Introduction
Neuroblastoma (NB) is the most common extracranial
solid tumor in children, accounting for 8–10% of all pediatric
cancers and 15% of cancer-related deaths worldwide (1,2).
According to the Global Burden of Disease 2021 report, the
incidence rate increased from 0.25 cases per 100,000 individuals in
1990 to 0.28 cases per 100,000 individuals in 2021, representing an
overall increase of 12% (3). NB,
which originates from neural crest cells of the sympathetic nervous
system, is a biologically and clinically heterogeneous tumor.
High-risk NB (HR-NB), which constitutes ~50% of new diagnoses,
typically presents with metastases (e.g. bone marrow [BM], bone and
lymph node metastases), MYCN proto-oncogene bHLH transcription
factor (MYCN) amplification or unfavorable histological features.
These factors are strongly associated with a poor prognosis, with a
5-year overall survival (OS) rate of <50% (4,5).
Despite intensive therapy, relapsed or refractory (R/R) disease
remains the primary cause of treatment failure, and ~60% of HR
patients experience disease recurrence. The survival rate following
relapse is <20%, highlighting the urgent need for novel and more
effective therapeutic strategies (6).
Immunotherapy, particularly targeting
disialoganglioside GD2, which is highly expressed on NB cells, has
emerged as a promising option for R/R NB. Dinutuximab beta, a
monoclonal GD2-targeting antibody, was approved in China in August
2021 for treating patients with HR-NB who were ≥12 months old,
including R/R patients (7). The
National Comprehensive Cancer Network guidelines recommend the use
of anti-GD2 immunotherapy in combination with chemotherapy to
enhance tumor remission and survival in patients with R/R NB
(8). An increasing number of
studies have also provided empirical medical evidence for the
treatment strategy of chemoimmunotherapy. The BEACON-Immuno phase
II trial showed that dinutuximab beta plus temozolomide/topotecan
improved overall response rate (ORR) by 17% and 1-year
progression-free survival (PFS) rate from 27 to 57% compared with
chemotherapy alone (9). Two other
studies have also provided supportive evidence for the efficacy of
chemoimmunotherapy in R/R NB. A phase II Children's Oncology Group
study demonstrated that dinutuximab combined with irinotecan,
temozolomide and granulocyte-macrophage colony-stimulating factor
(GM-CSF) was tolerable and resulted in clinically meaningful
responses in heavily pretreated patients (10), while a recent meta-analysis reported
that GD2-targeting monoclonal antibody therapy combined with
chemotherapy significantly improved tumor remission rates,
event-free survival and OS in this population (11). A retrospective study of dinutuximab
beta combined with chemotherapy in R/R NB showed 64% of ORR, with
32% achieving complete response (CR). Notably, 31% of responders
achieved a CR or partial response (PR) after 6 to 8 cycles, with
some benefiting from up to 10 cycles of treatment, suggesting that
extended immunotherapy may improve outcomes (12).
Further studies are needed to optimize the duration
of immunotherapy and enhance its efficacy in this
difficult-to-treat population. The present retrospective study
assessed the efficacy and safety of extended treatment cycles of
dinutuximab beta and chemotherapy in patients with R/R NB who had
undergone >5 cycles of dinutuximab beta immunotherapy.
Materials and methods
Study design and patients
The present study was a single-center, retrospective
study that included patients with R/R NB who were treated in the
Department of Pediatric Oncology, Shandong Cancer Hospital and
Institute, Shandong First Medical University and Shandong Academy
of Medical Sciences (Jinan, China), between January 2022 and
October 2024. Eligible patients were classified as HR according to
the International Neuroblastoma Risk Group (INRG) Staging System
(13), and were ≥12 months old at
the initiation of dinutuximab beta treatment. Patients with R/R NB
who received >5 cycles of dinutuximab beta were included in the
study. Refractory NB was defined as either the patient that failed
to achieve a PR or CR after induction chemotherapy or one in which
the residual lesion was confirmed as active disease following
induction and consolidation therapy [as assessed by
18F-NOTATATE positron emission tomography/computed
tomography (PET/CT), iodine-123 or iodine-131
meta-iodobenzylguanidine scintigraphy, or biopsy]. Relapsed NB was
defined as the patient that achieved a CR after previous intensive
multimodal treatment for 4 weeks, followed by the emergence of new
active lesions. Patients with concurrent malignancies other than NB
or those who had not ended dinutuximab beta immunotherapy were
excluded. The study was approved by the Ethics Committee of
Shandong First Medical University and Shandong Academy of Medical
Sciences (approval no. SDTHEC202503192).
Treatment regimen
The treatment regimen included dinutuximab beta
combined with chemotherapy, GM-CSF and isotretinoin. Dinutuximab
beta was administered through central venous access as a continuous
long-term infusion at a dose of 10 mg/m2/day from days 1
to 10 of each 35-day cycle. GM-CSF was administered at a dose of
250 µg/m2/day from days 6 to 12. GM-CSF was discontinued
if the absolute neutrophil count was >20×109/l.
Isotretinoin was orally administered at a dose of 160
mg/m2/day, in two divided doses from days 15 to 28.
Chemotherapy was also administered, and the regimens were mainly
temozolomide-based, including 1.5 mg/m2 vincristine on
day 1, 50 mg/m2 irinotecan on days 1 to 5, 100
mg/m2 temozolomide on days 1 to 5
(vincristine-irinotecan-temozolomide [VIT] regimen), or
platinum-based chemotherapy. The latter included the DE regimen,
consisting of cisplatin at 20 mg/m2 and etoposide at 100
mg/m2, both administered on days 1 to 4, the OPET
regimen, consisting of vincristine at 1.5 mg/m2 on day
1, cisplatin at 25 mg/m2, etoposide at 100
mg/m2 and temozolomide at 100 mg/m2, each
administered on days 1 to 3, and the OPEC regimen, consisting of
vincristine at 1.5 mg/m2 on day 1, cisplatin at 25
mg/m2, etoposide at 100 mg/m2 and
cyclophosphamide at 400 mg/m2, each administered on days
1 to 3. The treatment regimen is illustrated in Fig. 1.
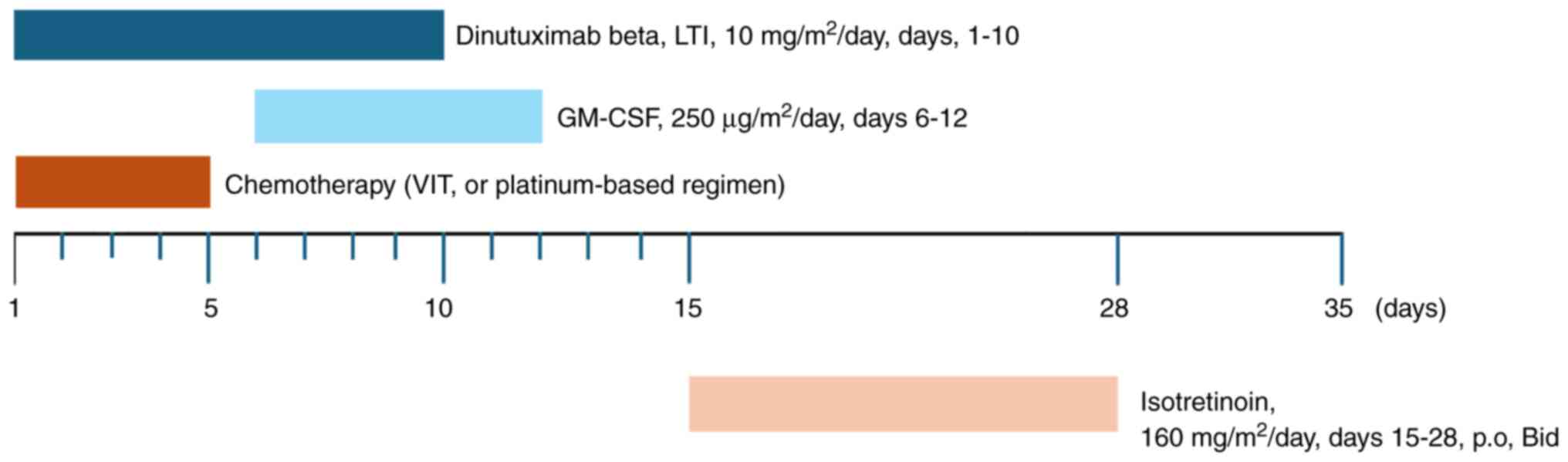 | Figure 1.Treatment regimens and dosing
schedules of chemoimmunotherapy used in relapsed or refractory
neuroblastoma. The VIT regimen consisted of 1.5 mg/m2
vincristine on day 1, 50 mg/m2 irinotecan on days 1–5
and 100 mg/m2 temozolomide on days 1–5. The
platinum-based regimens included DE (20 mg/m2 cisplatin
on days 1–4 and 100 mg/m2 etoposide on days 1–4), OPET
(1.5 mg/m2 vincristine on day 1, 25 mg/m2
cisplatin on days 1–3, 100 mg/m2 etoposide on days 1–3
and 100 mg/m2 temozolomide on days 1–3) or OPEC (1.5
mg/m2 vincristine on day 1, 25 mg/m2
cisplatin on days 1–3, 100 mg/m2 etoposide on days 1–3
and 400 mg/m2 cyclophosphamide on days 1–3). LTI,
long-term infusion; GM-CSF, granulocyte-macrophage
colony-stimulating factor; p.o., oral; Bid, twice a day. |
Regarding dinutuximab beta immunotherapy, if a CR
was achieved after 5 cycles, an additional 2 to 4 cycles could be
administered based on the clinical evaluation and patients
conditions, to maintain CR and eradicate minimal residual disease.
If a CR was not achieved, in consideration of the absence of other
treatment measures with greater advantages, it was recommended to
extend the immunotherapy cycles of dinutuximab beta and evaluate
once every 2 or 3 cycles until the best response was achieved, or
until disease progression or intolerable toxicity.
Assessment and outcomes
The overall tumor response was assessed using the
revised International Neuroblastoma Response Criteria (14). BM involvement was detected by the
presence of tumor cells in the BM by immunocytology and
immunohistochemistry. Bone and soft tissues were evaluated using
18F-NOTATATE-PET/CT imaging. Owing to the unavailability
of 68Ga, the 18F-NOTATATE-PET/CT imaging
technique is adopted for tumor assessment in Shandong Cancer
Hospital and Institute. The imaging mechanism is comparable with
that of 68Ga-DOTATATE-PET/CT (15,16),
as both involve the imaging of octreotide derivatives.
The primary outcome was the best ORR (most favorable
response achieved by a patient at any time during the treatment
period, calculated as the proportion of patients with best
response) during the immunotherapy period. The secondary outcomes
were ORR [proportion of patients achieving a tumor response (CR or
PR)], disease-control-rate (DCR), PFS, OS and safety. The best
response was defined as the most favorable tumor response observed
from the initiation of immunotherapy to the end of therapy. PFS
time was defined as the time from the initiation of dinutuximab
beta to disease progression or death from any cause, or until the
last contact with the patient. OS time was defined as the time from
the initiation of dinutuximab beta to death from any cause, or
until the last contact with the patient.
Patients were monitored for adverse events (AEs)
following the Common Terminology Criteria for Adverse Events
Version 5.0 (17), Grade ≥3
treatment-related AEs (TRAEs) were reported.
Statistical analysis
Patients with residual disease prior to
immunotherapy were included in the ORR and DCR analyses, assuming a
binomial distribution with 95% confidence intervals (CIs). PFS and
OS were estimated using the Kaplan-Meier method, with comparisons
via log-rank test. The 2-year PFS and OS rates were reported with
95% CIs. Stratified analysis assessed PFS and OS based on
pre-immunotherapy disease status (CR vs. non-CR). Cox proportional
hazards regression analysis was performed to assess the prognostic
impact of clinical outcomes. Median follow-up was estimated using
the inverse Kaplan-Meier method. All analyses were conducted using
SPSS v22.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 30 patients were included, with a median
age at diagnosis of 3.9 years (range, 0.6–9.7 years), and a median
age at the initiation of dinutuximab beta treatment of 5.1 years
(range, 2.0–11.1 years). Among them, 24 patients (80%) had
refractory NB and 6 patients (20%) had relapsed NB. Furthermore, 3
patients (10%) had MYCN amplification, 13 patients (43.3%) had 11q
deletion and 3 patients (10%) had 1p deletion. All patients were
diagnosed as stage 4 according to the International Neuroblastoma
Staging System, and stage M according to the INRG Staging System. A
total of 28 patients (93.3%) were observed with a primary tumor in
the abdomen. The baseline characteristics are presented in Table I.
 | Table I.Patient (n=30) and disease
characteristics. |
Table I.
Patient (n=30) and disease
characteristics.
| Characteristics | Value |
|---|
| Age, years |
|
| Median
age at diagnosis (range) | 3.9 (0.6–9.7) |
| Median
age at the initiation of dinutuximab beta treatment (range) | 5.1 (2.0–11.1) |
| Sex, n (%) |
|
|
Female | 13 (43.3) |
| Male | 17 (56.7) |
| Disease type, n
(%) |
|
|
Relapsed | 6 (20.0) |
|
Refractory | 24 (80.0) |
| MYCN status, n
(%) |
|
|
Amplified | 3 (10.0) |
|
Non-amplified | 27 (90.0) |
|
Unknown | 0 (0.0) |
| 11q deletion, n
(%) |
|
|
Yes | 13 (43.3) |
| No | 11 (36.7) |
|
Unknown | 6 (20.0) |
| 1p deletion, n
(%) |
|
|
Yes | 3 (10.0) |
| No | 21 (70.0) |
| Unknown | 6 (20.0) |
| INSS stage at
diagnosis, n (%) |
|
| Stage
4 | 30 (100.0) |
| INRG stage at
diagnosis, n (%) |
|
| Stage
M | 30 (100.0) |
| Primary tumor
localization, n (%) |
|
|
Abdomen | 28 (93.3) |
|
Chest | 1 (3.3) |
|
Neck | 0 (0.0) |
|
Other | 1 (3.3) |
Prior to dinutuximab beta treatment, 10 patients
(33.3%) had no residual disease and achieved a CR, 2 patients
(6.7%) achieved a PR, 14 patients (46.7%) achieved a minor response
and 4 patients (13.3%) experienced progressive disease (PD). Among
the 20 patients with residual disease, 12 (60%) had bone metastases
solely, 4 (20%) had both bone and soft tissue metastases, 2 (10%)
had bone and BM metastases, 1 patient (5%) had BM metastasis
solely, and 1 patient (5%) had bone, soft tissue and BM metastases
together. Additionally, 23 of the total patients (76.7%) had
undergone stem cell transplantation, and 28 patients (93.3%) had
undergone radiation therapy. All patients had received chemotherapy
in their previous treatment, and the median number of cycles of
previous chemotherapy was 10. All patients received dinutuximab
beta immunotherapy, which was combined with chemotherapy; 26
patients (86.7%) received a temozolomide-based chemotherapy
regimen, whereas 13 patients (43.3%) received a platinum-based
chemotherapy regimen (12 patients received both regimens). All
patients received immunotherapy, with the median number of cycles
of dinutuximab beta treatment being 7 cycles, ranging from 6 to 12
cycles. The median follow-up time was 18 months (range, 9–35
months). The details related to clinical treatment are presented in
Table II.
 | Table II.Clinical characteristics and disease
status of all patients (n=30) prior to immunotherapy. |
Table II.
Clinical characteristics and disease
status of all patients (n=30) prior to immunotherapy.
|
Characteristics | Value |
|---|
| Status prior to the
initiation of dinutuximab beta, n (%) |
|
| CR | 10 (33.3) |
| PR | 2 (6.7) |
| MR | 14 (46.7) |
| PD | 4 (13.3) |
| Metastases site
prior to dinutuximab beta treatment, n (%) |
|
| Bone
marrow only | 1 (3.3) |
| Bone
only | 12 (40.0) |
| Bone
and bone marrow | 2 (6.6) |
| Bone
and soft tissue | 4 (13.3) |
| Bone
and soft tissue and bone marrow | 1 (3.3) |
| Stem cell
transplantation, n (%) |
|
| Single
transplantation | 22 (73.3) |
| Tandem
transplantation | 1 (3.3) |
| No
transplantation | 7 (23.3) |
| Radiotherapy, n
(%) |
|
|
Yes | 28 (93.3) |
| No | 2 (6.7) |
| Cycles of previous
chemotherapy, n (%) |
|
|
8-9 | 10 (33.3) |
|
10-12 | 12 (40.0) |
|
13-24 | 8 (26.7) |
|
Median | 10 |
| Cycles of
dinutuximab beta treatment, n (%) |
|
| 6 | 10 (33.3) |
| 7 | 9 (30.0) |
| 8 | 5 (16.7) |
| 9 | 3 (10.0) |
| 10 | 2 (6.7) |
| 12 | 1 (3.3) |
| Median | 7 |
| Chemotherapy
regimena in
combination with dinutuximab beta, n (%) |
|
|
Temozolomide-based
chemotherapy | 26 (86.7) |
|
Platinum-based
chemotherapy | 13 (43.3) |
Efficacy
Tumor response
The response rates were evaluated in the 20 patients
with residual disease prior to immunotherapy. During the
immunotherapy period, the best ORR was observed to be 65% (95% CI,
40.8–84.6%), including 6 instances of a CR and 7 of a PR. The best
DCR was observed to be 100.0%, including 6 instances of a CR, 7 of
a PR and 7 of SD. At cycle 3 of dinutuximab beta immunotherapy, the
ORR was 25% (95% CI, 8.6–49.1%), DCR was 95% (95% CI, 75.1–99.9%)
and the CR rate was 15% (95% CI, 3.2–37.9%), while at cycle 5 or 6,
the ORR was 40% (95% CI, 19.1–63.9%), the DCR was 100.0% and the CR
rate was 20% (95% CI, 5.7–43.7%). At the end of dinutuximab beta
immunotherapy, the ORR was 55% (95% CI, 31.5–76.9%), the DCR was
90% (95% CI, 68.3–98.8%) and the CR rate was 30% (95% CI,
11.9–54.3%). It showed that ORR improved by 15% from 40% at the end
of cycle 5 or 6 to 55% at the end of the extended cycles of
dinutuximab beta immunotherapy; moreover, the CR rate improved by
10% from 20% at the end of cycle 5 or 6 to 30% at the end of
extended immunotherapy. The detailed data are presented in Table III.
 | Table III.Responses at cycles 3 and 5/6, and at
the end of dinutuximab beta immunotherapy in 20 patients with
residual active disease. |
Table III.
Responses at cycles 3 and 5/6, and at
the end of dinutuximab beta immunotherapy in 20 patients with
residual active disease.
| Category | After cycle 3 | After cycle
5/6 | After the end of
immunotherapy | Best response |
|---|
| CR, n (%) | 3 (15) | 4 (20) | 6 (30) | 6 (30) |
| PR, n (%) | 2 (10) | 4 (20) | 5 (25) | 7 (35) |
| MR, n (%) | 0 (0) | 0 | 0 | 0 (0) |
| SD, n (%) | 14 (70) | 12 (60) | 7 (35) | 7 (35) |
| PD, n (%) | 1 (5) | 0 (0) | 2 (10) | 0 (0) |
| ORR, % | 25 | 40 | 55 | 65 |
| DCR, % | 95 | 100 | 90 | 100 |
Notably, 13 patients showed the best tumor response,
5 patients achieved the best response after >5 cycles of
dinutuximab beta treatment, including 2 patients with SD at the end
of cycle 5 who improved to CR after cycles beyond the standard 5,
and 3 patients who converted from SD to PR, implying 38.5% (5/13
patients) showed improvement from cycles beyond the standard 5 of
immunotherapy. Additionally, among the 10 patients without residual
disease prior to immunotherapy, the CR maintenance rate was 90%
(95% CI, 55.5–99.7%) after cycles beyond the standard 5, with 9
patients achieving a CR and 1 experiencing PD. The disease status
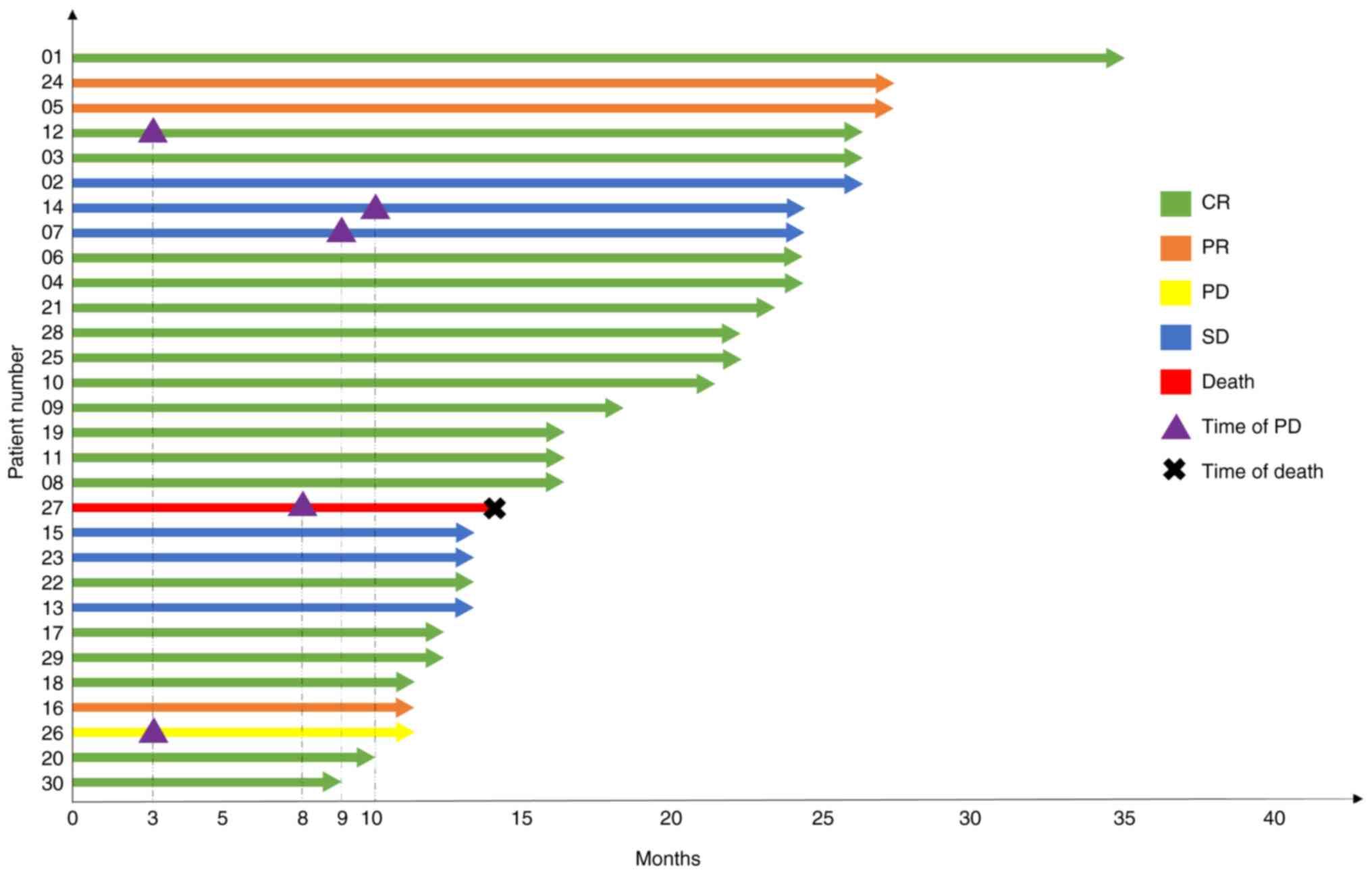
of individual patients is illustrated in Fig. 2, and the detailed individual
response is presented in Table
SI.
Tumor response in a subgroup of
patients
At the end of extended dinutuximab beta
immunotherapy, among the 6 patients with relapsed NB, the ORR was
33.3% (2/6 patients) and the DCR was 66% (4/6 patients). Among
these 6 patients, 1 achieved a CR, 1 achieved a PR, 2 reported SD
and 2 experienced PD. Among the 24 patients with refractory NB, the
ORR was 75% (18/24 patients) and the DCR was 95.8% (23/24
patients), including 14 patients who achieved a CR, 4 who achieved
a PR, 5 who reported SD and 1 who had developed PD. Numerically,
patients with refractory disease achieved a better tumor response
compared with those with relapsed disease.
Among the 12 patients with only bone metastasis
prior to the initiation of dinutuximab beta therapy, the ORR was
58.3% (7/12 patients) and DCR was 91.7% (11/12 patients). At the
end of dinutuximab beta treatment, 4 patients achieved a CR, 3
achieved a PR, 4 noted SD and 1 experienced PD. For 1 patient with
isolated BM metastasis, a SD state remained throughout the
treatment period. Notably, it was found that a CR was achieved at
the last follow-up examination after treatment cessation. It was
speculated that this was due to a delayed effect associated with
dinutuximab beta immunotherapy.
Additionally, 2 patients had concurrent active
lesions in both bone and BM. Of them, 1 patient who initially
experienced SD achieved a CR subsequently, whereas the other
patient experienced PD. Among the 4 patients with concurrent active
soft-tissue and bone metastases, 2 achieved a CR, 1 achieved a PR
and 1 experienced SD, with an ORR of 75% (3/4 patients) and a DCR
of 100.0% (4/4 patients).
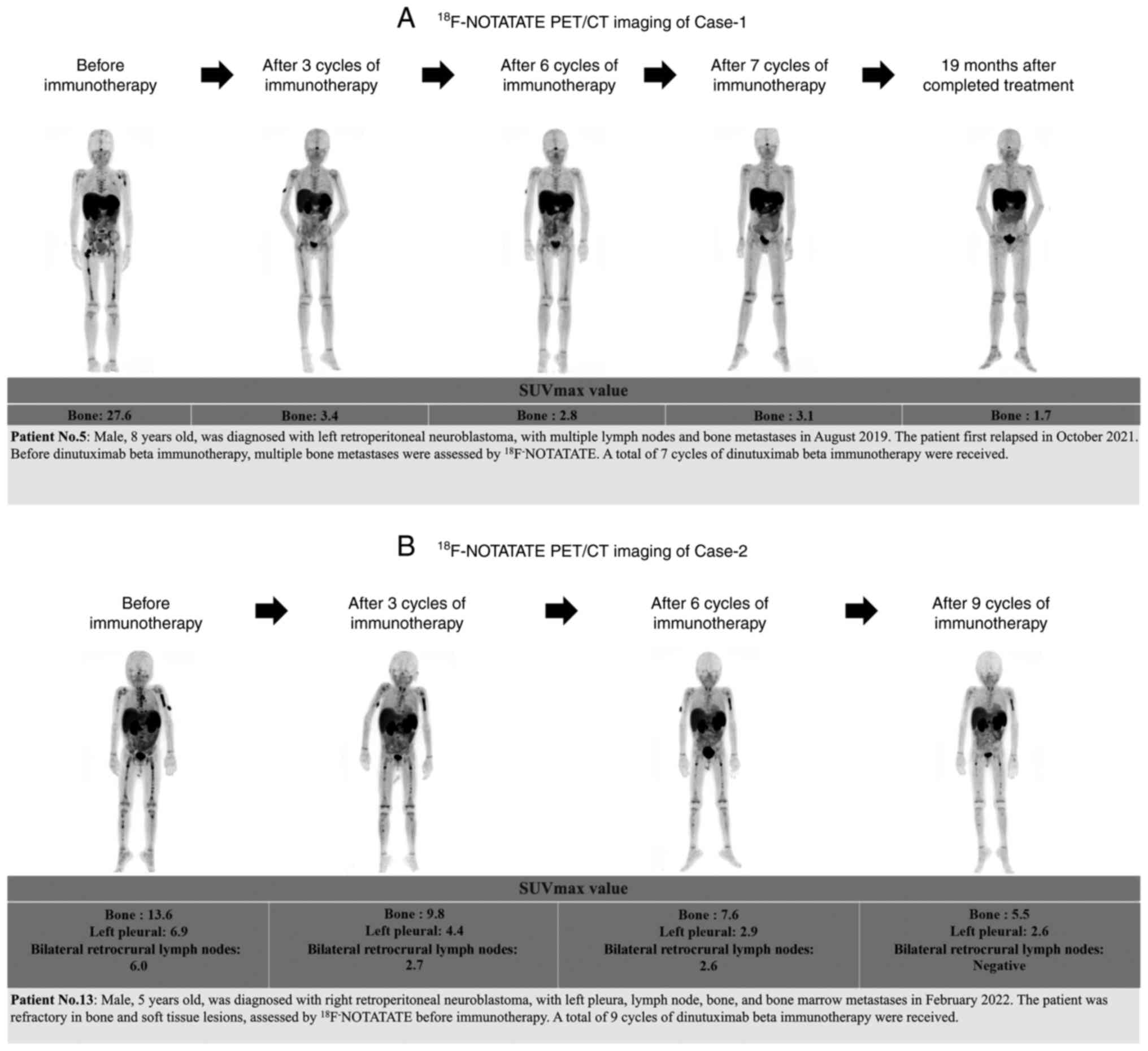
The tumor status at the center was assessed using
18F-NOTATATE-PET/CT imaging prior to dinutuximab beta
therapy, after 3 cycles, after 5/6 cycles and at the end of the
extended treatment cycles. A gradual decrease in tumor activity was
observed with the extension of dinutuximab beta treatment cycles.
Two typical cases and corresponding imaging data, which include
bone or pleura/lymph node metastasis, are presented for reference
in Fig. 3.
Furthermore, among the 23 patients who received a
stem cell transplantation, 13 patients achieved a CR, 4 achieved a
PR, 5 exhibited SD and 1 patient developed PD. The ORR and DCR were
73.9% (17/23 patients) and 95.7% (22/23 patients), respectively.
Among the 7 patients who did not receive a stem cell
transplantation, 2 patients achieved a CR, 1 achieved a PR, 2
exhibited SD and 2 developed PD. The ORR was 42.9% (3/7 patients)
and the DCR was 71.4% (5/7 patients). It appears that patients who
received a stem cell transplantation exhibited a more favorable
tumor response compared with those non-transplanted patients.
Of the 4 patients with PD who received
chemoimmunotherapy, 2 patients achieved a CR, 1 achieved a PR and 1
exhibited SD, with an ORR of 75% (3/4 patients) and a DCR of 100.0%
(4/4 patients), suggesting the beneficial effects of
chemoimmunotherapy for patients with PD. Furthermore, 2 patients
developed PD after receiving 3 cycles of dinutuximab beta
immunotherapy and they continued with co-therapy with a
chemotherapy regimen. One of these patients was assessed as
exhibiting SD after 6 cycles of dinutuximab beta treatment and
subsequently achieved a PR after receiving 10 cycles. However, the
other patient still had active disease after 6 cycles of
dinutuximab beta. Continuing with chemoimmunotherapy may be a
beneficial treatment option for patients with PD.
Survival analysis
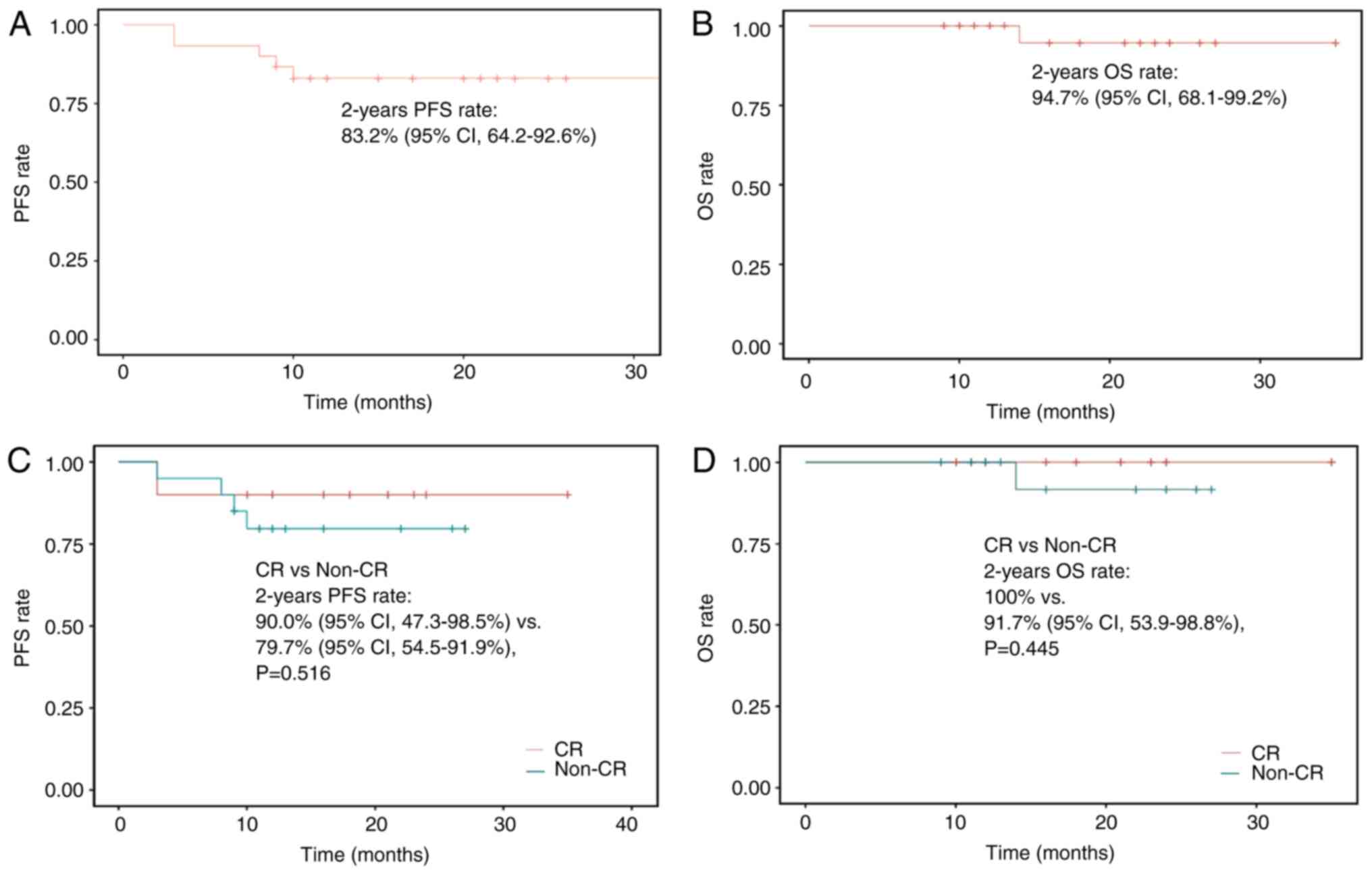
The 2-year PFS rate was 83.2% (95% CI, 64.2–92.6%)
and the 2-year OS rate was 94.7% (95% CI, 68.1–99.2%) for the
overall population (Fig. 4A and B).
Although not statistically significant, the 2-year PFS rate
[(90.0%; 95% CI, 47.3–98.5%) vs. (79.7%; 95% CI, 54.5–91.9%);
P=0.516] and the 2-year OS rate [(100.0%) vs. 91.7% (95% CI,
53.9–98.8%); P=0.445] were numerically higher in patients who
achieved a CR compared with those values in patients who did not
achieve a CR prior to dinutuximab beta immunotherapy (Fig. 4C and D).
The prognostic impact of clinical variables on PFS
was further assessed. The model included tumor status prior to
antibody therapy (CR vs. non-CR), disease type (refractory vs.
relapsed) and history of autologous stem cell transplantation. MYCN
amplification was excluded due to the absence of progression events
in this subgroup. The analysis identified disease type as a
significant predictor, with refractory NB associated with a reduced
risk of progression (hazard ratio, 0.14; 95% CI, 0.02–0.92;
P=0.04). Other variables did not reach statistical significance,
likely due to the limited sample size and number of events.
Detailed results are presented in Fig.
S1.
Safety
During the standard 5 cycles of treatment, the most
common grade ≥3 TRAEs were infections (n=26; 86.7%), neutropenia
(n=25; 83.3%), leukopenia (n=17; 56.7%) and pain (n=16; 53.3%).
These TRAEs persisted into the extended immunotherapy phase, with
higher incidence rates compared with the standard cycles
[infections, n=27 (90%); neutropenia, n=26 (86.7%); leukopenia,
n=19 (63.3%) and pain, n=16 (53.3%)]. Nonetheless, these events
were clinically manageable and did not result in treatment
discontinuation. Moreover, no long-term toxicities were observed
during the follow-up period. Detailed TRAEs are presented in
Table IV.
 | Table IV.Evaluation of treatment-related
adverse events (n=30). |
Table IV.
Evaluation of treatment-related
adverse events (n=30).
|
| Period during the
standard 5 cycles | Period during the
extended immunotherapy cycles |
|---|
|
|
|
|
|---|
| Adverse events | Grade 3, n (%) | Grade 4–5, n
(%) | Total, n (%) | Grade 3, n (%) | Grade 4–5, n
(%) | Total, n (%) |
|---|
| Anemia | 15 (50.0) | 0 (0.0) | 15 (50.0) | 15 (50.0) | 0 (0.0) | 15 (50.0) |
| Leukopenia | 16 (53.3) | 1 (3.3) | 17 (56.7) | 16 (53.3) | 3 (10.0) | 19 (63.3) |
| Neutropenia | 24 (80.0) | 1 (3.3) | 25 (83.3) | 24 (80.0) | 2 (6.7) | 26 (86.7) |
|
Thrombocytopenia | 10 (33.3) | 3 (10.0) | 13 (43.3) | 10 (33.3) | 6 (20.0) | 16 (53.3) |
| Elevated serum
transaminase levels | 6 (20.0) | 0 (0.0) | 6 (20.0) | 7 (23.3) | 0 (0.0) | 7 (23.3) |
| Infection | 26 (86.7) | 0 (0.0) | 26 (86.7) | 27 (90.0) | 0 (0.0) | 27 (90.0) |
| Pain | 16 (53.3) | / | 16 (53.3) | 16 (53.3) | / | 16 (53.3) |
| Fever | 9 (30.0) | 0 (0.0) | 9 (30.0) | 9 (30.0) | 0 (0.0) | 9 (30.0) |
| Capillary leak
syndrome | 2 (6.7) | 0 (0.0) | 2 (6.7) | 2 (6.7) | 0 (0.0) | 2 (6.7) |
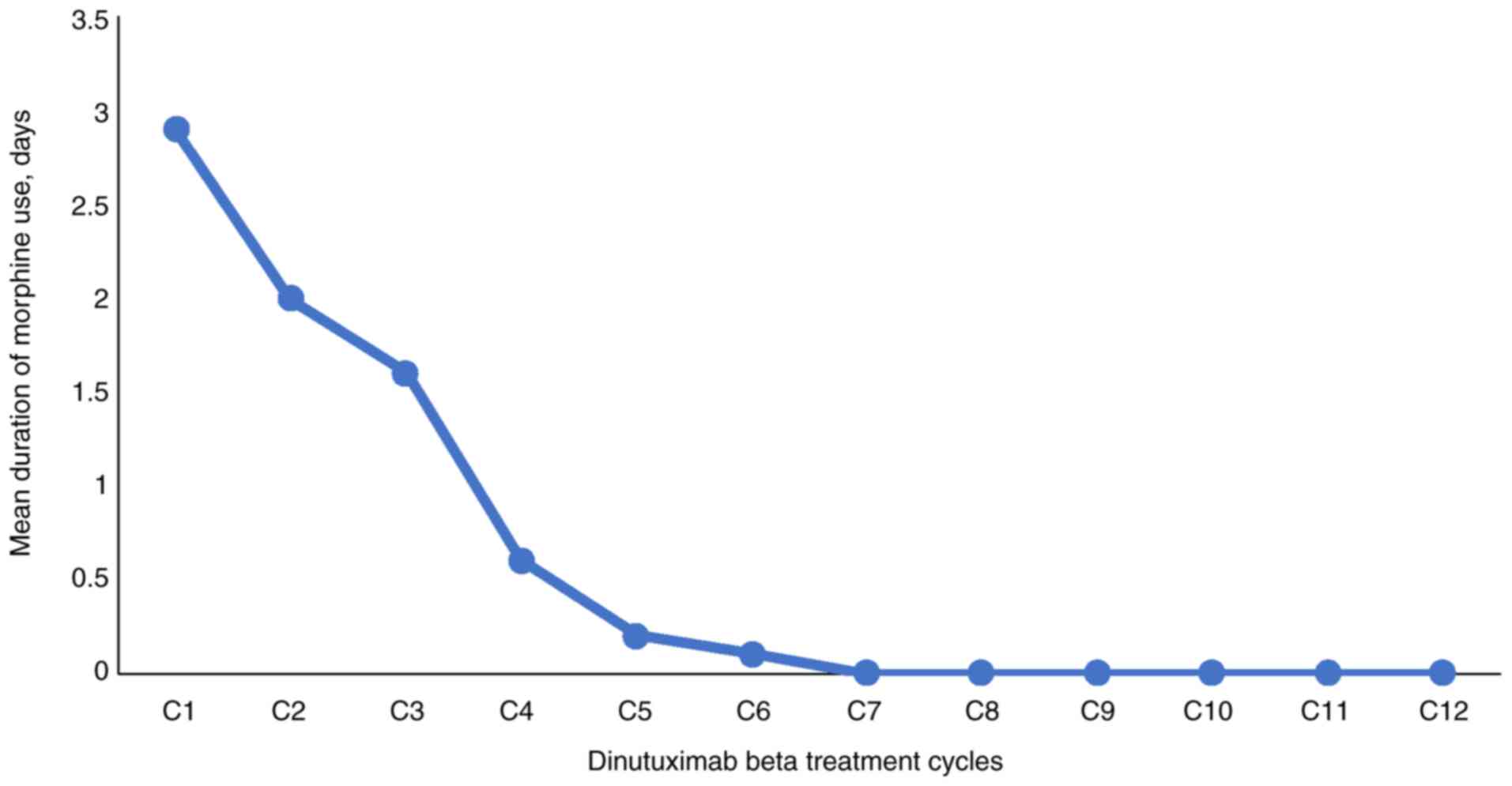
Notably, the use of morphine decreased from cycle 3,
showing reduced immune-related pain (Fig. 5). Furthermore, no immune-related
deaths were observed.
Discussion
The present study findings suggest that cycles
beyond the standard 5 cycles of dinutuximab beta treatment combined
with chemotherapy improve tumor responses and survival in patients
with R/R NB. A subset of patients achieved optimal responses after
undergoing extended dinutuximab beta treatment cycles, contributing
to favorable outcomes.
Anti-GD2 immunotherapy has shown promise for HR-NB.
Dinutuximab beta, dosed at 10 mg/m2/day for a fixed 5
cycles is recommended according to the Summary of Product
Characteristics (7,18). Given the poor prognosis of R/R cases
and limited effective options, extending therapy beyond 5 cycles
may be a viable strategy. In a retrospective study from Poland and
Germany, 31% of patients showed best responses after >5 cycles,
with treatment extended up to 10 cycles (12). A Turkish study similarly reported
treatment up to 14 cycles with positive outcomes (19). Consistent with these findings, the
present study showed that 38.5% of patients benefited from extended
treatment, with up to 12 cycles administered. Late responses were
observed post-cycle 5, suggesting that residual disease may still
respond to prolonged therapy. The low toxicity profile of
dinutuximab beta supports the feasibility of extended use.
The combination of anti-GD2 antibodies with
chemotherapy has enhanced efficacy in relapsed/progressive HR-NB,
as demonstrated in randomized trials BEACON-Immuno and ANBL1221
(9,20). Preclinical studies also reported up
to 17-fold increased cytotoxicity when dinutuximab beta was
combined with chemotherapy, attributed mainly to enhanced
antibody-dependent cellular cytotoxicity (21). The current study employed both
platinum-based and temozolomide-based regimens. Despite no
stratified analysis by regimen, dinutuximab beta showed consistent
activity across all combinations. Supporting this, the SACHA-France
study (clinical trial no. NCT04477681) reported comparable ORRs of
40–42% across different chemotherapy backbones, namely topotecan +
cyclophosphamide, temozolomide + topotecan, and temozolomide +
irinotecan when combined with dinutuximab beta, suggesting that the
choice of chemotherapy may not significantly influence efficacy
(22). These findings further
support the notion that the choice of chemotherapeutic drugs may
not significantly impact the overall efficacy of dinutuximab
beta-based chemoimmunotherapy.
Dinutuximab beta was generally well tolerated in the
present study, with a similar profile of grade ≥3 TRAEs as observed
in the SIOPEN trials (23,24). The TRAE profile of
chemoimmunotherapy in the present study, which was consistent with
previous findings, indicates that the majority of TRAEs are
hematological toxicities (12,25),
and that they are considered to be predominantly induced by
chemotherapy. Pain is one of the most common AEs associated with
anti-GD2 antibodies (26) and was
well managed in the present study. Additionally, no severe
neurological disturbances were observed. In the present study, a
higher incidence of AEs was observed during the extended
immunotherapy cycles compared with that in the standard 5-cycle
treatment period. Notable increases were seen with regard to
leukopenia, neutropenia, thrombocytopenia, transaminase elevations
and infections. Despite the increased frequency, these events were
clinically manageable with standard supportive measures, and no
long-term or irreversible toxicities were documented. These
findings support the feasibility and tolerability of extended
chemoimmunotherapy in patients with HR-NB when administered under
appropriate clinical oversight.
Although a multivariate analysis was performed to
explore potential prognostic factors for PFS, its interpretability
is constrained by the limited number of progression events and the
modest cohort size. As such, the analysis should be viewed as
exploratory and hypothesis-generating rather than confirmatory. To
maintain scientific transparency, the findings have been provided
as supplementary material. Ongoing follow-up and event accumulation
will be critical to support more robust multivariable modeling and
to validate potential prognostic indicators in this population.
Several limitations of the present study should be
acknowledged. The study was a retrospective, single-center and
non-randomized study, which introduces the potential for selection
bias that may limit the generalizability of the findings to a
broader population. The single-center nature of the study also
restricts the diversity of the sample, potentially limiting the
applicability of the results to other healthcare settings. The
absence of a well-defined control group, such as patients receiving
only the standard 5-cycle regimen, restricts the ability of the
study to definitively assess the incremental benefit of extended
treatment. As such, the observations should be interpreted with
caution.
In conclusion, cycles beyond the standard 5 cycles
of dinutuximab beta treatment in combination with chemotherapy
demonstrated feasibility, tolerability and potential clinical
benefit in patients with R/R NB. A subset of patients achieved
meaningful responses following extended treatment, supporting the
rationale for prolonged anti-GD2-based chemoimmunotherapy. However,
the retrospective, single-center design and absence of a control
arm limit the strength of causal inferences regarding treatment
efficacy. To substantiate these findings and determine optimal
treatment duration, future prospective, multi-center randomized
trials are essential.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Collaborative Academic
Innovation Project of Shandong Cancer Hospital (grant no.
FC005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZG was responsible for organizing, validating, and
maintaining data to ensure its accuracy, consistency, and usability
for analysis, statistical analysis, writing the original draft, and
reviewing and editing the manuscript. SK performed study
conceptualization, assisted with the methodology, and reviewed and
edited the manuscript. CB performed the statistical analysis and
helped to write the original draft. YL and XY performed data
curation, validation, statistical analysis, and manuscript review
and editing. KS and YS performed investigation, literature
research, study conception, and manuscript review and editing. FC
performed study supervision, study conception, and manuscript
review and editing. JW was responsible for conceptualization,
methodology, supervision, writing the original draft, and reviewing
and editing. JW and ZG confirm the authenticity of all raw data.
All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong First Medical University and Shandong Academy of Medical
Sciences (Jinan, China; approval no. SDTHEC202503192). The
requirement for informed consent was exempted due to the
retrospective nature of the data acquisition.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kholodenko IV, Kalinovsky DV, Doronin II,
Deyev SM and Kholodenko RV: Neuroblastoma origin and therapeutic
targets for immunotherapy. J Immunol Res. 2018:3942682018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JR, Eggert A and Caron H:
Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol
Clin North Am. 24:65–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nong J, Su C, Li C, Wang C, Li W, Li Y,
Chen P, Li Y, Li Z, She X, et al: Global, regional, and national
epidemiology of childhood neuroblastoma (1990–2021): A statistical
analysis of incidence, mortality, and DALYs. EClinicalMedicine.
79:1029642025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith V and Foster J: High-risk
neuroblastoma treatment review. Children (Basel).
5:1142018.PubMed/NCBI
|
|
5
|
Su Y, Qin H, Chen C, Wang S, Zhang S,
Zhang D, Jin M, Peng Y, He L, Wang X, et al: Treatment and outcomes
of 1041 pediatric patients with neuroblastoma who received
multidisciplinary care in China. Pediatr Investig. 4:157–167. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
London WB, Castel V, Monclair T, Ambros
PF, Pearson AD, Cohn SL, Berthold F, Nakagawara A, Ladenstein RL,
Iehara T and Matthay KK: Clinical and biologic features predictive
of survival after relapse of neuroblastoma: A report from the
international neuroblastoma risk group project. J Clin Oncol.
29:3286–3292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Medical Products Administration,
. Information on the pending collection of drug approval documents
released on August 16, 2021. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20210816154622110.htmlMay
5–2025
|
|
8
|
National Comprehensive Cancer Network.
Clinical Practice Guidelines in Oncology, . Neuroblastoma. Version
2.2024. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/neuroblastoma.pdfMay
5–2025
|
|
9
|
Gray J, Moreno L, Weston R, Barone G,
Rubio A, Makin G, Vaidya S and Wheatley W: BEACON-Immuno: Results
of the dinutuximab beta (dB) randomization of the
BEACON-Neuroblastoma phase 2 trial-A european innovative therapies
for children with cancer (ITCC-International Society of Paediatric
Oncology Europe Neuroblastoma Group (SIOPEN) trial. J Clin Oncol.
40:10002. 2022. View Article : Google Scholar
|
|
10
|
Mody R, Yu AL, Naranjo A, Zhang FF, London
WB, Shulkin BL, Parisi MT, Servaes SE, Diccianni MB, Hank JA, et
al: Irinotecan, temozolomide, and dinutuximab with GM-CSF in
children with refractory or relapsed neuroblastoma: A report from
the Children's oncology group. J Clin Oncol. 38:2160–2169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olgun N, Arayici ME, Kızmazoglu D and
Cecen RE: Assessment of Chemo-immunotherapy regimens in patients
with refractory or relapsed neuroblastoma: A systematic review with
Meta-analysis of critical oncological outcomes. J Clin Med.
14:9342025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wieczorek A, Zaniewska-Tekieli A, Ehlert
K, Pawinska-Wasikowska K, Balwierz W and Lode H: Dinutuximab beta
combined with chemotherapy in patients with relapsed or refractory
neuroblastoma. Front Oncol. 13:10827712023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monclair T, Brodeur GM, Ambros PF, Brisse
HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK,
Nuchtern JG, et al: The international neuroblastoma risk group
(INRG) staging system: An INRG task force report. J Clin Oncol.
27:298–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JR, Bagatell R, Cohn SL, Pearson AD,
Villablanca JG, Berthold F, Burchill S, Boubaker A, McHugh K,
Nuchtern JG, et al: Revisions to the international neuroblastoma
response criteria: A consensus statement from the national cancer
institute clinical trials planning meeting. J Clin Oncol.
35:2580–2587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pauwels E, Cleeren F, Tshibangu T, Koole
M, Serdons K, Dekervel J, Van Cutsem E, Verslype C, Van Laere K,
Bormans G and Deroose CM: [18F]AlF-NOTA-octreotide PET imaging:
biodistribution, dosimetry and first comparison with
[68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur J Nucl Med
Mol Imaging. 47:3033–3046. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou J, Long T, He Z, Zhou M, Yang N, Chen
D, Zeng S and Hu S: Evaluation of 18F-AlF-NOTA-octreotide for
imaging neuroendocrine neoplasms: Comparison with 68Ga-DOTATATE
PET/CT. EJNMMI Res. 11:552021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
U.S. Department of Health and Human
Services, . Common Terminology Criteria for Adverse Events (CTCAE)
Version 5.0. https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events/ctcae-v5-5×7.pdfOctober
15–2025
|
|
18
|
European Medicines Agency, . Qarziba
epar-product information. https://www.ema.europa.eu/en/documents/product-information/qarziba-epar-product-information_en.pdfOctober
15–2025
|
|
19
|
Olgun N, Cecen E, Ince D, Kizmazoglu D,
Baysal B, Onal A, Ozdogan O, Guleryuz H, Cetingoz R, Demiral A, et
al: Dinutuximab beta plus conventional chemotherapy for
relapsed/refractory high-risk neuroblastoma: A single-center
experience. Front Oncol. 12:10414432022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mody R, Naranjo A, Van Ryn C, Yu AL,
London WB, Shulkin BL, Parisi MT, Servaes SE, Diccianni MB, Sondel
PM, et al: Irinotecan-temozolomide with temsirolimus or dinutuximab
in children with refractory or relapsed neuroblastoma (COG
ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol.
18:946–957. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Troschke-Meurer S, Zumpe M, Meißner L,
Siebert N, Grabarczyk P, Forkel H, Maletzki C, Bekeschus S and Lode
HN: Chemotherapeutics used for High-risk neuroblastoma therapy
improve the efficacy of Anti-GD2 antibody dinutuximab beta in
preclinical spheroid models. Cancers (Basel). 15:9042023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raiser P, Schleiermacher G, Gambart M,
Dumont B, Defachelles AS, Thebaud E, Tandonnet J, Pasqualini C,
Proust S, Entz-Werle N, et al: Chemo-immunotherapy with dinutuximab
beta in patients with relapsed/progressive high-risk neuroblastoma:
Does chemotherapy backbone matter? Eur J Cancer. 202:1140012024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ladenstein R, Pötschger U, Valteau-Couanet
D, Luksch R, Castel V, Yaniv I, Laureys G, Brock P, Michon JM,
Owens C, et al: Interleukin 2 with anti-GD2 antibody ch14.18/CHO
(dinutuximab beta) in patients with high-risk neuroblastoma
(HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet
Oncol. 19:1617–1629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ladenstein RL, Poetschger U,
Valteau-Couanet D, Gray J, Luksch R, Balwierz W, Castel V and Lode
HN: Randomization of dose-reduced subcutaneous interleukin-2
(scIL2) in maintenance immunotherapy (IT) with anti-GD2
antibody dinutuximab beta (DB) long-term infusion (LTI) in
front-line high-risk neuroblastoma patients: Early results from the
HR-NBL1/SIOPEN trial. J Clin Oncol. 37:10013. 2019. View Article : Google Scholar
|
|
25
|
Lode HN, Ladenstein R, Troschke-Meurer S,
Struppe L, Siebert N, Zumpe M, Ehlert K, Huber S, Glogova E,
Hundsdoerfer P, et al: Effect and tolerance of N5 and N6
chemotherapy cycles in combination with dinutuximab beta in
relapsed High-risk neuroblastoma patients who failed at least one
second-line therapy. Cancers (Basel). 15:33642023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barone G, Barry A, Bautista F, Brichard B,
Defachelles AS, Herd F, Manzitti C, Reinhardt D, Rubio PM,
Wieczorek A and van Noesel MM: Managing adverse events associated
with dinutuximab beta treatment in patients with High-risk
neuroblastoma: Practical guidance. Pediatr Drugs. 23:537–548. 2021.
View Article : Google Scholar
|