Introduction
Endometrial cancer (EC) is the most common
gynecological malignancy in developed countries, with a rising
incidence and associated mortality (1,2). For a
number of years, EC has been classified dichotomously, including by
examining clinical prognostic factors combined with histological
characteristics and sex hormone receptor status (3). However, contemporary translational
research efforts have further characterized endometrial tumors to
identify risk groups and potential molecular sensitivities to
targeted therapies, which could improve overall prognoses. For
instance, The Cancer Genome Atlas (TCGA) Research Network
consortium have proposed a novel EC classification based on genomic
analysis that includes polymerase ε somatic (POLE)
mutations, TP53 mutations, copy number alterations and
microsatellite instability (MSI) (4). Despite these breakthrough molecular
discoveries, the complex crosstalk between the tumor milieu,
including the hormonal environment and immune system interactions,
is considered to be an important pathway for EC growth (5). Identifying the subpopulations of
patients with EC harboring unfavorable genomic and immune
predictive features and introducing targeted treatment could
improve the final therapeutic outcome (5).
Exposure to unopposed estrogen activity has been
recognized as the main trigger for EC development (6). Estrogen binds to estrogen receptors
(ERs) that exist in two main forms, ERα and ERβ, forming a complex
which then acts on the estrogen response element in the cell
nucleus, activating the transcription of target genes (7). ERα and ERβ are considered to have
different expression patterns and biological functions in
gynecological tumors (8–10). Specifically, ERα expression has been
shown to be markedly lower in endometrial stroma than in the
glands, indicating that stromal cells lose ERα expression when
tumor cells proliferate as result of EC progression. The absence of
ERα expression in EC tumor tissue has also been significantly
associated with advanced clinical stage and grade, as well as being
indicated as a strong predictor of lymph node involvement (11). At the beginning of EC development,
significant IL-17A-driven ERα upregulation by tumor-infiltrating
macrophages results in increased local estrogen sensitivity of the
endometrial lesion (12). During EC
progression to advanced stages, more ERα-negative cells appear due
to changes in the ERα sensitivity to estrogen, leading to the
significant decrease in the expression of this receptor (10). The lack of ERα expression in EC has
also been considered a robust predictive marker of
epithelial-mesenchymal transition (EMT) and associated with reduced
patient survival (13).
Additionally, it has been found that ERα-negative tumors are
associated with the activation of the phosphoinositide 3-kinase
(PI3K) pathways, which could implicate ERα expression as a
potential predictive biomarker of the therapeutic response to PI3K
pathway inhibitors (13). Another
important molecular mechanism underlying the role of ERα in
endometrial tumor development is the methylation of its genes.
Methylation of the ERα-C isoform is frequently found in EC
tissue compared with normal endometrium (14–16).
In a study, this methylation was detected in 94% of EC samples in
which the ERα-C gene was inactivated, suggesting a mechanism
for tumor development (14).
The expression of tumor-infiltrating lymphocytes
(TILs) in cancer tissue has been demonstrated to be a strong
prognostic factor in patients with EC (5,17–21).
In a study, the presence of a high number of CD8+ T
lymphocytes predicted an improved overall survival (OS) of the
entire EC cohort, while a high number of forkhead box P3
(FoxP3+) T cells was associated with a decrease in the
OS of patients with the endometrioid EC subtype and memory T
lymphocytes (CD45R0+) were independent predictors of an
increased OS in patients with non-endometrioid EC (18). Čermáková et al (19) demonstrated that CD3+ TIL
counts decrease with an advanced EC stage. Furthermore, a high
level of TILs has been shown to be negatively correlated with
histological grade, myometrial invasion and lymph node metastasis,
while increased densities of CD8+ and CD45R0+
T cells in EC tumors are associated with favorable outcomes
(21).
Considering the new molecular TCGA classification of
EC tumors and the variation in TIL activities and immunosuppressive
characteristics between the molecular EC subtypes, the assessment
of such an immune marker may improve the predicted response to
immunotherapy (22). MSI and
high-grade POLE wildtype/microsatellite-stable EC clusters
have been confirmed to be highly immunogenic subtypes with a
significant high-density TIL infiltrate, especially within the
invasive front of the tumor (23).
POLE-mutated and MSI EC tumors possess a high neoantigen
load and number of TILs, which is counterbalanced by the
upregulation of programmed cell death-1 (PD-1) and programmed cell
death ligand-1 (PD-L1), making these tumors excellent candidates
for PD-1-targeted immunotherapies (5,24).
Estradiol-mediated ERα signaling has been reported to be critical
for the regulation of TIL function in patients with cervical
cancer. Specifically, Adurthi et al (25) presented evidence that estradiol and
ERα interactions with the FOXP3 locus exerted potent effects
on gene expression and could modulate the suppressive function of
primary human tumor-infiltrating regulatory T cells in patients
with cervical cancer. This proposed model may be universal as ERα
has been shown to regulate genes in a similar manner in MCF-7
breast cancer cells (26); however,
to the best of our knowledge, this model has not been studied in an
EC population thus far.
In our previous research, decreased gene expression
of both ER isoforms was observed in the peripheral blood
lymphocytes isolated from patients with EC compared with
individuals of reproductive age with simple functional ovarian
cysts (27). However, the tumor
environment crosstalk between TILs and cancer cells may be
different due to distinct tissue conditions compared with the
peripheral blood.
Based on these findings, we hypothesized that ERα
expression in infiltrating lymphocytes from EC tissue may change
during disease progression and may depend on the EC molecular
subtype; therefore, the present study aimed to investigate ERα
manifestation in endometrial tumor-derived TILs. The potential
prognostic value of the ERα level in TILs from patients with EC may
help to translate the findings of previous studies into improved
diagnostic and therapeutic approaches.
Materials and methods
Patients
The study group was selected from a cohort of
patients with EC who underwent surgery as their primary treatment
at the Department of Oncological Gynecology within the Lower
Silesian Oncology, Pulmonology and Hematology Center (Wroclaw,
Poland) between September 2019 and June 2023. All patients
underwent hysterectomy with bilateral salpingo-oophorectomy
followed by pelvic and paraaortic lymph node dissection or a
sentinel lymph node biopsy procedure, depending on the preoperative
evaluation and assumed clinical stage. This study was approved by
the Wroclaw Medical University Bioethics Committee (registration
no. 166/2019; March 5, 2019). Written informed consent for study
participation was obtained from each qualified patient prior to
surgery, in accordance with the Declaration of Helsinki.
The inclusion criteria were as follows: i) The
diagnosis of primary EC (both endometrioid and non-endometrioid)
and a surgical intervention treatment plan; ii) endometrial tumor
size of at least 1 cm in the largest diameter to ensure sufficient
tissue both for routine pathological assessment and for the present
study; iii) no previous oncological treatment of any type and no
previous immunotherapy of any type for any reason; and iv) patients
aged >18 years old who provided written, informed consent.
The pathological assessments included a standard
institutional protocol for EC, such as immunohistochemical staining
tests for mismatch repair (MMR) status and p53 protein expression
and Sanger sequencing of EC tissues to determine the somatic
POLE mutation status (28),
the results from which were obtained from the medical records. All
cases were staged according to the 2018 International Federation of
Gynecology and Obstetrics classification based on the final
histopathological reports (29),
and divided into molecular clusters based on TCGA classification
(4). Additionally, the study
population was defined and stratified to specific risk groups due
to the presence of prognostic factors according to the European
Society of Gynecological Society (ESGO)/European Society for
Radiotherapy and Oncology (ESTRO)/European Society of Pathology
(ESP) 2021 guidelines (30). The
general study group characteristics are presented in Table I.
 | Table I.General clinical characteristics of
the study population (n=54). |
Table I.
General clinical characteristics of
the study population (n=54).
| Parameters | Value |
|---|
| Median age, years
(range) | 67 (29–82) |
| Median BMI,
kg/m2 (range) | 32.5 (23–55) |
| Median tumor size,
cm (range) | 3.0 (1–11) |
| FIGO stage, n
(%) |
|
| Stage
I | 40 (74.1) |
| Stage
>I | 14 (25.9) |
| Myometrial
infiltration, n (%) |
|
|
<50% | 30 (55.6) |
|
≥50% | 24 (44.4) |
| Parametria
infiltration, n (%) |
|
|
Negative | 52 (96.3) |
|
Positive | 2 (3.7) |
| Histological
gradea, n (%) |
|
| Low
grade | 39 (72.2) |
| High
grade | 15 (27.8) |
| Histological
typeb, n (%) |
|
| Type
I | 48 (88.9) |
| Type
II | 6 (11.1) |
| LVSI, n (%) |
|
| Not
identified | 37 (68.5) |
|
Present | 17 (31.5) |
| Clinical risk
group, n (%) |
|
|
Low | 21 (38.9) |
|
Intermediate | 9 (16.6) |
|
High-intermediate | 13 (24.1) |
|
High | 11 (20.4) |
| MMR, n (%) |
|
| MMR
proficient | 32 (59.2) |
| MMR
deficient | 22 (40.8) |
| TCGA, n (%) |
|
|
NSMP | 26 (48.1) |
|
MSI | 22 (40.8) |
| p53
mutant | 6 (11.1) |
The primary aim of the present study was to examine
ERα expression in all studied subgroups of TILs derived from
endometrial tumors compared with healthy endometrial tissue. The
secondary aims included examining the correlations of ERα
expression in TILs with the relevant clinicopathological features
(including molecular clusters) of the studied endometrial
tumors.
Sample collection
Immediately after the hysterectomy, the uterus was
halved to expose the endometrial tumor and the macroscopically
normal endometrium. Both EC tissue and control tissue (healthy
endometrium adjacent to the tumor tissue) were sampled by an
experienced gynecological oncologist and then immediately
transported to the Hirszfeld Institute of Immunology and
Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland
for further processing. The postoperative samples were thoroughly
examined by an experienced pathologist who also verified the sample
collection sites by visual assessment to confirm that the
macroscopic healthy endometrium was non-malignant.
A total of 82 samples were collected out of the 100
planned. However, lymphocyte isolation failed in some cases,
especially when specimens were very scant. Furthermore, the timing
of sample collection was related to the peak of the COVID-19
pandemic, which inferred logistical challenges. Therefore, only 54
cases (with sufficient tumor and control tissue samples) were
ultimately enrolled and investigated, which was a limitation of the
present study.
Enzymatic digestion
EC and control tissues (~300 mg) were roughly minced
with scissors into small pieces in Hanks' Balanced Salt Solution
with Ca2+/Mg2+ (Biowest) supplemented with 30
µg/ml DNase I (Roche Diagnostics) and 1 mg/ml collagenase type IV
(BioShop Canada, Inc.), then incubated in a water bath for 15 min
at 37°C. The tissue dispersing process was repeated, including a
further incubation for 15 min at 37°C. Then, the samples were
briefly shaken and incubated for 15 min at 37°C. This step was
repeated three times. The digested tissues were passed through 100
and 40 µm cell strainers. The purified cells were centrifuged at
300 × g for 4 min at 4°C, then the cell pellet was resuspended in
sorting buffer [phosphate-buffered saline (PBS) supplemented with 2
mM EDTA and 2% fetal bovine serum (Biowest)] and incubated for 30
min at 37°C. The samples were then centrifuged at 300 × g for 4 min
at 4°C and the cell pellet was resuspended in RBC Lysis Buffer
(BioLegend, Inc.). After 15 min of incubation at room temperature
in the dark, according to the manufacturer's instructions, the
cells were centrifuged at 300 × g for 5 min at 4°C and finally
washed with PBS buffer.
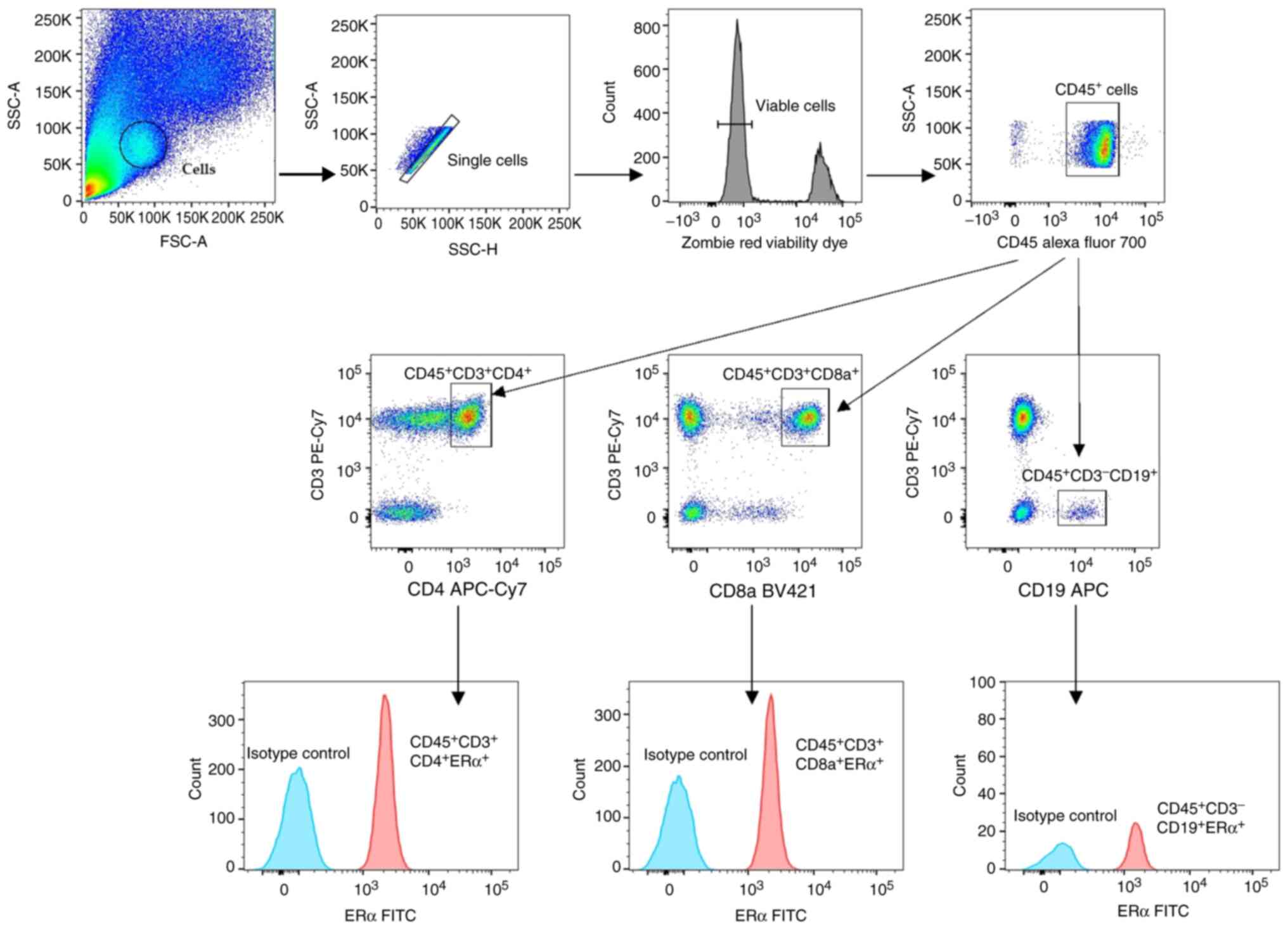
Flow cytometry
Isolated cells (1×106) were stained with
a cocktail of fluorescently labeled antibodies: PE/Cy7 Anti-CD3
(clone: UCHT1; cat. no. 30042), APC/Cy7 anti-CD4 (clone: RPA-T4;
cat. no. 300518), BV421 anti-CD8a (clone: RPA-T8; cat. no. 301036),
APC anti-CD19 (clone: HIB19; cat. no. 302212) and AF700 anti-CD45
(clone: HI30; cat. no. 304024) (all from BioLegend, Inc.) for 30
min at 4°C in the dark. Then, the cells were washed twice, with
staining buffer (0.5 mM EDTA, 0.002% sodium azide, 1% fetal bovine
serum), and intracellular staining was performed according to the
manufacturer's protocol (True-Nuclear Transcription Factor Buffer
Set; BioLegend, Inc.). Briefly, cells were fixed for 14 h at 4°C in
the dark, washed twice with permeabilization buffer and stained for
1 h at 4°C in the dark with a recombinant AF488 anti-ERα antibody
(clone: E115; cat. no. AB194150; Abcam) or appropriate isotype
control (AF488 rabbit IgG, clone: EPR25A; cat. no. AB199091; Abcam)
at the same concentration as the specific antibody. The samples
were washed twice with permeabilization buffer, fixed with buffered
1% paraformaldehyde at 4°C and then the cells were analyzed using a
BD LSR Fortessa™ flow cytometer (BD Biosciences). Only viable cells
(stained with the Zombie Red™ Fixable Viability Kit at room
temperature, in the dark, for 15 min, according to the
manufacturer's instructions; BioLegend, Inc.) were analyzed. Before
extracellular and intracellular staining, Fc receptor blocking with
Human TruStainFcX (5 µl in 100 µl staining volume, 10 min at room
temperature; BioLegend, Inc.) was performed according to the
manufacturer's instructions. The relative level of ERα is shown as
the specific median fluorescence intensity (MFI) based on the
difference between the median fluorescence intensity of the
specifically stained cells and the isotype-matched control cells
gated for the populations of interest. All analyses were performed
using FlowJo v10.7.2 (BD Biosciences). Representative dot plots
showing the gating strategy of the cells of interest are shown in
Fig. 1.
Statistical analysis
Continuous variables are expressed as mean ±
standard deviation and median with interquartile range. STATISTICA
version 13.3 (TIBCO Software, Inc.) and R statistical software
version 4.1.0 (The R Foundation; http://www.R-project.org) were used to perform the
statistical procedures. Differences in categorical factors were
determined with Fisher's exact test. Normality was assessed using
Shapiro-Wilk tests. Differences in continuous values between two
groups were assessed with non-parametric Mann-Whitney U tests for
non-normally distributed variables. Differences in continuous
variables among three or more groups were assessed with the
Kruskal-Wallis test. The Dunn test was used for adjustment for
multiple testing. To assess the correlation between risk factors
and the subsets of tested lymphocytes in the tumor tissue,
Spearman's rank correlation coefficient test (ρ) was performed. The
Wilcoxon signed-rank test was used to compare the level of the two
dependent parameters. For multiple comparisons, the Bonferroni
correction was used. All tests were two-sided. P<0.05 was
considered to indicate a statistically significant difference.
Results
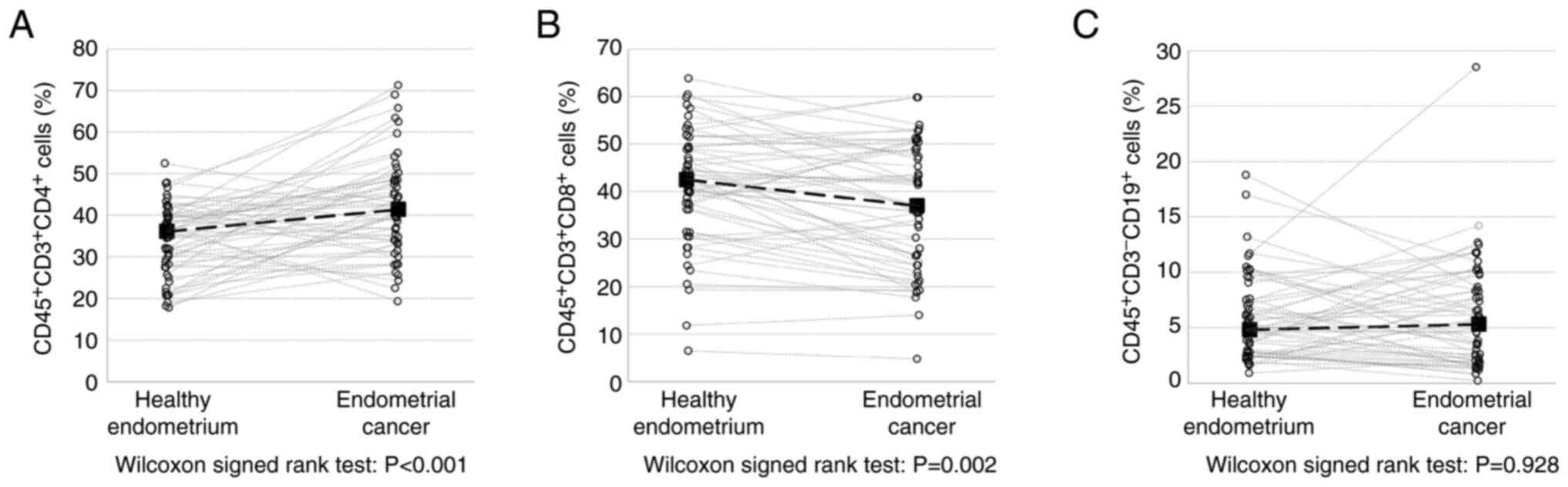
Frequencies of TILs in EC tissues vary
depending on the lymphocyte type
In the present study, three subpopulations of
lymphocytes were investigated: T helper lymphocytes (Th cells;
CD45+CD3+CD4+), cytotoxic T
lymphocytes (CTLs; CD45+CD3+CD8+)
and B lymphocytes subset (B cells;
CD45+CD3−CD19+). The frequency of
Th cells increased significantly in EC tissues compared with the
control tissues (41.4 vs. 36.1%; P<0.001). By contrast, the
frequency of CTLs was significantly lower in EC tissues (37.0 vs.
42.5%; P=0.002), while there were no differences in the B cells
frequencies between EC and control tissue (5.3 vs. 4.8%; P=0.928)
(Fig. 2).
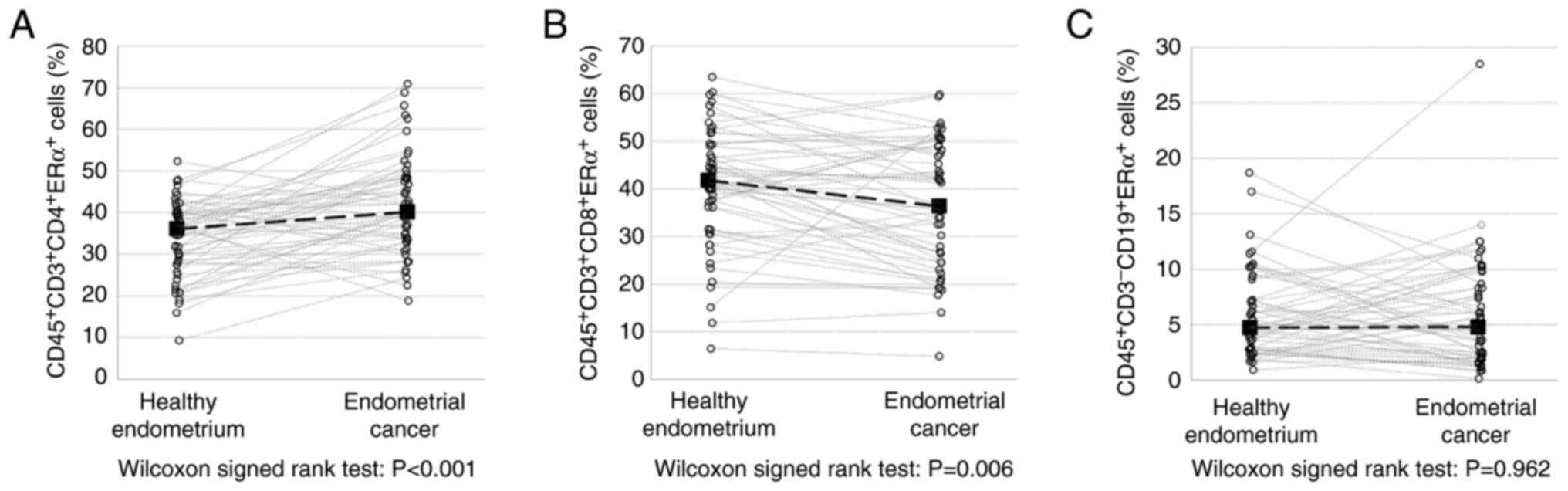
Similarly, the prevalence of ERα-expressing TILs was
dependent on the subpopulation. The number of ERα+ Th
cells was significantly higher in EC tissues compared with the
control tissue (40.9 vs. 33.7%; P<0.001), whereas the frequency
of ERα+ CTL cells was significantly decreased in EC
tissues (35.9 vs. 40.3; P<0.006). However, significant
differences in the frequency of ERα+ B cells were not
observed between the EC and control tissues (Fig. 3).
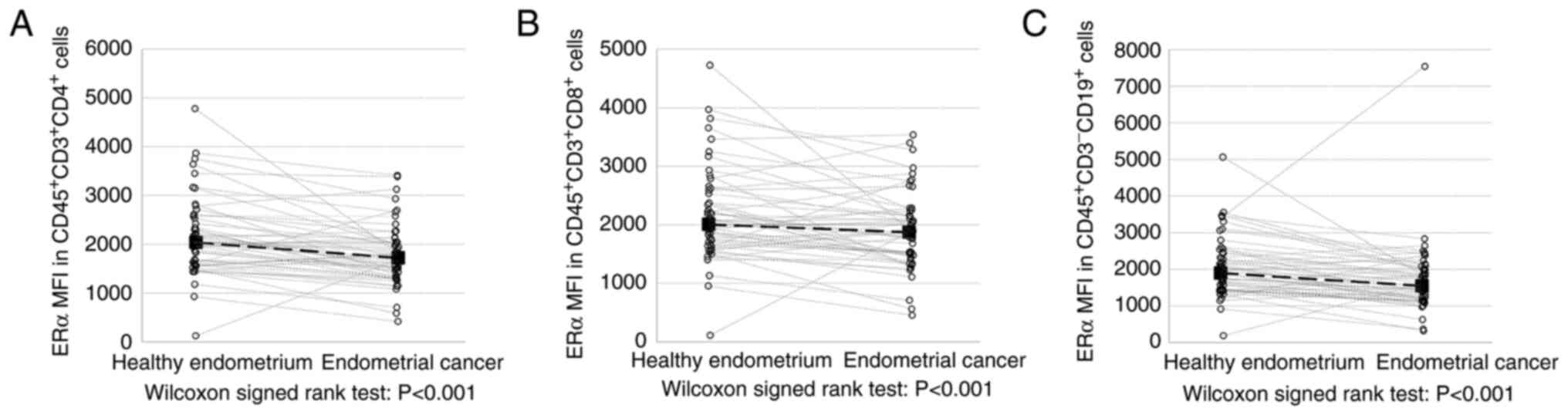
ERα expression is significantly
decreased in TILs from EC tissues
The main objective of the present study was to
quantify ERα expression in TILs based on MFI, which was its
absolute level, while the frequency of ERα-positive TILs only
reflects their presence in tumor tissue. The expression of ERα
(presented as MFI) of all tested endometrial tumor-derived TIL
subpopulations was significantly decreased compared with control
tissues of normal adjacent endometrium (ERα-MFI in Th cells was
1,721 vs. 2,036 in the control; P<0.001; ERα-MFI in CTLs was
1,871 vs. 2,006; P=0.001 and ERα-MFI in B cells was 1,546 vs.
1,898; P<0.001; Fig. 4).
Association of the TIL and
ERα+ TIL frequencies with the clinicopathological
characteristics of the patients
The associations between the measured TIL
subpopulation frequencies and important prognostic factors, such as
the clinical risk group according to ESGO/ESTRO/ESP recommendations
(27), myometrial infiltration (≤50
vs. >50%), parametrial involvement (present vs. absent),
lymphovascular space involvement (present vs. absent), histological
type (endometrioid vs. non-endometrioid), histological grading (low
vs. high Grade), stage (I vs. II and III), body mass index (BMI;
<30 vs. 30–35 vs. >35), tumor size (<2 cm vs. 2–4 cm vs.
>4 cm), p53 mutation (present vs. absent), MMR protein
expression (proficient vs. deficient) and type of TCGA molecular
clusters [POLE mutated tumors vs. MMR deficient tumors
(MMR-d) or with microsatellite instability (MSI) vs. non-specific
molecular profile tumors (NSMP) and p53 mutated tumors], were
estimated for every patient. However, no cases with POLE
mutations were found in the study cohort; therefore, analysis was
only possible in the three remaining cluster subpopulations. All
the data are presented in Tables
II and III. The B cell
frequency was significantly increased in high-grade EC tumors
compared with low grade (6.7 vs. 3.9; P<0.024), but no
significant difference in grade was observed in the Th and CTLs
subsets. MMR-d tumors showed an increased B cell frequency compared
with MMR proficient EC tissues (5.9 vs. 4.7; P<0.028). However,
the frequency of the examined Th cells was significantly decreased
in EC tissues with MSI (30.8 vs. 37.3; P<0.037). Furthermore,
the frequency of Th cells significantly decreased in obese patients
(BMI >35) compared with normal weight patients (BMI <30)
(29.8 vs. 37.9; P<0.048).
 | Table II.Frequencies of the three studied
tumor infiltrating lymphocyte subsets, Th cells
(CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), derived from the
tumor according to certain endometrial cancer prognostic
factors. |
Table II.
Frequencies of the three studied
tumor infiltrating lymphocyte subsets, Th cells
(CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), derived from the
tumor according to certain endometrial cancer prognostic
factors.
|
| Th cells (%) | CTLs (%) | B cells (%) |
|---|
|
|
|
|
|
|---|
| Prognostic
factor | Me | IQR | P-value | Me | IQR | P-value | Me | IQR | P-value |
|---|
| Clinical risk
group |
|
| 0.937 |
|
| 0.755 |
|
| 0.911 |
| Low
(n=21) | 34.7 | 11.6 |
| 41.2 | 17.8 |
| 4.7 | 7.1 |
|
|
Intermediate (n=9) | 36.0 | 8.0 |
| 40.1 | 8.3 |
| 3.7 | 3.0 |
|
|
High-intermediate (n=13) | 36.8 | 11.3 |
| 42.5 | 8.4 |
| 5.9 | 3.7 |
|
| High
(n=11) | 31.8 | 17.1 |
| 45.6 | 16.8 |
| 4.9 | 3.8 |
|
| Myometrial
infiltration |
|
| 0.774 |
|
| 0.589 |
|
| 0.676 |
| <50%
(n=30) | 35.6 | 12.4 |
| 40.8 | 12.8 |
| 4.7 | 7.1 |
|
| ≥50%
(n=24) | 36.1 | 10.5 |
| 44.2 | 16.2 |
| 4.9 | 4.3 |
|
| Parametria
involvement |
|
| 0.647 |
|
| 0.714 |
|
| 0.697 |
|
Negative (n= 52) | 36.1 | 12.0 |
| 42.5 | 13.2 |
| 4.8 | 4.8 |
|
|
Positive (n=2) | 36.8 | 13.3 |
| 38.5 | 14.2 |
| 5.5 | 1.4 |
|
| LVSI status |
|
| 0.230 |
|
| 0.608 |
|
| 0.288 |
| Not
identified (n=37) | 34.7 | 12.3 |
| 42.8 | 13.3 |
| 4.7 | 6.6 |
|
| Present
(n=17) | 37.3 | 11.6 |
| 41.2 | 17.5 |
| 5.9 | 2.5 |
|
| Histological
type |
|
| 0.611 |
|
| 0.752 |
|
| 0.474 |
| Type I
(n=48) | 36.1 | 12.0 |
| 42.5 | 12.9 |
| 4.7 | 4.7 |
|
| Type II
(n=6) | 35.6 | 13.3 |
| 42.9 | 20.4 |
| 5.5 | 4.3 |
|
| FIGO stage |
|
| 0.906 |
|
| 0.459 |
|
| 0.401 |
| I
(n=40) | 36.2 | 12.0 |
| 41.9 | 15.3 |
| 5.2 | 6.5 |
|
| >I
(n=14) | 34.0 | 12.1 |
| 44.0 | 16.8 |
| 4.3 | 3.5 |
|
| Histological
grade |
|
| 0.870 |
|
| 0.446 |
|
| 0.024 |
| Low
grade (n=39) | 36.0 | 11.7 |
| 41.2 | 17.8 |
| 3.9 | 4.5 |
|
| High
grade (n=15) | 36.2 | 17.1 |
| 45.6 | 14.6 |
| 6.7 | 5.4 |
|
| BMI,
kg/m2 |
|
| 0.048 |
|
| 0.309 |
|
| 0.367 |
| <30
(n=14) | 37.9 | 9.5 |
| 39.4 | 18.2 |
| 4.7 | 4.3 |
|
| 30-35
(n=24) | 34.0 | 11.9 |
| 42.6 | 8.2 |
| 4.7 | 4.0 |
|
| >35
(n=16) | 29.8 | 16.0 |
| 46.5 | 14.6 |
| 6.7 | 5.9 |
|
| TU, cm |
|
| 0.097 |
|
| 0.146 |
|
| 0.241 |
| <2
(n=11) | 36.1 | 12.7 |
| 39.2 | 24.4 |
| 4.8 | 5.6 |
|
| 2-4
(n=27) | 32.0 | 16.8 |
| 44.4 | 12.1 |
| 5.9 | 4.9 |
|
| >4
(n=16) | 39.2 | 7.7 |
| 39.8 | 13.0 |
| 2.9 | 3.1 |
|
| p53 |
|
| 0.277 |
|
| 0.912 |
|
| 0.527 |
| Normal
(n=48) | 35.4 | 12.3 |
| 42.5 | 13.2 |
| 4.7 | 4.8 |
|
| Mutant
(n=6) | 38.1 | 11.6 |
| 42.9 | 15.1 |
| 5.5 | 2.5 |
|
| MMR |
|
| 0.037 |
|
| 0.238 |
|
| 0.028 |
| MMR
proficient (n=32) | 37.3 | 10.4 |
| 39.8 | 17.9 |
| 4.7 | 4.0 |
|
| MMR
deficient (n=22) | 30.8 | 16.7 |
| 44.0 | 11.5 |
| 5.9 | 5.9 |
|
| TCGA |
|
| 0.086 |
|
| 0.536 |
|
| 0.062 |
| NSMP
(n=26) | 37.0 | 8.7 |
| 39.3 | 17.8 |
| 3.8 | 4.0 |
|
| MSI
(n=22) | 30.8 | 16.7 |
| 44.0 | 11.5 |
| 5.9 | 5.9 |
|
| p53
(n=6) | 38.1 | 11.6 |
| 42.9 | 15.1 |
| 5.5 | 2.5 |
|
 | Table III.Frequencies of the three studied
tumor infiltrating lymphocyte subsets, Th cells
(CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), expressing ERα
derived from the tumor according to certain endometrial cancer
prognostic factors. |
Table III.
Frequencies of the three studied
tumor infiltrating lymphocyte subsets, Th cells
(CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), expressing ERα
derived from the tumor according to certain endometrial cancer
prognostic factors.
|
| Th cells (%) | CTLs (%) | B cells (%) |
|---|
|
|
|
|
|
|---|
| Prognostic
factor | Me | IQR | P-value | Me | IQR | P-value | Me | IQR | P-value |
|---|
| Clinical risk
group |
|
| 0.916 |
|
| 0.744 |
|
| 0.792 |
| Low
(n=21) | 34.7 | 12.3 |
| 40.0 | 16.0 |
| 4.5 | 7.1 |
|
|
Intermediate (n=9) | 36.0 | 7.7 |
| 40.1 | 8.0 |
| 3.6 | 2.0 |
|
|
High-intermediate (n=13) | 36.8 | 11.3 |
| 42.5 | 8.4 |
| 5.9 | 3.6 |
|
| High
(n=11) | 31.8 | 17.1 |
| 44.2 | 16.4 |
| 4.8 | 3.4 |
|
| Myometrial
infiltration |
|
| 0.663 |
|
| 0.433 |
|
| 0.486 |
| <50%
(n=30) | 35.6 | 12.4 |
| 40.1 | 15.6 |
| 4.6 | 7.1 |
|
| ≥50%
(n=24) | 36.1 | 10.4 |
| 44.1 | 16.1 |
| 4.8 | 4.2 |
|
| Parametria
involvement |
|
| 0.614 |
|
| 0.819 |
|
| 0.630 |
|
Negative (n=52) | 36.1 | 12.3 |
| 41.8 | 15.4 |
| 4.7 | 4.4 |
|
|
Positive (n=2) | 36.7 | 13.4 |
| 38.5 | 14.1 |
| 5.5 | 1.5 |
|
| LVSI status |
|
| 0.196 |
|
| 0.716 |
|
| 0.174 |
| Not
identified (n=37) | 34.7 | 13.8 |
| 42.3 | 13.2 |
| 3.9 | 6.6 |
|
| Present
(n=17) | 37.0 | 11.4 |
| 41.1 | 15.1 |
| 5.9 | 2.5 |
|
| Histological
type |
|
| 0.554 |
|
| 0.620 |
|
| 0.401 |
| Type I
(n=48) | 36.1 | 12.3 |
| 41.8 | 14.3 |
| 4.6 | 4.4 |
|
| Type II
(n=6) | 35.6 | 13.4 |
| 42.8 | 20.3 |
| 5.5 | 4.3 |
|
| FIGO stage |
|
| 0.984 |
|
| 0.407 |
|
| 0.508 |
| I
(n=40) | 36.3 | 13.2 |
| 40.7 | 16.8 |
| 4.7 | 6.4 |
|
| >I
(n=14) | 34.0 | 12.1 |
| 43.3 | 16.4 |
| 4.3 | 3.5 |
|
| Histological
grade |
|
| 0.985 |
|
| 0.374 |
|
| 0.016 |
| Low
grade (n=39) | 36.0 | 12.4 |
| 41.1 | 16.6 |
| 3.9 | 3.8 |
|
| High
grade (n=15) | 36.2 | 17.1 |
| 44.4 | 14.5 |
| 6.7 | 5.4 |
|
| BMI,
kg/m2 |
|
| 0.087 |
|
| 0.177 |
|
| 0.270 |
| <30
(n=14) | 37.8 | 10.3 |
| 38.2 | 17.9 |
| 4.1 | 3.7 |
|
| 30-35
(n=24) | 34.0 | 11.9 |
| 42.6 | 6.0 |
| 4.6 | 3.8 |
|
| >35
(n=16) | 29.8 | 16.0 |
| 46.5 | 14.5 |
| 6.6 | 5.6 |
|
| TU, cm |
|
| 0.076 |
|
| 0.287 |
|
| 0.277 |
| <2
(n=11) | 36.1 | 12.8 |
| 39.2 | 24.4 |
| 4.8 | 5.5 |
|
| 2-4
(n=27) | 30.8 | 17.8 |
| 43.9 | 13.2 |
| 4.9 | 4.5 |
|
| >4
(n=16) | 39.1 | 7.7 |
| 39.8 | 13.0 |
| 2.9 | 3.1 |
|
| p53 |
|
| 0.253 |
|
| 0.731 |
|
| 0.441 |
| Normal
(n=48) | 35.4 | 13.2 |
| 41.8 | 15.4 |
| 4.6 | 4.4 |
|
| Mutant
(n=6) | 38.1 | 11.6 |
| 42.8 | 15.1 |
| 5.5 | 2.4 |
|
| MMR |
|
| 0.060 |
|
| 0.170 |
|
| 0.018 |
| MMR
proficient (n=32) | 37.0 | 12.0 |
| 38.7 | 16.6 |
| 3.9 | 3.9 |
|
| MMR
deficient (n=22) | 30.8 | 16.6 |
| 43.9 | 11.6 |
| 5.9 | 5.6 |
|
| TCGA |
|
| 0.124 |
|
| 0.380 |
|
| 0.033 |
| NSMP
(n=26) | 36.8 | 10.1 |
| 38.2 | 16.6 |
| 3.2 | 3.6 |
|
| MSI
(n=22) | 29.7 | 16.6 |
| 43.7 | 11.6 |
| 5.7 | 5.6 |
|
| p53
(n=6) | 38.1 | 11.6 |
| 42.8 | 15.1 |
| 5.5 | 2.4 |
|
Notably, the frequency of the examined B cell subset
expressing ERα demonstrated a significant increase in high-grade EC
tumors compared with low-grade tumors (5.4 vs. 3.8; P<0.016,
MMR-d tumors compared with MMR-p tumors (5.6 vs. 3.9; P<0.018)
and the TCGA MSI molecular cluster compared with the NSMP and p53
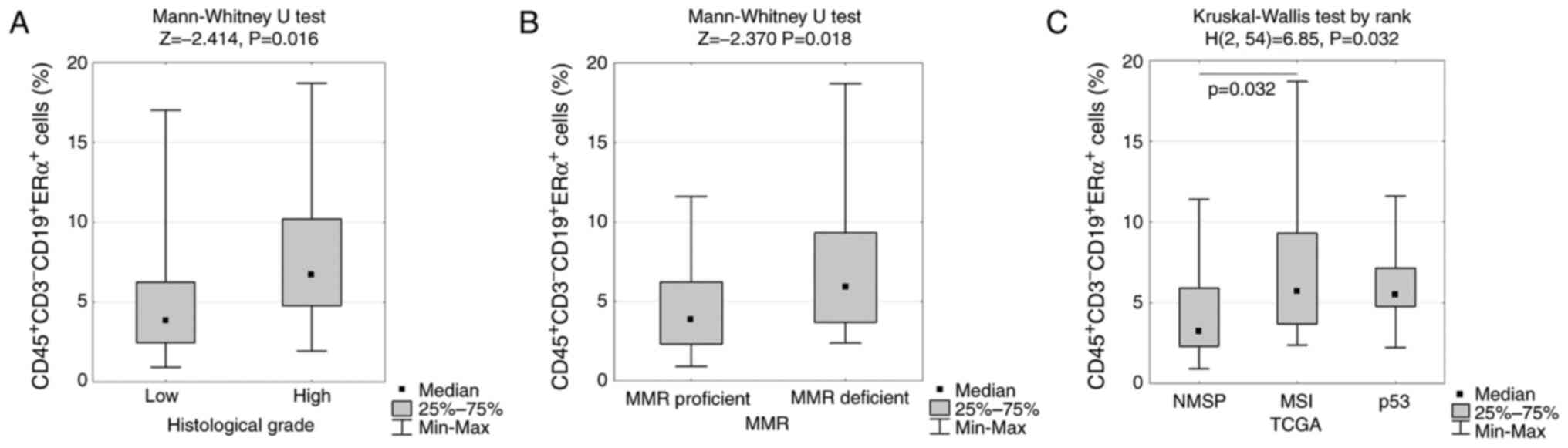
mutant clusters (5.6 vs. 3.6 vs. 2.4; P<0.033) (Table III and Fig. 5).
ERα expression in TILs from EC tissue
was not significantly associated with relevant prognostic factors,
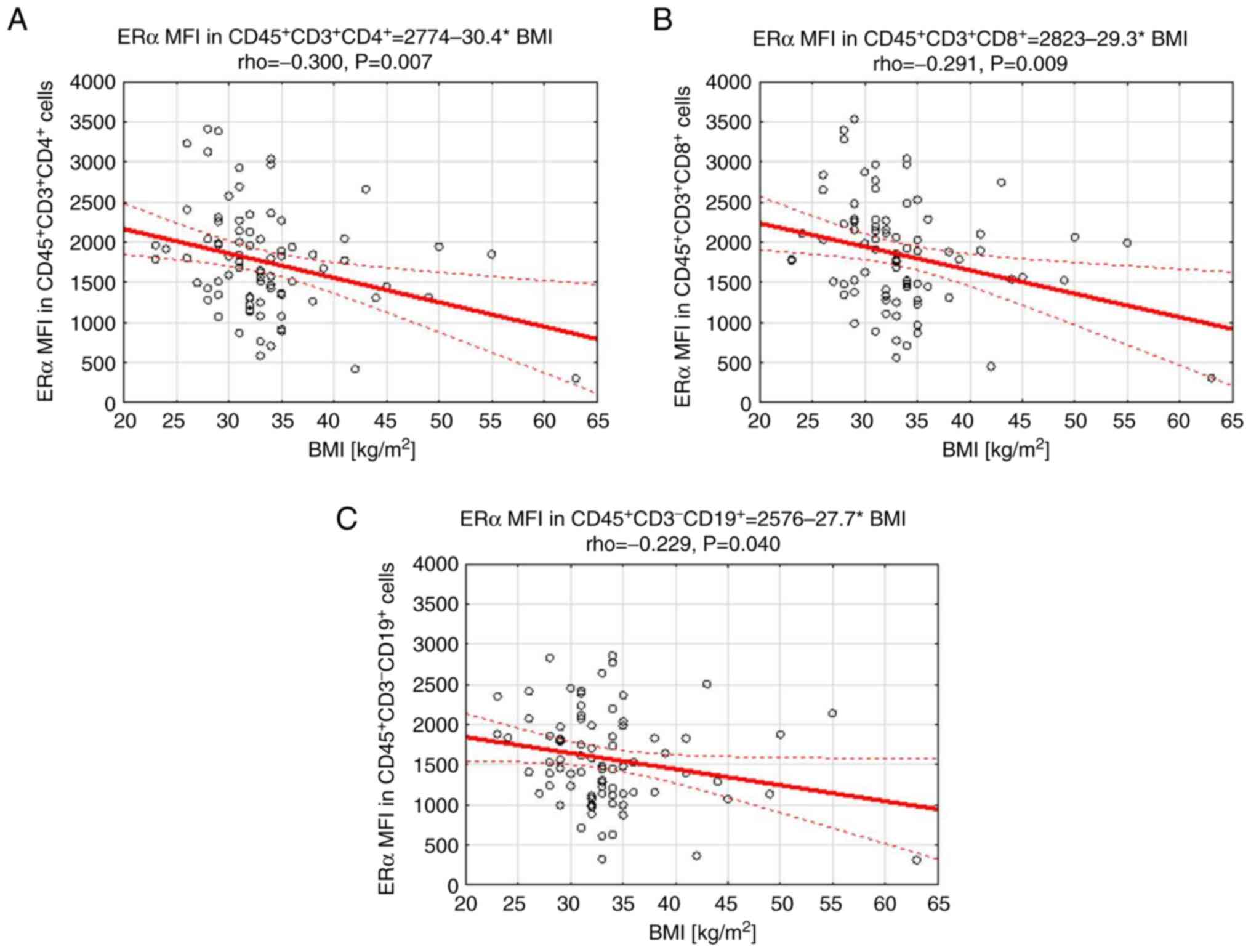
except BMI
ERα expression (when measured by MFI) in all the
studied TIL subgroups was not significantly associated with the
relevant clinicopathological prognostic factors, except for BMI
(Table IV). Furthermore, decreased
ERα expression in each TIL subset was inversely correlated with the
BMI of the studied patients (Fig.
6).
 | Table IV.ERα expression (presented as MFI) in
the three studied tumor infiltrating lymphocyte subpopulations, Th
cells (CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), derived from the
tumor according to certain endometrial cancer prognostic
factors. |
Table IV.
ERα expression (presented as MFI) in
the three studied tumor infiltrating lymphocyte subpopulations, Th
cells (CD45+CD3+CD4+), CTLs
(CD45+CD3+CD8a+) and B cells
(CD45+CD3−CD19+), derived from the
tumor according to certain endometrial cancer prognostic
factors.
|
| ERα MFI in Th
cells | ERα MFI in
CTLs | ERα MFI in B
cells |
|---|
|
|
|
|
|
|---|
| Prognostic
factor | Me | IQR | P-value | Me | IQR | P-value | Me | IQR | P-value |
|---|
| Clinical risk
group |
|
| 0.853 |
|
| 0.736 |
|
| 0.646 |
| Low
(n=21) | 1,648 | 795 |
| 1,785 | 779 |
| 1,615 | 867 |
|
|
Intermediate (n=9) | 1,989 | 846 |
| 2,144 | 913 |
| 1,750 | 597 |
|
|
High-intermediate (n=13) | 1,788 | 546 |
| 1,861 | 546 |
| 1,736 | 723 |
|
| High
(n=11) | 1,804 | 721 |
| 1,890 | 728 |
| 1,409 | 1,113 |
|
| Myometrial
infiltration |
|
| 0.186 |
|
| 0.213 |
|
| 0.444 |
| <50%
(n=30) | 1,744 | 795 |
| 1,901 | 772 |
| 1,630 | 777 |
|
| ≥50%
(n=24) | 1,690 | 747 |
| 1,824 | 746 |
| 1,447 | 889 |
|
| Parametria
involvement |
|
| 0.099 |
|
| 0.131 |
|
| 0.060 |
|
Negative (n=52) | 1,682 | 690 |
| 1,824 | 695 |
| 1,507 | 830 |
|
|
Positive (n=2) | 2,484 | 893 |
| 2,518 | 905 |
| 2,377 | 531 |
|
| LVSI status |
|
| 0.440 |
|
| 0.284 |
|
| 0.440 |
| Not
identified (n=37) | 1,687 | 700 |
| 1,913 | 748 |
| 1,564 | 780 |
|
| Present
(n=17) | 1,788 | 531 |
| 1,861 | 658 |
| 1,485 | 862 |
|
| Histological
type |
|
| 0.890 |
|
| 0.720 |
|
| 0.762 |
| Type I
(n=48) | 1,721 | 688 |
| 1,871 | 713 |
| 1,507 | 822 |
|
| Type II
(n=6) | 1,679 | 719 |
| 1,761 | 728 |
| 1,838 | 1,144 |
|
| FIGO stage |
|
| 0.890 |
|
| 0.767 |
|
| 0.407 |
| I
(n=40) | 1,662 | 737 |
| 1,787 | 790 |
| 1,590 | 828 |
|
| >I
(n=14) | 1,838 | 721 |
| 1,942 | 657 |
| 1,443 | 766 |
|
| Histological
grade |
|
| 0.609 |
|
| 0.735 |
|
| 0.434 |
| Low
grade (n=39) | 1,648 | 730 |
| 1,786 | 711 |
| 1,476 | 817 |
|
| High
grade (n=15) | 1,847 | 695 |
| 1,926 | 746 |
| 1,830 | 1,005 |
|
| BMI,
kg/m2 |
|
| 0.007 |
|
| 0.009 |
|
| 0.04 |
| <30
(n=14) | 1,979 | 902 |
| 2,194 | 1,127 |
| 1,806 | 1,068 |
|
| 30-35
(n=24) | 1,637 | 771 |
| 1,824 | 779 |
| 1,427 | 936 |
|
| >35
(n=16) | 1,594 | 559 |
| 1,663 | 633 |
| 1,360 | 785 |
|
| TU, cm |
|
| 0.872 |
|
| 0.870 |
|
| 0.881 |
| <2
(n=11) | 1,676 | 1,357 |
| 1,861 | 1,300 |
| 1,485 | 1,236 |
|
| 2-4
(n=27) | 1,687 | 685 |
| 1,787 | 750 |
| 1,528 | 894 |
|
| >4
(n=16) | 1,838 | 601 |
| 1,885 | 611 |
| 1,697 | 764 |
|
| p53 |
|
| 0.912 |
|
| 0.847 |
|
| 0.409 |
| Normal
(n=48) | 1,682 | 688 |
| 1,871 | 713 |
| 1,507 | 767 |
|
| Mutant
(n=6) | 1,818 | 719 |
| 1,891 | 728 |
| 2,127 | 1,355 |
|
| MMR |
|
| 0.694 |
|
| 0.902 |
|
| 0.656 |
| MMR
proficient (n=31) | 1,687 | 759 |
| 1,913 | 782 |
| 1,446 | 944 |
|
| MMR
deficient (n=23) | 1,788 | 696 |
| 1,861 | 680 |
| 1,564 | 750 |
|
| TCGA |
|
| 0.914 |
|
| 0.969 |
|
| 0.666 |
| NSMP
(n=26) | 1,682 | 662 |
| 1,849 | 720 |
| 1,428 | 845 |
|
| MSI
(n=22) | 1,727 | 696 |
| 1,871 | 680 |
| 1,546 | 636 |
|
| p53
(n=6) | 1,818 | 719 |
| 1,891 | 728 |
| 2,127 | 1,355 |
|
Discussion
In the present study, three main subsets of TILs
demonstrated a different frequency pattern in EC samples compared
with healthy endometrial tissues from the same patient: Th cells
were increased, CTLs were decreased and no significant change in B
cells was observed. The same results were observed for these TIL
subsets with ERα positivity. However, when the ERα level was
presented as MFI, all TIL subsets with ERα positivity were
significantly decreased in EC tissues compared with healthy
endometrial tissue. ERα expression (presented as MFI) in all
studied TILs was not associated with any clinically relevant
prognostic factors, except for BMI. Furthermore, an increase in BMI
was inversely correlated with a decrease in ERα levels. Moreover,
an increased frequency of ERα-positive B lymphocytes in patients
with high-grade endometrial tumors classified as the MSI molecular
group was observed. To the best of our knowledge, the present
preliminary study was the first to investigate ERα expression in
TILs derived from endometrial tumors.
The TIL frequency results from the present study are
in concordance with another study that used flow cytometry to
determine the Th cell and CTL distributions and levels in EC vs.
non-neoplastic endometrium (31). A
study by Jung et al (20),
observed a negative correlation between the CD8+ and
CD4+ infiltration rate (based on histopathological
examination) and histological grade, myometrial invasion and lymph
node metastasis (only CD8+ rate), suggesting their role
as important predictive factors. A number of studies have described
the prognostic role of TIL involvement in EC tissue, highlighting
the dominant positive correlation with high TIL density (especially
CD8+) in early stages and an overall good clinical
outcome (18,19,21).
Such prognostic correlations were not demonstrated in the present
study. However, flow cytometry was used as the main tool to
identify the prevalence of various TIL subsets in the present
study, which was different from the typical methods of
immunohistochemical staining and viewing whole microscopic slides
in multiple fields to estimate the average percentage of TILs used
by other investigators in their studies This methodological
difference in TIL rate assessment could also explain the ambiguous
differences observed in the lymphocyte groups examined in the
present study in terms of their frequency in MMR-d endometrial
tumors: Significant increase in B cells, significant decrease in Th
cells and a negligible decrease in CTLs. This observation is
contrary (except B cell frequency) to other studies that observed
generally high TIL levels within the endometrial tumors of patients
with POLE mutated and MMR-d EC (22,23,32).
Notably, CD3+ PD-L+ and CD3+
PD-L1+ T-cell subpopulations showed significantly higher
frequencies in EC tumors with MMR-d than with NSMP or p53
mutation-positive clusters (33).
Specifically, MMR-d patients (with genetic mutation) demonstrated a
significant increase in TILs compared with epigenetic MMR-d (MLH1
promoter hypermethylation) in EC tissues; however, no difference
was demonstrated for PD-L1 expression (17). The clinical importance of higher
TILs in mutated MMR-d EC tumors than in epigenetic MMR-d EC tumors
is unknown, but it may associated with an improved prognosis of
mutated MMR-d EC tumors and may be a favorable predictive marker
for an improved response to PD-L1 targeted immunotherapy (17).
In contrast to T cells, B cells are considered not
common in EC tumor epithelium, regardless of its genomic cluster
but generally, elevated densities of epithelial and stromal T cells
are accompanied by higher levels of B cells in the stromal
compartment, with the highest expression levels demonstrated in
MMR-d EC tumors (22). Moreover,
tumor-associated B cells and their antibodies have recently been
reported to be important factors of modulated immunity in the EC
tumor microenvironment (TME) (34).
Based on multiplex immunofluorescence and immunohistochemical
staining of EC tissue microarrays, the immune response in the EC
TME was found to be mediated by the binding of high levels of
dimeric IgA (produced by tumor-associated B cells and plasma cells)
to the polymeric immunoglobulin receptor, resulting in
cell-intrinsic inflammatory pathways that promote T-cell-mediated
immunity and the downregulation of DNA repair mechanisms, leading
to an improved clinical outcome (35). Regardless of antigen recognition,
irrelevant B-cell-derived IgA was shown to induce a series of
inflammatory changes, ER stress and proapoptotic transcriptional
pathways in EC cancer cells (35).
The precise mechanism of B cell activation in the TME and the
tumor-associated antigens specific for IgA remains unknown;
however, we hypothesize that an increase in ERα-expressing B cells
in the tumor milieu may play a role in this phenomenon, which
should be clarified in further studies.
ERα plays a crucial role in endometrial malignant
transformation as follows: i) Upstream regulators influence ERα
transcription activity and thus EC cell proliferation; ii) ERα
promotes EC occurrence with other co-regulators; and iii) ERα
influences and mediates tumor proliferation, metastasis and
apoptosis via downstream proteins or target genes (36). ERα has been shown to be a strong
prognostic marker for EMT in EC and potentially predictive of the
response to PI3K/mTOR pathway inhibitors (13). Kreizman-Shefer et al
(37) demonstrated that ERα
expression was significantly lower in EC compared with
non-malignant tissue. Particularly, this decreased expression was
observed in the stromal tissue of the EC tumor compared with the
epithelial tissue, which may indicate an invasive tumor
characteristic. In the present study, significantly decreased ERα
expression (measured by MFI) was observed in the studied lymphocyte
subpopulations derived from endometrial tumors compared with normal
endometrium. We hypothesize that, with progressive endometrial
tumor growth, TILs may exhibit decreased ERα levels compared with
normal endometrial tissue due to a paracrine mechanism triggered by
the development of tumor growth, with an increasing number of tumor
progression mechanisms; however, this possibility was not
investigated in the present preliminary study and the relationship
between TIL frequency and ERα expression remains unknown. In
contrast to the observation of decreased levels of ERα in TILs
derived from EC tumors, ERα expression was shown to be increased in
the tumor tissue itself. A study by Ning et al (12) observed that
CD68+CD163+ macrophages infiltrating EC
secrete a cytokine, IL17A, and thus enhance estrogen-dependent
neoplastic cell proliferation by upregulating the ERα level in the
tumor through ten-eleven translocation 1 mediated epigenetic gene
modulation. This observation of the ERα promoting role in EC
progression was supported by Hu et al (10), in which higher expression of ERα was
noted in well differentiated and early tumors compared with poorly
differentiated advanced EC tumors. The authors speculated that,
during the process of gradual tumor progression, more ERα negative
EC cells appear leading to a final low level of ERα, which is due
to tumor heterogeneity and changes in ERα sensitivity to
estrogen.
In a previous study, it was demonstrated that the
estradiol-mediated ERα signaling pathway, under the transcriptional
regulation of FOXP3, controls regulatory T-cell (Treg
cell) functions in patients with cervical cancer (25). The authors found high levels of both
estradiol and ERα in Treg cells
(CD4+CD25highCD127low cells)
isolated from cervical cancer tissues, suggesting an important role
of ERα in the biology of Treg cell subsets, whose
suppressive activity can be enhanced by increasing FOXP3
expression. Marked FOXP3 expression was observed in Treg
cells derived from the peripheral blood and tumor tissue in
patients with cervical cancer; however, minimal expression was
noted in effector T cells and a total lack of expression was noted
in naїve T cell populations from cervical cancer. To support this
finding, a specific ERα antagonist, fulvestrant, was used, which
significantly reduced the expression levels of both ERα and FOXP3
in cervical tumor Treg cells. The T-cell response to
estradiol is mainly mediated by the intracellular expression of ERα
and ERβ, but their levels may differ according to cell
distribution, including in FOXP3+ subsets, within the
tumor mass (25,38). In the present study, TIL subsets in
endometrial tissue showed decreased ERα levels, which is partially
consistent with the results of other immunofluorescence assays
where a lack of ERα expression in a CD45+
hematopoietic-lineage subset and in CD68+ macrophages
derived from cervical tumors was observed (39). ERα expression in the tumor cell
environment is mainly restricted to cells negative for FSP-1 and
CD34 (fibrocytes) and positive for α-smooth muscle actin,
demonstrating that ERα may act primarily through paracrine
stromal-to-tumor signaling (39).
ERα signaling may remodel the TME by exerting a
direct effect on proliferation and suppressing the activity of both
CD4+ and CD8+ T cells (39). The inhibition of ERα signaling could
enhance the response to immune check-point blockade (ICB) therapy,
which has been observed in a murine melanoma model (40). In this model, a tumor cell extrinsic
activity of ERα increased the accumulation of macrophages and
activated tumor-associated macrophages in the TME, suppressing
adaptive immunity and promoting tumor growth. This activity was not
observed in macrophages lacking ERα expression. Inhibition of ERα
expression with fulvestrant decreased myeloid cell growth and
increased the antitumor efficacy of ICB.
In the present study, a significant decrease in ERα
expression (presented as MFI) in all studied TIL populations
derived from endometrial tumors compared with normal endometrial
tissue was observed. However, no significant correlations were
observed between ERα expression and relevant clinicopathological
features, with the exception of ERα+ B cells, the
frequency of which was significantly higher in MMR-d tumors. The
functions of B cells in the TME of patients with EC remain
essentially unknown as there have been few relevant studies;
however, Guo et al (41)
observed a similar trend of increased B cell levels in MMR-d tumors
and noted that an improved prognosis was associated with higher
levels of CD20+ B cells. Nevertheless, the increased
frequency of ERα-positive B cells in the TME of patients with EC
classified as MSI cluster demonstrated in the present study, as
well as in high-grade endometrial tumors, requires further
investigation.
Obesity is well established as the leading
modifiable risk factor for the development of EC (42). After menopause (90% of the
population in the present study), ovarian estradiol synthesis stops
and adrenal androstenedione is converted, mostly in the fat tissue,
by aromatase into estrone (43).
Consequently, women with obesity present with 2 to 4-fold higher
estrone levels, which correlate with a greater risk of ER-positive
postmenopausal breast and endometrial cancer (44,45).
Previous studies have shown that an increasing BMI (>30) is
inversely correlated with CD8+ tumor infiltration (but
not with other immune cell subsets), and therefore obesity may
reduce cytotoxic T cell immune surveillance of the endometrium;
however, this mechanism can be reversed with weight loss (46,47).
This observation could not be confirmed in the present study as the
CD8+ subset was not decreased; however, the density of
Th cells (CD4+) was significantly decreased in obese
patients (BMI >35), suggesting a possible role for these cells
in cancer growth in this specific population prone to developing
EC. Notably, in the present study, a decrease in ERα expression in
TILs was observed, which was correlated with a higher BMI. The
significance of this phenomenon remains unknown, but the results
suggest that maintaining a lower BMI may reduce estrogen-induced
stimulation and modulate ERα expression in the TILs of the TME,
potentially limiting the risk of ERα-positive EC in postmenopausal
women.
The limitations of the present study include only
focusing on ERα expression, resulting in an incomplete picture of
the role of the ER family in TILs from endometrial tumors. ERβ may
also be expressed in TILs and thus requires further investigation.
The present study lacked functional validation experiments to
verify the effect of ERα downregulation on the antitumor functions
of TILs; however, the present study was designed as a pilot study
and its small sample size (only 54 cases included) prevented
further validation.
In conclusion, the results of the present study
emphasized that TILs derived from EC tissue exhibit decreased ERα
expression compared with healthy endometrial tissue. We hypothesize
that estrogenic stimulation may regulate the TME in patients with
EC, primarily through the ERα signaling pathway. This pathway
influences the immune system (namely TILs), which in turn may
modulate ERα expression through unknown paracrine effects. Future
validation studies on TILs showing decreased ERα expression in
terms of functions such as cytotoxicity and cytokine secretion, may
help to understand this phenomenon. Subsequent investigation of the
molecular mechanisms underlying ERα downregulation in TILs, such as
ERα gene methylation and transcription factors, may prove crucial
in translating this finding into clinical practice in the diagnosis
and treatment of endometrial tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by statutory subsidies of the
Polish Ministry of Health as part of the Department of Oncology
Wroclaw Medical University research grant (grant nos.
SUBZ.C280.24.063 and SUBZ.C280.25.016).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MJ, AS, PK, DL, AK, AC, RM and ACS confirm the
authenticity of all the raw data. MJ, AS, PK, DL, AK, AC, RM and
ACS contributed to the design of the study. MJ, AS, PK, DL, AK and
AC made substantial contributions to the acquisition of data. MJ,
AS, PK, DL, AK, AC, RM and ACS made substantial contributions to
the analysis and interpretation of data. MJ, AS and AK contributed
to data curation by carefully and thoroughly collecting and
reviewing key data. All authors read and approved to the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Wroclaw
Medical University Bioethics Committee (Wroclaw, Poland;
registration no. 166/2019, dated March 5, 2019), which reviews
studies at cooperating hospitals in the region. Written informed
consent for the participation in this study was obtained from each
qualified patient prior to surgery, in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EC
|
endometrial cancer
|
|
ER
|
estrogen receptor
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
TCGA
|
The Cancer Genome Atlas
|
|
POLE
|
polymerase ε
|
|
MSI
|
microsatellite instability
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
OS
|
overall survival
|
|
FoxP3
|
forkhead box P3
|
|
PD-1
|
programmed cell death-1
|
|
PD-L1
|
programmed cell death ligand-1
|
|
ESGO
|
European Society of Gynecological
Oncology
|
|
ESTRO
|
European Society for Radiotherapy and
Oncology
|
|
ESP
|
European Society of Pathology
|
|
Th cells
|
T helper lymphocytes
|
|
B cells
|
B lymphocytes
|
|
CTLs
|
cytotoxic T lymphocytes
|
|
MFI
|
median fluorescence intensity
|
|
BMI
|
body mass index
|
|
NSMP
|
non-specific molecular profile
|
|
MMR-d
|
mismatch repairs deficient
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Corr BR, Erickson BK, Barber EL, Fisher CM
and Slomovitz B: Advances in the management of endometrial cancer.
BMJ. 388:e0809782025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:101983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al:
Integrated genomic characterization of endometrial carcinoma.
Nature. 497:67–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mittica G, Ghisoni E, Giannone G, Aglietta
M, Genta S and Valarega G: Checkpoint inhibitors in endometrial
cancer: Preclinical rationale and clinical activity. Oncotarget.
8:90532–90544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Key TJ and Pike MC: The Dose-effect
relationship between ‘unopposed’ oestrogens and endometrial mitotic
rate: Its central role in explaining and predicting endometrial
cancer risk. Br J Cancer. 57:205–212. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klinge CM: miRNAs regulated by estrogens,
tamoxifen, and endocrine disruptors and their downstream gene
targets. Mol Cell Endocrinol. 418:273–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan KKL, Siu MKY, Jiang YX, Wang JJ, Wang
Y, Leung THY, Liu SS, Cheung ANY and Ngan HYS: Differential
expression of estrogen receptor subtypes and variants in ovarian
cancer: Effects on cell invasion, proliferation and prognosis. BMC
Cancer. 17:6062017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jameera Begam A, Jubie S and Nanjan MJ:
Estrogen receptor agonists/antagonists in breast cancer therapy: A
critical review. Bio Org Chem. 71:257–274. 2017.PubMed/NCBI
|
|
10
|
Hu G, Zhang J, Zhou X, Liu J, Wang Q and
Zhang B: Roles of estrogen receptor α and β in the regulation of
proliferation in endometrial carcinoma. Pathol Res Pract.
216:1531492020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Backes FJ, Walker CJ, Goodfellow P, Hade
EM, Agarwal G, Mutch D, Cohn DE and Suarez AA: Estrogen
receptor-alpha is a predictive biomarker in endometrioid
endometrial cancer. Gynecol Oncol. 141:312–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ning C, Xie B, Zhang L, Li C, Shan W, Yang
B, Luo X, Gu C, He Q, Jin H, et al: Infiltrating macrophages induce
ERα expression through an IL17A-mediated epigenetic mechanism to
sensitize endometrial cancer cells to estrogen. Cancer Res.
76:1354–1366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wik E, Ræder MB, Krakstad C, Trovik J,
Birkeland E, Hoivik EA, Mjos S, Werner HMJ, Mannelqvist M,
Stefansson IM, et al: Lack of estrogen receptor-α is associated
with epithelial-mesenchymal transition and PI3K alterations in
endometrial carcinoma. Clin Cancer Res. 19:1094–1105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki M, Kotcherguina L, Dharia A,
Fujimoto S and Dahiya R: Cytosine-phosphoguanine methylation of
estrogen receptors in endometrial cancer. Cancer Res. 15:3262–3266.
2001.PubMed/NCBI
|
|
15
|
Shiozawa T, Itoh K, Horiuchi A, Konishi I,
Fujii S and Nikaido T: Down-regulation of estrogen receptor by the
methylation of the estrogen receptor gene in endometrial carcinoma.
Anticancer Res. 22:139–143. 2002.PubMed/NCBI
|
|
16
|
Navari JR, Roland PY, Keh P, Salvesen HB,
Akslen LA, Iversen OE, Das S, Kothari R, Howey S and Phillips B:
Loss of estrogen receptor (ER) expression in endometrial tumors is
not associated with de novo methylation of the 5′end of the ER
gene. Clin Cancer Res. 6:4026–4032. 2000.PubMed/NCBI
|
|
17
|
Chavez JA, Wei L, Suarez AA, Parwani AV
and Li Z: Clinicopathologic characteristics, tumor infiltrating
lymphocytes and programmed cell death ligand-1 expression in 162
endometrial carcinomas with deficient mismatch repair function. Int
J Gynecol Cancer. 29:113–118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Jong RA, Leffers N, Boezen HM, Ten Hoor
KA, Van der Zee AG, Hollema H and Nijman HW: Presence of
Tumor-infiltrating Lymphocytes is an independent prognostic factor
in type I and II endometrial cancer. Gynecol Oncol. 114:105–110.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Čermáková P, Melichar B, Tomšová M, Zoul
Z, Kalábová H, Spaček J and Doležel M: Prognostic significance of
CD3+ tumorinfiltrating lymphocytes in patients with endometrial
carcinoma. Anticancer Res. 34:5555–5561. 2014.PubMed/NCBI
|
|
20
|
Jung IK, Kim SS, Suh DS, Kim KH, Lee CH
and Yoon MS: Tumor-infiltration of T-lymphocytes is inversely
correlated with clinicopathologic factors in endometrial
adenocarcinoma. Obstet Gynecol Sci. 57:266–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo F, Dong Y, Tan Q, Kong J and Yu B:
Tissue infiltrating immune cells as prognostic biomarkers in
endometrial cancer: A Meta-analysis. Dis Markers. 2020:18057642020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Talhouk A, Derocher H, Schmidt P, Leung S,
Milne K, Gilks CB, Anglesio MS, Nelson BH and McAlpine JN:
Molecular subtype not immune response drives outcomes in
endometrial carcinoma. Clin Cancer Res. 25:2537–2548. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willvonseder B, Stögbauer F, Steiger K,
Jesinghaus M, Kuhn PH, Brambs C, Engel J, Bronger H, Schmidt GP,
Haller B, et al: The immunologic tumor microenvironment in
endometrioid endometrial cancer in the morphomolecular context:
Mutual correlations and prognostic impact depending on molecular
alterations. Cancer Immunol Immunother. 70:1679–1689. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Howitt BE, Shukla SA, Sholl LM,
Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC,
D'Andrea AD, Wu CJ, et al: Association of polymerase e-Mutated and
Microsatellite-instable endometrial cancers with neoantigen load,
number of Tumor-infiltrating lymphocytes, and expression of PD-1
and PD-L1. JAMA Oncol. 1:1319–1323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adurthi S, Kumar MM, Vinodkumar HS,
Mukherjee G, Krishnamurthy H, Acharya KK, Bafna UD, Uma DK,
Abhishekh B, Krishna S, et al: Oestrogen Receptor-α binds the FOXP3
promoter and modulates regulatory T-cell function in human cervical
cancer. Sci Rep. 7:72892017. View Article : Google Scholar
|
|
26
|
Stender JD, Kim K, Charn TH, Komm B, Chang
KCN, Kraus WL, Benner C, Glass CK and Katzenellenbogen BS:
Genome-wide analysis of estrogen receptor alpha DNA binding and
tethering mechanisms identifies Runx1 as a novel tethering factor
in receptor-mediated transcriptional activation. Mol Cell Biol.
30:3943–3955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jedryka M, Chrobak A, Chelmonska-Soyta A,
Fijałkowska D and Matkowski R: Decreased expression of estrogen
receptors alpha and beta in peripheral blood lymphocytes from the
endometrial cancer patients and women with endometriosis. Eur J
Gynaecol Oncol. 42:375–379. 2020.
|
|
28
|
Laczmanska I, Michalowska D, Jedryka M,
Blomka D, Semeniuk M, Czykalko E, Abrahamowska M, Mlynarczykowska
P, Chrusciel A, Pawlak I and Maciejczyk A: Fast and reliable sanger
POLE sequencing protocol in FFPE tissues of endometrial cancer.
Pathol Res Pract. 242:1543152023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynecol Obstet. 143 (Suppl
2):S37–S50. 2018. View Article : Google Scholar
|
|
30
|
Concin N, Matias-Guiu X, Vergote I, Cibula
D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Int J Gynecol Cancer. 31:12–39. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pascual-Garcia M, Bertolo C, Nieto JC,
Serrat N, Espinosa I, D'Angelo E, Munoz R, Rovira R, Vidal S and
Prat J: CD8 Down-regulation on cytotoxic T lymphocytes of patients
with endometrioid endometrial carcinomas. Hum Pathol. 56:180–188.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong D, Lei H, Liu D, Bai H, Yang Y, Tang
B, Li K, Liu J, Xu G and Xiao X: POLE and mismatch repair status,
checkpoint proteins and tumor-infiltrating lymphocytes in
combination, and tumor differentiation: Identify endometrial
cancers for immunotherapy. Front Oncol. 11:6400182021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mendiola M, Pellinen T, Ramon-Patino JL,
Berjon A, Bruck O, Heredia-Soto V, Turrki R, Escudero J, Hemmes A,
Garcia de la Calle LE, et al: Prognostic implications of
tumor-infiltrating T cells in early-stageendometrial cancer. Mod
Pathol. 35:256–265. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osorio JC and Zamarin D: Beyond T cells:
IgA incites immune recognition in endometrial cancer. Cancer Res.
82:766–768. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mandal G, Biswas S, Anadon CM, Yu X,
Gatenbee CD, Prabhakaran S, Payne KK, Chaurio RA, Martin A,
Innamarato P, et al: IgA-dominated humoral immune responses govern
patients' outcome in endometrial cancer. Cancer Res. 82:859–871.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ge Y, Ni X, Li J, Ye M and Jin X: Roles of
estrogen receptor α in endometrial carcinoma (review). Oncol Lett.
26:5302023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kreizman-Shefer H, Pricop J, Goldman S,
Elmalah I and Shalev E: Distributions of estrogen and progesterone
receptor isoforms in endometrial cancer. Diagn Pathol. 9:772014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rae JM and Lippman ME: The role of
estrogen receptor signaling in suppressing the immune response to
cancer. J Clin Invest. 131:e1554762021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chakraborty B, Byemerwa J, Shepherd J,
Haines CN, Baldi R, Gong W, Liu W, Mukherjee D, Artham S, Lim F, et
al: Inhibition of estrogen signaling in myeloid cells increases
tumor immunity in melanoma. J Clin Invest. 131:e1513472021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo YE, Liu Y, Zhang W, Luo H, Shu P, Chen
G and Li Y: The clinicopathological characteristics, prognosis and
immune microenvironment mapping in MSI-H/MMR-D endometrial
carcinomas. Discov Oncol. 13:122022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van der Woude H, Hally KE, Currie MJ,
Gasser O and Henry CE: Importance of the endometrial immune
environment in endometrial cancer and associated therapies. Front
Oncol. 12:9752012022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siiteri PK: Review of studies on estrogen
biosynthesis in the human. Cancer Res. 42 (8 Suppl):3269S–3273S.
1982.PubMed/NCBI
|
|
44
|
Vincze B, Kapuvári B, Udvarhelyi N,
Horváth Z, Mátrai Z, Czeyda-Pommersheim F, Kohalmy K, Kovacs J,
Boldizsar M, Lang I, et al: Serum estrone concentration, estrone
sulfate/estrone ratio and BMI are associated with human epidermal
growth factor receptor 2 and progesterone receptor status in
postmenopausal primary breast cancer patients suffering invasive
ductal carcinoma. Springerplus. 4:3872015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Busch EL, Crous-Bou M, Prescott J, Chen
MM, Downing MJ, Rosner BA, Mutter GL and De Vivo I: Endometrial
cancer risk factors, hormone receptors, and mortality prediction.
Cancer Epidemiol Biomarkers Prev. 26:727–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dyck L, Prendeville H, Raverdeau M, Wilk
MM, Loftus RM, Douglas A, McCormack J, Moran B, Wilkinson M, Mills
EL, et al: Suppressive effects of the obese tumor microenvironment
on CD8 T cell infiltration and effector function. J Exp Med.
219:e202100422022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Naqvi A, MacKintosh ML, Derbyshire AE,
Tsakiroglou AM, Walker TDJ, McVey RJ, Bolton J, Fergie M, Bagley S,
Ashton G, et al: The impact of obesity and bariatric surgery on the
immune microenvironment of the endometrium. Int J Obes. 46:605–612.
2022. View Article : Google Scholar : PubMed/NCBI
|