Introduction
Historically, breast cancer management followed a
standard sequence of surgery, followed by chemotherapy and
radiotherapy (1). However, the use
of chemotherapy before surgery, in a neoadjuvant setting, shows
similar survival outcomes to adjuvant therapy (2), but it offers some clinical advantages.
Initially, it was used to downstage and render operable locally
advanced breast cancers that were inoperable from the outset, or to
enable conservative surgery for certain operable, locally advanced
forms (3). Indications of
neoadjuvant chemotherapy (NAC) have extended to include early-stage
triple-negative (TN) and HER2-positive subtypes, supported by the
positive predictive value of pathological complete response (pCR)
for survival (4,5). Studies have demonstrated that
pathological responses to NAC vary between the intrinsic molecular
subtypes (1,6,7).
Pathological response following neoadjuvant chemotherapy was also
used to guide adjuvant systemic. Patients who experience a pCR had
a good prognosis, and did not benefit from adjuvant therapy (except
endocrine therapy in endocrine receptor-positive breast cancer).
HER2-positive and triple negative cancer patients with incomplete
response would benefit from additional adjuvant systemic therapy
(8,9). Most of these studies come from
developed countries, and thus, mostly include White patients
(10,11). However, breast cancer presents
racial and ethnic disparities (12). Therefore, the results of these
studies cannot be generalized systematically to Black African
patients. Breast cancer is a heterogeneous disease with molecular
and phenotypical subtypes that vary across racial and ethnic groups
(13). These subtypes influence
prognosis and treatment outcomes (14). In the Republic of Côte d'Ivoire,
breast cancer is the most prevalent cancer among female patients,
with 3869 new cases in 2022 (33.5% of all new cancer cases)
according to the Globocan statistics (15). From 2008 to 2015, most cases were
diagnosed at advanced stages (nearly 65% at stages III or IV),
often requiring the use of NAC (16). To the best of our knowledge, pCR
following NAC and its impact on survival outcomes has not yet been
studied. Therefore, the present study aimed to evaluate the
pathological response to NAC and its influence on survival in
patients with breast cancer.
Patients and methods
Patient selection and data
collection
The present study was a retrospective analytical
study of patients treated from January 2017 to December 2024. The
present study included 238 female patients aged ≥18 years.
Inclusion criteria were: Pathologically confirmed, non-metastatic
breast carcinoma who were treated with NAC followed by surgery in
three tertiary hospitals in the Republic of Côte d'Ivoire (Alassane
Ouattara National Center of Medical Oncology and Radiotherapy of
Abidjan, Treichville Hospital and University Center of Abidjan, and
Bouaké Hospital and University Center, Bouaké); available
pathological examination of the surgical specimen.
Description of the chemotherapy protocols, the
number of cycles, and the subsequent treatments such as
radiotherapy, endocrine therapy or adjuvant chemotherapy. The
present study was approved by The National Ethical Committee of
Life and Health Sciences (approval no. 00068/25/MSHPCMU/CNESVS-km)
of Republic of Côte d'Ivoire. All patients gave their written
consent to participate to the study. Pathological and
immunohistochemistry analyses were performed on a breast core
biopsy before the chemotherapy course. Computed tomography scans of
the chest, abdomen and pelvis were performed to rule out distant
metastasis. Pathological, immunohistochemistry and computed
tomography scan data were obtained from the medical records of the
patients. Tumor staging was based on the eighth edition of the
American Joint Committee on Cancer/Union for International Cancer
Control (UICC) system (17).
The exclusion criteria were as follows: Patients who
received radiotherapy prior to surgery; patients with a delay
>12 weeks between NAC and surgery; patients with a delay >6
weeks between cycles of chemotherapy; patients with a follow-up
time <1 year; and patients lost to follow-up after the end of
treatment for whom post-treatment data were unavailable.
The collected data included: Age; tumor stage and
grade; estrogen receptor (ER) status; progesterone receptor (PR)
status; HER2 status; molecular subtype; chemotherapy regimen;
treatment following surgery (radiotherapy, hormonal therapy,
anti-HER2 therapy or adjuvant chemotherapy); pathological response;
and overall survival (OS) and progression-free survival (PFS).
Molecular subtypes were categorized as luminal,
HER2-positive or TN based on ER, PR and HER2 status. The ER, PR and
HER2 status was assessed by immunohistochemistry. Data were
extracted from the immunohistochemistry exam reports in the patient
medical records. ER and PR were considered positive if ≥10% of
cells were stained positive. Evaluation of HER2 expression was
based on the degree of membrane staining as follows: 0, no or
incomplete, faint/barely perceptible membrane staining in ≤10% of
invasive tumor cells; 1, incomplete, faint/barely perceptible
membrane staining in >10% of invasive tumor cells; 2, incomplete
and/or weak to moderate circumferential membrane staining in
>10% of invasive tumor cells or complete, intense,
circumferential membrane staining in ≤10% of invasive tumor
cells
Score 3 corresponds to complete, intense,
circumferential membrane staining in >10% of invasive tumor
cells.
HER2 was considered upregulated (HER2-positive) if
the score was 3; scores of 0 and 1 indicated an absence of HER2
upregulation. If the score was 2, an in situ hybridization
test was required.
Tumors were classified as follows: Luminal, ER-
and/or PR-positive, HER2-negative; HER2-positive, HER2-positive
with or without ER or PR expression; and TN, ER-, PR- and
HER2-negative.
pCR was defined as an absence of residual invasive
or micro-invasive disease in both the breast (the primary tumor
site) and axillary lymph nodes (ypT0/ypTis, ypN0 based on the
8th edition of breast cancer staging of the
AJCC)(17). The pathological
response was reported according to whether pCR was achieved
[absence of complete response group (no pCR) and complete response
group (pCR)].
PFS was defined as the time from diagnosis to
recurrence (locoregional and/or distant) or death from any cause.
OS was defined as the time from diagnosis until death from any
cause.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics version 27 (https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-27).
Baseline characteristics of patients, tumors and treatment were
presented as mean ± SD) for continuous variables, or as proportions
for categorical variables. Binary logistic regression analysis was
performed to detect associations between clinicopathological
variables (age, histological subtype, grade, molecular subtype and
stage) and pathological response. χ2 test was used for
comparison. There was a significant association between the
variable and pathological response if P-value was <0,05. Odd
ratio (OR) and 95% confident interval were reported for each
variable. Survival was assessed using the Kaplan-Meier method on an
intention-to-treat basis. Univariate and multivariate analyses were
performed to assess the influence of clinicopathological variables
on PFS and OS. The log-rank test was used for the univariate
analysis. Variables that were significant (P<0.05) in the
univariate analysis were included in the multivariate analysis
using a Cox regression model. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinicopathological
characteristics
A total of 195 patients met the selection criteria
and were included in the study. The mean age was 46.3±10.4 years
and 26.7% of patients were aged <40 years. Invasive carcinoma of
no special type was the predominant histology (91.3%). Most tumors
were grade 2 (67.7%) and stage III (69.8%). Immunohistochemistry
revealed that 39.5% of the tumors were positive for ER and/or PR,
8.7% were positive for both HER2 and ER and/or ER, 11.8% were
positive for HER2 only and 40.0% were TN (data not shown). The
molecular subtypes were luminal (39.5%), HER2-positive (20.5%) and
TN (40%). NAC was primarily based on sequential
anthracycline-taxane regimens (94.4%). The number of cycles ranged
from six to eight in 91.3% of patients (data not shown). Among
HER2-positive patients, 32.5% received anti-HER2 therapy
(trastuzumab) as part of the NAC regimen. In the remaining
HER2-positive patients (67.5%), trastuzumab was given in the
adjuvant setting (following surgery). Among TN patients, a platinum
agent (carboplatin) was added to the NAC regimen in 3.8% of cases.
Mastectomy was performed in 75.4% of patients and 95.9% received
postoperative radiotherapy. Endocrine therapy was administered to
all patients with hormone receptor-positive tumors, while adjuvant
capecitabine was given to patients with TN tumors without pCR (data
not shown). pCR was observed in 28.7% of patients based on
pathological examination of the surgical specimen (Table I).
 | Table I.Patient and tumor
characteristics. |
Table I.
Patient and tumor
characteristics.
| Variable | Luminal (%) | HER2-positive
(%) | Triple-negative n
(%) | Total (%) |
|---|
| Age, years |
|
|
|
|
|
<40 | 16 (8.2) | 15 (7.7) | 21 (10.8) | 52 (26.7) |
|
≥40 | 61 (31.3) | 25 (12.8) | 57 (29.2) | 143 (73.3) |
| Histological
subtype |
|
|
|
|
|
NST | 64 (32.8) | 39 (20.0) | 75 (38.5) | 178 (91.3) |
|
Other | 13 (6.7) | 1 (0.5) | 3 (1.5) | 17 (8.7) |
| Grade |
|
|
|
|
| 1 | 16 (8.2) | 3 (1.5) | 3 (1.5) | 22 (11.3) |
| 2 | 53 (27.2) | 29 (14.9) | 50 (25,6) | 132 (67.7) |
| 3 | 8 (4.1) | 8 (4.1) | 25 (12.8) | 41 (21.0) |
| Stage |
|
|
|
|
|
IIA | 8 (4.1) | 4 (2.1) | 4 (2.1) | 16 (8.2) |
|
IIB | 17 (8.7) | 6 (3.1) | 20 (10.3) | 43 (22.1) |
|
IIIA | 25 (12.8) | 11 (5.6) | 17 (8.7) | 53 (27.2) |
|
IIIB | 27 (13.8) | 19 (9.7) | 37 (19.0) | 83 (42.6 |
| Chemotherapy
regimen |
|
|
|
|
|
Anthracycline/taxane | 72 (36.9) | 26 (13.3) | 70 (35.9) | 168 (86.2) |
|
Anthracycline/taxane +
trastuzumab | - | 13 (6.7) | - | 13 (6.7) |
|
Anthracycline/taxane +
carboplatin | - | - | 3 | 3 (1.5) |
|
Anthracycline +
cyclophosphamide | 6 (3.1) | 1 (0.5) | 4 (2.0) | 11 (5.6) |
| Response |
|
|
|
|
|
pCR | 18 (9.2) | 17 (8.7) | 21 (10.8) | 56 (28.7) |
| No
pCR | 60 (30.8) | 22 (11.3) | 57 (29.2) | 139 (71.3) |
Association between pCR and
clinicopathological variables
Molecular subtype and tumor stage influenced the
pathological response. Patients with HER2-positive tumors were more
likely to achieve pCR than those with TN subtypes [odds ratio (OR),
2.62; 95% CI, 1.10–6.22). Patients with stage II tumors were also
more likely to achieve pCR than those with stage III tumors (OR,
3.67; 95% CI, 1.82–7.48). However, age, grade and pathological
subtype did not significantly influence the pathological response
(Table II).
 | Table II.Logistic regression analysis of the
association between pathological response and clinicopathological
variables. |
Table II.
Logistic regression analysis of the
association between pathological response and clinicopathological
variables.
| Variable | No pCR, n (%) | pCR, n (%) | P-value | OR | 95% CI |
|---|
| Age, years |
|
| 0.231 |
|
|
|
<40 | 33 (16.9) | 19 (9.7) |
| 1.57 | 0.75–3.33 |
|
≥40 | 106 (54.4) | 37 (19.0) |
| 1 | - |
| Pathological
subtype |
|
| 0.546 |
|
|
|
NST | 125 (64.1) | 53 (27.2) |
| 1.53 | 0.38–6.17 |
|
Other | 14 (7.2) | 3 (1.5) |
| 1 | - |
| Stage |
|
| <0.001 |
|
|
| II | 32 (16.4) | 27 (13.8) |
| 3.67 | 1.82–7.48 |
|
III | 107 (54.9) | 29 (14.9) |
| 1 | - |
| Grade |
|
| 0.382 |
|
|
| 1 | 19 (9.7) | 3 (1.5) |
| 0.35 | 0.08–1.56 |
| 2 | 93 (47.7) | 39 (20.0) |
| 0.71 | 0.31–1.64 |
| 3 | 27 (13.8) | 14 (7.2) |
| 1 | - |
| Molecular
subtype |
|
| 0.048 |
|
|
|
Luminal | 60 (30.8) | 17 (8.7) |
| 0.94 | 0.41–2.11 |
|
HER2-positive | 22 (11.3) | 18 (9.2) |
| 2.62 | 1.10–6.22 |
|
Triple-negative | 57 (29.2) | 21 (10.8) |
| 1 | - |
Univariate analysis
The 5-year PFS and OS rates were 68.4% (median,
40.23 months) and 78% (median, 42.13 month), respectively, with a
median follow-up of 49.4 months. Stage and pathological response
significantly influenced the 5-year PFS and OS rates. Patients with
stage II tumors had significantly higher 5-year PFS and OS rates
than patients with stage III tumors (81.9 vs. 62.7%; and 93 vs.
71.2%, respectively). pCR was associated with significantly higher
5-year PFS and OS rates than no pCR (96.1 vs. 57.7%; and 95 vs.
71.6%, respectively; Table
III).
 | Table III.Univariate analysis by the log-rank
test. |
Table III.
Univariate analysis by the log-rank
test.
| Variable | 5-year PFS (%) | P-value | 5-year OS (%) | P-value |
|---|
| Age, years |
| 0.452 |
| 0.562 |
|
≤40 | 69.5 |
| 84.6 |
|
|
>40 | 67.7 |
| 75.5 |
|
| Histological
subtype |
| 0.623 |
| 0.787 |
|
NST | 70.4 |
| 77.1 |
|
|
Other | 53.3 |
| 86.7 |
|
| Grade |
| 0.771 |
| 0.396 |
| 1 | 55.1 |
| 84.8 |
|
| 2 | 69.8 |
| 78.9 |
|
| 3 | 71.5 |
| 69.9 |
|
| Molecular
subtype |
| 0.389 |
| 0.371 |
|
Luminal | 70.7 |
| 83.1 |
|
|
HER2-positive | 68.9 |
| 79.9 |
|
|
Triple-negative | 64.7 |
| 71.9 |
|
| Stage |
| 0.005 |
| 0.006 |
| II | 81.9 |
| 93 |
|
|
III | 62.7 |
| 71.2 |
|
| Response |
| <0.001 |
| 0.002 |
|
pCR | 96.1 |
| 95 |
|
| No
pCR | 57.7 |
| 71.6 |
|
Multivariate analysis
Tumor stage significantly influenced the 5-year OS
but not the 5-year PFS [hazard ratio (HR), 0.32; 95% CI, 0.11–0.93
for OS; and HR, 0.47; 95% CI, 0.22–1.02 for PFS. Pathological
response significantly influenced both 5-year PFS and OS (HR, 6.54;
95% CI, 2.02–21.19 for PFS; HR, 5.84; 95% CI, 1.39–24.54 for OS;
Table IV).
 | Table IV.Multivariate analysis by the Cox
model. |
Table IV.
Multivariate analysis by the Cox
model.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Stage |
|
|
|
|
|
|
| II | 0.055 | 0.47 | 0.22–1.02 | 0.036 | 0.32 | 0.11–0.93 |
|
III |
| 1.00 | - |
| 1 | - |
| Response |
|
|
|
|
|
|
|
pCR | 0.002 | 6.54 | 2.02–21.19 | 0.016 | 5.84 | 1.39–24.54 |
| No
pCR |
| 1 | - |
| 1 | - |
Survival rate according to
pathological response in patients with different molecular
subtypes
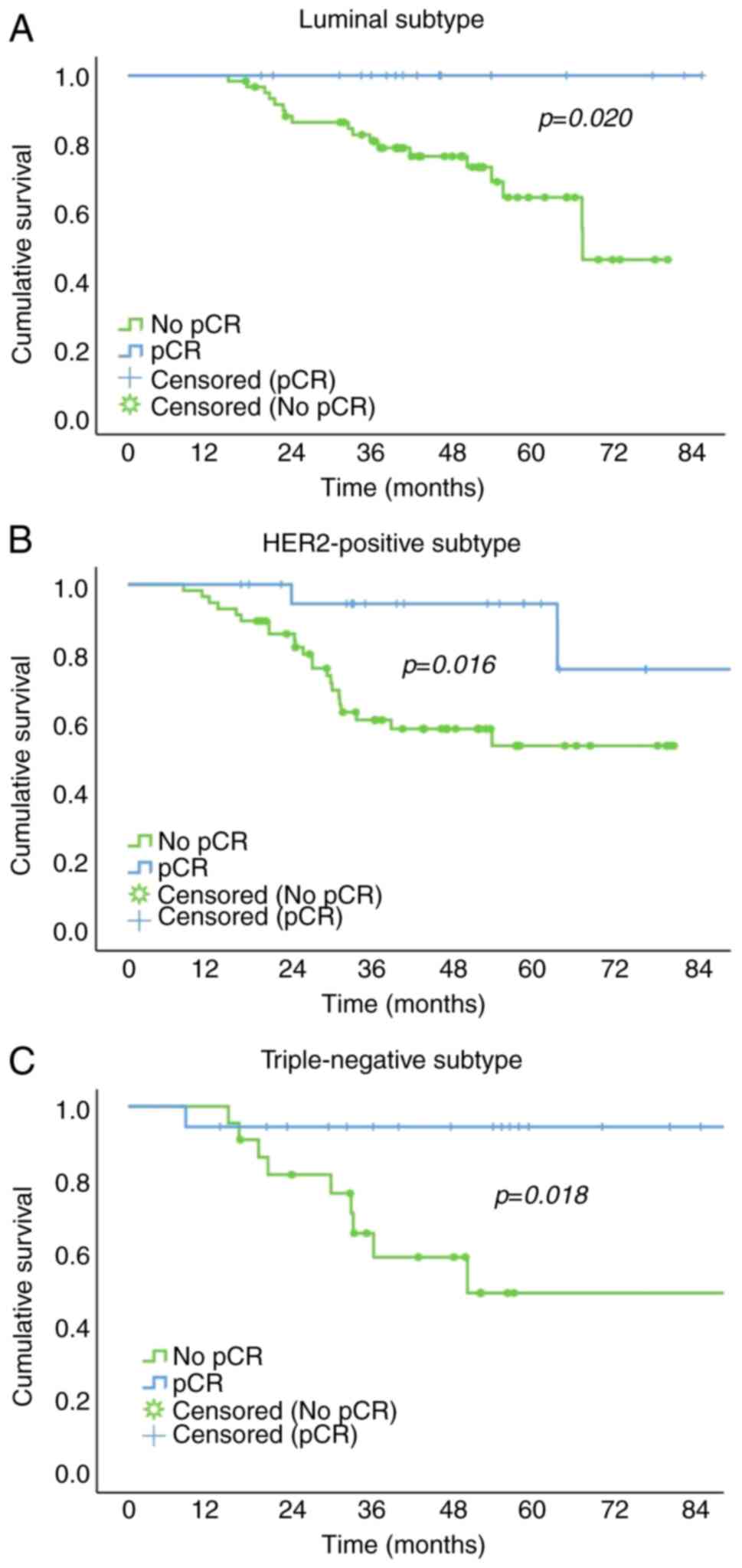
Within each molecular subtype group, the 5-year PFS
rate of the pCR group was significantly higher than that of the no
pCR group [100.0 vs. 64.2% for the luminal subtype; 94.4 vs. 49%
for the HER2-positive subtype; and 94.4 vs. 53.5% for the TN
subtype; Fig. 1).
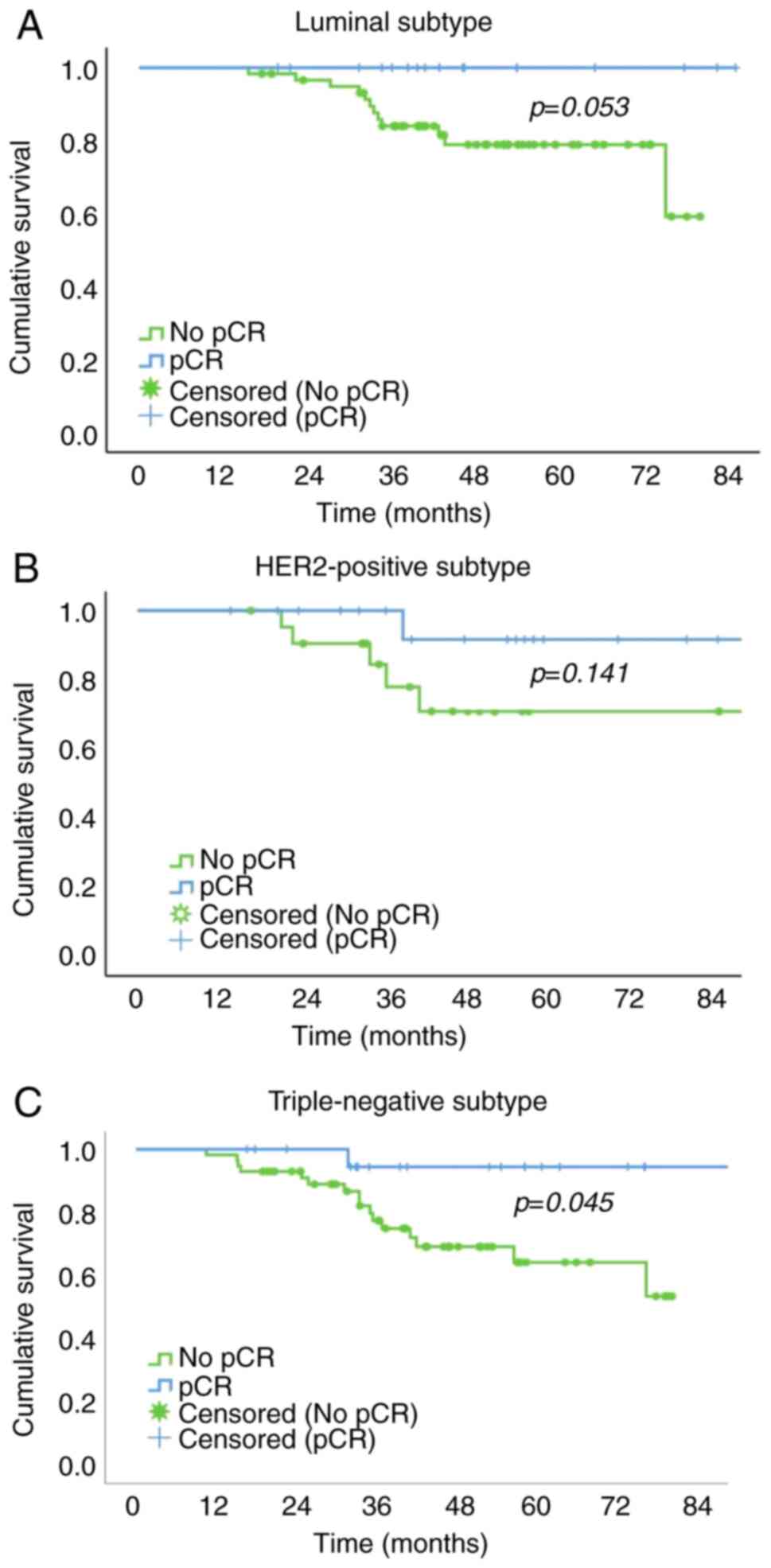
The pCR group had a significantly higher 5-year OS
rate than the no pCR group in patients with the TN subtype (64.3
vs. 94.4%;). In patients with the luminal and HER2-positive
subtypes, the 5-year OS rate of the pCR group was higher than that
of the no pCR group, but the difference was not significant (100.0
vs. 78.9% for luminal subtype; and 91.7 vs. 70.9% for HER2-positive
subtype, respectively; Fig. 2).
Discussion
The aim of the present study was to evaluate the
response to NAC and determine its impact on survival rates in
patients with breast cancer. Approximately one-third of patients
experienced pCR following NAC. This result is similar to that of
Farrukh et al (18), who
reported a pCR rate of 27.2%, using the same definition as the
present study (absence of residual invasive or micro-invasive
disease in both the breast and axillary lymph nodes, residual in
situ disease). Cirier et al (19) and Cortazar et al (20) obtained lower pCR rates of 16 and
13%, respectively. The definition of pCR in the aforementioned
studies (the absence of any invasive or in situ carcinoma)
may explain the low pCR rates. However, Müller et al
(21), using the same definition as
the present study, reported a higher pCR rate (47%) than the
present study. The chemotherapy regimen and drugs (anti-HER2) used
in the aforementioned study could explain the high pCR rate.
Studies have shown that the use of anti-HER2 therapy increases the
pCR in HER2-positive cancer (22–24).
In TN breast cancer, combination of platinum agents with
conventional chemotherapeutic agents (anthracyclines and taxanes)
has also been shown to be superior to sole conventional therapeutic
agents (25). Adding carboplatin to
anthracycline/taxane regimens improves pCR in early-stage TN breast
cancer (25). In the present study,
one-third of HER2-positive patients received anti-HER2 therapy as
part of their chemotherapy regimen, and a small percentage (1.5%)
of patients with TN breast cancer received carboplatin. This was
because immunohistochemistry results of most patients were not
available at the time of NAC.
In addition to the definition of pCR and the
chemotherapy regimen, other clinical and pathological factors
influence response rates. Several studies have identified tumor
biology as the strongest factor influencing the probability of
achieving pCR (6,26–29).
HER2-positive and TN breast cancers are more likely to respond to
NAC compared to hormone receptor-positive cancers (6,29). In
the present study, HER2-positive tumors were associated with a
higher probability of pCR compared to triple negative . The present
results were similar to those of Swain et al (7). TN breast cancer was an important
concern in the present study owing to its high frequency. In
contrast to occidental and east and south African series (12,30,31),
the present study reported a high rate of TN breast cancer (40%)
similar to other west African studies (32,33).
probability of pCR in TN tumors was not significantly different
from luminal subtype. This may be explained by the molecular
heterogeneity of TN breast cancer. According to Lehmann et
al (34), TN breast cancers can
be subdivided into four subtypes [basal-like 1 and 2, mesenchymal
and luminal androgen receptor (AR)], which have different clinical
and pathological characteristics. The basal-like 1 group
demonstrates the highest response rate to NAC, while the basal-like
2 group demonstrates the lowest response rate. Most studies that
have demonstrated a high rate of pCR in patients with TN breast
cancer mostly included non-Black or non-African patients (6,10,35).
The present sample was composed of Black African patients, whose
molecular characteristics differ from those of White or Asian
patients (10,36). However, a recent study by Rajagopal
et al (13) found that young
Black female patients have similar proportions of basal-like 1 and
mesenchymal subtypes to European or Asian patients, a smaller
relative proportion of luminal AR-type tumors and a larger
proportion of non-subtyped tumors. Independent of the Lehmann
classification of triple-negative breast cancers (TNBC), a distinct
subgroup lacking androgen receptor (AR) expression referred to as
quadruple-negative breast cancer (QNBC) has been identified
(37,38). This subtype is predominantly
observed in West African and African-American populations.
(36). Notably, the prognostic
impact of AR negativity appears to vary between ethnic and racial
groups (39). In West African
(particularly Nigerian), African-American, and Asian women, the
absence of AR expression has been associated with chemoresistance
and reduced overall survival (39–41).
In the present study, tumor stage influenced the
pathological response. Stage II was associated with a significantly
higher probability of pCR than stage III. The present results are
consistent with those of Goorts et al (42), who demonstrated that tumor stage was
an independent and strong predictor of pCR compared with HER2, ER
and PR status, and grade.
In the present study, pCR was the sole independent
predictive factor for improved PFS and OS. The molecular subtype
did not influence OS or PFS. However, achieving a pCR was
associated with a significantly improved 5-year PFS rate in each
molecular subtype. In the TN group, pCR was associated with
significantly improved 5-year OS. The present findings are
consistent with those of Cortazar et al (20), who found an increase in event-free
survival with pCR in each molecular subtype, although this was
weakest for hormone receptor-positive and low-grade tumors. Spring
et al (43) found that pCR
was associated with improved OS and event-free survival in the TN
and HER2-positive subtypes. von Minckwitz et al (5) demonstrated that pCR improved
disease-free survival in luminal B/HER2-negative,
HER2-positive/non-luminal and TN tumors, but not in luminal or
luminal B/HER2-positive cancer. When evaluating the long-term
results of the Alliance study (trial no. ACOZOG Z1071), Boughey
et al (26) found that
breast cancer-specific survival rates were higher in the pCR group
compared with no pCR (residual disease) for the hormone
receptor-positive and TN groups, but not for the HER2-positive
group.
To the best of our knowledge, the present study was
the first to evaluate the impact of pathological response to NAC on
breast cancer survival in patients from the Republic of Côte
d'Ivoire. However, it has limitations that should be noted,
including the retrospective nature of the study, the absence of
centralized pathological exams and the absence of Ki67 testing. The
pathological exams were performed in different laboratories with
different techniques and preparation conditions. A centralized
examination would allow the specimens to be subjected to the same
preparation conditions to ensure the robustness of the results. The
retrospective nature of the present study prevented control of
specimen handling and NAC administration. The proliferation index
Ki67 data, which distinguishes between low- and high-grade cancer,
were not available. In the Republic of Côte d'Ivoire,
immunohistochemistry testing of endocrine (estrogen and
progesterone) receptors and HER2, but not Ki67, is free for
patients with breast cancer. Therefore, Ki67 testing was not
systematically performed. Consequently, the intrinsic subtype
classification did not differentiate cancers into luminal A and B,
nor into HER2-positive/hormone receptor-positive and
HER2-positive/hormone receptor-negative.
In the present study, pCR after NAC in breast cancer
was found to be a positive predictor of progression-free and OS.
While previous studies found a higher pCR rate in the TN subgroup
(6,29), the present study found that the
probability to achieve pCR was not significantly different between
TN and luminal subgroups. This indicated that TN breast cancer was
less sensitive to chemotherapy, potentially due to predominant
expression of AR in TN tumors. Future studies should investigate
the genetics and phenotype of TN cancer.
Although the TN subtype was not significantly
associated with a higher probability of pCR rate than the other
subgroups, reaching a pCR resulted in significantly improved OS.
These findings support the use of intensify NAC regimens for this
molecular subgroup to improve survival rates. Immune checkpoint
inhibitors have proven efficacy with an acceptable toxicity profile
in neoadjuvant treatment of TN tumors (44). However, these chemotherapeutic
agents are not currently available in the Republic of Côte
d'Ivoire. Access to immune checkpoint inhibitors may improve
survival rates in patients with breast cancer. Pertuzmab in
combination with chemotherapy resulted in a higher response rate in
HER2-positive breast cancer (23).
In the Republic of Côte d'Ivoire, anti-HER2 therapies are available
free of charge (since 2018 for trastuzumab and 2024 for
pertuzumab). However, delays in immunohistochemistry testing due to
lack of facilities and qualified personnel hamper the use of
anti-HER2 therapy and platinum agents in HER2-positive and TN
patients, respectively. Centralization of pathology services and
personal training programs may address this problem.
In conclusion, NAC for breast cancer resulted in pCR
in approximately one-third of patients. Tumors positive for HER2
demonstrated the highest probability of pCR following neoadjuvant
chemotherapy. A pathological response following NAC was the most
relevant factor influencing PFS and OS in all patients. For each
molecular subtype, pCR was associated with a significantly higher
PFS rate. Therefore, the pathological response following NAC was
the strongest predictive factor of improved PFS, particularly in
the TN subtype.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ENS designed the study, analyzed and interpreted
data, and wrote the manuscript. DAT designed the study and wrote
the manuscript. CTS, AGT and KT analyzed and interpreted data. BD
conceived the study. AGT and DAT confirmed the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by The National
Ethical Committee of Life and Health Sciences (approval no.
00068/25/MSHPCMU/CNESVS-km; Côte d'Ivoire. All patients gave their
written consent to participate to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Leon-Ferre RA, Hieken TJ and Boughey JC:
The landmark series: Neoadjuvant chemotherapy for triple-negative
and HER2-positive breast cancer. Ann Surg Oncol. 28:2111–2119.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Long-term outcomes for neoadjuvant
versus adjuvant chemotherapy in early breast cancer: Meta-analysis
of individual patient data from ten randomised trials. Lancet
Oncol. 19:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King TA and Morrow M: Surgical issues in
patients with breast cancer receiving neoadjuvant chemotherapy. Nat
Rev Clin Oncol. 12:335–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Provenzano E: Neoadjuvant chemotherapy for
breast cancer: Moving beyond pathological complete response in the
molecular age. Acta Med Acad. 50:88–109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houssami N, Macaskill P, von Minckwitz G,
Marinovich ML and Mamounas E: Meta-analysis of the association of
breast cancer subtype and pathologic complete response to
neoadjuvant chemotherapy. Eur J Cancer. 48:3342–3354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swain SM, Tang G, Lucas PC, Robidoux A,
Goerlitz D, Harris BT, Bandos H, Geyer CE Jr, Rastogi P, Mamounas
EP and Wolmark N: Pathologic complete response and outcomes by
intrinsic subtypes in NSABP B-41, a randomized neoadjuvant trial of
chemotherapy with trastuzumab, lapatinib, or the combination.
Breast Cancer Res Treat. 178:389–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding YC, Steele L, Warden C, Wilczynski S,
Mortimer J, Yuan Y and Neuhausen SL: Molecular subtypes of
triple-negative breast cancer in women of different race and
ethnicity. Oncotarget. 10:198–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huo D, Hu H, Rhie SK, Gamazon ER,
Cherniack AD, Liu J, Yoshimatsu TF, Pitt JJ, Hoadley KA, Troester
M, et al: Comparison of breast cancer molecular features and
survival by african and european ancestry in the cancer genome
atlas. JAMA Oncology. 3:1654–1662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keenan T, Moy B, Mroz EA, Ross K,
Niemierko A, Rocco JW, Isakoff S, Ellisen LW and Bardia A:
Comparison of the genomic landscape between primary breast cancer
in African American versus white women and the association of
racial differences with tumor recurrence. J Clin Oncol.
33:3621–3627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajagopal PS, Reid S, Fan R, Venton L,
Weidner A, Roberson ML, Vadaparampil S, Wang X, Yoder S, Rosa M, et
al: Population-specific patterns in assessing molecular subtypes of
young black females with triple-negative breast cancer. NPJ Breast
Cancer. 11:282025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martini R, Newman L and Davis M: Breast
cancer disparities in outcomes; unmasking biological determinants
associated with racial and genetic diversity. Clin Exp Metastasis.
39:7–14. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Global Cancer Observatory, . Cancer Today.
Lyon, France: International Agency for Research on Cancer.
Available from. https://gco.iarc.who.int/media/globocan/factsheets/populations/384-cote-divoire-fact-sheetMay
2–2024
|
|
16
|
Joko-Fru WY, Miranda-Filho A,
Soerjomataram I, Egue M, Akele-Akpo MT, N'da G, Assefa M, Buziba N,
Korir A, Kamate B, et al: Breast cancer survival in sub-Saharan
Africa by age, stage at diagnosis and human development index: A
population-based registry study. Int J Cancer. 146:1208–1218. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teichgraeber DC, Guirguis MS and Whitman
GJ: Breast cancer staging: Updates in the AJCC cancer staging
manual, 8th edition, and current challenges for radiologists, from
the AJR special series on cancer staging. AJR Am J Roentgenol.
217:278–290. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farrukh N, Bano R, Naqvi SRQ and Latif H:
Assessment of pathological complete response in patients with
breast cancer receiving neoadjuvant systemic therapy. J Coll
Physicians Surg Pak. 32:746–750. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cirier J, Body G, Jourdan ML, Bédouet L,
Fleurier C, Pilloy J, Arbion F and Ouldamer L: Impact de la réponse
histologique complète à la chimiothérapie néo-adjuvante pour cancer
du sein selon le sous-type moléculaire. Gynécol Obstétrique
Fertilité Sénologie. 45:535–544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cortazar P, Zhang L, Untch ML, Bedouet L,
Fleurier C, Pilloy J, Arbion F and Ouldamer L: Pathological
complete response and Long-term clinical benefit in breast cancer:
The CTNeoBC pooled analysis. Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Müller C, Schmidt G, Juhasz-Böss I, Jung
L, Huwer S, Solomayer EF and Juhasz-Böss S: Influences on
pathologic complete response in breast cancer patients after
neoadjuvant chemotherapy. Arch Gynecol Obstet. 304:1065–1071. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foldi J, Mougalian S, Silber A, Lannin D,
Killelea B, Chagpar A, Horowitz N, Frederick C, Rispoli L, Burrello
T, et al: Single-arm, neoadjuvant, phase II trial of pertuzumab and
trastuzumab administered concomitantly with weekly paclitaxel
followed by 5-fluoruracil, epirubicin, and cyclophosphamide (FEC)
for stage I–III HER2-positive breast cancer. Breast Cancer Res
Treat. 169:333–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Untch M, Rezai M, Loibl S, Fasching PA,
Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B,
et al: Neoadjuvant treatment with trastuzumab in HER2-positive
breast cancer: results from the GeparQuattro study. J Clin Oncol.
28:2024–2031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma P, Connolly RM, Roussos Torres ET
and Thompson A: Best foot forward: Neoadjuvant systemic therapy as
standard of care in triple-negative and HER2-positive breast
cancer. Am Soc Clin Oncol Educ Book. 40:1–16. 2020. View Article : Google Scholar
|
|
26
|
Boughey JC, Ballman KV, McCall LM,
Mittendorf EA, Symmans WF, Julian TB, Byrd D and Hunt KK: Tumor
biology and response to chemotherapy impact breast cancer-specific
survival in node-positive breast cancer patients treated with
neoadjuvant chemotherapy: Long-term follow-up from ACOSOG Z1071
(Alliance). Ann Surg. 266:667–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hieken TJ, Murphy BL, Boughey JC, Degnim
AC, Glazebrook KN and Hoskin TL: Influence of biologic subtype of
inflammatory breast cancer on response to neoadjuvant therapy and
cancer outcomes. Clin Breast Cancer. 18:e501–e506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prat A, Fan C, Fernández A, Hoadley KA,
Martinello R, Vidal M, Viladot M, Pineda E, Arance A, Muñoz M, et
al: Response and survival of breast cancer intrinsic subtypes
following multi-agent neoadjuvant chemotherapy. BMC Med.
13:3032015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hadgu E, Seifu D, Tigneh W, Bokretsion Y,
Bekele A, Abebe M, Sollie T, Merajver SD, Karlsson C and Karlsson
MG: Breast cancer in Ethiopia: Evidence for geographic difference
in the distribution of molecular subtypes in Africa. BMC Womens
Health. 18:402018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brandão M, Guisseve A, Bata G, Alberto M,
Ferro J, Garcia C, Zaqueu C, Lorenzoni C, Leitão D, Come J, et al:
Breast cancer subtypes: implications for the treatment and survival
of patients in Africa-a prospective cohort study from Mozambique.
ESMO Open. 5:e0008292020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adeniji AA, Dawodu OO, Habeebu MY, Oyekan
AO, Bashir MA, Martin MG, Keshinro SO and Fagbenro GT: Distribution
of breast cancer subtypes among nigerian women and correlation to
the risk factors and clinicopathological characteristics. World J
Oncol. 11:165–172. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seshie B, Adu-Aryee NA, Dedey F,
Calys-Tagoe B and Clegg-Lamptey JN: A retrospective analysis of
breast cancer subtype based on ER/PR and HER2 status in Ghanaian
patients at the Korle Bu Teaching Hospital, Ghana. BMC Clin Pathol.
15:142015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen XS, Wu JY, Huang O, Chen CM, Wu J, Lu
JS, Shao ZM, Shen ZZ and Shen KW: Molecular subtype can predict the
response and outcome of Chinese locally advanced breast cancer
patients treated with preoperative therapy. Oncol Rep.
23:1213–1220. 2010.PubMed/NCBI
|
|
36
|
Jiagge E, Jibril AS, Chitale D,
Bensenhaver JM, Awuah B, Hoenerhoff M, Adjei E, Bekele M, Abebe E,
Nathanson SD, et al: Comparative analysis of breast cancer
phenotypes in African American, White American, and West versus
east African patients: Correlation between African ancestry and
triple-negative breast cancer. Ann Surg Oncol. 23:3843–3849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davis M, Tripathi S, Hughley R, He Q, Bae
S, Karanam B, Martini R, Newman L, Colomb W, Grizzle W and Yates C:
AR negative triple negative or ‘quadruple negative’ breast cancers
in African American women have an enriched basal and immune
signature. PLoS One. 13:e01969092018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saini G, Bhattarai S, Gogineni K and Aneja
R: Quadruple-negative breast cancer: An uneven playing field. JCO
Glob Oncol. 6:233–237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhattarai S, Klimov S, Mittal K,
Krishnamurti U, Li XB, Oprea-Ilies G, Wetherilt CS, Riaz A,
Aleskandarany MA, Green AR, et al: Prognostic role of androgen
receptor in triple negative breast cancer: A Multi-institutional
study. Cancers (Basel). 11:9952019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Asano Y, Kashiwagi S, Goto W, Tanaka S,
Morisaki T, Takashima T, Noda S, Onoda N, Ohsawa M, Hirakawa K and
Ohira M: expression and clinical significance of androgen receptor
in Triple-negative breast cancer. Cancers (Basel). 9:42017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thike AA, Yong-Zheng Chong L, Cheok PY, Li
HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J and Tan PH: Loss
of androgen receptor expression predicts early recurrence in
triple-negative and basal-like breast cancer. Mod Pathol.
27:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goorts B, van Nijnatten TJA, de Munck L,
Moossdorff M, Heuts EM, de Boer M, Lobbes MB and Smidt ML: Clinical
tumor stage is the most important predictor of pathological
complete response rate after neoadjuvant chemotherapy in breast
cancer patients. Breast Cancer Res Treat. 163:83–91. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Spring LM, Fell G, Arfe A, Sharma C,
Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ,
et al: Pathologic complete response after neoadjuvant chemotherapy
and impact on breast cancer recurrence and survival: A
comprehensive Meta-analysis. Clin Cancer Res. 26:2838–2848. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Villacampa G, Navarro V, Matikas A,
Ribeiro JM, Schettini F, Tolosa P, Martínez-Sáez O, Sánchez-Bayona
R, Ferrero-Cafiero JM, Salvador F, et al: Neoadjuvant immune
checkpoint inhibitors plus chemotherapy in early breast cancer: A
systematic review and Meta-analysis. JAMA Oncol. 10:1331–1341.
2024. View Article : Google Scholar : PubMed/NCBI
|