) | 22-25 | 5 | / |
|
| Biochemical Co.,
Ltd. |
|
|
|
|
|
IHC staining protocol
Bone marrow biopsy specimens were fixed in 10%
neutral buffered formalin at room temperature for 40 min, followed
by routine dehydration and clearing before embedding in paraffin.
Tissue blocks were sectioned at a 3-µm thickness and mounted on
glass slides. After deparaffinization and rehydration, endogenous
peroxidase activity was blocked by incubation with 3% hydrogen
peroxide at room temperature for 8 min. The antibodies used in this
study and their corresponding specifications are listed in Table III. Sections were subsequently
incubated with primary antibodies. Following thorough washing, the
sections were treated with appropriate secondary antibody detection
systems. Diaminobenzidine was employed as the chromogenic
substrate, followed by counterstaining with hematoxylin,
dehydration, clearing and final mounting. All stained sections were
examined under an Olympus BX43 light microscope, with
representative images captured at ×40 and ×100 magnification.
Discussion
The present case report illustrated the substantial
diagnostic challenges posed by PBM-DLBCL. The initial presentation
characterized by pancytopenia and BM fibrosis and the presence of a
clonal T-cell population, initially suggested a T-cell
lymphoproliferative disease. The second biopsy revealed a
proliferation of medium-to-large lymphocytes that had not been
detected in the initial fibrotic sample. Crucially, IHC
demonstrated that these large cells were strongly positive for CD20
and CD79a (B-cell markers), with an elevated Ki-67 index,
confirming a dominant monoclonal B-cell process. T-cell populations
were present but merely constituted a background component. The
markedly different finding on the second BM biopsy underscores the
pivotal role of repeat marrow evaluation in patients with
refractory cytopenia or a progressive clinical course.
The initial clonal T-cell population represented an
enigmatic finding. Its absence on the second BM evaluation suggests
it may have reflected an incidental clonal T-cell expansion of
uncertain clinical significance or a reactive process that was
subsequently overtaken by the more aggressive DLBCL clone. Although
the presence of a true composite lymphoma is conceivable, it
appears less likely. Comparative TCR-γ gene rearrangement analysis
or more comprehensive molecular profiling on the second sample were
not performed due to patient refusal, leaving this question
unresolved.
The patient received supportive care and regular
monitoring in an outpatient setting. Following the initial biopsy,
the working diagnosis was a T-cell lymphoproliferative disorder
with associated MF. However, the patient failed to respond to
first-line immunosuppressive therapy (cyclosporine) and subsequent
glucocorticoid treatment, a finding that fundamentally challenged
the initial diagnostic impression. Glucocorticoid resistance is
highly unusual in classical T-cell lymphomas and effectively ruled
out steroid-responsive conditions such as autoimmune cytopenia or
certain forms of aplastic anemia. Treatment failure thus became the
key catalyst prompting re-evaluation and a definitive second BM
biopsy. In effect, the lack of treatment response served as the
most critical ‘diagnostic test’ at this stage, directing
investigation toward a more aggressive underlying pathology.
Lack of response to glucocorticoids is
characteristic of high-grade lymphomas, including DLBCL. Although
glucocorticoids exhibit lympholytic effects and are commonly used
in combination chemotherapy (for example, R-CHOP), they are largely
ineffective as long-term monotherapy in DLBCL. Their action
primarily induces apoptosis in mature lymphocytes, but aggressive
malignant clones such as DLBCL rapidly develop resistance (11). Thus, the persistent clinical and
hematological deterioration despite glucocorticoid therapy was
inconsistent with an indolent T-cell disorder or immune-mediated
cytopenia and instead aligned with the profile of an aggressive
lymphoma such as DLBCL. This clinical course provided critical
corroborative evidence for the ultimate diagnosis and reinforced
the histopathological findings of the second biopsy.
Secondary MF is frequently a reactive process to an
underlying malignancy, analogous to the MF observed in metastatic
cancer (12). However, its
occurrence in the rare context of PBM-DLBCL generates a complex
clinical scenario that can obscure the underlying diagnosis. In the
present case, the secondary MF was hypothesized to be
cytokine-mediated. The DLBCL cells likely secreted profibrotic
cytokines such as TGF-β and PDGF, which subsequently activated the
JAK/STAT and SMAD pathways in BM stromal cells, ultimately leading
to collagen deposition and marrow fibrosis, as confirmed by the
reticulin staining. This cytokine-driven remodeling of the BM
microenvironment represents a plausible central mechanism (13,14).
According to the 2024 CSCO guidelines for PMF, diagnosis requires
the presence of all three major criteria, including megakaryocyte
hyperplasia with atypia, grade ≥2 reticulin fibrosis and
JAK2/CALR/MPL mutation, along with at least one minor criterion
(for example, splenomegaly, anemia or increased serum LDH level).
Βy contrast, establishing a diagnosis of secondary MF requires the
exclusion of PMF or other MPNs with differentiation by BM pathology
and mutation analysis for JAK2/CALR/MPL (15). In the last decade, two targeted
therapies have been approved for the treatment of MF, both JAK2
inhibitors, namely ruxolitinib and fedratinib (16). While the majority of patients with
DLBCL present with advanced-stage disease at diagnosis, >60% can
be cured with R-CHOP (rituximab, cyclophosphamide, doxorubicin,
vincristine and prednisone) immunochemotherapy (11). In the present case report, the clear
evidence of a B-cell lymphomatous process supports the
classification as secondary MF. Although molecular testing for
JAK2, CALR and MPL mutations would have been invaluable to formally
exclude an underlying MPN, the patient's refusal precluded this
analysis, presenting a significant limitation.
The review by Zamò et al (17) demonstrated that molecular
characterization of rare hematological malignancies necessitates
multicenter collaboration to accrue sufficient sample sizes. For
PBM-DLBCL with secondary MF, further studies can be conducted in
the following aspects: i) Molecular mechanisms: Delineating the
microenvironmental differences between patients with PBM-DLBCL with
versus without MF by single-cell sequencing or spatial
transcriptomics to identify fibrosis-driving factors. ii)
Prognostic impact: Evaluating whether the presence of MF
exacerbates BM failure or contributes to treatment resistance in
PBM-DLBCL requires long-term follow-up data support. iii) Clinical
management: Assessing the feasibility and efficacy of combined
therapeutic approaches, including immunochemotherapy (for example,
R-CHOP), targeted agents (JAK inhibitors) and antifibrotic
interventions (for example, TGF-β inhibitors).
Currently, the efficacy of conventional DLBCL
regimens (for example, R-CHOP) in PBM- DLBCL with secondary MF
remains uncertain. Although using rituximab markedly improves the
prognosis of patients with CD20-positive B-cell lymphomas, its
efficacy has not been rigorously validated in PBML due to the lack
of randomized controlled clinical trials (18,19).
The potential utility of incorporating a JAK inhibitor (for
example, ruxolitinib) to target the fibrotic morrow
microenvironment in this setting remains unexplored and warrants
future investigation. In conclusion, the extreme rarity of
PBM-DLBCL with MF creates substantial gaps about its
pathophysiology and optimal therapeutic strategies. In the future,
cases could be collected through international registry systems
(for example, NIH or ESMO rare tumor databases) and combined with
multi-omics analyses to facilitate personalized treatment
approaches. The patient's refusal of treatment precluded any
assessment of therapeutic response and resulted in a loss of
valuable prognostic data.
PBM-DLBCL represents a diagnostically challenging
malignancy, underscoring the importance of heightened clinical
awareness. PBM-DLBCL should be considered in patients presenting
with unexplained pancytopenia. The present case report has several
limitations, primarily due to the patient's refusal of further
testing. The lack of PET/CT imaging precludes definitive exclusion
of occult extramedullary disease, though it was not detected on
available CT scans. Additionally, the absence of molecular data
(JAK2/CALR/MPL and MYC/BCL2 analysis by FISH) and an extended IHC
panel (for example, MUM-1, BCL-2, BCL-6 and C-MYC) hampers accurate
prognostic stratification for both the MF and the DLBCL. The
11-month interval between the initial steroid-refractory
presentation and the second BM biopsy, although ultimately leading
to a definitive diagnosis, represents a potential delay; however,
it was the progression of symptoms and cytopenias that necessitated
the repeat procedure. In conclusion, accurate diagnosis of
PBM-DLBCL requires a comprehensive clinical and laboratory
evaluation, the utilization of appropriate diagnostic modalities in
cases of unexplained cytopenia, and a meticulous clinical approach
to ensure optimal patient safety and outcomes. PBM-DLBCL is a
diagnostic chameleon capable of mimicking T-cell malignancies,
aplastic anemia and other causes of marrow failure. The present
case report highlights that the presence of a clonal T-cell
population does not necessarily confirm a T-cell lymphoma diagnosis
and may represent a misleading secondary finding. Unexplained and
persistent pancytopenia, especially when accompanied with systemic
symptoms and elevated LDH, warrants a low threshold for repeat BM
evaluation. Moreover, the complex interplay between lymphoma and
the marrow microenvironment, leading to fibrosis, represents a
critical avenue for further research to elucidate pathogenesis and
identify potential therapeutic targets. Overall, this case
underscores the diagnostic challenges and the imperative for
thorough and at times, repeated diagnostic assessment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JY, SKT, HP, YYP, ML and KS conceived and designed
the study. JY, SKT, HP and YYP collected and interpreted all
relevant clinical and laboratory data. JY, SKT, HP, YYP, ML and KS
prepared the manuscript. ML and KS revised the manuscript. All
authors have read and approved the final manuscript. JY, SKT, HP,
YYP, ML and KS confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li S, Young KH and Medeiros LJ: Diffuse
large B-cell lymphoma. Pathology. 50:74–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinez A, Ponzoni M, Agostinelli C,
Hebeda KM, Matutes E, Peccatori J, Campidelli C, Espinet B, Perea
G, Acevedo A, et al: Primary bone marrow lymphoma: An uncommon
extranodal presentation of aggressive non-hodgkin lymphomas. Am J
Surg Pathol. 36:296–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang H, Hung YS, Lin TL, Wang PN, Kuo MC,
Tang TC, Wu JH, Dunn P and Shih LY: Primary bone marrow diffuse
large B cell lymphoma: A case series and review. Ann Hematol.
90:791–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang G, Chang Y, Wu X, Li X, Li L, Zhang
L, Fu X, Sun Z, Zhang X and Zhang M: Clinical features and
prognostic factors of primary bone marrow lymphoma. Cancer Manag
Res. 11:2553–2563. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakagawa N, Yamano R, Kajikawa S, Kondo Y
and Okumura H: Successful bridging therapy with tirabrutinib before
ASCT for relapsed primary DLBCL of the CNS complicated with PBC,
cirrhosis, and pancytopenia. Leuk Res Rep. 17:1003312022.PubMed/NCBI
|
|
6
|
Tefferi A: Primary myelofibrosis: 2023
update on diagnosis, risk-stratification, and management. Am J
Hematol. 98:801–821. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Passamonti F and Mora B: Myelofibrosis.
Blood. 141:1954–1970. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Somasundaram E and Abramson JS: Double hit
lymphoma: Contemporary understanding and practices. Leuk Lymphoma.
66:26–33. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J and Ma J: Chinese Society of
Clinical Oncology (CSCO) diagnosis and treatment guidelines for
malignant lymphoma 2021 (English version). Chin J Cancer Res.
33:289–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W: The 5th Edition of the World Health
Organization Classification of Hematolymphoid Tumors. Leukemia.
Exon Publications; Brisbane (AU): 2022, View Article : Google Scholar
|
|
11
|
Sehn LH and Salles G: Diffuse large B-cell
lymphoma. N Engl J Med. 384:842–858. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu YL, Wang WJ and Wang XN: Pathological
characteristics of bone marrow in Non-Hodgkin's lymphoma patients
with secondary myelofibrosis and their relationship with prognosis.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 23:674–678. 2015.(In Chinese).
PubMed/NCBI
|
|
13
|
Benbassat J: Myelofibrosis with myeloid
metaplasia. N Engl J Med. 343:6592000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JN and Li Y: Exploring the molecular
mechanisms between lymphoma and myelofibrosis. Am J Transl Res.
16:730–737. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Sullivan JM and Harrison CN:
Myelofibrosis: Clinicopathologic features, prognosis, and
management. Clin Adv Hematol Oncol. 16:121–131. 2018.PubMed/NCBI
|
|
16
|
Waksal JA, Harrison CN and Mascarenhas JO:
Novel therapeutics and targets in myelofibrosis. Leuk Lymphoma.
63:1020–1033. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamò A, Johnston P, Attygalle AD, Laurent
C, Arber DA and Fend F: Aggressive B-cell lymphomas with a primary
bone marrow presentation. Histopathology. 77:369–379. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai L, Stauder MC, Zhang YJ, Poortmans P,
Li YX, Constantinou N, Thariat J, Kadish SP, Nguyen TD, Kirova YM,
et al: Early-stage primary bone lymphoma: A retrospective,
multicenter rare cancer network (RCN) study. Int J Radiat Oncol
Biol Phys. 83:284–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhu J, Song Y, Ping L and Zheng
W: Clinical characterization and outcome of primary bone lymphoma:
a retrospective study of 61 Chinese patients. Sci Rep. 6:288342016.
View Article : Google Scholar : PubMed/NCBI
|