Introduction
Colorectal cancer is the fourth most commonly
diagnosed solid malignancy worldwide. Although rectal cancer is
often grouped together with colon cancer, growing evidence supports
its distinct clinical behavior, anatomical considerations, and
therapeutic management strategies (1).
For patients with locally advanced rectal cancer
(stages II–III), neoadjuvant chemoradiotherapy has become the
cornerstone of treatment. In recent years, novel therapeutic
regimens, including total neoadjuvant therapy (TNT), have
demonstrated improved local control and pathological response rates
in multiple randomized trials (2).
Clinical studies such as PRODIGE 23, RAPIDO, and
OPRA have shifted the treatment paradigm by positioning TNT as the
preferred initial strategy in eligible patients. However, not all
patients are candidates for this approach. Contraindications to
chemotherapy-such as ongoing infection, frailty, or significant
comorbidities-remain a major clinical challenge. These scenarios
are particularly difficult to manage from a multidisciplinary
standpoint and are often associated with limited curative potential
for affected patients. Current treatment guidelines offer limited
recommendations for managing these complex conditions (3,4).
Here, we present the case of a patient with locally
advanced rectal cancer and concurrent pelvic infection who was
ineligible for systemic chemotherapy. The patient underwent
short-course radiotherapy as the sole neoadjuvant modality.
Following radiotherapy, a marked reduction in both tumor size and
inflammatory signs was observed, enabling a safe surgical
intervention. The postoperative course was uneventful, and
histopathological examination confirmed a significant treatment
response with clear resection margins.
Case report
A 46-year-old male with no significant past medical
history presented to the Emergency Department with fever and severe
proctalgia. He reported a 6-month history of constitutional
symptoms, including weight loss and fatigue. On physical
examination, a digital rectal exam revealed a painful, indurated
mass in the perianal region, suggestive of a left ischiorectal
abscess. The examination was limited due to patient discomfort.
Laboratory tests showed leukocytosis and elevated inflammatory
markers.
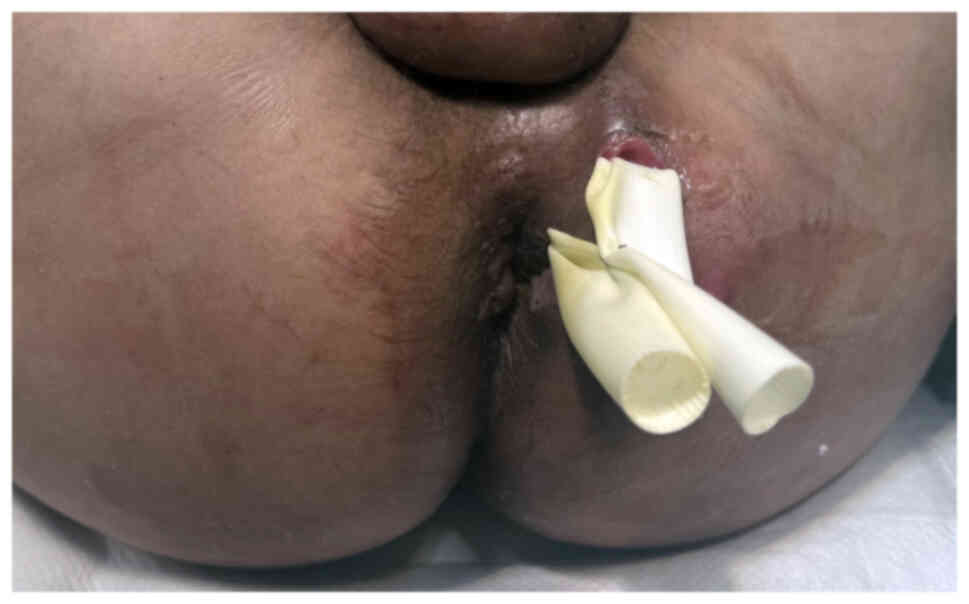
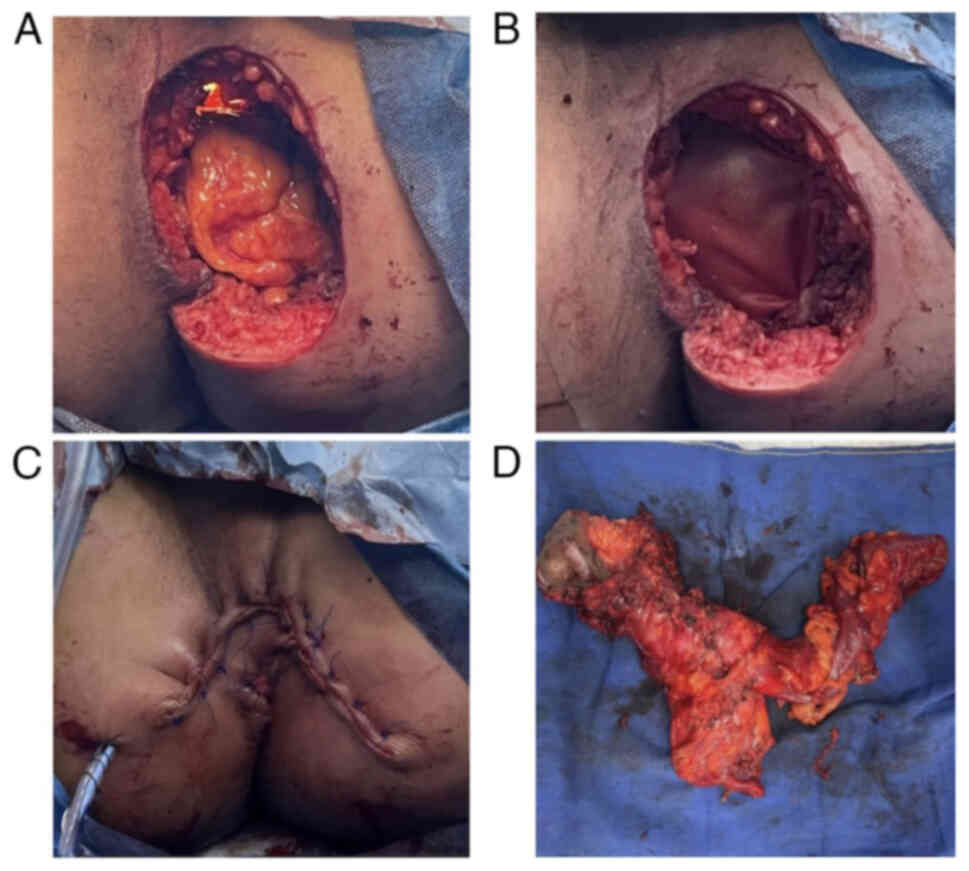
Emergency surgical drainage of the abscess was
performed (Fig. 1).
Intraoperatively, a friable, exophytic anal mass was identified in
the 4 o'clock position in lithotomy. A biopsy was taken. Initially,
clinical examination suggested that the most likely diagnosis was a
perianal infection. However, subsequent surgical findings revealed
that the origin of the infection appeared to be a tumor mass.
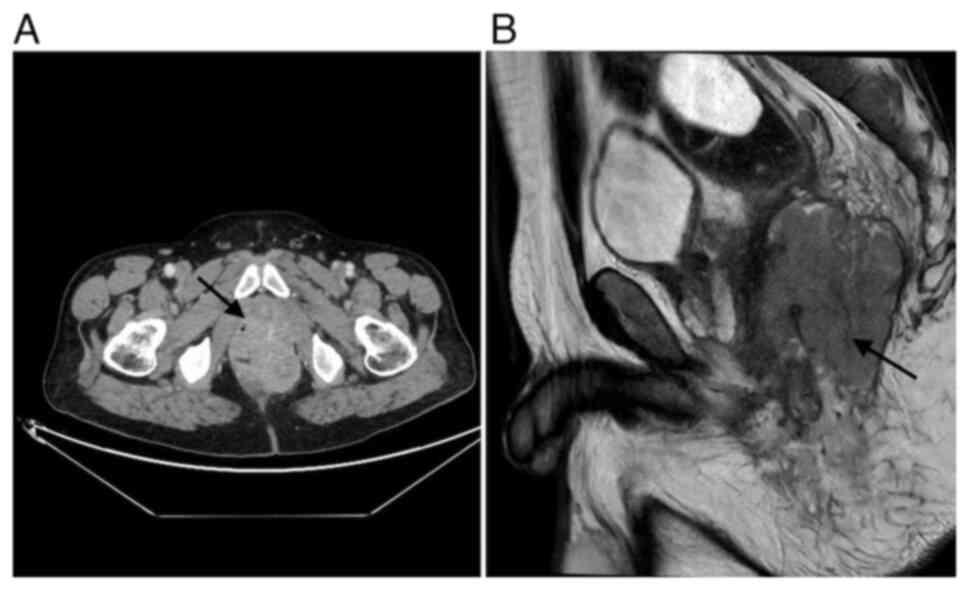
Postoperative computed tomography (CT) (Fig. 2) revealed an 8×6.7×8 cm presacral
mass extending into the left ischiorectal fossa and infiltrating
the gluteus maximus muscle. The mass was inseparable from the
anorectal junction, puborectalis muscle, internal obturator muscle,
seminal vesicles, and prostate, although no distant metastases were
identified.
Magnetic resonance imaging (MRI) (Fig. 2) confirmed a large tumor in the mid
and lower rectum (81×75×84 mm, APxTRxCC), with extension through
the muscularis propria into the left levator ani muscle, ischioanal
space, and subcutaneous gluteal tissues. There was contact with the
left seminal vesicle and peripheral prostate, without clear
invasion. Two pathologic lymph nodes were noted. The tumor was
staged as T4bN1.
Colonoscopy visualized the rectal mass, and biopsies
confirmed a moderately differentiated adenocarcinoma with low
microsatellite instability and positive CK20 and CDX2
immunostaining.
According to NCCN guidelines, locally advanced
rectal cancer such as this would typically require initiation of
neoadjuvant treatment combining radiotherapy and chemotherapy to
achieve a resectable stage. However, due to the ongoing pelvic
infection, systemic chemotherapy was contraindicated. A diverting
loop colostomy was performed to reduce fecal contamination and
facilitate infection control. This intervention aimed to manage the
local infection and potentially allow the patient to complete
oncological treatment. The stoma became functional by postoperative
day two.
Despite these measures, the patient required four
additional surgical debridements due to persistent pelvic infection
and abscess formation, which extended into the left pararectal
space.
The case was reviewed at a multidisciplinary tumor
board. The oncology team continued to advise against initiating
chemotherapy, as the patient still required wound care due to
persistent local infection. Owing to the extensive size of the
tumor, surgical resection at that stage could not guarantee
complete tumor removal. A decision was therefore made to initiate
short-course radiotherapy (500 cGy ×4 sessions over one week).
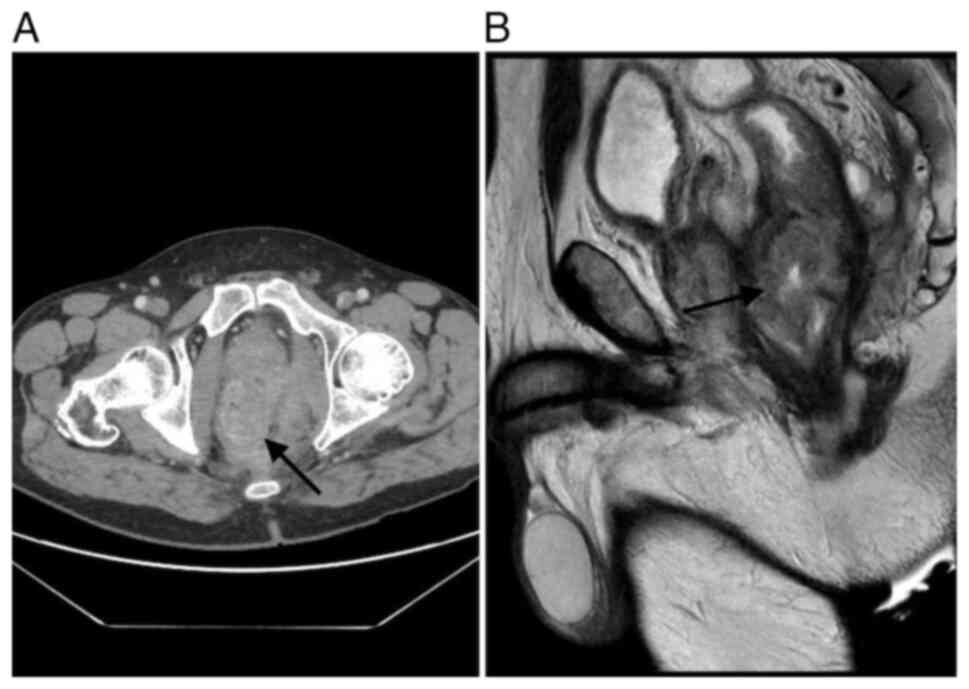
Repeat imaging demonstrated significant tumor
regression, although a residual abscess persisted (Fig. 3). The mass remained inseparable from
adjacent structures but showed no involvement of the bladder. Based
on radiological improvement and the absence of metastatic disease,
the tumor board recommended surgical resection with curative
intent.
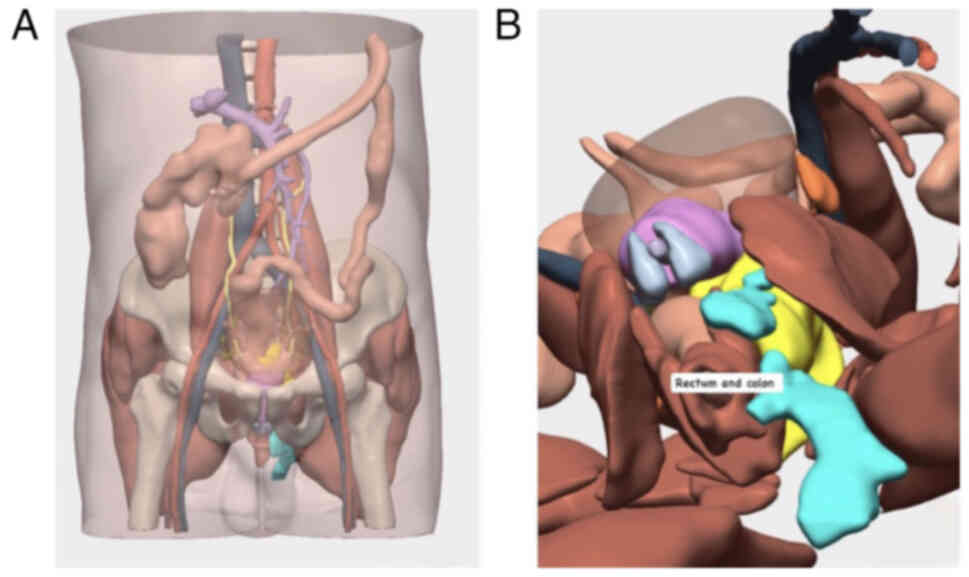
Preoperative 3D modeling using Cella Medical
Solutions® software provided detailed anatomical
visualization, including tumor extent and its relationship with
pelvic organs, enabling precise surgical planning (Fig. 4).
A total pelvic exenteration with Bricker-type
urinary diversion was performed in collaboration with the Urology
team. The procedure was conducted via laparotomy in the Lloyd-Davis
position, beginning with the abdominal phase and concluding with
the perineal dissection. R0 resection was achieved. Perineal
reconstruction involved placement of an omental flap over the bowel
loops, followed by a Vicryl mesh and layered closure of muscle and
skin (Fig. 5).
Histopathological examination staged the tumor as
ypT3, ypN0. A complete mesorectal excision was performed.
Perineural and lymphovascular invasion were absent, as were tumor
deposits. The distal and proximal margins were free of tumor,
although the circumferential margin contained a focal area in which
involvement of the posterior margin could not be excluded. A total
of 74 lymph nodes were examined, all of which were negative (0/74).
Between the rectal wall and adherent structures, including the
bladder, seminal vesicles, and prostate, abundant fibrotic tissue
was present without evidence of malignancy.
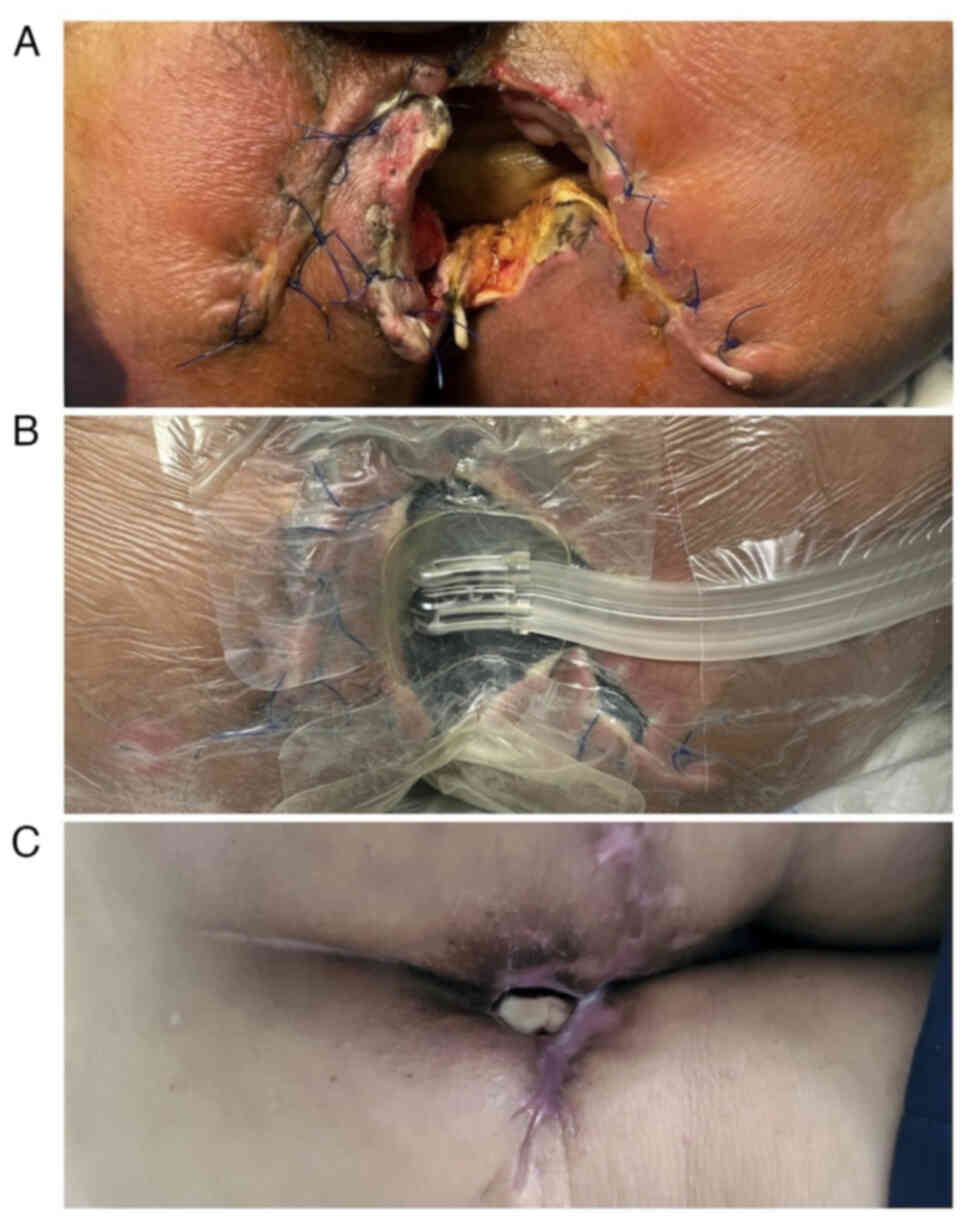
Postoperatively, the patient developed perineal
wound dehiscence, which was successfully managed with
vacuum-assisted closure (VAC) therapy. Dressings were changed every
72 h, and the wound gradually closed without the need for further
surgical intervention (Fig. 6).
The patient received continuous support from the
surgical team throughout the hospital stay. Although wound care was
prolonged, he remained optimistic due to the favorable outcome of
the intervention. He was discharged in stable condition with
scheduled outpatient follow-up. At subsequent evaluations, there
was no clinical or radiological evidence of disease recurrence.
Discussion
Rectal cancer accounts for approximately 30% of
newly diagnosed colorectal malignancies each year. Accurate
mortality data are often limited, as deaths due to rectal cancer
are sometimes misclassified under colon cancer statistics (3).
The National Comprehensive Cancer Network (NCCN)
provides comprehensive treatment algorithms for rectal cancer, with
neoadjuvant chemoradiotherapy or total neoadjuvant therapy (TNT)
representing standard approaches for locally advanced disease.
However, a subset of patients is ineligible for systemic
chemotherapy due to factors such as active infections, as in the
present case (5).
In this patient, chemotherapy was contraindicated
due to recurrent perineal sepsis. Surgical resection was initially
deferred because of the tumor's large size, anatomical complexity,
and the inability to guarantee an R0 resection. Given the limited
therapeutic options, short-course radiotherapy was selected as the
sole neoadjuvant modality. Although this approach is rarely used in
isolation, it proved effective in achieving significant tumor
regression, ultimately enabling curative surgery.
Several clinical trials have investigated
radiotherapy-only protocols. The Trans-Tasman Radiation Oncology
Group (TROG 01.04) randomized trial, which compared short-course
radiotherapy with long-course chemoradiotherapy, found no
significant differences in local recurrence, distant metastasis, or
overall survival. Notably, patients who underwent short-course
radiotherapy experienced fewer severe toxicities, although they
demonstrated a higher rate of permanent stoma formation (6,7). These
findings support the viability of radiotherapy-alone strategies in
selected high-risk patients.
Another critical aspect of this case was the
incorporation of three-dimensional (3D) reconstruction for
preoperative planning. 3D modeling has gained recognition as a
valuable adjunct in complex oncologic surgeries, enabling surgeons
to visualize tumor boundaries, assess involvement of adjacent
structures, and simulate the surgical approach. In this case, 3D
models facilitated meticulous preoperative planning, resulting in
complete resection with negative margins. Previous studies have
demonstrated that this technology improves surgical precision and
reduces intraoperative uncertainty (8–12). In
our experience, the use of Cella provided the surgical team with a
detailed understanding of the patient's anatomy, enabling them to
achieve an R0 resection. This tool allowed us to study the tumor
and its margins in advance, offering valuable insights into what to
expect during surgery. Moreover, during the most challenging stages
of the procedure, the software provided an external reference that
helped the surgeons maintain the correct dissection plane.
Conventional imaging modalities, such as CT or MRI, do not provide
sufficient detail to ensure this level of precision.
Perineal closure following pelvic exenteration
presents a significant challenge due to the risk of wound
complications and herniation. Biological and absorbable meshes have
shown promise in reducing postoperative perineal hernias without
significantly increasing the risk of infection (13–15).
In our case, a Vicryl mesh combined with an omental flap provided
an effective barrier between the bowel and the closure site.
Although wound dehiscence occurred, it was managed conservatively
with vacuum-assisted closure (VAC) therapy, avoiding the need for
further surgical intervention (16).
Moreover, systemic assessment of inflammatory and
nutritional status is increasingly recognized as a key component in
surgical decision-making and prognostic stratification of patients
with colorectal cancer. Several studies have demonstrated that
composite inflammatory markers, such as the pan-immune-inflammatory
value (PIV) and the albumin-to-globulin ratio (AGR), are
significantly associated with overall and disease-free survival in
stage I–III colorectal cancer (17,18).
These tools may help to individualize management in high-risk
patients who, as in the present case, cannot receive total
neoadjuvant therapy.
Likewise, the Onodera prognostic nutritional index
(PNI) has shown utility in the early prediction of postoperative
complications, such as anastomotic leakage after rectal cancer
surgery (19), which is
particularly relevant in the context of the perineal wound
evolution described in this case. The integration of these
parameters could complement conventional preoperative assessment
and optimize surgical selection.
Finally, evidence derived from other solid tumors
reinforces the applicability of these markers. For example, the
modified Glasgow prognostic score (mGPS) has been correlated with
prognosis in breast cancer, highlighting the potential of
inflammatory and nutritional indicators as universal tools for
tailoring therapeutic strategies beyond standard treatment pathways
(20). Incorporating these
assessments into clinical practice may be particularly valuable in
complex scenarios such as ours, where conventional treatment is not
feasible and decisions must rely on a comprehensive systemic
characterization of the patient.
This case highlights the importance of
individualized treatment strategies when conventional protocols are
not feasible. Radiotherapy alone, combined with advanced surgical
tools such as 3D reconstruction and thoughtful reconstructive
techniques, can provide curative outcomes even in complex and
initially unfavorable scenarios. However, it is important to
acknowledge that this is a single case report, and further studies
are required to determine whether these findings can be
generalized.
Patients who fall outside standard rectal cancer
treatment algorithms due to clinical contraindications-such as
active pelvic infections-pose a significant therapeutic challenge.
In such cases, short-course radiotherapy may offer a viable
alternative to initiate treatment and enable curative surgical
resection.
This case demonstrates that radiotherapy alone can
effectively downstage advanced rectal tumors in selected patients
unfit for chemotherapy. The integration of 3D reconstruction into
surgical planning significantly enhanced anatomical visualization,
allowing for precise and safe resection. Additionally, perineal
reconstruction using absorbable mesh and omental interposition
helped minimize complications associated with wound healing.
In summary, individualized treatment approaches,
supported by modern imaging and reconstructive strategies, can lead
to favorable outcomes even in complex clinical scenarios.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SESDTG, FMM, PUS, FMJ, YAM, LCG, PBB, AJLM, MML,
AVT, MDA and AGC contributed to the diagnosis and treatment of the
patient, and in the design of the study. PUS was a major
contributor to the writing of the manuscript. PUS and FMM confirm
the authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study followed international and
national regulations and was performed in agreement with The
Declaration of Helsinki and ethical principles. The patient signed
an informed consent form before the surgery was performed.
Patient consent for publication
The patient provided written informed consent for
the publication of any data and/or accompanying images before the
surgery was performed. Patients have a right to anonymity and
privacy, and authors have a legal and ethical responsibility to
respect this right.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pinheiro M, Moreira DN and Ghidini M:
Colon and rectal cancer: An emergent public health problem. World J
Gastroenterol. 30:644–651. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boublikova L, Novakova A, Simsa J and
Lohynska R: Total neoadjuvant therapy in rectal cancer: The
evidence and expectations. Crit Rev Oncol Hematol. 192:1041962023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lotfollahzadeh S, Kashyap S, Tsoris A,
Recio-Boiles A and Babiker HM: Rectal cancer. [Updated 2023 Jul 4].
StatPearls [Internet] Treasure Island (FL): StatPearls Publishing;
2025
|
|
4
|
Garcia-Aguilar J, Patil S, Gollub MJ, Kim
JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M and Dunne RF:
Organ preservation in patients with rectal adenocarcinoma treated
with total neoadjuvant therapy. J Clin Oncol. 40:2546–2556. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benson AB, Venook AP, Adam M, Chang G,
Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna
I, et al: NCCN guidelines® insights: rectal cancer,
version 3.2024. J Natl Compr Canc Netw. 22:366–375. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ansari N, Solomon MJ, Fisher RJ, Mackay J,
Burmeister B, Ackland S, Heriot A, Joseph D, McLachlan SA, McClure
B and Ngan SY: Acute adverse events and postoperative complications
in a randomized trial of preoperative short-course radiotherapy
versus long-course chemoradiotherapy for T3 adenocarcinoma of the
rectum: Trans-tasman radiation oncology group trial (TROG 01.04).
Ann Surg. 265:882–888. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McLachlan SA, Fisher RJ, Zalcberg J,
Solomon M, Burmeister B, Goldstein D, Leong T, Ackland SP,
McKendrick J, McClure B, et al: The impact on health-related
quality of life in the first 12 months: A randomised comparison of
preoperative short-course radiation versus long-course
chemoradiation for T3 rectal cancer (trans-tasman radiation
oncology group trial 01.04). Eur J Cancer. 55:15–26. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Granero A, Pellino G, Giner F,
Frasson M, Fletcher-Sanfeliu D, Primo Romeguera V, Flor Lorente B,
Gamundi M, Brogi L, Garcia-Calderón D, et al: A video demonstration
of three-dimensional imaging to assess the circumferential
resection margin in locally advanced rectal cancer and recurrent
rectal cancer-a video vignette. Colorectal Dis. 22:2340–2341. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pellino G, García-Granero A,
Fletcher-Sanfeliu D, Navasquillo-Tamarit M, Frasson M,
García-Calderon D, García-Gausi M, Valverde-Navarro AA,
Garcia-Armengol J, Roig-Vila JV and García-Granero E: Preoperative
surgical planning based on cadaver simulation and 3D imaging for a
retrorectal tumour: Description and video demonstration. Tech
Coloproctol. 22:709–713. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
García-Granero Á, Jerí-McFarlane S,
Torres-Marí N, Brogi L, Ferrà-Canet M, Navarro Zoroa MÁ,
Gamundí-Cuesta M and González-Argenté FX: 3D-reconstruction printed
models and virtual reality improve teaching in oncological
colorectal surgery. Tech Coloproctol. 29:242024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rusli SM, Kim JS, Choo JM, Cheong JY,
Piozzi GN and Kim SH: Robotic-assisted mesh pelvic closure for
prevention of small bowel descent after surgery for recurrent
rectal cancer. Tech Coloproctol. 26:309–310. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jerí-McFarlane S, García-Granero Á,
Pellino G, Torres-Marí N, Ochogavía-Seguí A, Rodríguez-Velázquez M,
Gamundí-Cuesta M and González-Argenté FX: Prospective observational
non-randomized trial protocol for surgical planner 3D image
processing & reconstruction for locally advanced colon cancer.
BMC Surg. 24:2922024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gutiérrez Delgado MDP, Mera Velasco S,
Miron Fernandez I, González-Poveda I, Ruiz-López M, Mata JAT,
Carrasco Campos J and Santoyo JS: Prophylactic use of perineal and
peristomal mesh in laparoscopic abdominoperineal amputation-A video
vignette. Colorectal Dis. 24:1253–1254. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dijkstra EA, Kahmann NLE, Hemmer PHJ,
Havenga K and van Etten B: A low incidence of perineal hernia when
using a biological mesh after extralevator abdominoperineal
excision with or without pelvic exenteration or distal sacral
resection in locally advanced rectal cancer patients. Tech
Coloproctol. 24:855–861. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devulapalli C, Jia Wei AT, DiBiagio JR,
Baez ML, Baltodano PA, Seal SM, Sacks JM, Cooney CM and Rosson GD:
Primary versus flap closure of perineal defects following oncologic
resection: A systematic review and meta-analysis. Plast Reconstr
Surg. 137:1602–1613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Z, Wang Y, Zeng Q, Rong W, Wang Z,
Zhai Z, Ding C, An K, Gao Q, Niu P, et al: Perineal defect
reconstruction after surgery for advanced or locally recurrent
rectal cancer involving organ resection: Multiple flaps combined
with lining repair. Colorectal Dis. 25:2087–2092. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li K, Zeng X, Zhang Z, Wang K, Pan Y, Wu
Z, Chen Y and Zhao Z: Pan-immune-inflammatory values predict
survival in patients after radical surgery for non-metastatic
colorectal cancer: A retrospective study. Oncol Lett. 29:1972025.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li K, Chen Y, Zhang Z, Wang K, Sulayman S,
Zeng X, Ababaike S, Guan J and Zhao Z: Preoperative
pan-immuno-inflammatory values and albumin-to-globulin ratio
predict the prognosis of stage I–III colorectal cancer. Sci Rep.
15:115172025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang ZY, Li KJ, Zeng XY, Wang K, Sulayman
S, Chen Y and Zhao ZL: Early prediction of anastomotic leakage
after rectal cancer surgery: Onodera prognostic nutritional index
combined with inflammation-related biomarkers. World J Gastrointest
Surg. 17:1028622025.PubMed/NCBI
|
|
20
|
Wang D, Duan L, Tu Z, Yan F, Zhang C, Li
X, Cao Y and Wen H: The Glasgow Prognostic Score Predicts Response
to Chemotherapy in Patients with Metastatic Breast Cancer.
Chemotherapy. 61:217–222. 2016. View Article : Google Scholar : PubMed/NCBI
|