Introduction
According to the latest cancer statistics from the
International Agency for Research on Cancer, 434,419 new cases and
155,702 deaths from renal cancer were reported in 2022 (1). Renal cell carcinoma (RCC) includes
histological subtypes such as clear cell RCC (ccRCC), papillary
RCC, and chromophobe RCC (2). Among
these, ccRCC represents the highest proportion, accounting for
70–75% of all RCCs (2). Compared
with non-ccRCC, ccRCC is associated with higher mortality and lower
survival rates, indicating the poorest prognosis among RCCs
(2,3). Furthermore, ccRCC originates from
proximal tubule epithelial cells and typically exhibits an
expansive growth pattern (4).
Histologically, it is marked by abundant clear cytoplasm resulting
from lipid and glycogen accumulation (2). The Fuhrman grade (5) and the WHO/ISUP grading system
(6) are generally used to grade the
nuclear features of ccRCC. Higher nuclear grades correlate with
poorer prognosis; therefore, these nuclear grading systems have
been used in clinical practice. However, recent studies have
indicated that the Fuhrman grade has moderate interobserver
agreement (7). Because of
subjectivity in how pathologists emphasize specific criteria and
assess nuclear size, it has been suggested that grading beyond
Grade 4 should primarily focus on nucleolar evaluation (7,8).
Dagher et al (8) suggested
that the WHO/ISUP grading system correlates more strongly with
patient survival than the Fuhrman grade.
Changes in nuclear morphology are reported to be
influenced by changes in the expression of nuclear envelope
(NE)-associated proteins, including Lamin, Emerin, SUN, and Nesprin
(9). In particular, Lamins are
classified into two types: A-type and B-type Lamin (10). Lamin A, which is on the
nucleoplasmic side, forms a dense meshwork (10). Lamin B1, which is situated between
Lamin A and the inner nuclear membrane (INM), forms a relatively
loose meshwork (10). Lamin B1
prevents nuclear bleb formation caused by Lamin A protrusions
(10). Lamin influences nuclear and
cellular morphologies. Vahabikashi et al (11) reported that Lamin A deficiency leads
to nuclear distortion and increased nuclear volume, whereas Lamin
B1 or B2 deficiency causes no evident distortion. In Lamin
B1-deficient cells, nuclear volume is smaller than in wild-type
cells, whereas in Lamin B2 deficiency, it is nearly identical to
wild-type. Furthermore, Lamin interacts with the Linker of
Nucleoskeleton and Cytoskeleton (LINC) complex and intermediate
filaments, thereby regulating cytoplasmic stiffness (11). The effects of Lamin also vary by
tumor type. Moss et al (12)
reported that in esophageal squamous cell carcinoma, Lamin A/C is
reduced while Lamin B1 is retained, whereas in colon tumors and
gastric dysplasia, both Lamin A/C and B1 are decreased. Conversely,
pancreatic cancer retains the expression of both Lamin proteins
(12). Although reduced Lamin
expression is common in cancer, it is not observed in all
epithelial tumors (12). Previous
studies have also reported findings related to Emerin (13,14).
Vaughan et al (14) reported
that Lamin A, C, and B1 interact with Emerin; when Lamin A/C is
absent from the NE, Emerin relocates from the NE to the endoplasmic
reticulum. Hence, Lamin A is necessary to anchor Emerin to the NE.
Lammerding et al (13)
reported that Emerin deficiency induces abnormalities in the
nuclear shape; however, these are milder than those caused by Lamin
A deficiency, and nuclear rigidity remains comparable to that of
wild type. Furthermore, SUN and Nesprin interact with both Lamin
and Emerin as well as the cytoskeleton, thereby influencing both
the nucleoskeleton and cytoskeleton (15,16).
Haque et al reported that the C-terminals of SUN1 and SUN2
interact with the KASH domains of Nesprin-1 and −2, linking the
nucleus to actin, whereas their N-terminals interact with nuclear
Lamin, Emerin, and short Nesprin-2 isoforms. These interactions
bridge the nucleoskeleton and the cytoskeleton, playing a crucial
role in maintaining nuclear and cellular integrity (15). Banerjee et al (17) reported that deficiency of either
Nesprin-1 or Nesprin-2 increases nuclear area and perimeter and
reduces roundness; when both are deficient, the localization of
Lamin A/C and Emerin is disrupted. Ketema et al (16) demonstrated that Nesprin-3, similar
to Nesprin-1 and −2, localizes to the outer nuclear membrane
through interactions with SUN1 and SUN2. Furthermore, Nesprin-3α
binds to plectin dimers, linking intermediate filaments to the
nucleus, whereas the interaction between SUN and Lamin A forms the
Nesprin-3-LINC complex that bridges the cytoskeleton and
nucleoskeleton (16). Heffler et
al (18) also reported that
Nesprin-3 deficiency increases the number of nuclear wrinkles and
indentations.
Several studies have reported the contribution of
altered expression levels of Lamin, Emerin, SUN, and Nesprin to
nuclear and cellular morphologies. Therefore, examining the
expression of these NE-associated proteins may help identify
potential indicators of nuclear grade. Regarding kidney cancer, Xin
et al reported that reduced Lamin A expression contributes
to abnormal nuclear morphology (19). Radspieler et al (20) also reported that the gene expression
levels of Lamin B1 serve as a marker of poor prognosis.
Furthermore, Fukushima et al (21) demonstrated that reduced Nesprin-1
gene expression is a poor prognostic factor and that its knockdown
enhances invasive potential. However, to our knowledge, no studies
have directly compared nuclear grade with the expression of
NE-associated proteins to elucidate the mechanisms underlying
nuclear morphological changes in ccRCC. Therefore, we conducted
this study to investigate the relationship between nuclear grade
and nine NE-associated proteins: Lamin A, Lamin B1, Lamin B2,
Emerin, SUN1, SUN2, Nesprin-1, Nesprin-2, and Nesprin-3, which have
been implicated in alterations of nuclear and cellular
morphology.
Materials and methods
Samples
This study included 199 patients who underwent
surgical resection for ccRCC at Gunma University Hospital between
January 2005 and July 2012. The Gunma University Ethical Review
Board for Medical Research Involving Human Subjects approved this
study (approval no. HS2024-068). We used formalin-fixed and
paraffin-embedded tissue blocks used for routine pathological
diagnosis. Sections were cut at 4 µm thickness for
immunohistochemical staining.
Evaluation of nuclear grade
Hematoxylin-eosin-stained diagnostic specimens,
stored in the Department of Pathology at Gunma University Hospital,
were reviewed by pathologists. The nuclear grades of four patient
groups (G1-G4) were determined according to the Fuhrman grade
(5) and WHO/ISUP grading systems
(6).
Immunohistochemical staining
Four micrometer-thick sections were deparaffinized
through three 5-min xylene treatments. For rehydration, sections
were sequentially immersed in 100, 99, 95, and 70% ethanol for 1
min each, followed by a 1-min rinse under running water. For
immunohistochemical staining, the following antibodies were used at
a concentration of 2 µg/ml: anti-Lamin A antibody (clone 133A2;
mouse monoclonal antibody; ab8980; Abcam), anti-Lamin B1 antibody
(clone 3C10G12; mouse monoclonal antibody; 66095-1-Ig; Proteintech,
Rosemont, USA), anti-Lamin B2 antibody (clone GT144; mouse
monoclonal antibody; GTX628803; GeneTex, California, USA),
anti-Emerin antibody (clone CL0201; mouse monoclonal antibody;
NBP2-52876; Novus Biologicals, Centennial, CO, USA), anti-SUN1
antibody (clone EPR6554; rabbit monoclonal antibody; ab124770;
Abcam), anti-SUN2 antibody (clone EPR6557; rabbit monoclonal
antibody; ab124916; Abcam), anti-Nesprin-1 antibody (clone
EPR14196; rabbit monoclonal antibody; ab192234; Abcam),
anti-Nesprin-2 antibody (clone EPR28137-54; rabbit monoclonal
antibody; ab186746; Abcam), and anti-Nesprin-3 antibody (clone
EPR15623; rabbit monoclonal antibody; ab314872; Abcam). First,
sections were placed in an electric pot containing Immunosaver
(Nisshin EM Co. Ltd., Shinjuku-ku, Tokyo, Japan) diluted 200-fold
with distilled water for antigen retrieval. Next, they were boiled,
maintained at 98°C for 40 min, and left in the pot for 30 min after
turning off the heat. Subsequently, they were transferred to a
container with 0.01 M phosphate-buffered saline (PBS) solution.
Following antigen retrieval, immunohistochemical staining was
performed using an automated staining system (Histostainer 36A;
Nichirei Biosciences, Chuo-ku, Tokyo, Japan). We treated the
specimens with hydrogen peroxide solution (code 715242; Nichirei
Bioscience) for 5 min to block endogenous peroxidase activity.
After the specimens were washed with PBS (code 715224; Nichirei
Bioscience), 2% goat serum (ab7481; Abcam) was added and incubated
for 15 min. Primary antibodies (clones 133A2, 3C10G12, GT144,
CL0201, EPR6554, EPR6557, EPR14196, EPR28137-54, and EPR15623) were
applied and incubated at room temperature for 60 min. After
washing, peroxidase-labeled anti-mouse IgG polyclonal antibody
(Histofine Simple Stain MAX-PO(M); code 724132; Nichirei
Bioscience) was applied as the secondary antibody for 30 min to
specimens treated with primary antibodies, including clones 133A2,
3C10G12, GT144, and CL0201. For clones EPR6554, EPR6557, EPR14196,
EPR28137-54, and EPR15623, peroxidase-labeled anti-rabbit IgG
polyclonal antibody (Histofine Simple Stain MAX-PO(R); code 724142;
Nichirei Bioscience) was used as the secondary antibody for 30 min.
For color development, the specimens were incubated with the DAB
substrate kit (code 725191; Nichirei Bioscience) twice for 10 min.
After washing, the nucleus was stained with hematoxylin (code
715081; Nichirei Bioscience) for 1 min. Subsequently, the specimens
were removed from the automatic staining machine and washed once
with distilled water. Next, sections were dehydrated in 70, 95, and
100% ethanol (two baths) for 1 min each. For clearing, xylene was
applied in three consecutive baths for 5 min, followed by mounting.
Subsequently, the sections were examined using an optical
microscope (BX51; Evident, Tokyo, Japan) to confirm staining.
Acquisition of specimen images using a
virtual slide scanner
Whole-slide images of immunohistochemically stained
specimens for Lamin A, B1, and B2 were obtained using a virtual
slide scanner (Nano Zoomer 2.0-HT Virtual Slide Scanner; C9600-13;
Hamamatsu Photonics K.K., Shizuoka, Japan). The scanning conditions
were as follows: observation lens, 20×; resolution, 0.75; scan
mode, 40×; maximum capture size, 26×76 mm; pixel size, 0.23
µm/pixel; light source, halogen lamp; and image storage format,
JPEG.
Computer-assisted image analysis
For analyzing Lamin A-, B1-, and B2-stained
specimens, five representative regions per specimen were selected
at 40× magnification from whole-slide images and saved as TIFF
files. The images were then analyzed using the NE measurement
software e-Nucmen3 (ver. 1.8; E-path Co. Ltd, Fujisawa, Kanagawa,
Japan). To focus on tumor cell nuclei, nontumor regions were
excluded using the annotation function. Nuclei of nontumor cells
within the tumor region, overlapping nuclei, and nuclei not
correctly recognized by the software were also excluded. Analysis
conditions are provided in Table
SI.
Evaluation of Emerin, SUN, and Nesprin
expression
Specimens stained with each primary antibody were
examined under a microscope. To evaluate expression changes,
staining intensity in the tumor region was compared with that of
the nuclear membrane in normal proximal tubules from the same
patient. Expression levels of Emerin, SUN1, SUN2, and Nesprin-2
were classified into three categories: decreased, no change, and
increased. For Nesprin-1, the expression level was classified into
decreased, no change, and nucleoplasm. For Nesprin-3, because no
expression was detected in the NE of normal proximal tubules,
expression was classified as no change (absent) or increased
(present).
Statistical analysis
All statistical data were analyzed using JMP Pro
ver. 18. 2.0 (SAS Japan, Tokyo, Japan). For multiple comparisons
involving unequal group sample sizes (unbalanced data), one-way
ANOVA was initially performed to check variance among groups, after
which, the Tukey-Kramer HSD test was used for pair-wise
comparisons, referencing Lee and Lee (22) for its validity. Because part of the
statistical evaluation involved qualitative assessment, Fisher's
exact test was used for 2×2 group comparisons to examine the
presence or absence of distribution differences (23). Comparisons involving more than two
groups were analyzed using the Fisher-Freeman-Halton exact
conditional test (24). Association
between two continuous variables were assessed using linear
regression analysis. Association strength was classified according
to the correlation coefficient (r) as follows: 0.200–0.399,
weak; 0.400–0.699, moderate; and ≥0.700, strong. P<0.05 was
considered to indicate a statistically significant difference
(25).
Results
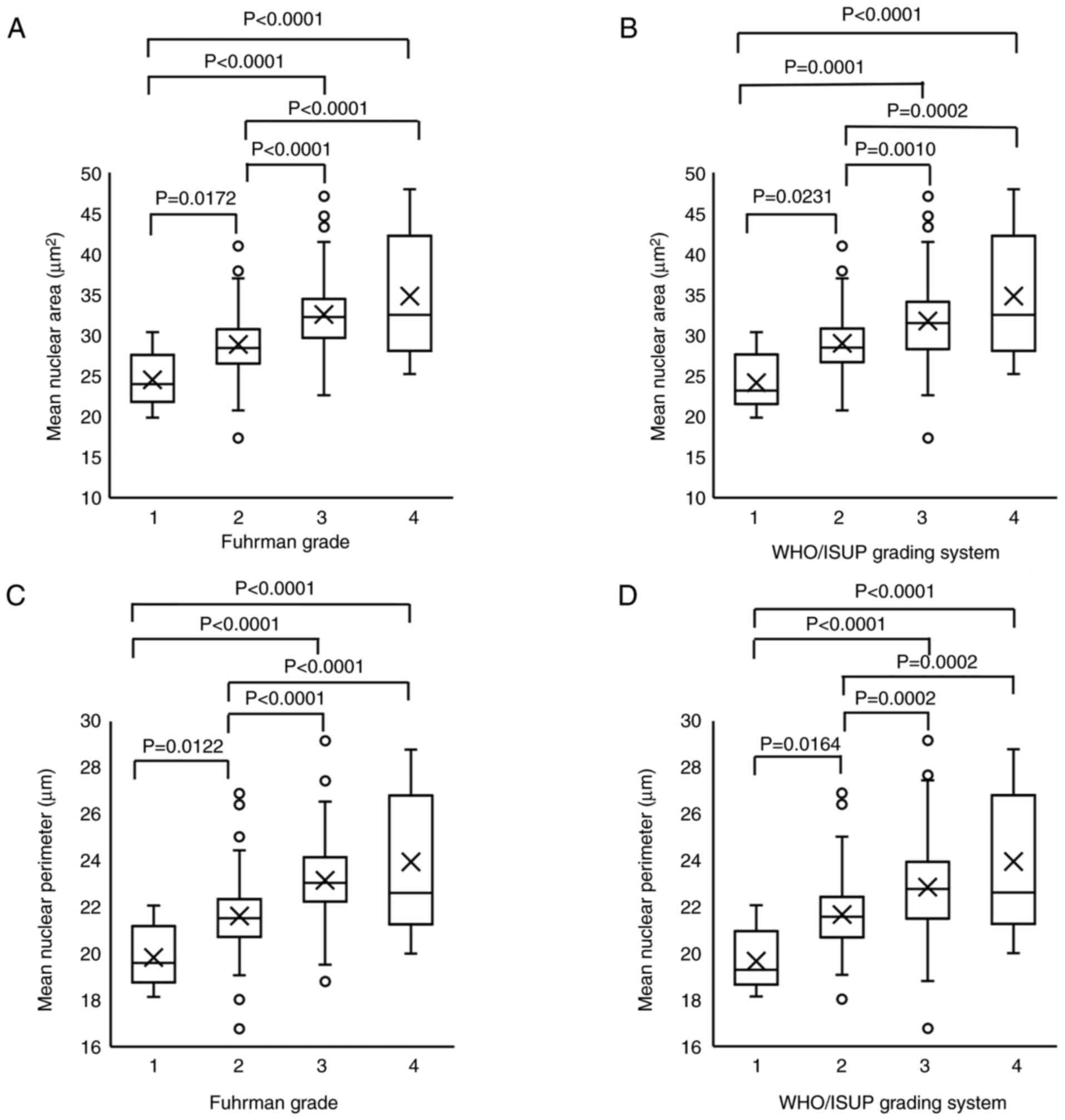
Nuclear size increased from G1 to
G3
Table I summarizes
the clinicopathological characteristics of the patients (26). First, the mean nuclear area and
perimeter were compared with Fuhrman and WHO/ISUP grades. G1-G3
showed a significant increase in mean nuclear area and perimeter
across both grading systems. However, neither grading system showed
a significant difference between G3 and G4 (Fig. 1A-D). Therefore, nuclear size may not
adequately represent G4, and other factors likely contribute to
nuclear and cellular morphology in this grade. The representative
G1 to G4 grade histological images of HE staining for both Fuhrman
and WHO/ISUP grades are shown in Fig.
S1.
 | Table I.Clinicopathological characteristics
of patients. |
Table I.
Clinicopathological characteristics
of patients.
| Characteristic | Value |
|---|
| Total number of
patients, n (%) | 199 (100) |
| Mean age,
years | 63.0 |
| Sex, n (%) |
|
|
Male | 142 (71) |
|
Female | 57 (29) |
| Fuhrman grade, n
(%) |
|
| 1 | 10 (5) |
| 2 | 117 (59) |
| 3 | 59 (30) |
| 4 | 13 (6) |
| WHO/ISUP grading
system, n (%) |
|
| 1 | 8 (4) |
| 2 | 109 (55) |
| 3 | 69 (35) |
| 4 | 13 (6) |
| pT status, n
(%) |
|
|
pT1 | 169 (85) |
|
pT2 | 10 (5) |
|
pT3 | 19 (10) |
|
pT4 | 1 (0.5) |
| pN status, n
(%) |
|
|
pN0 | 196 (85) |
|
pN1 | 3 (15) |
| pM status, n
(%) |
|
|
pM0 | 194 (97) |
|
pM1 | 5 (3) |
| Stage, n (%) |
|
| I | 165 (83) |
| II | 10 (5) |
|
III | 16 (8) |
| IV | 8 (4) |
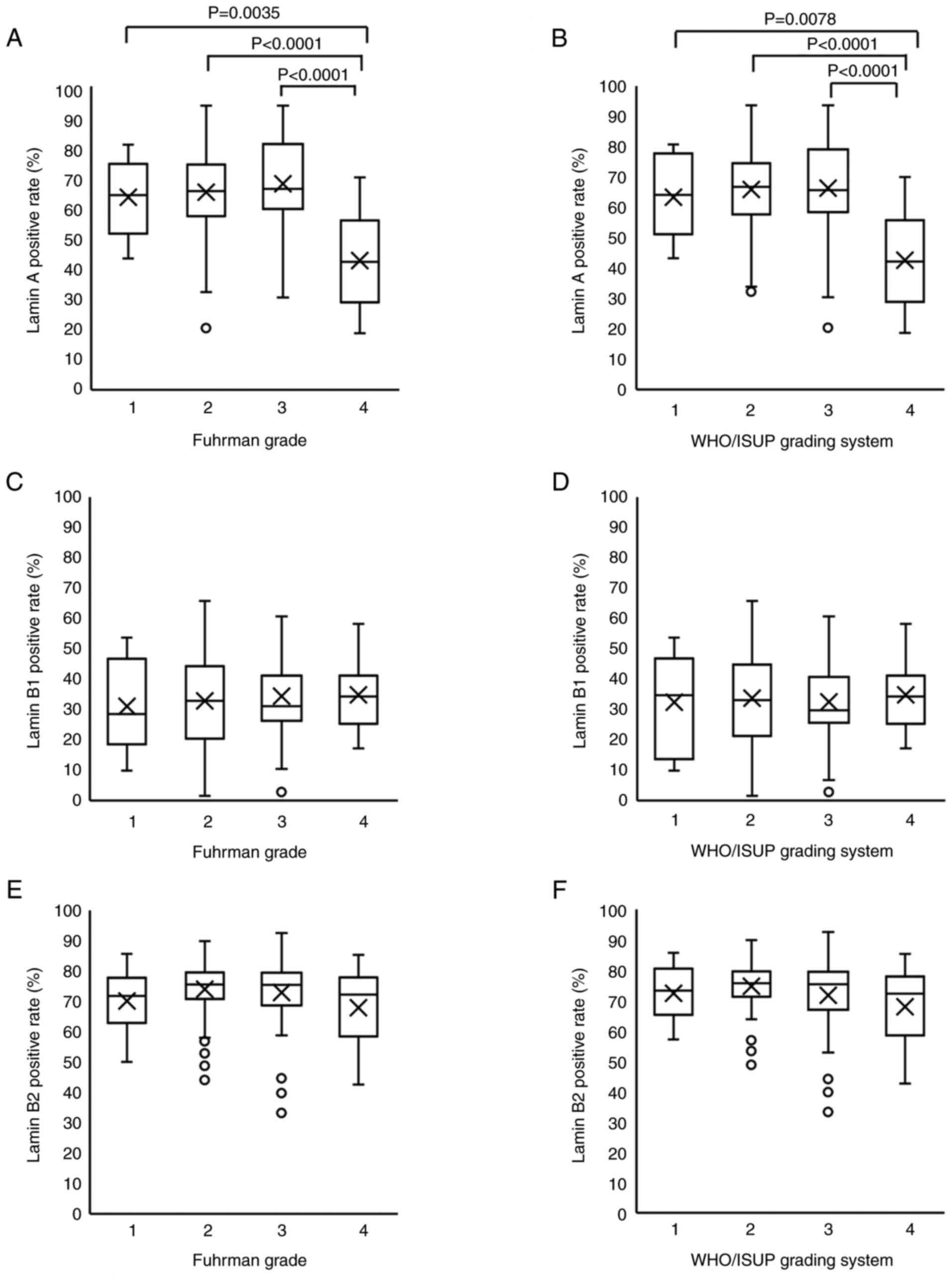
Decreased Lamin A expression
influences nuclear shape changes in G4
Next, we examined the positive rates for Lamin A,
B1, and B2 according to the two grading systems. Mean Lamin A
positive rates by grade were 63.4, 65.2, 68, and 42.6% for G1, G2,
G3, and G4 in the Fuhrman grade and 63.4, 65.9, 66.3, and 42.6% in
the WHO/ISUP grading system, respectively. Thus, the Lamin A
positive rate was significantly lower in G4 than in G1-G3 in both
grading systems (Fig. 2A and B).
Meanwhile, the mean Lamin B1 positive rates were 31.1, 32.8, 34.4,
and 34.9% for G1, G2, G3, and G4 cases in Fuhrman grade and 32.4,
33.7, 32.5, and 34.9% in the WHO/ISUP grading system, respectively.
For Lamin B2, mean positive rates by grade were 70.1, 74, 73, and
67.9% for G1, G2, G3, and G4 in the Fuhrman grade and 72.3, 74.7,
71.7, and 67.9% in the WHO/ISUP grading system, respectively.
Positive rates of Lamin B1 or B2 did not differ significantly
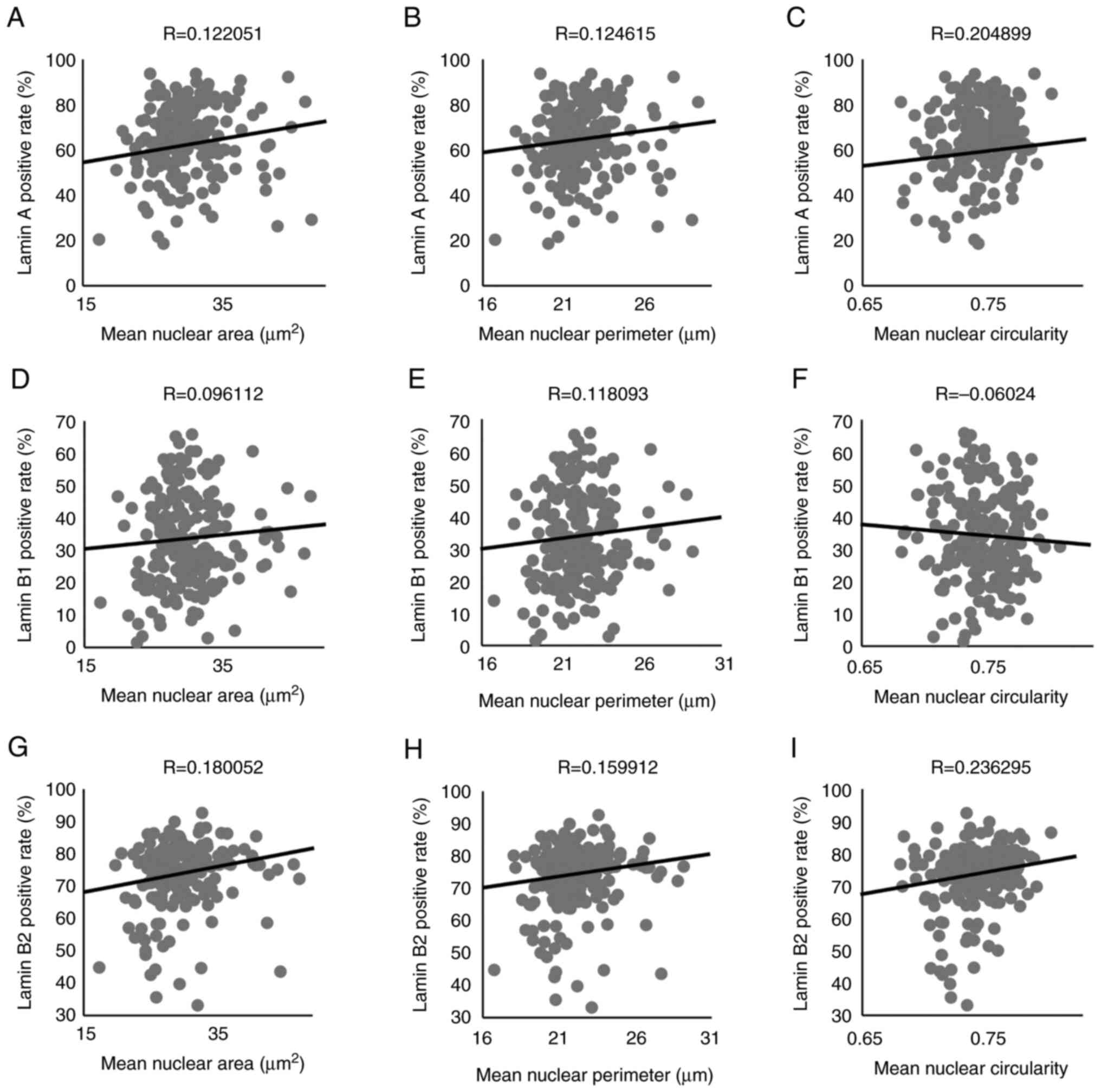
between groups in either grading systems (Fig. 2C-F). We compared the positive rates
for each Lamin with the mean nuclear area, perimeter, and
circularity. The positive rate for Lamin A showed no association
with mean nuclear area or perimeter (Fig. 3A and B) but exhibited a weak
association with mean circularity (Fig.
3C). Furthermore, the positive rate for Lamin B1 did not
associate with any of the nuclear shape indicators (Fig. 3D-F). The positive rate of Lamin B2
was not associated with the mean nuclear area or mean nuclear
perimeter (Fig. 3G and H) but
weakly associated with the mean circularity (Fig. 3I). Therefore, only Lamin A exhibited
a significant decrease in expression in G4 cases. Furthermore,
Lamin A and B2 were weakly associated with the maintenance of
nuclear shape across all grades. The representative images of Lamin
A, B1 and B2 based on the WHO/ISUP grading system was shown in
Fig. S2.
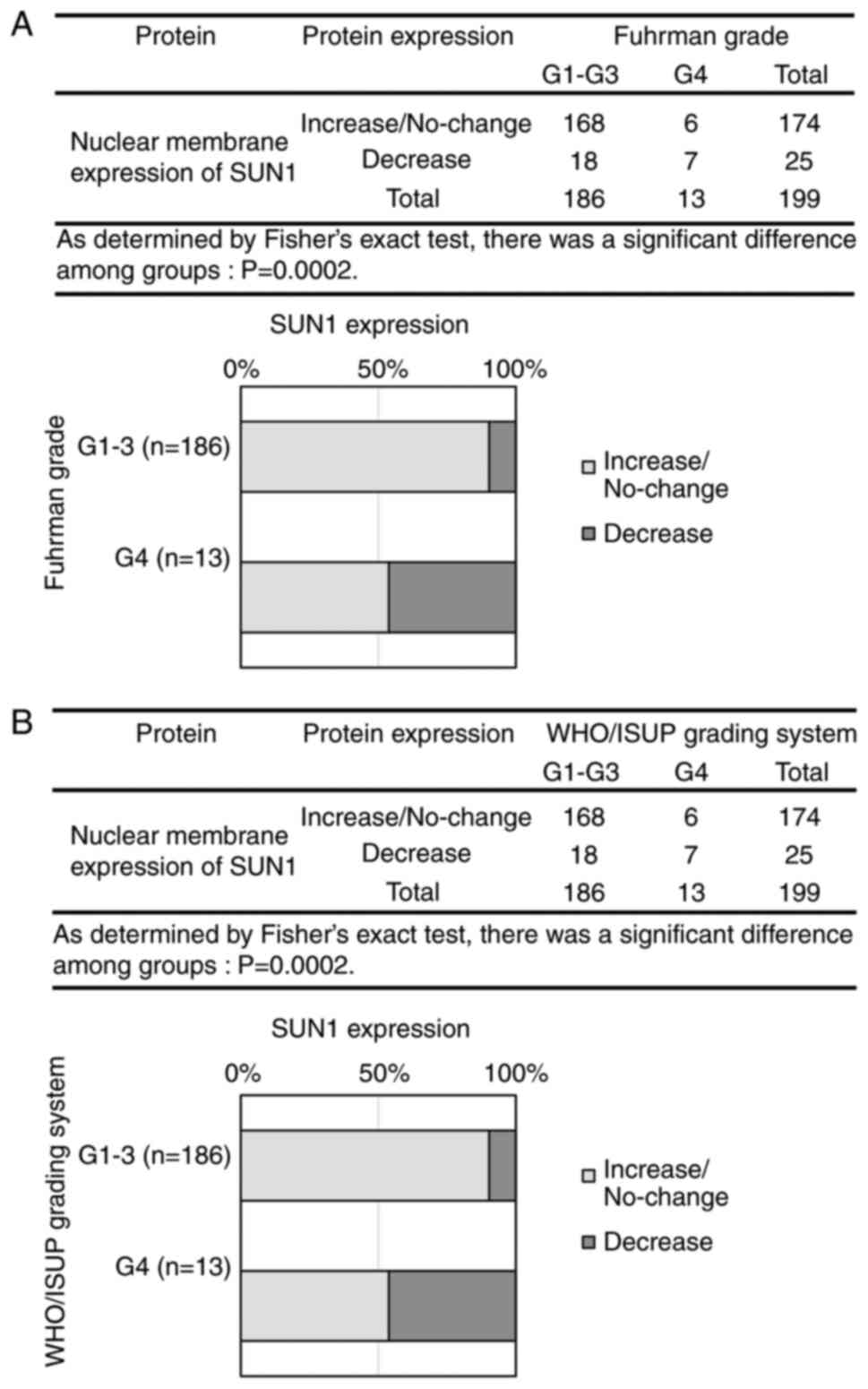
SUN1 reduction in G4 cases correlates
with nuclear grade
In addition to Lamin, we examined the association of
expression changes in Emerin, SUN, and Nesprin with nuclear grade
(Fig. 4A-L). In the
Fisher-Freeman-Halton exact conditional test, Emerin showed a
significant difference between groups in the Fuhrman grade
(P=0.0488) but not in the WHO/ISUP grading system (P=0.0690)
(Fig. 4A and D). For SUN1,
significant differences were observed between groups in both
nuclear grading systems (P<0.0001) (Fig. 4B and E). Similarly, SUN2 showed
significant between-group differences in the Fuhrman grade
(P=0.0289) and the WHO/ISUP grading system (P=0.0143) (Fig. 4C and F). In contrast, Nesprin-1 and
Nesprin-2 showed no significant changes between groups in either
grading system (Fig. 4G, H, J and
K). In all 199 cases, Nesprin-3 expression was negative in the
nuclear membranes of normal proximal tubules and that of tumor
cells (Fig. 4I and L). SUN1, which
demonstrated the most significant changes, showed reduced
expression was increased in G4 cases; in other grades, expression
either increased or remained unchanged. Therefore, a Fisher's exact
test was performed by dividing the cases into two groups: no
change/increased vs. decreased, and G1-G3 vs. G4. The results
revealed a significant reduction in SUN1 expression in G4 cases
(Fig. 5A and B), suggesting that
SUN1 reduction correlates with the progression of nuclear atypia to
G4. Regarding the significant differences detected in Emerin and
SUN2 expression by the Fisher-Freeman-Halton exact conditional
test, the difference among groups would be exist. The
representative image of SUN1 based on the WHO/ISUP grading system
is shown in Fig. S3.
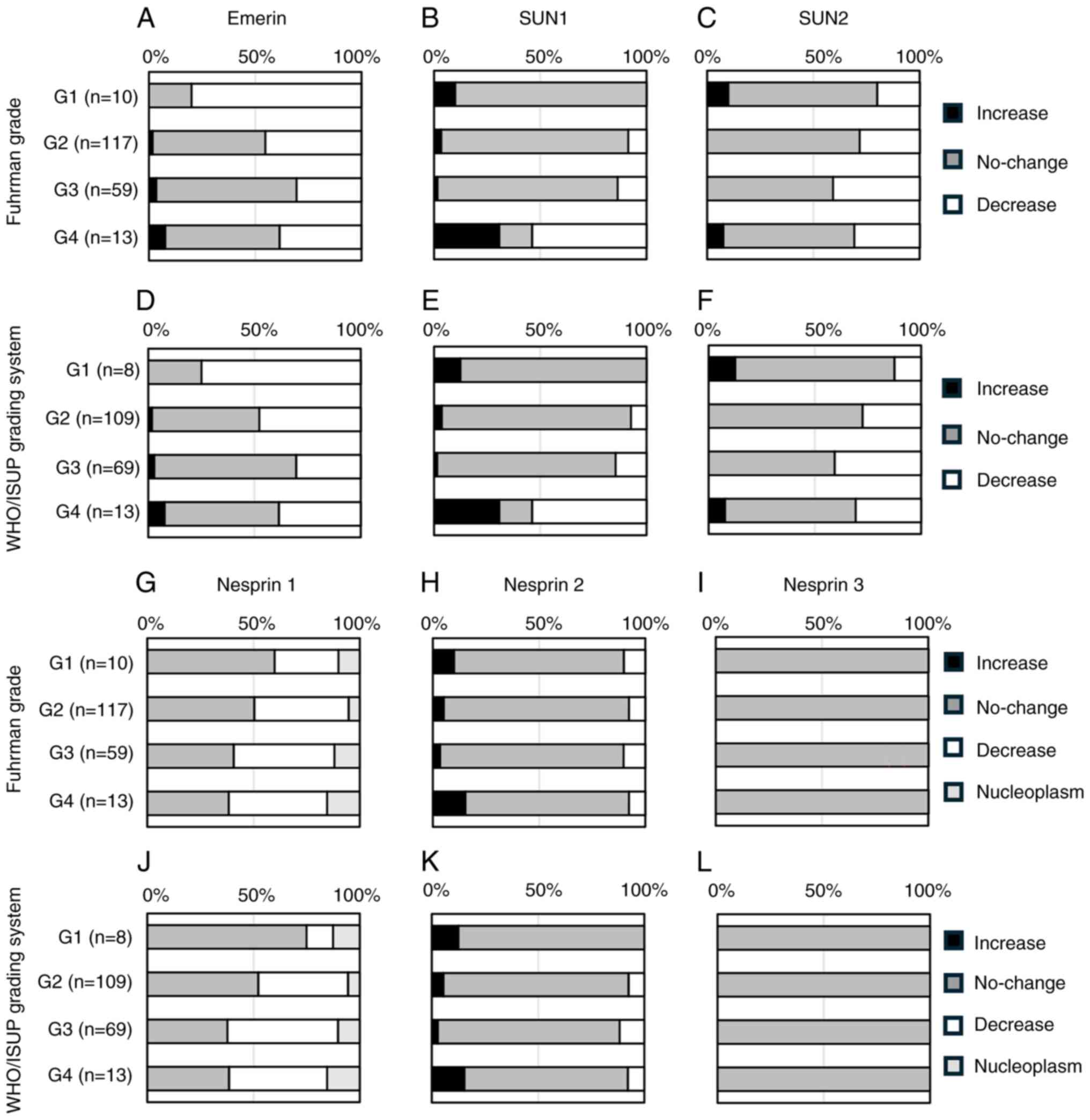 | Figure 4.Comparison of nuclear
envelope-associated protein expression and nuclear grade. (A)
Emerin expression vs. Fuhrman grade, (B) SUN1 expression vs.
Fuhrman grade, (C) SUN2 expression vs. Fuhrman grade. (D) Emerin
expression vs. WHO/ISUP grading system, (E) SUN1 expression vs.
WHO/ISUP grading system, (F) SUN2 expression vs. WHO/ISUP grading
system. (G) Nesprin1 expression vs. Fuhrman grade, (H) Nesprin2
expression vs. Fuhrman grade, (I) Nesprin3 expression vs. Fuhrman
grade. (J) Nesprin1 expression vs. WHO/ISUP grading system, (K)
Nesprin2 expression vs. WHO/ISUP grading system, (L) Nesprin3
expression vs. WHO/ISUP grading system. WHO, World Health
Organization; ISUP, International Society of Urologic
Pathologists. |
Discussion
In the WHO/ISUP grading system, G1-G3 are determined
solely by nucleolar size, without reference to nuclear size or
shape. In contrast, G4 is based on nuclear and cellular
morphological changes, including marked nuclear atypia,
multinucleated giant cells, sarcomatoid features, and rhabdoid
changes (6). Because nuclear grade
correlates with prognosis (8),
investigating its underlying mechanisms is essential. Accordingly,
in this study, we compared the expression of NE-associated proteins
with nuclear grade. To our knowledge, no studies have compared
nuclear grade with NE-associated protein expression to elucidate
the mechanism of nuclear morphological changes in ccRCC.
Capo-chichi et al (27)
reported that many cells in cervical smear specimens negative for
Lamin A/C contained enlarged, irregularly shaped nuclei.
Furthermore, Xin et al (19)
reported that knockdown of the LMNA gene encoding Lamin A in
kidney cancer cell lines resulted in NE invaginations and
abnormalities in nuclear contours. Therefore, we first investigated
Lamin's contribution to nuclear grade, followed by an examination
of the mechanisms underlying nuclear morphological changes in
ccRCC. We found that Lamin A expression levels were reduced in G4
cases, whereas those of Lamin B1 and B2 showed no significant
changes. Lammerding et al reported that in mouse embryonic
fibroblasts, reduced Lamin A decreased nuclear stiffness, whereas
Lamin B1 or B2 had no effect (28).
Furthermore, although Lamin B1 reduction increased nuclear bleb
formation, it did not alter overall nuclear rigidity or shape
stability (28). In a study by
Pujadas Liwag et al (29)
using human colon adenocarcinoma cell lines, reduced B-type
Laminincreased nuclear volume and nuclear bleb formation. Based on
these findings, B-type Lamin contributes to nuclear bleb formation,
but its effect on overall nuclear morphology is less pronounced
than that of Lamin A. Harborth et al (30) reported that silencing Lamin B1 and
B2 in HeLa cells and rat fibroblasts induced growth arrest and
apoptosis. These findings suggest that Lamin A is involved in
maintaining nuclear morphology and Lamin B1 and Lamin B2 are
involved in other functions, including cell proliferation. This
interpretation does not appear to contradict the results of the
present study.
Reis-Sobreiro et al (31) reported that Emerin deficiency leads
to irregular nuclear shapes, reduced roundness, and impaired
deformability in prostate and breast cancer cell lines.
Furthermore, Lamin A/C downregulation contributes to nuclear shape
instability and destabilization such as Emerin mislocalization
(31). Based on this report,
considering that Emerin may influence nuclear morphology, we
investigated its effect on nuclear pleomorphism classification. In
G4 nuclei, cellular morphological changes such as sarcomatoid and
rhabdoid features are also considered indicators (6). NE-associated proteins such as SUN and
Nesprin interact to form the LINC complex (32). Lombardi et al (33) reported that LINC complex disruption
causes defects in nuclear positioning, cytoskeletal organization,
and cell/nuclear deformation. The mechanisms underlying both
nuclear and cellular morphological changes were investigated by
examining the relationship between changes in SUN and Nesprin
expression and nuclear grade. In this study, SUN1 expression
decreased in G4 cases, similar to Lamin A. Emerin and SUN2 also
showed significant intergroup differences. Although the proportion
of G1 cases with reduced Emerin expression was higher than in other
grades, the difference was statistically significant only in the
Fuhrman grade, not in the WHO/ISUP classification. Therefore, the
small number of G1 cases may have influenced the observed
significance. For SUN2, between-group differences were significant
in the Fuhrman grade and the WHO/ISUP grading system, although they
looked unclear in Fig. 4E and F.
Thus, despite the possibility of differences in Emerin and SUN2
among groups, describing a specific association between nuclear
grade and protein expression changes in ccRCC was difficult in this
study. For Nesprin-1, Nesprin-2, or Nesprin-3, the changes were not
significant in relation to nuclear grade. Matsumoto et al
(34) reported that in breast
cancer specimens, Lamin A, SUN1, SUN2, and Nesprin-2 expression was
downregulated in cancerous areas compared with that in noncancerous
ones. Accordingly, their findings for SUN2 and Nesprin-2 do not
align with our results, possibly caused by organ-specific
differences. Ueda et al (35) reported that SUN1 deficiency in HeLa
cells, with no actin reduction observed in SUN2-deficient cells led
to reduced actin, decreased cytoskeletal force, and diminished
contractile force, whereas SUN2 deficiency had no effect on actin,
indicating a specific role for SUN1 in cytoskeletal regulation
(35). Therefore, SUN1 deficiency
may contribute to reduced tumor differentiation. The observed
reduction of SUN1 expression in G4 cases in our study is consistent
with the findings of Ueda et al (35). In our study, SUN1 was reduced only
in G4 cases, similar to Lamin A; thus, Lamin A may be related to
SUN1. Östlund et al (36)
demonstrated that Lamin A is more closely associated with SUN1 than
with SUN2. Haque et al (37)
reported that although SUN1 interacts with Lamin A, it localizes to
the NE even in the absence of Lamin A, indicating that Lamin A is
not required for the NE localization of SUN1. Nishioka et al
(38) reported that SUN1 interacts
with Lamin B1 and B2, whereas SUN2 shows no interaction with B-type
Lamin. Therefore, SUN1 may be associated with Lamin A, B1, and B2.
However, the present results suggest that only Lamin A is
associated with SUN1 in ccRCC. Sharma et al (39) reported that SUN1/2 knockdown in rat
mammary carcinoma cell lines caused irregular nuclear morphology.
As Lamin expression and localization remained unchanged, the
nuclear defects of SUN1/2 were not attributable to off-target
effects on Lamin. In our study, both Lamin A and SUN1 expression
were reduced in G4 cases. These findings suggest that Lamin A and
SUN1 are interrelated or independently contribute to nuclear
morphological alterations. Considering that Nesprin-1 was expressed
in the nucleoplasm, we examined the basis for this localization
pattern. Mislow et al (40)
reported that Nesprin-1α can bind both Lamin and Emerin, acting as
a structural cross-linker that anchors these proteins to the INM.
Duong et al (41) reported
that Nesprin-1α-1 was undetectable at very low levels in all
tissues, whereas Nesprin-1α and −2 were highly expressed
exclusively in cardiac and skeletal muscles. The proportions of the
Nesprin isoforms Nesprin-2-Giant and Nesprin-1-Giant in the kidney
were 81 and 15%, respectively (41). Furthermore, in cells lacking the
KASH domain of Nesprin-2, this protein exhibited a speckled pattern
within the nucleoplasm (41).
Therefore, the nucleoplasmic staining of Nesprin-1 observed in this
study may reflect the absence of the KASH domain, considering the
very low levels of Nesprin-1α in the kidney. Moreover, Sur-Erdem
et al (42) reported that
Nesprin-1 overexpression restored abnormalities in tumor cell
nuclear structure, NE organization, centrosome positioning, and
genomic instability. Nesprin-1 interacts with Lamin and SUN
proteins, contributing to the maintenance of nuclear structure and
cytoskeletal organization (42).
However, we found no significant correlation between Nesprin-1
expression changes and nuclear grade, suggesting that Nesprin-1
slightly influences ccRCC. Furthermore, Nesprin-3 was negative in
all cases, indicating that it was not associated with nuclear
grade. Wilhelmsen et al (43) showed that Nesprin-3 binds to
intermediate filaments via plectin. Morgan et al (44) reported that silencing Nesprin-3
induces cell elongation in human aortic endothelial cells. This was
accompanied by a marked reduction in the nuclear periphery
localization of plectin and the cytoskeletal protein vimentin,
highlighting the importance of Nesprin-3 in maintaining the
cytoskeletal structure of the nuclear periphery (44). However, our study showed that in the
kidney, Nesprin-3 expression was detected only in stromal
components, including mesangial cells and fibroblasts, but was
absent in the ccRCC regions derived from the proximal tubules,
similar to normal proximal tubules. Collectively, these findings
suggest that Nesprin-3 has no role in ccRCC.
Next, we discuss the rationale for using only
nuclear and cellular morphological characteristics to classify G4
in the WHO/ISUP grading system, whereas G1-G3 are defined primarily
by the nucleolar size. In this study, the mean nuclear area and
perimeter significantly increased with higher grades from G1 to G3
in both grading systems, whereas no significant differences were
observed between G3 and G4. Therefore, factors different from those
affecting G1-G3 may influence the transition to G4. In this study,
comparison of NE-associated proteins with nuclear grade revealed
that only Lamin A and SUN1 showed significantly reduced expression
in G4. Thus, decreased Lamin A and SUN1 expression may contribute
to nuclear and cellular morphological alterations associated with
progression to G4. Diegmiller et al (45) reported that the total nuclear volume
and total nucleolar volume in nurse cells exhibit a linear
proportional relationship. Therefore, observing nucleolar size can
be considered equivalent to observing nuclear size. In other words,
our finding that nuclear size increases with grade from G1 to G3
likely reflects proportional changes between nucleolar and nuclear
sizes. Conversely, our results indicate that G4 relies on nuclear
and cellular morphology as diagnostic indicators, as alterations in
NE-associated proteins such as Lamin A and SUN1 are observed only
at this stage.
Although this study did not evaluate prognostic or
therapeutic applications, we considered the potential diagnostic
and therapeutic implications of Lamin A and SUN1 based on previous
reports. Chiarini et al (46) reported that Lamin A functions as a
tumor suppressor in Ewing sarcoma, where its introduction reduces
invasive potential and high expression correlates with improved
5-year survival rates. Therefore, in ccRCC, reintroducing Lamin A
in patients with reduced tumor cell Lamin A expression may improve
survival outcomes. Regarding SUN, existing reports are primarily
related to SUN2. In lung cancer, SUN2 suppresses cell proliferation
and migration, and its overexpression enhances chemotherapy
sensitivity (47). In contrast, low
SUN2 expression is associated with shorter survival times (47). Although no studies have examined
treatment or survival outcomes related to SUN1, Nishioka et
al (38) reported that SUN1
promotes cell migration when overexpressed. Collectively, these
findings suggest that SUN2 upregulation and SUN1 inhibition may
have therapeutic potential. However, neither has been studied in
ccRCC, indicating that this topic warrants future
investigation.
Finally, this study has several limitations. First,
the numbers of G1 and G4 cases were small, each accounting for
approximately 5 and 6% of the 199 cases, respectively. Therefore, a
larger cohort and more detailed analyses are necessary. Second,
although Lamin expression was quantitatively evaluated through
image analysis, Emerin, SUN, and Nesprin expression relied on
manual evaluation, potentially limiting analytical precision. Some
proteins exhibited nucleoplasmic or cytoplasmic staining, resulting
in varied patterns that could not be measured using the e-Nucmen3
nuclear membrane analysis software. Therefore, future research
should focus on developing advanced analytical tools capable of
handling diverse staining patterns to enable automated image-based
quantification. Third, the study analyzed only human samples,
providing limited evidence that reduced the expression of Lamin A
or SUN1 directly causes changes in nuclear morphology. Therefore,
cell or animal models should be used in future research to verify
the underlying mechanisms.
In conclusion, in ccRCC, alterations in
NE-associated protein expression were observed exclusively in G4
cases. Among Lamin, Emerin, SUN, and Nesprin proteins, only Lamin A
and SUN1 showed altered expression. Although the nuclear for G1-G3
is determined based on the nucleolar size, this parameter also
indirectly reflects nuclear size in image analysis. Notably, the
influence of NE-associated proteins appears confined to G4 tumors.
These findings provide important insight into why the WHO/ISUP
grading system exclusively employs nuclear and cellular
morphological features for G4 assessment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RS collected clinicopathological data, and
contributed to sectioning, immunohistochemistry, staining
evaluation, image and statistical analyses, and manuscript
preparation. MS planned and conducted the experiments, performed
histological diagnosis of carcinoma cases, and performed image
analysis, staining evaluation, statistical analysis and manuscript
preparation. SK performed immunohistochemistry, conducted image
analysis and reviewed the literature. YN performed whole-slide
imaging and reviewed the manuscript. MK and RK performed
whole-slide image data acquisition. MN and KS collected some
clinicopathological data from electrical records, graded and staged
some cases, and reviewed the clinicopathological data which RS
initially had collected, then corrected any incorrect data and
filled in missing data. The final clinicopathological data were
then verified by MN and KS to ensure the accuracy of the data. HI
and HY performed histological diagnosis of carcinoma cases. RS and
MS confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted after obtaining approval
from Gunma University Ethical Review Board for Medical Research
Involving Human Subjects (approval no. HS2024-068). Informed
consent for this study was obtained by an opt-out method according
to the ‘Ethical Guidelines for Medical and Health Research
Involving Human Subjects’ established by Ministry of Education,
Culture, Sports, Science and Technology and Ministry of Health,
Labour and Welfare in Japan. Because the FFPE samples were used
secondarily after their initial diagnostic purpose, we published a
notification about this study on the Gunma University Hospital
website instead of obtaining individual informed consent. The
notice provided an outline of the research plan, details of the
patient information to be used, the storage period and methods for
the samples, contact information, and assurance of the right to
freely withdraw from the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Shuch B, Amin A, Armstrong AJ, Eble JN,
Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI and Kutikov A:
Understanding pathologic variants of renal cell carcinoma:
Distilling therapeutic opportunities from biologic complexity. Eur
Urol. 67:85–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sepp T, Poyhonen A, Uusküla A, Kotsar A,
Veitonmäki T, Tammela TLJ, Baburin A and Murtola TJ: Renal cancer
survival in clear cell renal cancer compared to other types of
tumor histology: A population-based cohort study. PLoS One.
20:e03290002025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muglia VF and Prando A: Renal cell
carcinoma: Histological classification and correlation with imaging
findings. Radiol Bras. 48:166–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montironi R, Santinelli A, Pomante R,
Mazzucchelli R, Colanzi P, Filho AL and Scarpelli M: Morphometric
index of adult renal cell carcinoma. Comparison with the Fuhrman
grading system. Virchows Arch. 437:82–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delahunt B, Srigley JR, Judge MJ, Amin MB,
Billis A, Camparo P, Evans AJ, Fleming S, Griffiths DF,
Lopez-Beltran A, et al: Data set for the reporting of carcinoma of
renal tubular origin: recommendations from the international
collaboration on cancer reporting (ICCR). Histopathology.
74:377–390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lang H, Lindner V, de Fromont M, Molinié
V, Letourneux H, Meyer N, Martin M and Jacqmin D: Multicenter
determination of optimal interobserver agreement using the Fuhrman
grading system for renal cell carcinoma: Assessment of 241 patients
with > 15-year follow-up. Cancer. 103:625–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dagher J, Delahunt B, Rioux-Leclercq N,
Egevad L, Srigley JR, Coughlin G, Dunglinson N, Gianduzzo T, Kua B,
Malone G, et al: Clear cell renal cell carcinoma: Validation of
World Health Organization/International Society of Urological
Pathology grading. Histopathology. 71:918–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de las Heras JI and Schirmer EC: The
nuclear envelope and cancer: A diagnostic perspective and
historical overview. Adv Exp Med Biol. 773:5–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nmezi B, Xu J, Fu R, Armiger TJ,
Rodriguez-Bey G, Powell JS, Ma H, Sullivan M, Tu Y, Chen NY, et al:
Concentric organization of A- and B-type lamins predicts their
distinct roles in the spatial organization and stability of the
nuclear lamina. Proc Natl Acad Sci USA. 116:4307–4315. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vahabikashi A, Sivagurunathan S, Nicdao
FAS, Han YL, Park CY, Kittisopikul M, Wong X, Tran JR, Gundersen
GG, Reddy KL, et al: Nuclear lamin isoforms differentially
contribute to LINC complex-dependent nucleocytoskeletal coupling
and whole-cell mechanics. Proc Natl Acad Sci USA.
119:e21218161192022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moss SF, Krivosheyev V, de Souza A, Chin
K, Gaetz HP, Chaudhary N, Worman HJ and Holt PR: Decreased and
aberrant nuclear lamin expression in gastrointestinal tract
neoplasms. Gut. 45:723–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lammerding J, Hsiao J, Schulze PC, Kozlov
S, Stewart CL and Lee RT: Abnormal nuclear shape and impaired
mechanotransduction in emerin-deficient cells. J Cell Biol.
170:781–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaughan A, Alvarez-Reyes M, Bridger JM,
Broers JL, Ramaekers FC, Wehnert M, Morris GE, Whitfield WGF and
Hutchison CJ: Both emerin and lamin C depend on lamin A for
localization at the nuclear envelope. J Cell Sci. 114:2577–2590.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haque F, Mazzeo D, Patel JT, Smallwood DT,
Ellis JA, Shanahan CM and Shackleton S: Mammalian SUN protein
interaction networks at the inner nuclear membrane and their role
in laminopathy disease processes. J Biol Chem. 285:3487–3498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ketema M, Wilhelmsen K, Kuikman I, Janssen
H, Hodzic D and Sonnenberg A: Requirements for the localization of
nesprin-3 at the nuclear envelope and its interaction with plectin.
J Cell Sci. 120:3384–3394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banerjee I, Zhang J, Moore-Morris T,
Pfeiffer E, Buchholz KS, Liu A, Ouyang K, Stroud MJ, Gerace L,
Evans SM, et al: Targeted ablation of nesprin 1 and nesprin 2 from
murine myocardium results in cardiomyopathy, altered nuclear
morphology and inhibition of the biomechanical gene response. PLoS
Genet. 10:e10041142014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heffler J, Shah PP, Robison P, Phyo S,
Veliz K, Uchida K, Bogush A, Rhoades J, Jain R and Prosser BL: A
balance between intermediate filaments and microtubules maintains
nuclear architecture in the cardiomyocyte. Circ Res. 126:e10–e26.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xin H, Tang Y, Jin YH, Li HL, Tian Y, Yu
C, Zhao ZJ, Wu MS and Pan YF: Knockdown of LMNA inhibits
Akt/β-catenin-mediated cell invasion and migration in clear cell
renal cell carcinoma cells. Cell Adh Migr. 17:1–14. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radspieler MM, Schindeldecker M, Stenzel
P, Forsch S, Tagscherer KE, Herpel E, Hohenfellner M, Hatiboglu G,
Roth W and Macher-Goeppinger S: Lamin-B1 is a senescence-associated
biomarker in clear-cell renal cell carcinoma. Oncol Lett.
18:2654–2660. 2019.PubMed/NCBI
|
|
21
|
Fukushima T, Kobatake K, Miura K, Takemoto
K, Yamanaka R, Tasaka R, Kohada Y, Miyamoto S, Sekino Y, Kitano H,
et al: Nesprin1 deficiency is associated with poor prognosis of
renal cell carcinoma and resistance to sunitinib treatment.
Oncology. 102:868–879. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S and Lee DK: What is the proper way
to apply the multiple comparison test? Korean J Anesthesiol.
71:353–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosner B: Fisher's exact test.
Fundamentals of Biostatistics. Cengage; Boston: pp. 387–394.
2019
|
|
24
|
Lydersen S, Pradhan V, Senchaudhuri P and
Laake P: Choice of test for association in small sample unordered r
× c tables. Stat Med. 26:4328–4343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guilford JP: Correlation. Fundamental
Statistics in Psychology and Education. McGraw-Hill Book Company;
New York: pp. 154–173. 1950
|
|
26
|
Gospodarowicz M and Mason M: Kidney. TNM
Classification of malignant tumours. O'Sullivan B, Mason M, Asamura
H, et al: Wiley Blackwell; Oxford: pp. 199–203. 2017, PubMed/NCBI
|
|
27
|
Capo-chichi CD, Aguida B, Chabi NW, Cai
QK, Offrin G, Agossou VK, Sanni A and Xu XX: Lamin A/C deficiency
is an independent risk factor for cervical cancer. Cell Oncol
(Dordr). 39:59–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lammerding J, Fong LG, Ji JY, Reue K,
Stewart CL, Young SG and Lee RT: Lamins A and C but not lamin B1
regulate nuclear mechanics. J Biol Chem. 281:25768–25780. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pujadas Liwag EM, Wei X, Acosta N, Carter
LM, Yang J, Almassalha LM, Jain S, Daneshkhah A, Rao SSP,
Seker-Polat F, et al: Depletion of lamins B1 and B2 promotes
chromatin mobility and induces differential gene expression by a
mesoscale-motion-dependent mechanism. Genome Biol. 25:772024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harborth J, Elbashir SM, Bechert K, Tuschl
T and Weber K: Identification of essential genes in cultured
mammalian cells using small interfering RNAs. J Cell Sci.
114:4557–4565. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reis-Sobreiro M, Chen JF, Novitskaya T,
You S, Morley S, Steadman K, Gill NK, Eskaros A, Rotinen M, Chu CY,
et al: Emerin deregulation links nuclear shape instability to
metastatic potential. Cancer Res. 78:6086–6097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stewart-Hutchinson PJ, Hale CM, Wirtz D
and Hodzic D: Structural requirements for the assembly of LINC
complexes and their function in cellular mechanical stiffness. Exp
Cell Res. 314:1892–1905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lombardi ML, Jaalouk DE, Shanahan CM,
Burke B, Roux KJ and Lammerding J: The interaction between nesprins
and sun proteins at the nuclear envelope is critical for force
transmission between the nucleus and cytoskeleton. J Biol Chem.
286:26743–26753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsumoto A, Hieda M, Yokoyama Y, Nishioka
Y, Yoshidome K, Tsujimoto M and Matsuura N: Global loss of a
nuclear lamina component, lamin A/C, and LINC complex components
SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med.
4:1547–1557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ueda N, Maekawa M, Matsui TS, Deguchi S,
Takata T, Katahira J, Higashiyama S and Hieda M: Inner nuclear
membrane protein, SUN1, is required for cytoskeletal force
generation and focal adhesion maturation. Front Cell Dev Biol.
10:8858592022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Östlund C, Folker ES, Choi JC, Gomes ER,
Gundersen GG and Worman HJ: Dynamics and molecular interactions of
linker of nucleoskeleton and cytoskeleton (LINC) complex proteins.
J Cell Sci. 122:4099–4108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haque F, Lloyd DJ, Smallwood DT, Dent CL,
Shanahan CM, Fry AM, Trembath RC and Shackleton S: SUN1 interacts
with nuclear lamin A and cytoplasmic nesprins to provide a physical
connection between the nuclear lamina and the cytoskeleton. Mol
Cell Biol. 26:3738–3751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishioka Y, Imaizumi H, Imada J, Katahira
J, Matsuura N and Hieda M: SUN1 splice variants, SUN1_888,
SUN1_785, and predominant SUN1_916, variably function in
directional cell migration. Nucleus. 7:572–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharma VP, Williams J, Leung E, Sanders J,
Eddy R, Castracane J, Oktay MH, Entenberg D and Condeelis JS:
SUN-MKL1 crosstalk regulates nuclear deformation and fast motility
of breast carcinoma cells in fibrillar ECM microenvironment. Cells.
10:15492021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mislow JM, Holaska JM, Kim MS, Lee KK,
Segura-Totten M, Wilson KL and McNally EM: Nesprin-1alpha
self-associates and binds directly to emerin and lamin A in vitro.
FEBS Lett. 525:135–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duong NT, Morris GE, Lam le T, Zhang Q,
Sewry CA, Shanahan CM and Holt I: Nesprins: Tissue-specific
expression of epsilon and other short isoforms. PLoS One.
9:e943802014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sur-Erdem I, Hussain MS, Asif M, Pinarbasi
N, Aksu AC and Noegel AA: Nesprin-1 impact on tumorigenic cell
phenotypes. Mol Biol Rep. 47:921–934. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilhelmsen K, Litjens SH, Kuikman I,
Tshimbalanga N, Janssen H, van den Bout I, Raymond K and Sonnenberg
A: Nesprin-3, a novel outer nuclear membrane protein, associates
with the cytoskeletal linker protein plectin. J Cell Biol.
171:799–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morgan JT, Pfeiffer ER, Thirkill TL, Kumar
P, Peng G, Fridolfsson HN, Douglas GC, Starr DA and Barakat AI:
Nesprin-3 regulates endothelial cell morphology, perinuclear
cytoskeletal architecture, and flow-induced polarization. Mol Biol
Cell. 22:4324–4334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diegmiller R, Doherty CA, Stern T, Imran
Alsous J and Shvartsman SY: Size scaling in collective cell growth.
Development. 148:dev1996632021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chiarini F, Paganelli F, Balestra T,
Capanni C, Fazio A, Manara MC, Landuzzi L, Petrini S, Evangelisti
C, Lollini PL, et al: Lamin A and the LINC complex act as potential
tumor suppressors in ewing sarcoma. Cell Death Dis. 13:3462022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lv XB, Liu L, Cheng C, Yu B, Xiong L, Hu
K, Tang J, Zeng L and Sang Y: SUN2 exerts tumor suppressor
functions by suppressing the warburg effect in lung cancer. Sci
Rep. 5:179402015. View Article : Google Scholar : PubMed/NCBI
|