Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth
most prevalent cancer globally and the fourth leading cause of
cancer-related mortality. There were an estimated 905,000 new cases
and 830,000 associated deaths worldwide in 2020 (1). The primary curative treatment options
for HCC include liver transplantation (LT) and liver resection
(LR). Currently, LR is the most commonly employed treatment, aimed
at tumor eradication and extending overall survival (OS) time in
patients with compensated liver function (2). However, HCC resectability is
contingent on several factors, including liver function, cirrhosis
status with portal hypertension, tumor size and number, tumor
location, clinical staging and the overall health of the patient.
LR is generally more effective in patients with HCC who have
Child-Pugh A liver function, with or without cirrhosis and portal
hypertension (3). However, the
recurrence rate (RR) of HCC after LR can approach 50% within the
first few years, likely due to the presence of occult HCC foci in
the residual liver, circulating cancer cells returning to the liver
to form novel tumors or de novo HCC development due to the
underlying liver disease in the remnant liver (4).
By contrast, the HCC RR following LT has been
recorded as ~17% (range, 15–19%) (5), with a >30% reduction in absolute RR
post-LT compared with that observed in LR (4). LT not only removes the tumor but also
addresses the underlying chronic liver disease and associated
complications, such as portal hypertension (6). However, LT has its limitations,
including organ donor shortages, extended waiting times, the need
for lifelong immunosuppressive therapy and the associated risk of
infections. Therefore, the debate continues regarding which
treatment, LR or LT, should be the preferred initial option for
HCC.
The core requirements of Milan criteria are as
follows: A single tumor with a diameter ≤5 cm, or up to 3 tumors
each ≤3 cm in diameter, with no vascular invasion or extrahepatic
metastasis, represent the first internationally recognized
guidelines for LT in liver cancer (7). Patients with HCC meeting these
criteria who undergo LT achieve a 4-year OS rate of up to 85% and a
disease-free survival (DFS) rate of 92%. The efficacy of LT within
the Milan criteria has been validated by multiple transplant
centers globally. Previous studies have indicated that 20–25% of
patients with liver cancer are eligible for both LT and LR, making
the decision for first-line treatment more complex (8–11). Due
to the numerous factors that complicate direct comparisons between
these two surgical approaches, their respective advantages and
prognostic differences remain contentious. Therefore, the present
study utilized a meta-analysis to compare the prognosis of patients
with HCC undergoing LT and LR within the Milan criteria,
specifically focusing on differences in OS and DFS rates, to
provide additional evidence-based guidance for clinical
decision-making in the future.
Materials and methods
Search strategy
A systematic search was conducted across multiple
databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (http://www.embase.com/), Cochrane (http://www.cochranelibrary.com/), Web of Science
(Medline) (https://www.webofscience.com/), OVID (https://www.ovid.com/), Scopus (https://www.scopus.com/), China National Knowledge
Infrastructure (http://www.cnki.net/), Value-Added
Information Provider (https://qikan.cqvip.com/), Wanfang (https://www.wanfangdata.com.cn/index.html) and China
Biology Medicine (http://www.sinomed.ac.cn/zh/), to identify studies
evaluating the prognosis of LT and LR in patients with HCC meeting
the Milan criteria. The search, which spanned from the inception of
each database to June 2024, utilized a combination of index terms
and text words. Key search terms included ‘liver cancer’,
‘hepatocellular carcinoma’, ‘HCC’, ‘liver transplantation’, ‘LT’,
‘liver resection’ and ‘LR’ (the terms HCC, LT and LR were also
searched for separately as the abbreviations), among others
(Data S1). Furthermore, references
cited in relevant studies were also reviewed to ensure
comprehensive retrieval.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Studies
comparing the efficacy and prognosis of LT and LR for HCC; ii)
patients with HCC meeting the Milan criteria, with pathological
confirmation; iii) interventions involving LR or LT; iv) studies
providing relevant outcome data, including 1-, 3-, 5- and 10-year
OS, DFS and RR; and v) full-text articles available in either
Chinese or English. The exclusion criteria were as follows: i)
Animal studies; ii) meta-analyses, systematic reviews, reviews,
case reports, degree thesis, editorials and conference abstracts;
iii) studies lacking primary research indicators or duplicated from
Surveillance, Epidemiology and End Results Program database data
(https://seer.cancer.gov/); iv) studies with
<10 cases in either the LT or LR groups; and v) studies where
full text could not be accessed.
Data extraction and quality
assessment
Data extraction was performed independently by two
reviewers using a standardized table, with discrepancies resolved
by a third reviewer. Extracted data included: i) Study details
[author, publication date, country, sample size for LT and LR
groups, transplant center name, treatment intent, sex, liver
function classification (12),
vascular invasion and follow-up period]; and ii) outcome measures
(OS and DFS rates, and RR, at 1-, 3-, 5- and 10-years
post-treatment). The quality of retrospective cohort studies was
assessed using the Newcastle-Ottawa Scale (NOS) (13), evaluating study subject selection,
group comparability and outcome measurement. The total score of the
scale was 9 points, with a score of ≥7 points indicating
high-quality studies.
Statistical analysis
Statistical analysis was performed using Review
Manager software (version 5.4; The Cochrane Collaboration). Odds
ratios (OR) were calculated for binary variables, with 95%
confidence interval (CI) provided for each estimate. Heterogeneity
among studies was assessed using the χ2 test, with
I2 quantifying the degree of heterogeneity. High
heterogeneity was defined by P<0.05 and I2>50%,
while low heterogeneity was indicated by P≥0.05 and
I2≤50%. A random effects model was used for all
comparisons, in line with previous evidence suggesting its greater
reliability compared with the fixed effects model (14). Sensitivity analysis was conducted to
explore sources of heterogeneity and publication bias was evaluated
using a funnel plot. The χ2 test was also used to
analyze differences in cirrhosis severity, sex and microvascular
invasion (MVI), with P<0.05 considered to indicate a
statistically significant difference.
Results
Characteristics of the included
studies and quality assessment
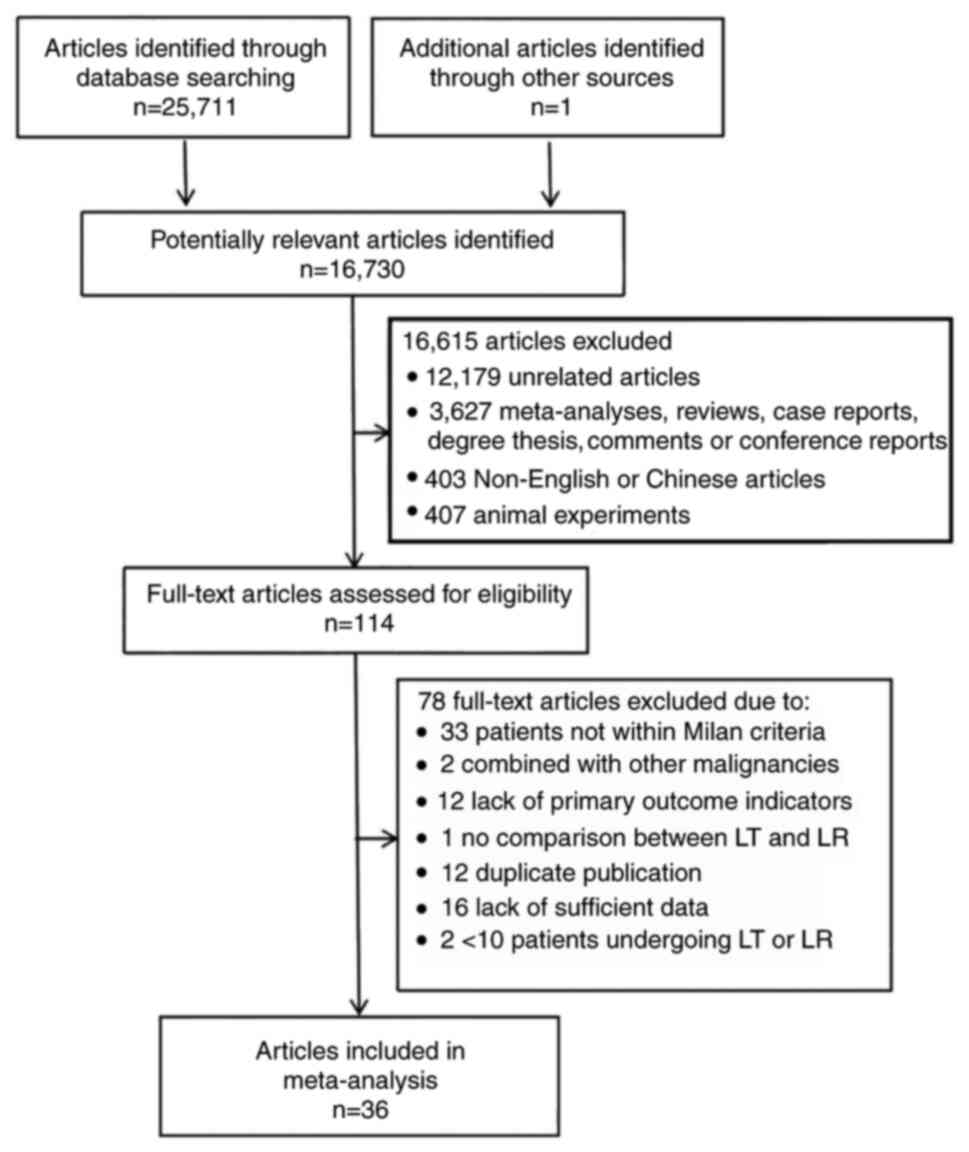
As illustrated in Fig.
1, a total of 25,712 articles were retrieved. After applying
the inclusion and exclusion criteria, 36 articles were selected,
comprising 30 English-language studies (15–43)
and 6 Chinese-language studies (44–49),
all of which were retrospective cohort studies. The included
studies involved a total of 6,839 patients, with 3,894 in the LR
group and 2,945 in the LT group. The basic characteristics of these
studies are summarized in Tables I
and II, while the quality
assessment results are presented in Table III. All studies were of high
quality, scoring ≥7 according to the NOS. Table IV provides a detailed description
of the basic characteristics of the patients with HCC. For patients
with liver cancer undergoing LR or LT, sex, liver function and
tumor vascular invasion status are closely related to the
prognosis. As the hormone levels influence tumor progression, and
microvascular invasion reflects the tumor's aggressiveness and is
directly linked to the risk of recurrence, liver function reserve
determines treatment tolerance and recovery capacity. There were no
statistically significant differences between the LR and LT groups
in terms of sex distribution (P=0.697). However, a significant
difference was observed in the severity of cirrhosis and MVI
distribution between the two groups, (P<0.001 and P=0.006,
respectively) (Table V). In
addition, among the 36 included articles, 5 did not provide a
detailed description of the dropout rate (18,25,32,36,49), 4
had dropout rates (15,16,33,44)
and the remaining 27 did not have dropout rates.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
|
|
|
| OS (LR/LT), % | DFS (LR/LT), % | Number of
recurrences |
|
|---|
|
|
|
|
|
|
|
|
|---|
| First author,
year | LR, n | LT, n | 1-year | 3-year | 5-year | 10-year | 1-year | 3-year | 5-year | 10-year | LR | LT | (Refs.) |
|---|
| Margarit et
al, 2005 | 37 | 36 | 92.0/78.0 | NA | 70.0/65.0 | 50.0/60.0 | 84.0/77.0 | NA | 39.0/64.0 | 18.0/56.0 | 22 | 4 | (19) |
| Jiang et al,
2014 | 33 | 34 | 84.8/94.1 | 64.0/91.2 | 51.2/76.5 | NA | NA | 35.6/72.0 | 19.8/41.0 | NA | 22 | 6 | (15) |
| Foltys et
al, 2014 | 30 | 31 | NA | NA | 57.9/42.0 | NA | NA | NA | NA | NA | NA | NA | (20) |
| Bigourdan et
al, 2003 | 20 | 17 | NA | 67.0/87.0 | 36.0/71.0 | NA | NA | 52.0/87.0 | 40.0/80.0 | NA | NA | NA | (21) |
| Sogawa et
al, 2013 | 56 | 52 | 85.1/75.0 | 65.4/59.4 | 51.6/51.3 | NA | NA | NA | NA | NA | 34 | 12 | (22) |
| Moon et al,
2007 | 100 | 17 | 92.9/94.1 | 79.0/94.1 | 66.5/94.1 | NA | 78.1/100 | 65.4/75.0 | 54.5/75.0 | NA | 36 | 1 | (23) |
| Bellavance et
al, 2008 | 245 | 134 | 93.0/91.0 | 71.0/79.0 | 46.0/66.0 | NA | 88.0/96.0 | 62.0/89.0 | 40.0/82.0 | NA | 122 | 19 | (24) |
| Squires et
al, 2014 | 45 | 131 | NA | NA | 43.8/65.7 | NA | NA | NA | 22.7/85.3 | NA | NA | NA | (25) |
| Michelakos et
al, 2019 | 95 | 89 | NA | 76.0/82.0 | 62.0/77.0 | 41.0/53.0 | NA | 48.0/96.0 | 44.0/94.0 | 31.0/94.0 | NA | NA | (18) |
| Meyerovich et
al, 2019 | 30 | 54 | 92.1/81.2 | 78.1/56.4 | 55.2/47.0 | NA | 73.4/89.4 | 53.6/62.4 | 27.8/56.2 | NA | 4 | 15 | (26) |
| Krenzien et
al, 2018a | 30 | 123 | NA | NA | 36.0/77.0 | NA | NA | NA | 29.0/76.0 | NA | NA | NA | (27) |
| Krenzien et
al, 2018b | 29 | 91 | NA | NA | 61.0/73.0 | NA | NA | NA | 40.0/70.0 | NA | NA | NA | (27) |
| Park et al,
2017 | 199 | 137 | NA | NA | NA | NA | 73.9/92 | 54.6/87.1 | NA | NA | 79 | 19 | (14) |
| Li et al,
2017 | 61 | 31 | 93.4/100 | 63.1/91.1 | 51.2/85.4 | NA | 68.9/100 | 48.4/87.4 | 38.6/80.1 | NA | NA | NA | (16) |
| Huang et al,
2016 | 254 | 49 | NA | NA | NA | NA | 82.0/86.0 | 61.0/80.0 | 45.0/80.0 | 31.0/68.0 | 140 | 8 | (28) |
| Li et al,
2014 | 243 | 39 | 96.3/100 | 83.9/85.2 | 76.2/81.0 | NA | 79.8/89.7 | 59.7/83.5 | 50.9/79.3 | NA | NA | NA | (29) |
| Dai et al,
2014 | 25 | 13 | 100/100 | 93.3/91.7 | 93.3/91.7 | NA | 92.0/92.3 | 71.7/92.3 | 64.5/92.3 | NA | NA | NA | (30) |
| Poon et al,
2007 | 204 | 43 | NA | NA | 68.0/81.0 | NA | NA | NA | 44.0/84.0 | NA | NA | NA | (31) |
| Baccarani et
al, 2008 | 38 | 48 | 82.0/85.0 | 61.0/79.0 | 26.0/74.0 | NA | 79.0/82.0 | 41.0/74.0 | 11.0/74.0 | NA | 13 | 1 | (32) |
| Sung et al,
2017 | 89 | 67 | NA | NA | 63.0/78.0 | 43.0/75.0 | NA | NA | 57.0/88.0 | 37.0/86.0 | 50 | 8 | (33) |
| Hsueh et al,
2016 | 184 | 65 | 94.9/96.9 | 82.2/86.7 | 71.4/76.4 | NA | 81.2/92.2 | 58.4/80.9 | 46.6/70.5 | NA | NA | NA | (17) |
| Lee et al,
2010 | 82 | 48 | 87.7/85.1 | 74.9/78.1 | 58.4/78.1 | NA | 74.3/91.5 | 59.3/89.1 | 57.4/89.1 | NA | NA | NA | (34) |
| Shabahang et
al, 2002 | 44 | 65 | NA | 57.0/66.0 | NA | NA | NA | 36.0/66.0 | NA | NA | NA | NA | (35) |
| Chapman et
al, 2015 | 248 | 496 | NA | NA | 52.8/74.3 | 21.7/53.7 | NA | NA | 30.1/71.8 | 11.7/53.4 | NA | NA | (36) |
| Koniaris et
al, 2011 | 33 | 205 | 94.0/87.0 | NA | 59.0/63.0 | NA | 94.0/84.0 | NA | NA | NA | NA | NA | (37) |
| Sotiropoulos et
al, 2009 | 26 | 26 | NA | NA | 26.0/56.0 | NA | NA | NA | NA | NA | NA | NA | (38) |
| Shah et al,
2007 | 121 | 140 | 89.0/90.0 | 70.0/75.0 | 56.0/64.0 | NA | NA | NA | NA | NA | 53 | 17 | (39) |
| Fan et al,
2011 | 287 | 50 | 93.4/92.0 | 82.1/86.0 | 72.8/81.0 | NA | 77.4/92.0 | 59.5/83.0 | 54.7/83.0 | NA | 135 | 5 | (40) |
| Del Gaudio et
al, 2008 | 80 | 147 | 90.0/90.0 | NA | 66.0/73.0 | NA | 72.0/85.0 | NA | 41.0/71.0 | NA | 7 | 1 | (41) |
| Adam et al,
2012 | 97 | 101 | NA | 67.0/79.0 | 52.0/75.0 | 36.0/65.0 | NA | 33.0/76.0 | 20.0/72.0 | 12.0/64.0 | 60 | 10 | (42) |
| Llovet et
al, 1999 | 77 | 87 | NA | 85.0/82.0 | 62.0/69.0 | 51.0/69.0 | NA | NA | NA | NA | NA | NA | (43) |
| Xia et al,
2021 | 285 | 90 | 96.3/95.4 | 87.1/79.4 | 76.9/77.4 | 54.7/71.7 | NA | NA | NA | NA | 136 | 13 | (44) |
| Yu et al,
2014 | 227 | 36 | 96.9/100 | 83.8/87.5 | 76.1/83.1 | NA | 80.6/91.7 | 59.8/85.3 | 50.8/81.0 | NA | 100 | 6 | (45) |
| Huang et al,
2014 | 55 | 33 | 87.3/90.9 | 69.3/87.7 | 57.3/70.1 | NA | 71.0/96.7 | 52.6/85.3 | 40.6/64.6 | NA | 29 | 6 | (46) |
| Xu et al,
2013 | 31 | 22 | 87.0/86.0 | 71.0/68.0 | NA | NA | 84.0/77.0 | 74.0/59.0 | NA | NA | 7 | 5 | (47) |
| Xia et al,
2012 | 89 | 32 | 86.0/87.0 | 63.0/70.0 | 44.0/62.0 | NA | 68.0/80.0 | 44.0/65.0 | 26.0/52.0 | NA | NA | NA | (48) |
| Zhu et al,
2011 | 65 | 66 | 92.3/93.9 | 67.7/87.9 | NA | NA | NA | NA | NA | NA | 15 | 4 | (49) |
 | Table II.Characteristics of the included
studies. |
Table II.
Characteristics of the included
studies.
| First author,
year | Region | Transplant
centers | ITT | (Refs.) |
|---|
| Margarit et
al, 2005 | Spain | Single | No | (19) |
| Jiang et al,
2014 | China | Single | No | (15) |
| Foltys et
al, 2014 | USA | Single | Yes | (20) |
| Bigourdan et
al, 2003 | France | Single | No | (21) |
| Sogawa et
al, 2013 | USA | Single | Yes | (22) |
| Moon et al,
2007 | Korea | Single | No | (23) |
| Bellavance et
al, 2008 | USA | Multiple | Yes | (24) |
| Squires et
al, 2014 | USA | Single | No | (25) |
| Michelakos et
al, 2019 | USA | Single | Yes | (18) |
| Meyerovich et
al, 2019 | Israel | Single | Yes | (26) |
| Krenzien et
al, 2018a | Germany | Single | No | (27) |
| Krenzien et
al, 2018b | Germany | Single | No | (27) |
| Park et al,
2017 | Korea | Single | No | (14) |
| Li et al,
2017 | China | Single | No | (16) |
| Huang et al,
2016 | China | Multiple | No | (28) |
| Li et al,
2014 | China | Single | No | (29) |
| Dai et al,
2014 | China | Single | No | (30) |
| Poon et al,
2007 | China | Single | No | (31) |
| Baccarani et
al, 2008 | Italy | Single | Yes | (32) |
| Sung et al,
2017 | Korea | Single | No | (33) |
| Hsueh et al,
2016 | China | Multiple | No | (17) |
| Lee et al,
2010 | Korea | Single | No | (34) |
| Shabahang et
al, 2002 | USA | Single | No | (35) |
| Chapman et
al, 2015 | USA | Multiple | No | (36) |
| Koniaris et
al, 2011 | USA | Multiple | Yes | (37) |
| Sotiropoulos et
al, 2009 | Germany | Single | No | (38) |
| Shah et al,
2007 | Canada | Single | Yes | (39) |
| Fan et al,
2011 | China | Single | No | (40) |
| Del Gaudio et
al, 2008 | Italy | Single | Yes | (41) |
| Adam et al,
2012 | France | Single | Yes | (42) |
| Llovet et
al, 1999 | Spain | Single | Yes | (43) |
| Xia et al,
2021 | China | Single | No | (44) |
| Yu et al,
2014 | China | Single | No | (45) |
| Huang et al,
2014 | China | Single | No | (46) |
| Xu et al,
2013 | China | Single | No | (47) |
| Xia et al,
2012 | China | Single | No | (48) |
| Zhu et al,
2011 | China | Multiple | No | (49) |
 | Table III.Quality assessment results
(Newcastle-Ottawa Scale). |
Table III.
Quality assessment results
(Newcastle-Ottawa Scale).
|
| Selection | Comparability |
|
|
|
|
|
|---|
|
|
|
| Outcome |
|
|
|---|
| First author,
year | Representation of
the exposed cohort | Selection of the
non-exposed cohort | Ascertainment of
exposure | Demonstration that
outcome of interest was not present at start of study | Study controls to
select the most key factor | Study controls for
any additional factor |
|
|
|
|---|
| Assessment of
outcome | Whether follow-up
was long enough for outcomes to occur | Integrity of
follow-up | Score | (Refs.) |
|---|
| Margarit et
al, 2005 | * | * | * | * | * | * | * | * | / | 8 | (19) |
| Jiang et al,
2014 | * | * | * | * | * | * | * | * | * | 9 | (15) |
| Foltys et
al, 2014 | * | * | * | * | * | / | * | * | * | 8 | (20) |
| Bigourdan et
al, 2003 | * | * | * | * | * | / | * | * | * | 8 | (21) |
| Sogawa et
al, 2013 | * | * | * | * | / | / | * | * | * | 7 | (22) |
| Moon et al,
2007 | * | * | * | * | * | * | * | * | * | 9 | (23) |
| Bellavance et
al, 2008 | * | * | * | * | * | / | * | * | / | 7 | (24) |
| Squires et
al, 2014 | * | * | * | * | / | * | * | * | / | 7 | (25) |
| Michelakos et
al, 2019 | * | * | * | * | * | / | * | * | / | 7 | (18) |
| Meyerovich et
al, 2019 | * | * | * | * | * | / | * | * | / | 7 | (26) |
| Krenzien et
al, 2018 | * | * | * | * | * | / | * | * | * | 8 | (27) |
| Park et al,
2017 | * | * | * | * | * | / | * | / | * | 7 | (14) |
| Li et al,
2017 | * | * | * | * | / | * | * | * | * | 8 | (16) |
| Baccarani et
al, 2008 | * | * | * | * | * | / | * | * | / | 7 | (32) |
| Huang et al,
2016 | * | * | * | * | / | * | * | * | * | 8 | (28) |
| Li et al,
2014 | * | * | * | * | * | / | * | * | * | 8 | (29) |
| Dai et al,
2014 | * | * | * | * | * | * | * | * | * | 9 | (30) |
| Poon et al,
2007 | * | * | * | * | / | / | * | * | * | 7 | (31) |
| Sung et al,
2017 | * | * | * | * | * | / | * | * | * | 8 | (33) |
| Hsueh et al,
2016 | * | * | * | * | / | * | * | * | / | 7 | (17) |
| Lee et al,
2010 | * | * | * | * | * | * | * | * | / | 8 | (34) |
| Shabahang et
al, 2002 | * | * | * | * | * | / | * | / | * | 7 | (35) |
| Chapman et
al, 2015 | * | * | * | * | * | / | * | * | / | 7 | (36) |
| Koniaris et
al, 2011 | * | * | * | * | * | * | * | * | / | 8 | (37) |
| Sotiropoulos et
al, 2009 | * | * | * | * | / | / | * | * | * | 7 | (38) |
| Shah et al,
2007 | * | * | * | * | / | * | * | * | / | 7 | (39) |
| Fan et al,
2011 | * | * | * | * | * | * | * | / | * | 8 | (40) |
| Adam et al,
2012 | * | * | * | * | / | * | * | * | * | 8 | (42) |
| Del Gaudio et
al, 2008 | * | * | * | * | * | / | * | * | / | 7 | (41) |
| Llovet et
al, 1999 | * | * | * | * | / | * | * | * | * | 8 | (43) |
| Xia et al,
2021 | * | * | * | * | / | * | * | * | * | 8 | (44) |
| Yu et al,
2014 | * | * | * | * | * | / | * | * | * | 8 | (45) |
| Huang et al,
2014 | * | * | * | * | / | * | * | * | / | 7 | (46) |
| Xu et al,
2013 | * | * | * | * | * | * | * | / | * | 8 | (47) |
| Xia et al,
2012 | * | * | * | * | * | * | * | * | * | 9 | (48) |
| Zhu et al,
2011 | * | * | * | * | * | * | * | / | / | 7 | (49) |
 | Table IV.Basic characteristics of patients
with hepatocellular carcinoma. |
Table IV.
Basic characteristics of patients
with hepatocellular carcinoma.
|
| Sex | Child-Pugh
score |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| LR | LT | LR | LT | MVI | Follow-up,
monthsa |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Male | Female | Male | Female | A | B | C | A | B | C | LR | LT | LR | LT | (Refs.) |
|---|
| Adam et al,
2012 | 84 | 13 | 85 | 16 | 80 | 10 | 1 | 19 | 45 | 37 | NA | NA | 36 | 83 | (42) |
| Baccarani et
al, 2008 | 28 | 10 | 42 | 6 | 28 | 10 | 0 | 19 | 20 | 8 | 29 | 8 | 36±25 | 28±26 | (32) |
| Bellavance et
al, 2008 | 203 | 42 | 110 | 24 | 233 | 12 | 0 | 75 | 59 | 0 | 60 | 11 | 27.60 | 39.60 | (24) |
| Bigourdan et
al, 2003 | NA | NA | NA | NA | 20 | 0 | 0 | 17 | 0 | 0 | 3 | 0 | 55 | 55 | (21) |
| Chapman et
al, 2015 | 170 | 78 | 372 | 124 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (36) |
| Dai et al,
2014 | 22 | 3 | 12 | 1 | 25 | 0 | 0 | 13 | 0 | 0 | 3 | 4 | NA | NA | (30) |
| Del Gaudio et
al, 2008 | 63 | 17 | 126 | 21 | 66 | 14 | 0 | 66 | 53 | 88 | NA | NA | NA | NA | (41) |
| Fan et al,
2011 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (40) |
| Foltys et
al, 2014 | NA | NA | NA | NA | 30 | 0 | 0 | 31 | 0 | 0 | NA | NA | 22.30 | 47.50 | (20) |
| Hsueh et al,
2016 | 139 | 45 | 49 | 16 | 179 | 5 | 0 | 20 | 24 | 21 | 45 | 13 | NA | NA | (17) |
| Huang et al,
2016 | 227 | 29 | 43 | 8 | 241 | 15 | 0 | 23 | 15 | 13 | NA | NA | 62.40 | 30 | (28) |
| Jiang et al,
2014 | 28 | 5 | 29 | 5 | 33 | 0 | 0 | 34 | 0 | 0 | 13 | 10 | 31.40±15.50 | 43.50±22.10 | (15) |
| Koniaris et
al, 2011 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (37) |
| Krenzien et
al, 2018b | 18 | 12 | 101 | 22 | NA | NA | NA | NA | NA | NA | 20 | 89 | NA | NA | (27) |
| Krenzien et
al, 2018c | 21 | 8 | 72 | 19 | NA | NA | NA | NA | NA | NA | 7 | 11 | NA | NA | (27) |
| Lee et al,
2010 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 66.50 | 49.10 | (34) |
| Li et al,
2017 | NA | NA | NA | NA | 61 | 31 | 0 | NA | NA | NA | NA | NA | NA | NA | (16) |
| Li et al,
2014 | 211 | 32 | 35 | 4 | 243 | 0 | 0 | 39 | 0 | 0 | 44 | 12 | 40.26±19.72 | 40.26±19.72 | (29) |
| Llovet et
al, 1999 | 48 | 29 | 65 | 22 | 74 | 3 | 0 | 37 | 38 | 12 | 11 | 22 | 32 | 26 | (43) |
| Margarit et
al, 2005 | 29 | 8 | 22 | 14 | 37 | 0 | 0 | 36 | 0 | 0 | 6 | 2 | 50 | 44 | (19) |
| Meyerovich et
al, 2019 | 21 | 9 | 37 | 17 | NA | NA | NA | NA | NA | NA | 15 | 4 | 27.70 | 23.30 | (26) |
| Michelakos et
al, 2019 | 74 | 21 | 76 | 13 | 95 | 0 | 0 | 89 | 0 | 0 | NA | NA | NA | NA | (18) |
| Moon et al,
2007 | 78 | 22 | 16 | 1 | 100 | 0 | 0 | 17 | 0 | 0 | 9 | 0 | 22 | 77 | (23) |
| Park et al,
2017 | 150 | 49 | 107 | 30 | 194 | 5 | 0 | 32 | 60 | 45 | 47 | 21 | 28.70 | 37.80 | (14) |
| Poon et al,
2007 | 165 | 39 | 35 | 8 | 195 | 4 | 0 | 8 | 15 | 20 | 61 | 6 | 49 | 53 | (31) |
| Shabahang et
al, 2002 | 26 | 18 | 45 | 20 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (35) |
| Shah et al,
2007 | 56 | 65 | 76 | 64 | NA | NA | NA | NA | NA | NA | 25 | 22 | NA | 35 | (39) |
| Sogawa et
al, 2013 | 41 | 15 | 60 | 15 | 55 | 1 | 0 | 28 | 47 | 0 | 34 | 22 | 58.30 | 74.30 | (22) |
| Sotiropoulos et
al, 2009 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (38) |
| Squires et
al, 2014 | NA | NA | NA | NA | 39 | 6 | 0 | 37 | 75 | 19 | 14 | 33 | 45 | 40.20 | (25) |
| Sung et al,
2017 | 71 | 18 | 54 | 13 | 87 | 2 | 0 | 18 | 35 | 14 | 9 | 12 | NA | NA | (33) |
| Huang et al,
2014 | 51 | 4 | 28 | 5 | 49 | 6 | 0 | 15 | 10 | 8 | 10 | 7 | NA | NA | (46) |
| Xia et al,
2021 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (44) |
| Xia et al,
2012 | 80 | 9 | 26 | 6 | 89 | 0 | 0 | 32 | 0 | 0 | 25 | 8 | 37 | 37 | (48) |
| Xu et al,
2013 | 22 | 9 | 19 | 3 | 27 | 4 | 0 | 11 | 11 | 0 | NA | NA | 35 | 35 | (47) |
| Yu et al,
2014 | 197 | 30 | 32 | 4 | 227 | 0 | 0 | 36 | 0 | 0 | 41 | 11 | 41.84±19.37 | 41.84±19.37 | (45) |
| Zhu et al,
2011 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | (49) |
 | Table V.Comparisons of sex and Child-Pugh
scores calculated using the χ2 test. |
Table V.
Comparisons of sex and Child-Pugh
scores calculated using the χ2 test.
|
Characteristics | LR, n | LT, n | P-value |
|---|
| Sex
(male/female) | 2,323/639 | 1,774/501 | 0.697 |
| MVI (yes/no) | 531/1,378 | 328/1,063 | 0.006 |
| Child-Pugh
score |
|
|
|
| A | 2,507 | 752 | <0.001 |
| B | 128 | 507 |
|
| C | 1 | 285 |
|
Results of meta-analysis
OS rate analysis
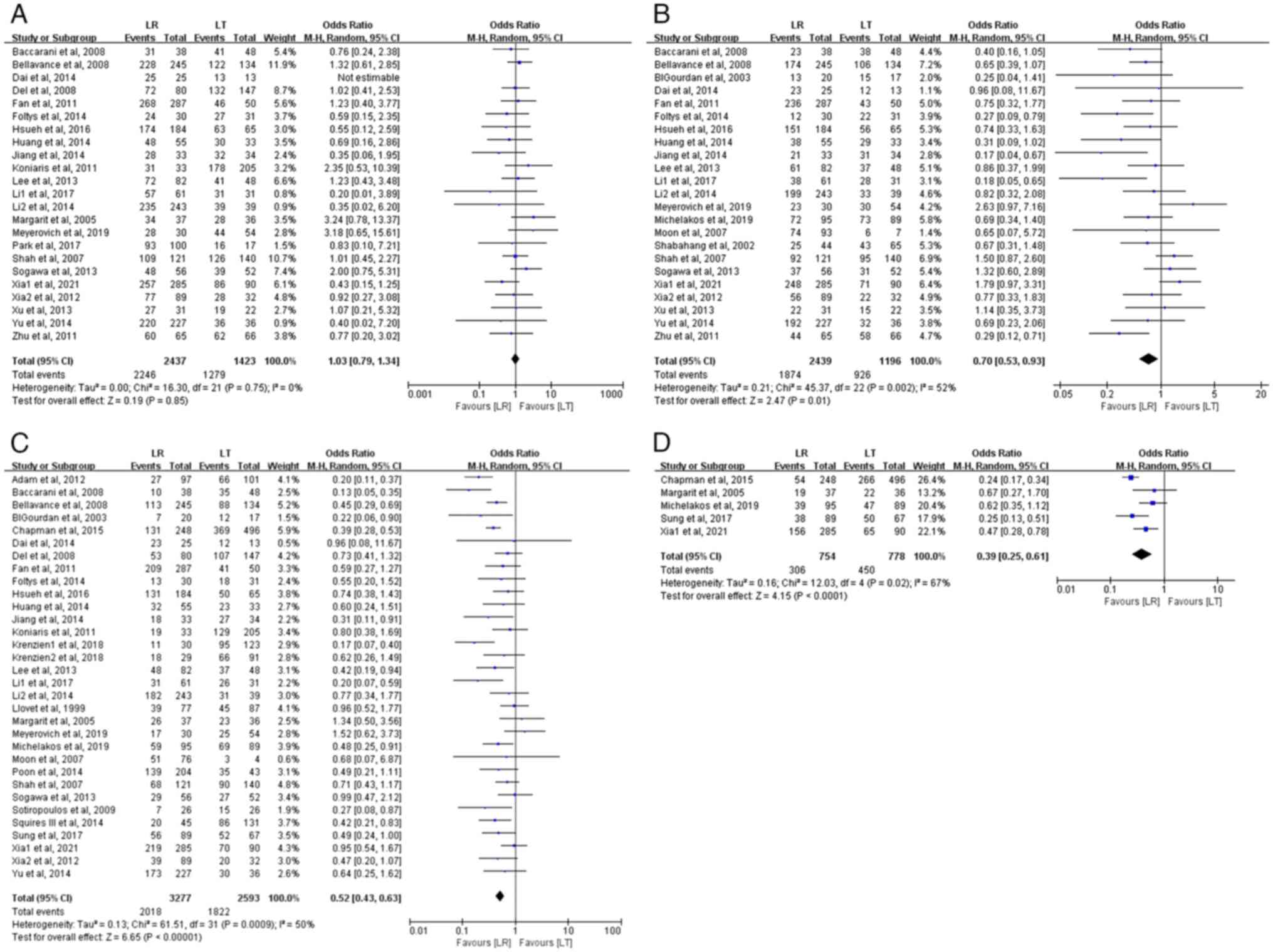
i) 1-year OS rate. In total, 23 studies assessed the
1-year OS rate, comprising 3,860 patients with HCC (2,437 in the LR
group and 1,423 in the LT group). The meta-analysis revealed no
significant difference in the 1-year OS rates between the LR and LT
groups, which were 92.2 and 89.9%, respectively (OR, 1.03; 95% CI,
0.79–1.34; P=0.85). No heterogeneity was observed among the studies
(P=0.75; I2=0%) (Fig.
2A).
ii) 3-year OS rate. In total, 23 studies evaluated
the 3-year OS rate, involving 3,635 patients with HCC (2,439 in the
LR group and 1,196 in the LT group). The meta-analysis demonstrated
a statistically significant difference in the 3-year OS rates
between the LR and LT groups, with patients with LT demonstrating a
slightly higher 3-year OS rate (77.4 vs. 76.8%) (OR, 0.70; 95% CI,
0.53–0.93; P=0.01). There was moderate heterogeneity among the
studies (P=0.002; I2=52%) (Fig. 2B).
iii) 5-year OS rate. In total, 32 studies assessed
the 5-year OS rate, involving 5,870 patients with HCC (3,277 in the
LR group and 2,593 in the LT group). The meta-analysis revealed a
significant difference in the 5-year OS rate between the two
groups, with the LT group having a higher 5-year OS rate (70.3 vs.
61.6%) (OR, 0.52; 95% CI, 0.43–0.63; P<0.00001). There was
moderate heterogeneity among the studies (P=0.0009;
I2=50%) (Fig. 2C).
iv) 10-year OS rate. In total, 5 studies evaluated
the 10-year OS rate, involving 1,532 patients with HCC (754 in the
LR group and 778 in the LT group). The meta-analysis indicated a
significantly higher 10-year OS rate for the LT group compared with
that in the LR group (57.8 vs. 40.6%) (OR, 0.39; 95% CI, 0.25–0.61;
P<0.0001). There was significant heterogeneity among the studies
(P=0.02; I2=67%) (Fig.
2D).
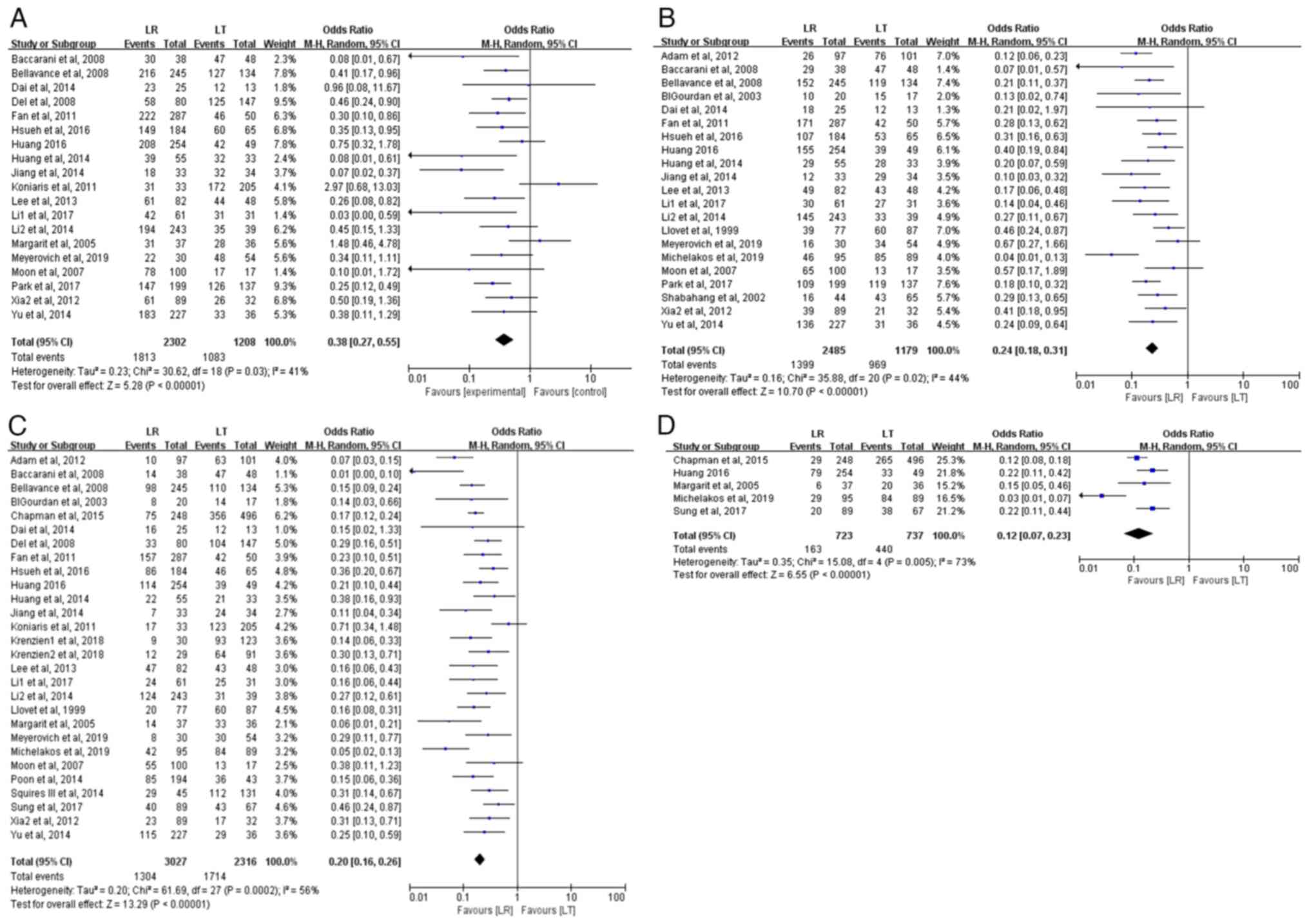
DFS rate analysis
i) 1-year DFS rate. In total, 19 studies evaluated
the 1-year DFS rate, including 3,510 patients with HCC (2,302 in
the LR group and 1,208 in the LT group). The meta-analysis revealed
that the 1-year DFS rate was significantly higher in the LT group
compared with the LR group (89.7 vs. 78.8%) for patients with HCC
meeting the Milan criteria (OR, 0.38; 95% CI, 0.27–0.55;
P<0.00001). Statistical heterogeneity was present among the
studies (P=0.03; I2=41%) (Fig. 3A).
ii) 3-year DFS rate. In total, 21 studies assessed
the 3-year DFS rate, involving 3,664 patients with HCC (2,485 in
the LR group and 1,179 in the LT group). The meta-analysis
indicated a statistically significant difference in the 3-year DFS
rates between the LR and LT groups (OR, 0.24; 95% CI, 0.18–0.31;
P<0.00001), with the LT group exhibiting a significantly higher
3-year DFS rate (82.2 vs. 56.3%). Statistical heterogeneity was
present (P=0.02; I2=44%) (Fig. 3B).
iii) 5-year DFS rate. In total, 28 studies assessed
the 5-year DFS rate, comprising 5,343 patients with HCC (3,027 in
the LR group and 2,316 in the LT group). The meta-analysis
indicated a significant difference in the 5-year DFS rates between
the LR and LT groups (OR, 0.20; 95% CI, 0.16–0.26; P<0.00001),
with the LT group demonstrating a considerably higher 5-year DFS
rate (74.0 vs. 43.1%). There was statistical heterogeneity among
the studies (P=0.0002; I2=56%) (Fig. 3C).
iv) 10-year DFS rate. In total, 5 studies evaluated
the 10-year DFS rate, including 1,460 patients with HCC (723 in the
LR group and 737 in the LT group). The meta-analysis demonstrated
that the 10-year DFS rate was significantly higher in the LT group
compared with that in the LR group (59.7 vs. 22.5%) (OR, 0.12; 95%
CI, 0.07–0.23; P<0.00001). There was high heterogeneity among
the studies (P=0.005; I2=73%) (Fig. 3D).
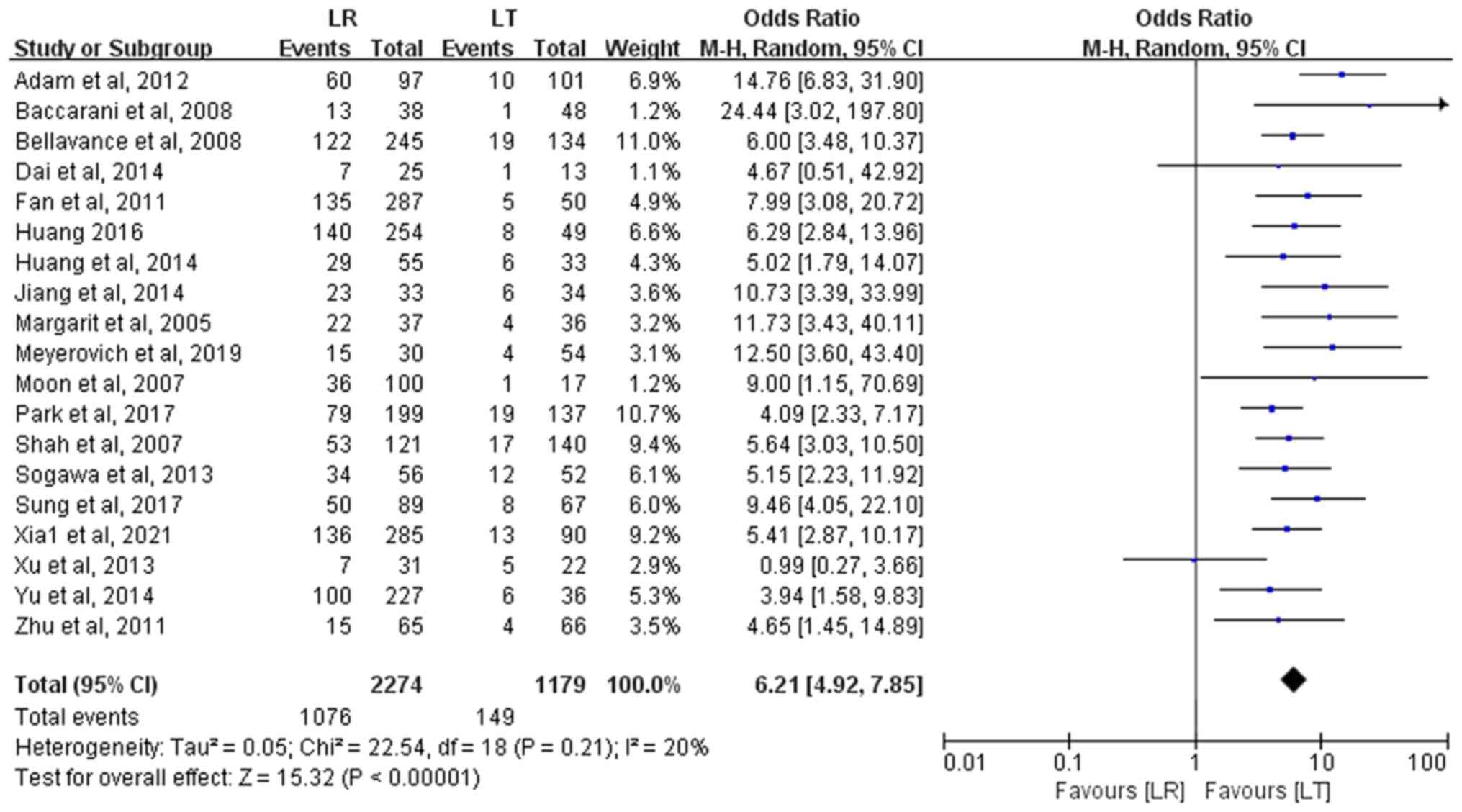
RR analysis
A total of 19 studies with 3,453 patients with HCC
(2,274 in the LR group and 1,179 in the LT group) assessed RR. The
meta-analysis revealed that the RR after LT was significantly lower
compared with that after LR (12.6 vs. 47.3%), with a statistically
significant difference (OR, 6.21; 95% CI, 4.92–7.85; P<0.00001).
No heterogeneity was observed among the studies (P=0.21;
I2=20%) (Fig. 4).
Sensitivity analysis and publication
bias
A sensitivity analysis was conducted by sequentially
excluding each study with high heterogeneity for outcome
indicators. The results revealed that the combined effect of the
outcome indicators did not change notably compared with the
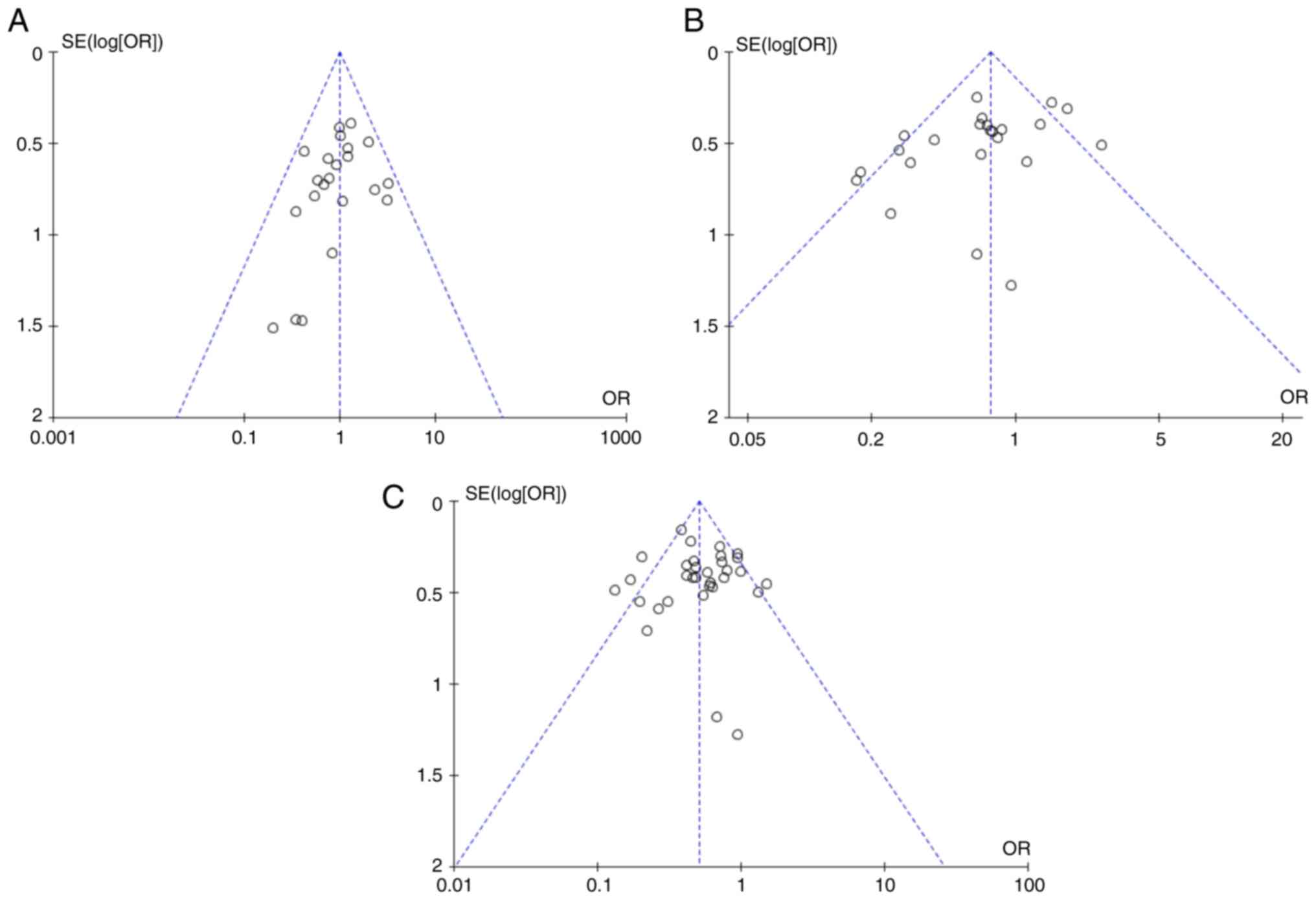
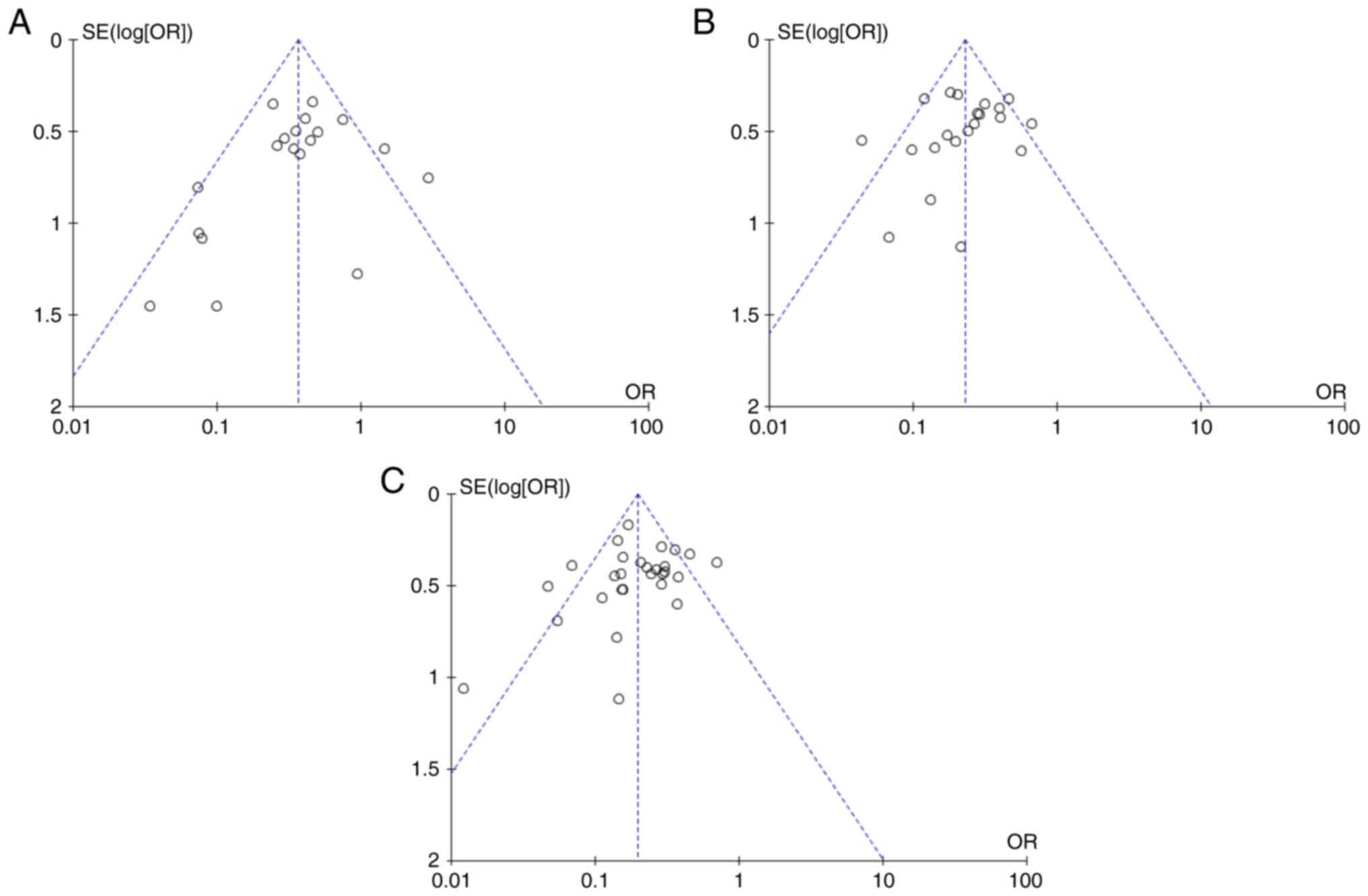
previous analysis. Publication bias was assessed using funnel plots
and all plots displayed varying degrees of asymmetry, indicating
potential publication bias (Figs. 5
and 6). These results reflected the
limitation of the present analysis due to the lack of published RCT
studies.
Discussion
The present meta-analysis compared the prognosis of
patients with HCC within the Milan criteria who received either LT
or LR. The results demonstrated no significant difference in the
1-year OS rate between the LR and LT groups. However, the LT group
demonstrated significantly higher 3-, 5- and 10-year OS rates
compared with the LR group. Similarly, the DFS rates were
consistently higher in the LT group at 1-, 3-, 5- and 10-year
intervals. The present study updates the literature on the
prognosis of patients with HCC within the Milan criteria undergoing
LT and LR, offering more precise information and clinical outcomes
for patients with early-stage HCC.
Varying degrees of heterogeneity were observed among
the studies included in the present meta-analysis. The studies by
Meyerovich et al (26) and
Xia et al (44) were notable
contributors to the high heterogeneity observed in the 3- and
5-year OS rates. A previous study by Meyerovich et al
(26), conducted in Israel,
reported a median waiting time of 304 days for LT, with a 24%
dropout rate, which suggested that LR provided a higher 5-year OS
rate compared with LT. This result contrasts with the present study
findings, possibly due to the lower organ donation rates in Israel,
which result in longer waiting times for LT. Shorter waiting times
might reduce dropout rates and improve post-transplant survival.
Similarly, Xia et al (44)
reported that the 3-year OS rate in the LT group was lower compared
with that in the LR group, although the difference was not
statistically significant. The heterogeneity in the 10-year OS rate
was primarily influenced by the study by Chapman et al
(36), which identified the type of
surgery as an independent risk factor for prognosis in multivariate
analysis. The present study, which retrospectively analyzed data
from five transplant centers, noted marked variation in surgical
approaches and its large sample size further contributed to the
observed heterogeneity in the 10-year OS rate.
The present analysis indicated that the 1-, 3-, 5-
and 10-year DFS rates were all significantly higher in the LT group
compared with those in the LR group for patients with HCC meeting
the Milan criteria. This finding is consistent with the report by
Michelakos et al (18),
which indicated that patients with LT had smaller maximum tumor
diameters and included more patients with T0 stage. By contrast,
Koniaris et al (37)
reported a higher 1-year DFS rate in the LR group compared with
that in the LT group, although the difference was not statistically
significant. This discrepancy may be attributed to the technical
challenges and complications arising from immunosuppressive therapy
in transplantation surgery. The recurrence of HCC within the first
year after LT may be influenced by several factors. First, despite
meeting the Milan criteria, certain tumors may exhibit high
invasiveness or micrometastasis that current diagnostic methods
(such as computed tomography and magnetic resonance imaging) cannot
detect prior to surgery (50).
Second, the use of immunosuppressants in LT recipients may impair
the immune system, creating an environment conducive to tumor
recurrence and growth. Furthermore, delayed postoperative follow-up
or failure to detect and treat recurrent tumors early could
increase the RR. A recently proposed deep learning model has
exhibited notable accuracy in the prediction of postoperative
recurrence compared with the Milan criteria and could potentially
identify high-risk subgroups, thereby improving recurrence
management after LT (51).
The present study included patients with HCC with
Child-Pugh B/C liver cirrhosis, revealing significant differences
in cirrhosis severity between the two groups. Most patients in the
LR group had Child-Pugh A liver function, whereas the LT group
predominantly consisted of patients with Child-Pugh B/C liver
function, a factor that may have contributed to the observed
heterogeneity. Cirrhosis severity serves a key role in the
prognosis of patients with HCC. For those with mild or
non-cirrhotic liver disease (Child-Pugh A), both LR and LT yield
favorable therapeutic outcomes. However, LT is typically
recommended for patients with moderate to severe cirrhosis.
The present study findings indicated that LT
resulted in significantly higher 1-, 3-, 5- and 10-year DFS rates
compared with LR for patients with HCC meeting the Milan criteria.
This may be attributed to the fact that LT addresses both the tumor
and the underlying liver disease (52). By contrast, residual liver tissue
following LR may harbor micrometastases, increasing the risk of
tumor recurrence or liver decompensation. While no significant
difference was found in the 1-year OS rate between LR and LT for
patients within the Milan criteria in the present study, LT
demonstrated improved 3-, 5- and 10-year OS outcomes. The minimal
difference in 1-year OS rate could be attributed to complications
such as acute graft rejection (53), infections due to immunosuppressants
and renal failure (54), rather
than the cancer itself. Long-term survival outcomes (3-, 5- and
10-year) were predominantly influenced by tumor recurrence. The
present analysis demonstrated that LT reduced RR by 29.2% compared
with LR, further supporting its long-term survival benefits. The
risk of death within 5 years post-LT in elderly patients was not
higher compared with that in younger patients and the OS rate in
elderly patients with LT was higher compared with those who
underwent LR. Thus, while elderly patients >70 may experience
decline in organ function, poor surgical tolerance, slower recovery
and coexisting chronic conditions, age alone should not exclude
patients from liver transplant consideration (55–57).
Due to factors such as organ shortage, liver donor
waiting times, hospitalization durations and associated costs,
certain studies have recommended LR as the initial treatment for
patients with HCC eligible for either LR or LT, with LT being
considered as a salvage method for tumor recurrence (58,59).
Several studies reported that the median hospitalization time for
patients with HCC undergoing LT was 9–14 days longer compared with
that for those patients who underwent LR (28,31).
Michelakos et al (18)
highlighted that the average cost of LT was markedly higher
compared with that of LR, considering the expenses associated with
preoperative bridging therapy and postoperative recurrence
management (18). Shah et al
(39) suggested that LT offers
improved survival outcomes compared with LR only if the waiting
time for a liver transplant is <4 months, as patients with HCC
may lose the opportunity for transplantation due to tumor
progression during the waiting period. However, certain studies
have noted that the prognosis of salvage LT after recurrence
post-resection is worse compared with that of primary LT, and the
risks associated with sequential LT following resection should be
carefully considered (60,61).
The present meta-analysis provided a comprehensive
evaluation of the prognostic outcomes of LT and LR for patients
with HCC within the Milan criteria, offering the latest reliable
data and insights key for guiding the selection of LT candidates in
liver cancer treatment. Nonetheless, several limitations should be
acknowledged. First, due to ethical considerations, all included
studies were retrospective, with no randomized controlled trials
available, preventing assessment of blinding and potentially
introducing selection bias. Second, most studies did not adequately
report loss-to-follow-up rates, limiting the ability to evaluate
attrition bias. Third, funnel plots indicated the presence of
publication bias, as studies with statistically significant
positive results were more likely to be published, which may have
influenced the present study findings. Lastly, relatively few
studies that had smaller sample sizes compared the 10-year OS and
DFS rates. These limitations present opportunities for future
research. To address these issues, strategies such as mandatory
clinical trial registration on public platforms before study
initiation, ensuring transparency, establishing cross-regional or
cross-institutional research networks to increase sample size and
improve generalizability, and utilizing propensity score matching
to correct for confounding factors could enhance the robustness and
applicability of future studies.
LT is the preferred treatment option for patients
with HCC within the Milan criteria. However, in clinical practice,
it faces multiple challenges across various dimensions.
Technically, the LT procedure is complex and demanding, which
requires a highly skilled and experienced surgical team to ensure
optimal patient outcomes. Economically, LT is associated with
notable costs, including high surgical expenses and the long-term
financial burden of immunosuppressive therapy. The severe shortage
of donor organs is a major limiting factor for the widespread
adoption of LT, and the lack of a global, comprehensive and
equitable organ allocation system further complicates the process
of securing suitable donors for patients. For example, in
Singapore, patients with liver cancer have to meet the University
of California San Francisco criteria (includes i) a single tumor
≤6.5 cm; ii) ≤3 lesions and maximum lesion diameter ≤4.5 cm,
cumulative diameter ≤8 cm; and iii) no intrahepatic vascular
infiltration or extrahepatic metastasis) to be eligible for the
deceased donor liver transplant waiting list, which makes donor
acquisition even more challenging (57). As a result, living donor LT has
emerged as a key strategy to address the shortage of deceased donor
organs (62).
Despite the benefits of LT, the 1-year OS rates
post-transplant are typically ~90%, with ~10% mortality rates
following both LT and LR. The high 1-year mortality rate after LR
can be predicted preoperatively by factors such as multinodularity,
Child-Pugh class and MVI (63).
Therefore, a comprehensive and accurate preoperative assessment of
patients with HCC is essential to guide the selection of the most
appropriate treatment. In certain cases, LT or LR may not be the
only viable options for treatment. Alternative approaches, such as
transarterial chemoembolization (TACE) combined with radiofrequency
ablation (RFA), may also offer curative outcomes. While the 3-year
and 5-year DFS rates are higher in the LR group compared with those
in the TACE + RFA group, no significant differences in the 1-, 3-
and 5-year OS rates have been observed (64). Therefore, TACE + RFA is also
considered a safe and effective treatment for early-stage HCC.
In conclusion, the present study demonstrated that
LT provides significantly improved long-term OS and DFS rates, as
well as a lower RR, compared with LR for patients with HCC meeting
the Milan criteria. Therefore, LT is recommended as the preferred
initial treatment for these patients, provided that a suitable
liver donor is available. However, each case should be evaluated
through multidisciplinary consultations to optimize patient
outcomes while ensuring efficient use of limited donor resources.
Although this conclusion is based on a comprehensive analysis of
existing studies, clinical decisions must also consider specific
patient circumstances. A notable limitation of the Milan criteria
is its focus solely on tumor size and number, without incorporating
liver function and cirrhosis in a more holistic evaluation. In
cases of organ shortage, the degree of liver cirrhosis and liver
reserve function should guide the decision between LT and LR. This
approach would prioritize LR for patients with HCC with preserved
liver function. The development of a more refined, comprehensive
evaluation criteria for LT remains an area for future research.
Furthermore, with the rapid advancements in cancer-targeted
immunotherapy, the outcomes of LT and LR should be reassessed. It
remains to be determined whether the combination of surgical
resection and targeted immunotherapy can provide notably improved
or similar results to LT, how immunotherapy should be managed
post-LT and how HCC recurrence can be addressed after LT under
immunosuppressive therapy, all of which warrant further
investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the International
Cooperation Fund of the Science and Technology Bureau of Jilin
Province (grant no. 20220402075GH), the Jilin Province Health
Research Talent Project (grant no. 2022SCZ06) and the NSFC Regional
Innovation and Development Fund (grant no. U20A20360).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JW and YW conducted database searching and data
analysis. JW wrote the manuscript. ZP conducted data analysis and
revised the manuscript. YY conducted database searching, and data
identification and analysis. WL participated in research design and
wrote the manuscript. JW and YW confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DFS
|
disease-free survival
|
|
HCC
|
hepatocellular carcinoma
|
|
LR
|
liver resection
|
|
LT
|
liver transplantation
|
|
MVI
|
microvascular invasion
|
|
OS
|
overall survival
|
|
RR
|
recurrence rate
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Taefi A, Abrishami A, Nasseri-Moghaddam S,
Eghtesad B and Sherman M: Surgical resection versus liver
transplant for patients with hepatocellular carcinoma. Cochrane
Database Syst Rev. 6:CD0069352013.PubMed/NCBI
|
|
3
|
Vibert E, Schwartz M and Olthoff KM:
Advances in resection and transplantation for hepatocellular
carcinoma. J Hepatol. 72:262–276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aufhauser DD Jr, Sadot E, Murken DR,
Eddinger K, Hoteit M, Abt PL, Goldberg DS, DeMatteo RP and Levine
MH: Incidence of occult intrahepatic metastasis in hepatocellular
carcinoma treated with transplantation corresponds to early
recurrence rates after partial hepatectomy. Ann Surg. 267:922–928.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bzeizi KI, Abdullah M, Vidyasagar K,
Alqahthani SA and Broering D: Hepatocellular carcinoma recurrence
and mortality rate post liver transplantation: Meta-analysis and
systematic review of real-world evidence. Cancers (Basel).
14:51142022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klupp J, Kohler S, Pascher A and Neuhaus
P: Liver transplantation as ultimate tool to treat portal
hypertension. Dig Dis. 23:65–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhoori S, Schiavo M, Russo A and
Mazzaferro V: First-line treatment for hepatocellular carcinoma:
Resection or transplantation? Transplant Proc. 39:2271–2273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
European Association for the Study of the
Liver, . EASL clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sala M, Fuster J, Llovet JM, Navasa M,
Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C, et
al: High pathological risk of recurrence after surgical resection
for hepatocellular carcinoma: An indication for salvage liver
transplantation. Liver Transpl. 10:1294–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cherqui D, Laurent A, Mocellin N, Tayar C,
Luciani A, Van Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A
and Duvoux C: Liver resection for transplantable hepatocellular
carcinoma: Long-term survival and role of secondary liver
transplantation. Ann Surg. 250:738–746. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deeks JJ, Dinnes J, D'Amico R, Sowden AJ,
Sakarovitch C, Song F, Petticrew M and Altman DG; International
Stroke Trial Collaborative Group; European Carotid Surgery Trial
Collaborative Group, : Evaluating non-randomised intervention
studies. Health Technol Assess. 7:3–10. 1–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park MS, Lee KW, Kim H, Choi YR, Hong G,
Yi NJ and Suh KS: Primary living-donor liver transplantation is not
the optimal treatment choice in patients with early hepatocellular
carcinoma with poor tumor biology. Transplant Proc. 49:1103–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang L, Liao A, Wen T, Yan L, Li B and
Yang J: Living donor liver transplantation or resection for
Child-Pugh A hepatocellular carcinoma patients with multiple
nodules meeting the Milan criteria. Transpl Int. 27:562–569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Liu JY, Peng W, Wen TF, Yan LN, Yang
JY, Li B, Wang WT and Xu MQ: Liver resection versus transplantation
for multiple hepatocellular carcinoma: A propensity score analysis.
Oncotarget. 8:81492–81500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsueh KC, Lee TY, Kor CT, Chen TM, Chang
TM, Yang SF and Hsieh CB: The role of liver transplantation or
resection for patients with early hepatocellular carcinoma. Tumour
Biol. 37:4193–4201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michelakos T, Xourafas D, Qadan M,
Pieretti-Vanmarcke R, Cai L, Patel MS, Adler JT, Fontan F, Basit U,
Vagefi PA, et al: Hepatocellular carcinoma in transplantable
Child-Pugh A cirrhotics: Should cost affect resection vs
transplantation? J Gastrointest Surg. 23:1135–1142. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Margarit C, Escartín A, Castells L, Vargas
V, Allende E and Bilbao I: Resection for hepatocellular carcinoma
is a good option in Child-Turcotte-Pugh class A patients with
cirrhosis who are eligible for liver transplantation. Liver
Transpl. 11:1242–1251. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foltys D, Zimmermann T, Kaths M, Strempel
M, Heise M, Hoppe-Lotichius M, Weiler N, Scheuermann U, Ruckes C,
Hansen T, et al: Hepatocellular carcinoma in Child's A cirrhosis: A
retrospective analysis of matched pairs following liver
transplantation vs liver resection according to the
intention-to-treat principle. Clin Transplant. 28:37–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bigourdan JM, Jaeck D, Meyer N, Meyer C,
Oussoultzoglou E, Bachellier P, Weber JC, Audet M, Doffoël M and
Wolf P: Small hepatocellular carcinoma in Child A cirrhotic
patients: Hepatic resection versus transplantation. Liver Transpl.
9:513–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sogawa H, Shrager B, Jibara G, Tabrizian
P, Roayaie S and Schwartz M: Resection or transplant-listing for
solitary hepatitis C-associated hepatocellular carcinoma: An
intention-to-treat analysis. HPB (Oxford). 15:134–141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon DB, Lee SG and Hwang S: Liver
transplantation for hepatocellular carcinoma: Single nodule with
Child-Pugh class A sized less than 3 cm. Dig Dis. 25:320–328. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bellavance EC, Lumpkins KM, Mentha G,
Marques HP, Capussotti L, Pulitano C, Majno P, Mira P,
Rubbia-Brandt L, Ferrero A, et al: Surgical management of
early-stage hepatocellular carcinoma: Resection or transplantation?
J Gastrointest Surg. 12:1699–1708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Squires MH III, Hanish SI, Fisher SB,
Garrett C, Kooby DA, Sarmiento JM, Cardona K, Adams AB, Russell MC,
Magliocca JF, et al: Transplant versus resection for the management
of hepatocellular carcinoma meeting Milan Criteria in the MELD
exception era at a single institution in a UNOS region with short
wait times. J Surg Oncol. 109:533–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meyerovich G, Goykhman Y, Nakache R,
Nachmany I, Lahat G, Shibolet O, Menachem Y, Katchman H, Wolf I,
Geva R, et al: Resection vs transplant listing for hepatocellular
carcinoma: An intention-to-treat analysis. Transplant Proc.
51:1867–1873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krenzien F, Schmelzle M, Struecker B,
Raschzok N, Benzing C, Jara M, Bahra M, Öllinger R, Sauer IM,
Pascher A, et al: Liver transplantation and liver resection for
cirrhotic patients with hepatocellular carcinoma: Comparison of
long-term survivals. J Gastrointest Surg. 22:840–848. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang ZY, Liang BY, Xiong M, Dong KS,
Zhang ZY, Zhang EL, Li CH and Chen XP: Severity of cirrhosis should
determine the operative modality for patients with early
hepatocellular carcinoma and compensated liver function. Surgery.
159:621–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C, Zhu WJ, Wen TF, Dai Y, Yan LN, Li B,
Yang JY, Wang WT and Xu MQ: Child-Pugh A hepatitis B-related
cirrhotic patients with a single hepatocellular carcinoma up to 5
cm: Liver transplantation vs resection. J Gastrointest Surg.
18:1469–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai Y, Li C, Wen TF and Yan LN: Comparison
of liver resection and transplantation for Child-pugh A cirrhotic
patient with very early hepatocellular carcinoma and portal
hypertension. Pak J Med Sci. 30:996–1000. 2014.PubMed/NCBI
|
|
31
|
Poon RTP, Fan ST, Lo CM, Liu CL and Wong
J: Difference in tumor invasiveness in cirrhotic patients with
hepatocellular carcinoma fulfilling the Milan criteria treated by
resection and transplantation: Impact on long-term survival. Ann
Surg. 245:51–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baccarani U, Isola M, Adani GL, Benzoni E,
Avellini C, Lorenzin D, Bresadola F, Uzzau A, Risaliti A, Beltrami
AP, et al: Superiority of transplantation versus resection for the
treatment of small hepatocellular carcinoma. Transpl Int.
21:247–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sung PS, Yanf H, Na GH, Hwang S, Kang D,
Jang JW, Bae SH, Choi JY, Kim DG, Yoon SK and You YK: Long-term
outcome of liver resection versus transplantation for
hepatocellular carcinoma in a region where living donation is a
main source. Ann Transplant. 22:276–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee KK, Kim DG, Moon IS, Lee MD and Park
JH: Liver transplantation versus liver resection for the treatment
of hepatocellular carcinoma. J Surg Oncol. 101:47–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shabahang M, Franceschi D, Yamashiki N,
Reddy R, Pappas PA, Aviles K, Flores S, Chaparro A, Levi JU,
Sleeman D, et al: Comparison of hepatic resection and hepatic
transplantation in the treatment of hepatocellular carcinoma among
cirrhotic patients. Ann Surg Oncol. 9:881–886. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chapman WC, Klintmalm G, Hemming A,
Vachharajani N, Majella Doyle MB, DeMatteo R, Zaydfudim V, Chung H,
Cavaness K, Goldstein R, et al: Surgical treatment of
hepatocellular carcinoma in North America: Can hepatic resection
still be justified? J Am Coll Surg. 220:628–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koniaris LG, Levi DM, Pedroso FE,
Franceschi D, Tzakis AG, Santamaria-Barria JA, Tang J, Anderson M,
Misra S, Solomon NL, et al: Is surgical resection superior to
transplantation in the treatment of hepatocellular carcinoma? Ann
Surg. 254:527–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sotiropoulos GC, Drühe N, Sgourakis G,
Molmenti EP, Beckebaum S, Baba HA, Antoch G, Hilgard P, Radtke A,
Saner FH, et al: Liver transplantation, liver resection, and
transarterial chemoembolization for hepatocellular carcinoma in
cirrhosis: Which is the best oncological approach? Dig Dis Sci.
54:2264–2273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah SA, Cleary SP, Tan JC, Wei AC,
Gallinger S, Grant DR and Greig PD: An analysis of resection vs
transplantation for early hepatocellular carcinoma: Defining the
optimal therapy at a single institution. Ann Surg Oncol.
14:2608–2614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan ST, Poon RT, Yeung C, Lam CM, Lo CM,
Yuen WK, Ng KK, Liu CL and Chan SC: Outcome after partial
hepatectomy for hepatocellular cancer within the Milan criteria. Br
J Surg. 98:1292–1300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Del Gaudio M, Ercolani G, Ravaioli M,
Cescon M, Lauro A, Vivarelli M, Zanello M, Cucchetti A, Vetrone G,
Tuci F, et al: Liver transplantation for recurrent hepatocellular
carcinoma on cirrhosis after liver resection: University of Bologna
experience. Am J Transplant. 8:1177–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Adam R, Bhangui P, Vibert E, Azoulay D,
Pelletier G, Duclos-Vallée JC, Samuel D, Guettier C and Castaing D:
Resection or transplantation for early hepatocellular carcinoma in
a cirrhotic liver: Does size define the best oncological strategy?
Ann Surg. 256:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Llovet JM, Fuster J and Bruix J:
Intention-to-treat analysis of surgical treatment for early
hepatocellular carcinoma: Resection versus transplantation.
Hepatology. 30:1434–1440. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia YX, Zhang F, Li XC, Kong LB, Zhang H,
Li DH, Cheng F, Pu LY, Zhang CY, Qian XF, et al: Surgical treatment
of primary liver cancer: A report of 10 966 cases. Zhonghua Wai Ke
Za Zhi. 59:6–17. 2021.(In Chinese). PubMed/NCBI
|
|
45
|
Yu Y, Li C and Wen T: Child-Pugh A Class
cirrhotic patients with a single hepatocellular carcinoma up to 5
cm in diameter: Liver transplantation versus resection. Chin J
Bases Clin General Surg. 21:406–409. 2014.(In Chinese).
|
|
46
|
Huang JH and Zhou J: Factors for
predicting outcomes of liver transplantation and liver resection
for hepatocellular carcinoma meeting Milan criteria. J South Med
Univ. 34:406–409. 2014.
|
|
47
|
Xu XS, Qu K, Zhou L, Song YZ, Zhang YL and
Liu C: Selection of surgical procedure in the treatment of early
hepatocellular carcinoma. Chin J Hepat Surg (Electronic Edition).
2:80–85. 2013.
|
|
48
|
Xia Y, Jiang Y, Cai Q, Pan F, Zhang X and
Lü L: Comparison of efficacies of hepatectomy and liver
transplantion for patients with hepatocellular carcinoma fulfilling
the Milan criteria. Chin J Dig Surg. 11:526–529. 2012.(In
Chinese).
|
|
49
|
Zhu X, He X, Chen M, Yuan Y and Cui S: A
multi-center comparative study of the effectiveness of three
radical therapies on hepatocellular carcinoma. Chin J Hepatobiliary
Surg. 17:372–375. 2011.(In Chinese).
|
|
50
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD practice guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ko SH, Cao J, Yang YK, Xi ZF, Han HW, Sha
M and Xia Q: Development of a deep learning model for predicting
recurrence of hepatocellular carcinoma after liver transplantation.
Front Med (Lausanne). 11:13730052024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Clavien PA, Lesurtel M, Bossuyt PM, Gores
GJ, Langer B and Perrier A; OLT for HCC Consensus Group, :
Recommendations for liver transplantation for hepatocellular
carcinoma: An international consensus conference report. Lancet
Oncol. 13:e11–e22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Matinlauri IH, Nurminen MM, HÖckerstedt KA
and Isoniemi HM: Changes in liver graft rejections over time.
Transplant Proc. 38:2663–2666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tinti F, Umbro I, Giannelli V, Merli M,
Ginanni Corradini S, Rossi M, Nofroni I, Poli L, Berloco PB and
Mitterhofer AP: Acute renal failure in liver transplant recipients:
Role of pretransplantation renal function and 1-year follow-up.
Transplant Proc. 43:1136–1138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
European Association for the Study of the
Liver, . Electronic address: simpleeasloffice@easloffice.eu.
EASL clinical practice guidelines: Liver transplantation. J
Hepatol. 64:433–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mousa OY, Nguyen JH, Ma Y, Rawal B, Musto
KR, Dougherty MK, Shalev JA and Harnois DM: Evolving role of liver
transplantation in elderly recipients. Liver Transpl. 25:1363–1374.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chow PKH, Choo SP, Ng DCE, Lo RH, Wang ML,
Toh HC, Tai DW, Goh BK, Wong JS, Tay KH, et al: National cancer
centre singapore consensus guidelines for hepatocellular carcinoma.
Liver Cancer. 5:97–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chan DL, Alzahrani NA, Morris DL and Chua
TC: Systematic review of efficacy and outcomes of salvage liver
transplantation after primary hepatic resection for hepatocellular
carcinoma. J Gastroenterol Hepatol. 29:31–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
De Carlis L, Di Sandro S, Giacomoni A,
Mangoni I, Lauterio A, Mihaylov P, Cusumano C and Rampoldi A: Liver
transplantation for hepatocellular carcinoma recurrence after liver
resection: Why deny this chance of cure? J Clin Gastroenterol.
47:352–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zheng Z, Liang W, Milgrom DP, Zheng Z,
Schroder PM, Kong NS, Yang C, Guo Z and He X: Liver transplantation
versus liver resection in the treatment of hepatocellular
carcinoma: A meta-analysis of observational studies.
Transplantation. 97:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adam R, Azoulay D, Castaing D, Eshkenazy
R, Pascal G, Hashizume K, Samuel D and Bismuth H: Liver resection
as a bridge to transplantation for hepatocellular carcinoma on
cirrhosis: A reasonable strategy? Ann Surg. 238:508–519. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Goldaracena N and Barbas AS: Living donor
liver transplantation. Curr Opin Organ Transplant. 24:131–137.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sheriff S, Madhavan S, Lei GY, Chan YH,
Junnarkar SP, Huey CW, Low JK and Shelat VG: Predictors of
mortality within the first year post-hepatectomy for hepatocellular
carcinoma. J Egypt Natl Canc Inst. 34:142022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gui CH, Baey S, D'cruz RT and Shelat VG:
Trans-arterial chemoembolization + radiofrequency ablation versus
surgical resection in hepatocellular carcinoma-A meta-analysis. Eur
J Surg Oncol. 46:763–771. 2020. View Article : Google Scholar : PubMed/NCBI
|