Introduction
Tumor-to-tumor metastasis is diagnosed when the
recipient lesion is a true primary neoplasm and the donor lesion is
an authentic metastatic deposit (1). Direct invasion of one tumor into
another or the presence of isolated tumor emboli within a recipient
neoplasm must be excluded from the diagnosis of tumor-to-tumor
metastasis, because they do not represent true metastatic spread
from the donor tumor to the recipient primary tumor but reflect
local infiltration or merely the passive retention of tumor cells
within the recipient tumor without the formation of lesions with
metastatic biological characteristics. Tumor-to-tumor metastasis is
rare (2), with <200 cases
documented cases reported in the literature. Although the
mechanisms of tumor-to-tumor metastasis remain unclear, proposed
contributors include disruption of the vascular barrier and
impaired immune surveillance within recipient thyroid lesions.
The primary malignancies that most often metastasize
to the thyroid are lung carcinoma, renal cell carcinoma (RCC),
colorectal carcinoma and breast carcinoma. Lung adenocarcinoma (LC,
the most common subtype of lung carcinoma, frequently spreads to
distant organs. Among thyroid neoplasms, follicular adenoma (FA)
and papillary thyroid carcinoma (PTC) represent the most frequent
benign (38%) and malignant recipients (26.5%) of metastatic tumor
cells, respectively (3). The
clinical presentation of thyroid metastasis is variable, typically
presenting with metastatic lesions as the initial manifestation
(4). Consequently, thyroid
metastasis may present substantial diagnostic difficulties, with
frequent misclassification as primary thyroid carcinoma.
Tumor-to-tumor metastasis involving thyroid
neoplasms-particularly cases where LC affects FTC-remains rare in
clinical practice, with limited evidence available to guide
diagnostic and management strategies for such unusual
presentations. Against this background, the primary aims of the
present case report were as follows: i) To detail the clinical,
imaging and pathological characteristics of a thyroid
neoplasm-associated case with complex diagnostic considerations, to
highlight key challenges in distinguishing secondary from primary
thyroid malignancies; ii) to collate the existing literature and
contextualize current understanding of tumor-to-tumor metastasis in
thyroid neoplasms; and iii) provide practical insights for
clinicians navigating the evaluation and management of similar
cases.
Case report
A 46-year-old Chinese woman was admitted to the 960
Hospital of the Chinese People's Liberation Army in March 2020
after detection of a sizable nodule in the left thyroid lobe during
a routine physical examination conducted by a general practitioner.
The patient reported no pain or other symptoms, had no risk factors
such as smoking, and no prior history of malignancy. The patient
also had no family history of cancer-including thyroid cancer, lung
cancer, or other malignant tumors-nor any family history of
hereditary conditions. Thyroid function tests were within normal
limits at presentation. Ultrasonography revealed a solid hypoechoic
mass with a relatively clear margin and regular contour, measuring
~60×40 mm and occupying most of the left lobe (TI-RADS III)
(Fig. S1A). However, no additional
imaging beyond ultrasound (for example, CT, chest X-ray or PET) was
performed preoperatively.
The patient underwent thyroidectomy with
intraoperative frozen section evaluation 4 days after admission in
March 2020. Histopathological analysis, performed as previously
described (5), indicated an
epithelial neoplasm, but its benign or malignant nature remained
indeterminate (Fig. S1B). In
addition, a contralateral nodule in the right thyroid lobe was
definitively diagnosed as papillary thyroid carcinoma (PTC) based
on intraoperative frozen section analysis, which revealed classic
and unambiguous PTC features (for example, nuclear groves, powdery
chromatin, and focal papillary architecture) (Fig. S1C). Due to the large size of the
lesion, the suspicious left-lobe lesion, and the confirmed
malignancy in the right lobe, total thyroidectomy with
comprehensive neck exploration was performed. Gross examination of
the surgical specimen demonstrated a solitary solid mass in the
left lobe, measuring 60×40 mm, grey-red in color, firm to the touch
and encapsulated by fibrous connective tissue on cut section,
together with a small solid nodule, grey in color, measuring 3×2
mm, in the right lobe.
Microscopically, the solid mass in the left lobe was
surrounded by a markedly thickened fibrous capsule. Serial
sectioning demonstrated neoplastic cell infiltration of the capsule
with focal transgression (Fig. 1A).
The tumor comprised three morphologically distinct carcinoma
components (Fig. 1B). The first
component (T1) displayed a follicular growth pattern with
medium-sized follicles containing variable amounts of colloid
(Fig. 1C). The second component
(T2) was characterized by solid, trabecular, or nested arrangements
with only scant colloid in follicles (Fig. 1D). The third component (T3)
exhibited high-grade adenocarcinoma morphology, including marked
nuclear pleomorphism and prominent nucleoli, arranged in glandular,
trabecular, papillary and hobnail patterns (Fig. 1E). Mitotic figures were readily
observed, and tumor necrosis was evident (Fig. 1F). This component infiltrated both
T1 and T2, producing indistinct boundaries between them.
Immunohistochemical analysis was performed as previously described
(6) and indicated that all three
carcinoma components were positive for thyroid transcription
factor-1 (TTF-1), cytokeratin 19 (CK19) and negative for BRAF-1,
calcitonin (CT) and parathyroid hormone (PTH) (Fig. S1D-G). Both T1 and T2 showed weak
CK19 expression and positivity for cluster of differentiation 56
(CD56), findings consistent with FTC rather than PTC (PTC typically
demonstrates strong CK19 positivity and weak or absent CD56)
(Fig. 2A and B). T1 and T2 also
expressed thyroglobulin (TG) and paired box gene 8 (PAX-8), whereas
T3 was negative for both these markers and CD56 (Fig. 2C and D). By contrast, NapsinA
(Fig. 2E) and carcinoembryonic
antigen (CEA) (Fig. S1H) were
strongly expressed in T3 but absent in T1 and T2. The Ki-67 index
reached 25% in T3, compared with 1% in T1 and T2 (Fig. 2F). No significant difference was
observed in the cyclin D1 index across the three components, and
this index was ~70% (Fig. S1I).
Furthermore, all components displayed wild-type expression of P53,
while the index was slightly higher in the T3 component than in the
T1 and T2 component (Fig. S1J).
The immunophenotypes of the three morphologically distinct
carcinoma components are summarized in Table I. T1 and T2 components were
classified as FTC, whereas T3 was identified as LC. The final
diagnosis indicated LC metastasis to a pre-existing FTC nodule.
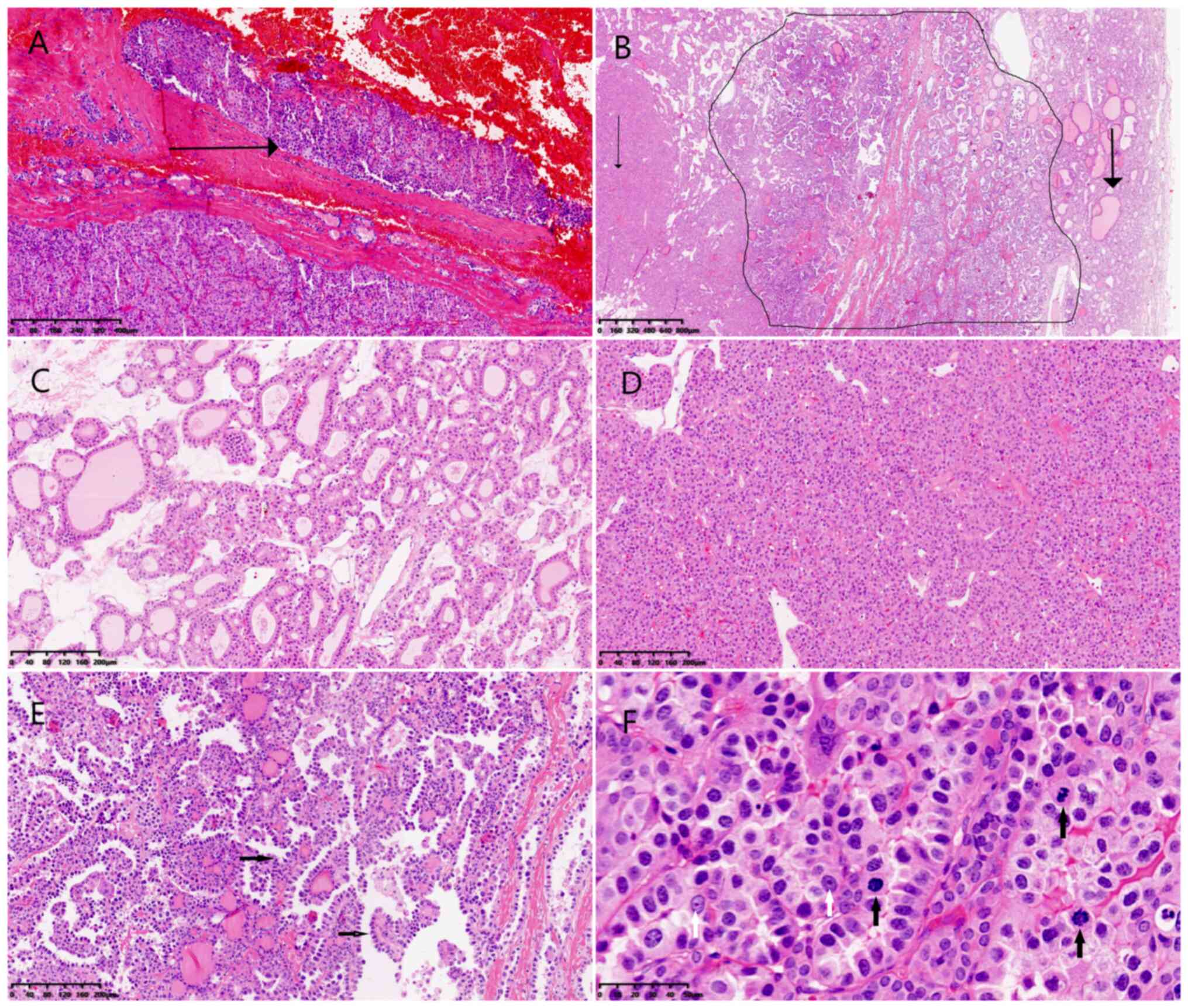 | Figure 1.Microscopic examination of the
recipient thyroid lesion. (A) Carcinoma was surrounded by a
thickened fibrous capsule with neoplastic cells infiltrated the
capsule and a focal area of penetration was identified after serial
sectioning (black arrow) (H&E stain; magnification, ×20). (B)
Morphologically distinct components of carcinoma labeled as T1
(thick black arrow), T2 (inside the black line) and T3 (thin black
arrow) (H&E stain; magnification, ×20). (C) The T1 component
revealed the follicular pattern features with medium size and a
variable amount of colloid in the follicles when examined at a
higher power, (H&E stain; magnification, ×200). (D) The T2
component revealed solid, trabecular or nested growth pattern and
little colloid in the follicles when examined at a higher power
(H&E stain; magnification, ×200). (E) T3 component revealed
glandular, papillary and hobnail pattern (black arrow) when
examined at a higher power (H&E stain; magnification, ×200).
(F) The T3 component revealed nuclear pleomorphism, conspicuous
nucleoli (white arrow) and the mitotic figures (black arrow) were
common (H&E stain; magnification, ×400). H&E, hematoxylin
and eosin. |
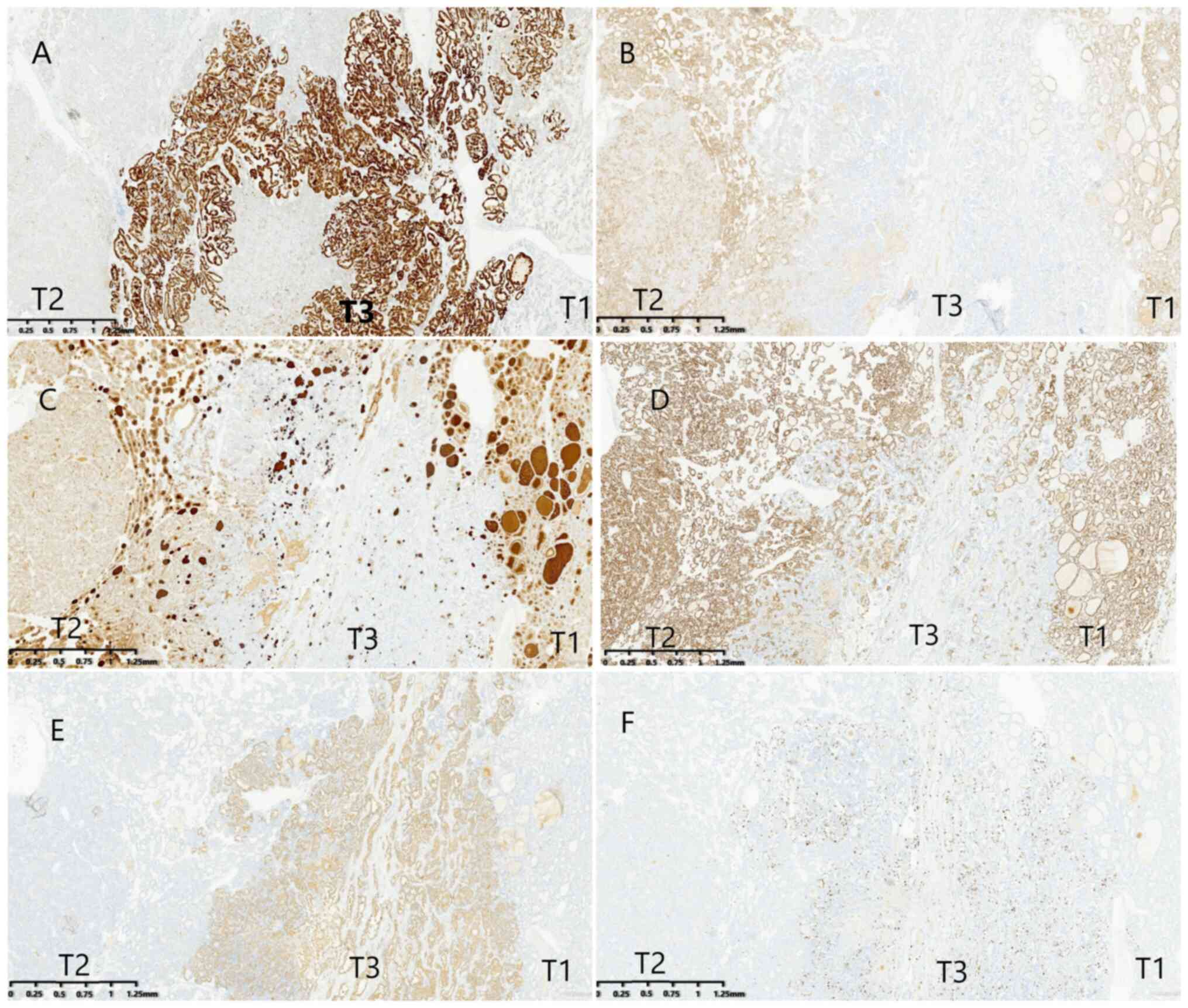 | Figure 2.Immunohistochemistry examination of
the three morphologically distinct components of carcinoma. (A)
Both the T1 and T2 component weakly expressed CK19, whereas the T3
component was strong positive (magnification, ×20). (B) Both the T1
and T2 component expressed CD56, whereas the T3 component was
negative (magnification, ×20). (C) Both the T1 and T2 component
expressed TG, whereas the T3 component was negative (magnification,
×20). (D) Both the T1 and T2 component strong expressed PAX8,
whereas the T3 component was negative (magnification, ×20). (E)
Both the T1 and T2 component was negative for Napsin-A, whereas the
T3 component was strong positive (magnification, ×20). (F) The
Ki-67 index was significantly higher in the T3 component than in
the T1 and T2 component (magnification, ×20). CK19, cytokeratin 19;
CD56, cluster of differentiation 56; PAX8, paired box gene 8. |
 | Table I.Immunohistochemical staining results
of different tumor cell populations and the nodule in the right
lobe. |
Table I.
Immunohistochemical staining results
of different tumor cell populations and the nodule in the right
lobe.
| Antibody | T1 | T2 | T3 |
|---|
| TG | + | + | - |
| TTF-1 | + | + | + |
| Napsin A | - | - | + |
| PAX-8 | + | + | - |
| CK19 | + | + | + |
| CyclinD1 | + | + | + |
| CD56 | + | + | - |
| BRAF-1 | - | - | - |
| CEA | - | - | + |
| CT | - | - | - |
| PTH | - | - | - |
| P53 | Wild-type | Wild type | Wild type |
| Ki-67 | 1% | 1% | 25% |
To substantiate this diagnosis, the mutational
profile of LC was analyzed using next generation sequencing (NGS).
Genomic DNA was isolated from the paraffin-embedded sections using
Human EGFR/KRAS/BRAF/ALK/ROS1 Genetic Test CDx (Sequencing By
Reversible Terminators) kit (cat. no. KS301-FA16; Guangzhou
Jinqirui Biotechnology Co., Ltd.). Fluorometric quantification was
performed with the Qubit dsDNA HS Assay Kit to verify the quality
and integrity of processed samples. Sequencing was performed on the
Illumina MiniSeq platform using the KM MiniSeq Dx-CN Mid Output kit
(300 cycles; cat. no. KS107-CXM; Guangzhou Jinqirui Biotechnology
Co. Ltd.) following the manufacturer's instructions, with 2×150 bp
paired-end sequencing. paired end according to the manufacturer's
instructions. The final library (quantified via the Roche KAPA
Library Quantification Kit) was loaded at a concentration of 1.4
pM. Sequence alignment was carried out using the Tumor Gene Data
Analysis and Management System (V3; Guangzhou Jinqirui
Biotechnology Co., Ltd.). The analysis revealed the L858R mutation
in exon 21 of EGFR, a recognized driver mutation predominantly
associated with non-small cell lung cancer (especially
adenocarcinoma) and rarely observed in other tumor types. Detection
of this lung-specific mutation provided strong molecular evidence
for a pulmonary origin. A subsequent chest computed tomography scan
revealed a 23 mm heterogeneously enhanced tumor in the left upper
lobe (data not shown). Given the presence of the L858R mutation,
targeted therapy with EGFR tyrosine kinase inhibitors was
recommended. However, the patient declined lung biopsy and died 16
months after surgery, in July 2021.
Discussion
Tumor-to-tumor metastasis is a rare phenomenon.
Garneau et al (3) reported
four cases involving thyroid neoplasms and conducted a
comprehensive literature review of English-language publications
from 1962 to 2022. Since that time, to the best of our knowledge,
13 additional cases of tumor-to-tumor metastasis (3,4,7–11)
(including the present case) have been documented between 2022 and
2025 (Table II). In total, 66
cases of tumor-to-tumor metastasis with primary thyroid neoplasms
as recipients have been recorded, including 43 cases of
cancer-to-cancer metastasis (Table
III). RCC was initially regarded as the most frequent donor
tumor to thyroid neoplasms (12).
However, more recent data indicate that LC (16/66 cases) has become
the most common donor tumor in tumor-to-tumor metastasis (Table IV), followed by renal carcinoma
(15/66 cases), colon carcinoma (15/66 cases) and breast carcinoma
(12/66 cases) (3,13–25).
Among benign recipient tumors, FA represents the majority (20/66
cases). PTC is the most frequent malignant recipient tumor,
comprising half of all reported cases (33/66). Of these 33 cases,
22 involve classic PTC and 11 involve follicular PTC. Additional
recipient tumor types are listed in Table III. This distribution corresponds
with the general incidence of thyroid tumors.
 | Table II.Cases of tumor-to-tumor metastasis
involving a primary thyroid neoplasm as the recipient reported from
2022 to September 2025 (n=13). |
Table II.
Cases of tumor-to-tumor metastasis
involving a primary thyroid neoplasm as the recipient reported from
2022 to September 2025 (n=13).
| Case | Author | Publication year | Age/sex | Primary tumor | Recipient | (Refs.) |
|---|
| 1 | Garneau et
al | 2022 | 56/F | LC | PTC | (3) |
| 2 |
|
| 76/F | BC | PTC |
|
| 3 |
|
| 54/F | Tonsillar SCC | PTC |
|
| 4 |
|
| 71/F | Colon NET | PTC |
|
| 5 | Ghossein et
al | 2021 | 72/F | EAC | FA | (4) |
| 6 |
|
| 66/F | CRC | NIEPTC |
|
| 7 |
|
| 65/F | RCC | NIFTP |
|
| 8 | Gawlik et
al | 2023 | 63/F | RCC | PTC | (7) |
| 9 | Maestri et
al | 2024 | 70/M | HCC | HC | (8) |
| 10 | Tadisina et
al | 2024 | 65/M | RCC | PTC | (9) |
| 11 | Gvazava et
al | 2025 | 63/F | BC | HC | (10) |
| 12 | Jbali et
al | 2025 | 63/F | BC | PTC | (11) |
| 13 | Present case | - | 46/F | LC | FTC | - |
 | Table III.Distribution of all the 66 cases
involving a primary thyroid neoplasm as the recipient in a
tumor-to-tumor metastasis. |
Table III.
Distribution of all the 66 cases
involving a primary thyroid neoplasm as the recipient in a
tumor-to-tumor metastasis.
|
|
| Recipient
tumor |
|---|
| Primary tumor | Total |
|
|---|
| FA | PTC | FVPTC | HA | HC | FTC | MTC | NIFTP |
|---|
| Lung | 16 | 7 | 4 | 4 | 0 | 0 | 1 | 0 | 0 |
| Renal | 15 | 3 | 5 | 4 | 1 | 1 | 0 | 0 | 1 |
| Breast | 12 | 3 | 6 | 1 | 1 | 1 | 0 | 0 | 0 |
| Colon | 15 | 4 | 7 | 0 | 0 | 1 | 1 | 2 | 0 |
| Esophagus | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tonsillar | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Pancreatic | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gastric | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prostatic | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total | 66 | 20 | 22 | 11 | 3 | 5 | 2 | 2 | 1 |
 | Table IV.Cases of intrathyroid metastases from
primary lung carcinoma (n=16). |
Table IV.
Cases of intrathyroid metastases from
primary lung carcinoma (n=16).
| Case | Author | Publication
year | Age/sex | Primary tumor | Recipient | (Refs.) |
|---|
| 1 | Mizukami et
al | 1990 | 75/F | LC | FA | (13) |
| 2 | Akamatsu et
al | 1994 | 46/F | LC | FA | (14) |
| 3 | Baloch and
Livolsi | 1999 | 75/F | SCLC | FVPTC | (15) |
| 4 | Kameyama et
al | 2000 | 51/M | LC | FA | (16) |
| 5 | Mori et
al | 2008 | 54/M | LC | FVPTC | (17) |
| 6 | Hashimoto et
al | 2011 | 60/F | LC | FVPTC | (18) |
| 7 | Stevens et
al | 2011 | 65/M | LPDC | FA | (19) |
| 8 | Matsukuma et
al | 2013 | 67/F | SCLC | PTC | (20) |
| 9 | Pusztaszeri et
al | 2015 | N/A | LC | FA | (21) |
| 10 | Wey and Chang | 2015 | 64/M | SCLC | FA | (22) |
| 11 |
| 2015 | 71/F | LASC | PTC |
|
| 12 | Gowda et
al | 2017 | 52/M | SCLC | FA | (23) |
| 13 | Ozoran et
al | 2018 | 53/F | LC | PTC | (24) |
| 14 | Clara et
al | 2022 | 70/M | LCC | FVPTC | (25) |
| 15 | Garneau et
al | 2022 | 56/F | LC | PTC | (3) |
| 16 | Present case | - | 46/F | LC | FTC | - |
FTC is recognized as an uncommon subtype of thyroid
carcinoma, accounting for 5–15% of all thyroid malignancies
(26). The present report
describes, to the best of our knowledge, the first case of LC
metastasizing to FTC, accompanied by PTC in the contralateral lobe.
This case contributes to addressing current knowledge gaps
regarding LC metastasis to FTC and provides reference material for
diagnostic and therapeutic considerations by clinicians and
pathologists.
Metastasis to the thyroid presents considerable
diagnostic challenges, often resulting in its misinterpretation as
primary thyroid carcinoma (4).
Tumor-to-tumor metastasis is particularly uncommon, especially when
both donor and recipient neoplasms are malignant. Diagnostic
imaging generally reveals no distinct clinical features or yields
limited characteristic findings. In the present case, a thyroid
mass represented the initial manifestation of LC. Metastatic
involvement of the thyroid usually appears as a solitary lesion,
which most clinical pathologists are inclined to regard as primary
in origin (4). In tumor-to-tumor
metastases, the metastatic component typically constitutes only a
minor fraction of the donor tumor and exhibits close admixture with
recipient tumor cells, without a discernible host response
(desmoplastic, inflammatory, or myxoid) (1).
In the present case, LC accounted for a small
portion of the FTC and demonstrated intimate intermingling with it.
Although FNA remains a widely applied method for the preliminary
assessment of thyroid nodules, it carries inherent limitations in
detecting potential metastatic disease. The limited and fragmented
nature of FNA specimens may preclude capturing the complete
histological structure of a tumor, thereby restricting evaluation
of growth patterns or differentiation between morphologically
similar tumors, such as poorly differentiated thyroid carcinoma
(PDTC) and metastatic LC in the present case. Distinguishing
primary from metastatic tumors based solely on histological
characteristics remains difficult due to overlapping growth
patterns. In the present study, the metastatic tumor displayed
predominantly solid, trabecular, papillary or nested growth with
scant colloid in follicles, leading to its initial classification
as PDTC. Both PDTC and metastatic lung cancer commonly exhibit
overlapping features, including solid, trabecular or nested
patterns and pleomorphic cells with high nuclear-to-cytoplasmic
ratios. Thus, the preliminary interpretation of the present case as
encapsulated FTC with concurrent PTC and PDTC was justified. The
consultant further noted that PTC (predominantly boot-spike type
with some high-cell type) was embedded within FTC. The present case
illustrates the diagnostic challenge posed by incomplete clinical
information and highlights the importance of considering
tumor-to-tumor metastasis when a lesion demonstrates a bimorphic
appearance with distinct morphological components.
Although the present case posed diagnostic
challenges, tumor-to-tumor metastasis should be considered whenever
atypical cytological and histological features are encountered. In
this instance, as metastatic deposits largely preserved the
morphology of the primary tumors, accurate identification was
possible. The metastatic tumor partially resembled primary PDTC but
also exhibited characteristics of a high-grade adenocarcinoma,
including marked nuclear pleomorphism, prominent nucleoli and
necrosis, suggesting the likelihood of metastasis.
Immunohistochemical staining resolved these diagnostic
uncertainties. In this report, immunostains for TG, PAX-8, TTF-1,
CEA and NapsinA proved valuable in differentiating primary thyroid
carcinoma from metastatic LC. PDTC, a follicular-derived primary
thyroid tumor, showed positivity for TG, PAX-8 and TTF-1, but
negativity for NapsinA and CEA. By contrast, the metastatic
adenocarcinoma demonstrated positivity for TTF-1, NapsinA and CEA,
with negativity for TG and PAX-8. The Ki-67 index was considerably
higher in the adenocarcinoma component (25%) compared with the FTC
component (1%). Pathological evaluation was performed using stains
specific to both thyroid and lung origins. TTF-1, although
expressed in tumors of both sites, has limited diagnostic value
without additional markers such as Napsin A and TG. In
diagnostically complex cases, molecular testing can be informative,
as tumor-specific mutations and rearrangements (for example, BRAF
V600E, EGFR, ALK and ROS) help determine the site of origin
(12). The mutational spectrum of
LC was further examined with NGS, which revealed the L858R
substitution in EGFR exon 21. This well-characterized driver
mutation, strongly associated with non-small cell lung cancer
(particularly adenocarcinoma) and uncommon in other malignancies,
provided compelling molecular evidence for the pulmonary origin of
the metastatic component (27).
Recognition of tumor-to-tumor metastasis should be considered when
bimorphic structure and divergent morphologies are encountered.
Accurate diagnosis requires the application of appropriate
immunohistochemical markers and molecular assays, which also guide
the selection of targeted therapeutic strategies.
Malignant tumors with distant metastasis generally
carry a poor prognosis. The role of surgical resection in thyroid
metastasis has been widely debated in the literature. Current
evidence indicates that removal of isolated thyroid metastases
yields superior survival compared with conservative management,
with subsite analyses highlighting benefits particularly for
metastases originating from the kidney, colon or lung (6,28,29).
Ghossein et al (4) reported
that surgical intervention with curative intent may be appropriate
in cases of oligometastasis to the thyroid gland, potentially
improving survival and even achieving long-term remission.
Accordingly, surgical treatment is advised for solitary metastatic
lesions (30). Surgical
decision-making additionally depends on factors such as the
histological subtype of the primary tumor, anatomical location of
the intrathyroid metastasis, biological behavior of the primary
malignancy, and classification of metastasis as oligometastatic or
polymetastatic. For patients with metastasis, targeted therapy
represents an effective treatment strategy, with drug selection
guided by specific genetic alterations (BRAF V600E, EGFR, ALK, ROS
and others). In the present case, no evidence of metastasis was
detected outside the thyroid, and surgical excision of the
metastatic tumor was achieved. Given the presence of the L858R
mutation, targeted therapy with EGFR tyrosine kinase inhibitors was
advised. However, the patient declined lung biopsy and subsequent
therapy, and died 16 months after surgery.
In conclusion, to the best of our knowledge, the
present case represents the first report of LC metastasizing to FTC
with concurrent PTC in the contralateral lobe. It illustrates the
diagnostic challenge encountered in the absence of a prior clinical
history of malignancy and stresses the necessity for pathologists
to maintain strong suspicion for cancer-to-cancer metastasis when a
tumor exhibits bimorphic structure and distinct morphological
characteristics. Considering the markedly poor prognosis associated
with metastatic tumors, early and accurate diagnosis remains
essential for timely and appropriate management. Despite the
generally unfavorable outcome, surgical intervention in selected
patients with metastases may contribute to improved survival.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All original NGS data generated in this study have
been deposited in the Sequence Read Archive database of the
National Center for Biotechnology Information (accession no.
SRR35762829; http://www.ncbi.nlm.nih.gov/sra). The rest of the data
generated in the present study may be requested from the
corresponding author.
Authors' contributions
CW and XL acquired the data. CW, YY, LB and XL
analyzed and interpreted the data and confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures complied with applicable laws and
institutional regulations and were approved by the relevant
institutional committee.
Patient consent for publication
The privacy rights of the subject were respected,
and waiver of informed consent was obtained for participation and
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fadare O, Parkash V, Fiedler PN, Mayerson
AB and Asiyanbola B: Tumor-to-tumor metastasis to a thyroid
follicular adenoma as the initial presentation of a colonic
adenocarcinoma. Pathol Int. 55:574–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hota SK, Mishra S, Dash S, Samantaray S
and Mallik RN: Intracranial tumor-to-tumor metastasis in an elderly
female: An unusual case report. J Cancer Res Ther. 19:1480–182.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garneau M, Alyzadneh E, Lal G and Rajan Kd
A: Metastatic disease to a concurrent thyroid neoplasm: A case
series and review of the literature. Head Neck Pathol. 17:447–459.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghossein CA, Khimraj A, Dogan S and Xu B:
Metastasis to the thyroid gland: A Single-institution 16-year
experience. Histopathology. 78:508–519. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie B, Huang K and Zhu S: Multiple primary
malignant neoplasms and long-term survival: A case report. Oncol
Lett. 30:6052025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li T, Ma T, Zhao Y, Zhang S, Yang G, Wang
X, Wang X, Wang M, Zhao X, Liu H, et al: Submandibular adenoid
cystic carcinoma presenting with liver metastasis as the initial
symptom: A case report. Oncol Lett. 30:5962025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gawlik C, Lane J and Horattas M:
Tumor-to-tumor spread: A case report and literature review of renal
cell carcinoma metastasis into thyroid cancer. World J Surg Oncol.
21:3622023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maestri M, Cicerone O, Messina A, Gallotti
A, Corallo S, Mauramati S, Canzi P, Fiandrino G, Paulli M and
Vanoli A: Pink-on-pink: Hepatocellular carcinoma metastatic to
oncocytic carcinoma of the thyroid. Pathologica. 116:158–162. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tadisina S, Sami F, Mettman D and Ridella
M: A rare case of Tumor-to-Tumor metastasis: Renal cell carcinoma
metastasis to papillary thyroid carcinoma. JCEM Case Rep.
2:luae0812024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gvazava N, Stevens TM and Rader RK: A case
of Tumor-to-Tumor metastasis: Breast carcinoma metastatic to
oncocytic carcinoma of thyroid. Kans J Med. 18:46–48. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jbali S, Amri A, Dhaha M, Jeridi L,
Houcine Y and Kedous S: Double trouble: Tumor-to-tumor metastasis
of breast cancer into a thyroid papillary Carcinoma-case report and
review of literature. Int J Surg Case Rep. 132:1114802025.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nixon IJ, Coca-Pelaz A, Kaleva AI,
Triantafyllou A, Angelos P, Owen RP, Rinaldo A, Shaha AR, Silver CE
and Ferlito A: Metastasis to the thyroid gland: A critical review.
Ann Surg Oncol. 24:1533–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizukami Y, Saito K, Nonomura A,
Michigishi T, Hashimoto T, Nakanuma Y, Matsubara F and Takasakura
E: Lung carcinoma metastatic to microfollicular adenoma of the
thyroid. A case report. Acta Pathol Jpn. 40:602–608.
1990.PubMed/NCBI
|
|
14
|
Akamatsu H, Amano J, Suzuki A and
Kikushima Y: A case of micro-metastatic lung adenocarcinoma into
adenoma of the thyroid. Kyobu Geka. 47:319–321, (In Japanese).
PubMed/NCBI
|
|
15
|
Baloch ZW and Livolsi VA: Tumor-to-tumor
metastasis to follicular variant of papillary carcinoma of thyroid.
Arch Pathol Lab Med. 123:703–706. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kameyama K, Kamio N, Okita H and Hata J:
Metastatic carcinoma in follicular adenoma of the thyroid gland.
Pathol Res Pract. 196:333–338. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori K, Kitazawa R, Kondo T and Kitazawa
S: Lung adenocarcinoma with micropapillary component presenting
with metastatic scrotum tumor and cancer-to-cancer metastasis: A
case report. Cases J. 1:1622008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashimoto K, Yamamoto H, Nakano T, Oyama
M, Shiratsuchi H, Nakashima T, Tamiya S, Komune S and Oda Y:
Tumor-to-tumor metastasis: Lung adenocarcinoma metastasizing to a
follicular variant of papillary thyroid carcinoma. Pathol Int.
61:435–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stevens TM, Richards AT, Bewtra C and
Sharma P: Tumors metastatic to thyroid neoplasms: A case report and
review of the literature. Patholog Res Int.
2011:2386932011.PubMed/NCBI
|
|
20
|
Matsukuma S, Kono T, Takeo H, Hamakawa Y
and Sato K: Tumor-to-tumor metastasis from lung cancer: A
clinicopathological postmortem study. Virchows Arch. 463:525–534.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pusztaszeri M, Wang H, Cibas ES, Powers
CN, Bongiovanni M, Ali S, Khurana KK, Michaels PJ and Faquin WC:
Fine-needle aspiration biopsy of secondary neoplasms of the thyroid
gland: A multi-institutional study of 62 cases. Cancer Cytopathol.
123:19–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wey SL and Chang KM: Tumor-to-tumor
metastasis: Lung carcinoma metastasizing to thyroid neoplasms. Case
Rep Pathol. 2015:1539322015.PubMed/NCBI
|
|
23
|
Gowda KK, Bal A, Agrawal P, Verma R and
Das A: Tumor-to-tumor metastasis: Small cell carcinoma lung
metastasising into a follicular adenoma of the thyroid. Indian J
Pathol Microbiol. 60:133–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozoran E, Güzel M, Piraliyev E, Destek S
and Aysan E: Tumor-to-tumor metastasis: A case of lung carcinoma
metastasizing to thyroid neoplasm. AACE Clin Case Rep. 4:112–114.
2018.
|
|
25
|
Clara U, Rossella DF, Giulio R, Gabriele M
and Virginia L: Tumor-to-tumor metastasis: Lung typical carcinoid
metastatic to follicular variant of papillary thyroid carcinoma.
Endocr Pathol. 33:330–332. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlegel N: Update on follicular thyroid
Cancer-what is relevant for surgeons? Chirurgie (Heidelb).
96:544–550. 2025.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma XL, Jia RN, Han K and Zhang YX:
Correlation between common driver gene variations and
clinicopathological typing in lung adenocarcinoma. Zhonghua Bing Li
Xue Za Zhi. 53:578–584. 2024.(In Chinese). PubMed/NCBI
|
|
28
|
Nguyen M, He G and Lam AK:
Clinicopathological and molecular features of secondary cancer
(Metastasis) to the thyroid and advances in management. Int J Mol
Sci. 23:32422022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Russell JO, Yan K, Burkey B and Scharpf J:
Nonthyroid metastasis to the thyroid gland: Case series and review
with observations by primary pathology. Otolaryngol Head Neck Surg.
155:961–968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sindoni A, Rizzo M, Tuccari G, Ieni A,
Barresi V, Calbo L, Cucinotta E, Trimarchi F and Benvenga S:
Thyroid metastases from renal cell carcinoma: Review of the
literature. ScientificWorldJournal. 10:590–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
















