Introduction
Colorectal cancer is a common malignancy of the
digestive system, and the liver is its most frequent site of
distant metastasis (1). The
relatively high incidence of colorectal liver metastasis (CRLM) can
be attributed in part to the liver's unique anatomical and
physiological environment. Specifically, ~70% of the liver's blood
supply is delivered via the portal venous system, which carries a
rich and slowly circulating volume of blood from the
gastrointestinal tract. Combined with the liver's inherent
immune-tolerant properties, these factors create a conducive milieu
for metastatic colonization (2).
Additionally, alterations in the tumor microenvironment, such as
the suppression of local immune responses and the influence of
liver-specific factors, play a significant role in promoting the
growth and survival of metastatic tumor cells (3). Furthermore, the expression of certain
molecular markers has been associated with an increased risk of
liver metastasis. For example, the upregulation of CD44, CD133,
epithelial cell adhesion molecule, Nanog and Sox2 enhances tumor
cell self-renewal, proliferation and tumorigenicity, thereby
fostering the cells invasive and metastatic potential (4).
The management of CRLM, encompassing both systemic
and local therapies, is tailored to the patient's overall condition
and the number, size and location of the liver lesions. Systemic
treatments primarily comprise chemotherapy, targeted therapy and
immunotherapy, while local therapies mainly include surgical
resection, transarterial embolization/transarterial
chemoembolization (TAE/TACE), radiofrequency ablation (RFA),
microwave ablation (MWA), selective internal radiation therapy,
stereotactic body radiation therapy and HIFU. Surgical resection
remains the primary treatment for CRLM, aiming to completely remove
all visible tumors and offering potential curative prospects
(5). However, only 10–20% of
patients with CRLM are eligible for resection, with a 5-year
postoperative survival rate of only 30% (3).
Systemic chemotherapy is the standard treatment for
patients with unresectable or widespread liver metastases. Common
regimens include FOLFOX and FOLFIRI, often combined with targeted
agents. Notably, the FOLFOXIRI plus bevacizumab regimen has
demonstrated significant survival benefits, albeit with a higher
incidence of adverse events (6).
Emerging immunotherapies, such as those utilizing
lipopolysaccharide-targeted fusion proteins and nanoparticle
systems, show potential for enhanced efficacy but require further
validation regarding their safety profiles (7). TAE/TACE is a local control approach
for metastatic liver cancer that improves both response and
survival rates (8). Other local
treatments include RFA and MWA, which can decrease tumor size,
alleviate pain and improve survival rates. However, their
effectiveness is limited by tumor size and location; larger tumors
or those near major hepatic vessels are not suitable for RFA or MWA
therapy (9).
HIFU has emerged as a powerful, non-invasive and
non-ionizing technique for the targeted ablation of tumors. Guided
by ultrasound or magnetic resonance imaging (MRI), HIFU enables
precise ablation of lesions, including those adjacent to major
hepatic vessels, without causing harm to them (10). The most extensively studied
therapeutic application of HIFU is thermal tissue ablation, which
has demonstrated both palliative and curative potential (11). Over the past two decades, a growing
body of preclinical and clinical literature has emerged in support
of the capacity of HIFU to enhance nascent antitumor immune
responses and to potentiate the effects of cancer immunotherapies
(for example, checkpoint inhibitors) through mechanisms such as
immune modulation and drug delivery (12–14).
Case report
A 78-year-old woman was admitted to Suining Central
Hospital (Suining, China) in June 2013 presenting with repeated
bloody stools for >1 month that had worsened 1 day prior to
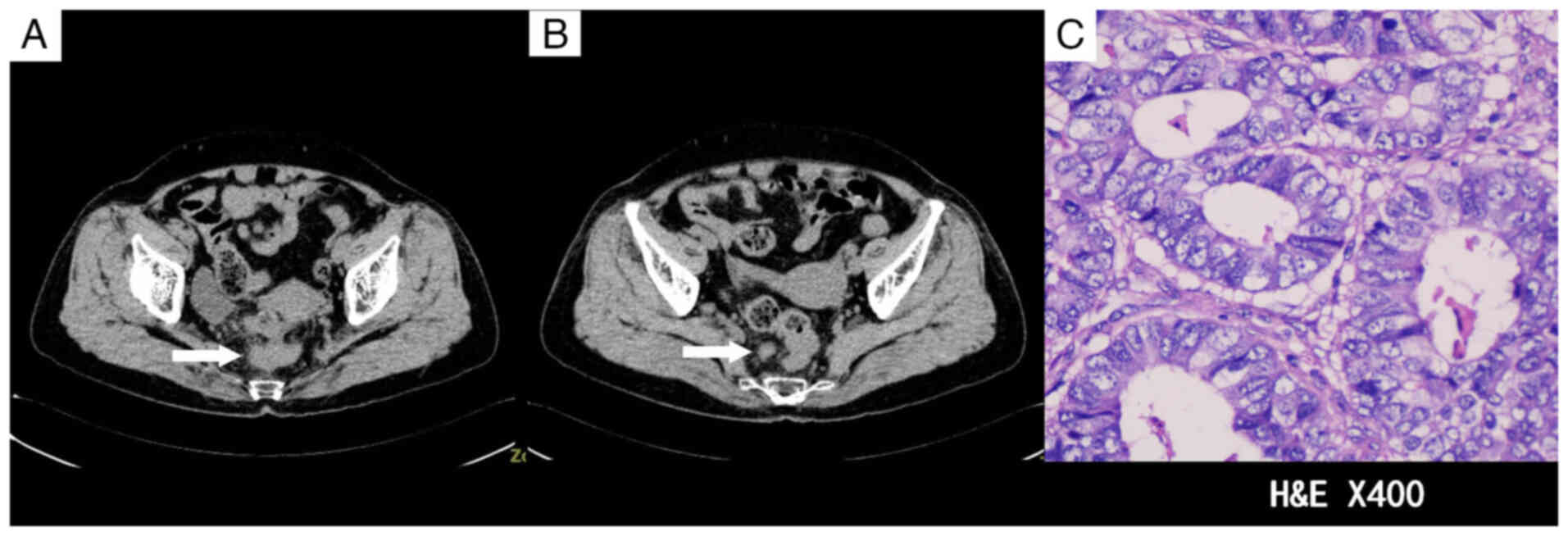
admission. An abdominal CT scan upon admission revealed wall
thickening at the rectosigmoid junction and enlargement of the
perirectal and bilateral inguinal lymph nodes, raising suspicion of
a malignant tumor (Fig. 1A and B).
The medical team recommended a colonoscopy for further diagnostic
clarification. After being informed of the risks and benefits of
the procedure, the patient declined colonoscopy and requested
direct surgical intervention. Following a multidisciplinary team
discussion, it was concluded that the probability of colon cancer
was high and that proceeding directly to surgical exploration was
appropriate given the patient's refusal of a colonoscopy. The
patient underwent laparoscopic radical surgery for rectal cancer
under general anesthesia 2 days after admission. Tissues were fixed
in 10% neutral buffered formalin at 15–25°C for 12–24 h and then
sectioned to 4-µm thick. Hematoxylin and eosin staining was used at
15–25°C for 45 min, and the results were assessed under an Olympus
BX43 optical microscope (Olympus Corporation). The postoperative
pathological diagnosis confirmed a moderately to
well-differentiated ulcerative adenocarcinoma (Fig. 1C), but the patient subsequently
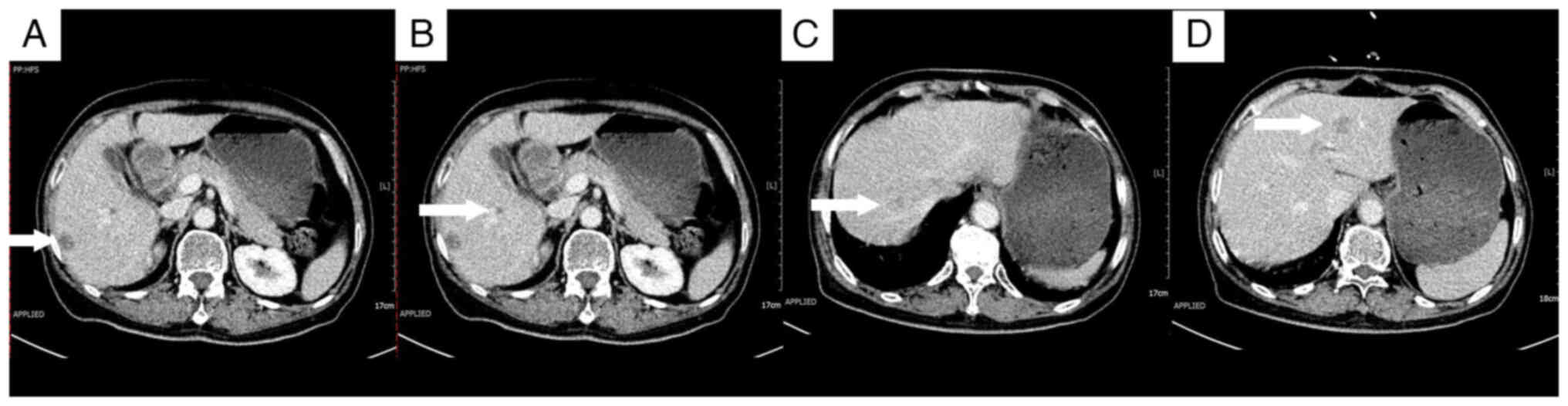
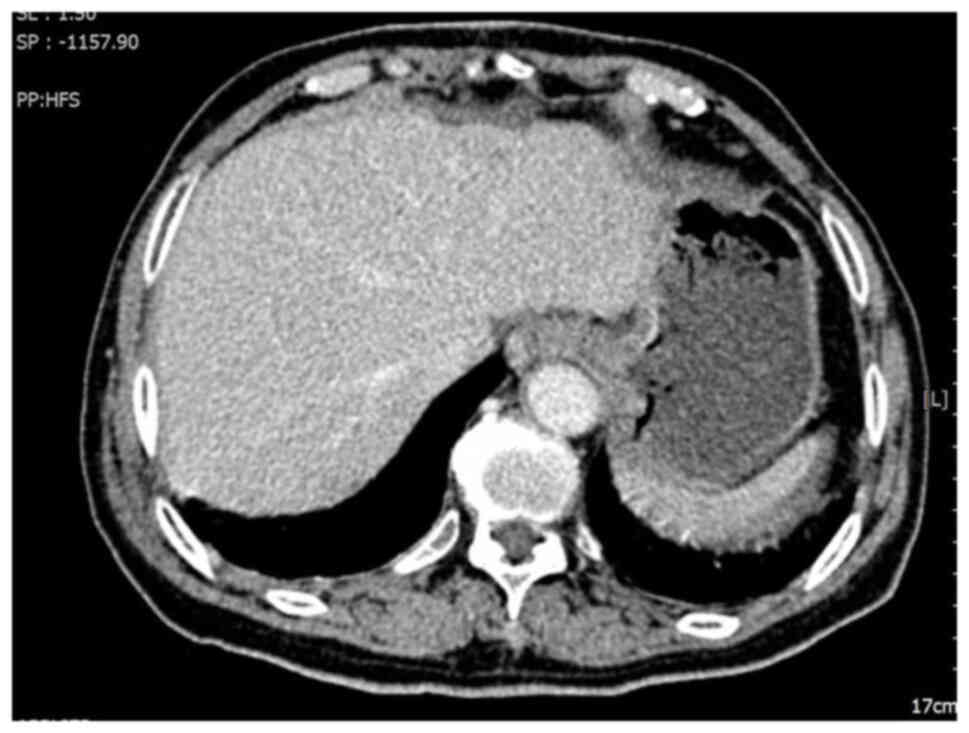
declined adjuvant chemotherapy. A follow-up abdominal CT scan 3
months after surgery demonstrated multiple liver nodules,
suggesting the presence of metastatic tumors (Fig. 2).
As a standard approach for multiple small lesions,
systemic chemotherapy was initiated 3 months after surgery, using
the FOLFOX regimen (100 mg oxaliplatin by intravenous infusion on
day 1; 200 mg calcium folinate by intravenous infusion on days 1–2;
500 mg fluorouracil by intravenous bolus on day 1, followed by
1,500 mg via continuous intravenous infusion over 44 h). However,
the treatment was discontinued due to poor patient tolerance. A
multidisciplinary consultation then recommended the use of RFA.
However, the pre-procedural assessment identified the following
notable challenges for RFA: The lesion beneath the liver capsule in
the right lobe of the liver was relatively superficial, which could
potentially lead to incomplete coverage by the radiofrequency probe
and impact treatment efficacy; the lesion adjacent to the right
portal vein posed a heightened risk for RFA due to its proximity to
major blood vessels; and the lesion at the apex of the right lobe
was too deep for adequate radiofrequency coverage.
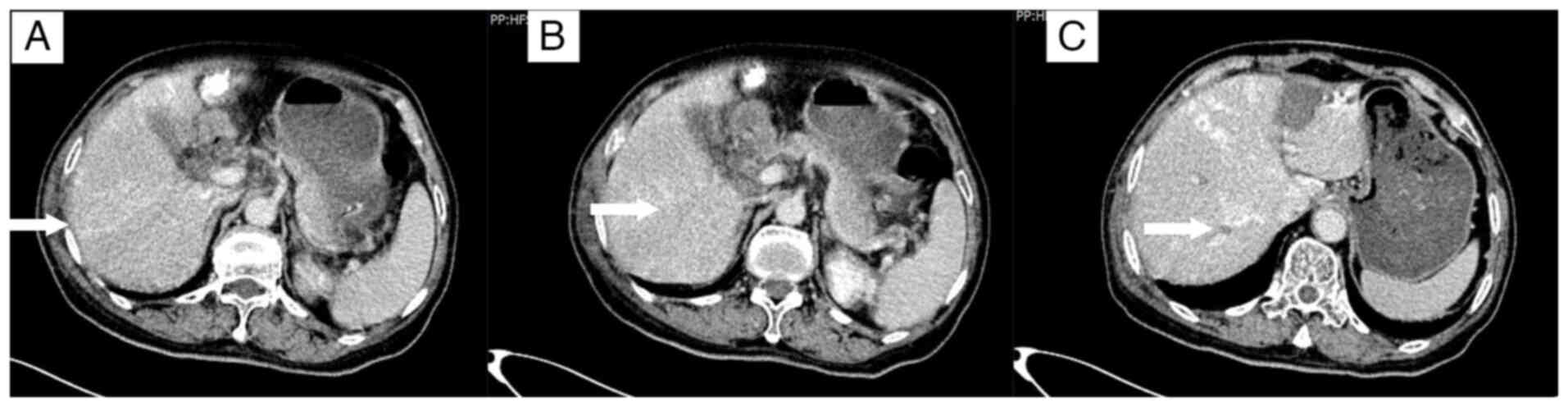
A total of 5 days after the initial chemotherapy
treatment, the patient was admitted to the in the Department of
Hepatobiliary Surgery and underwent RFA with a concurrent biopsy of
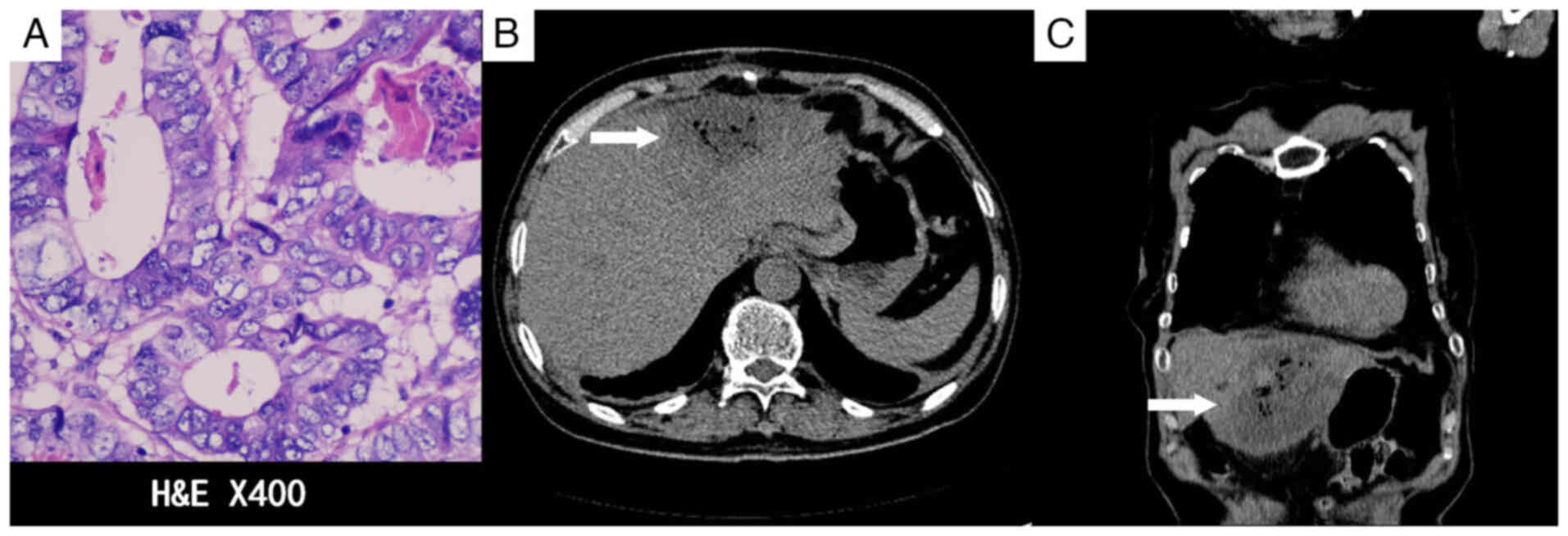
the left hepatic lobe lesion. The postoperative pathology,
performed as aforementioned, confirmed liver metastasis of
colorectal cancer. The cells were arranged in irregular glandular
structures with infiltrative growth, and the nuclei were enlarged,
with prominent nucleoli (Fig. 3A).
The patient developed abdominal pain and a fever following the
operation. A CT scan 1 week after RFA revealed signs of infection
(Fig. 3B and C), which recovered
after antibiotic treatment. In October 2013, the chemotherapy
regimen was switched to XELOX (1,500 mg capecitabine orally twice
daily on days 1–14; 180 mg oxaliplatin by intravenous infusion on
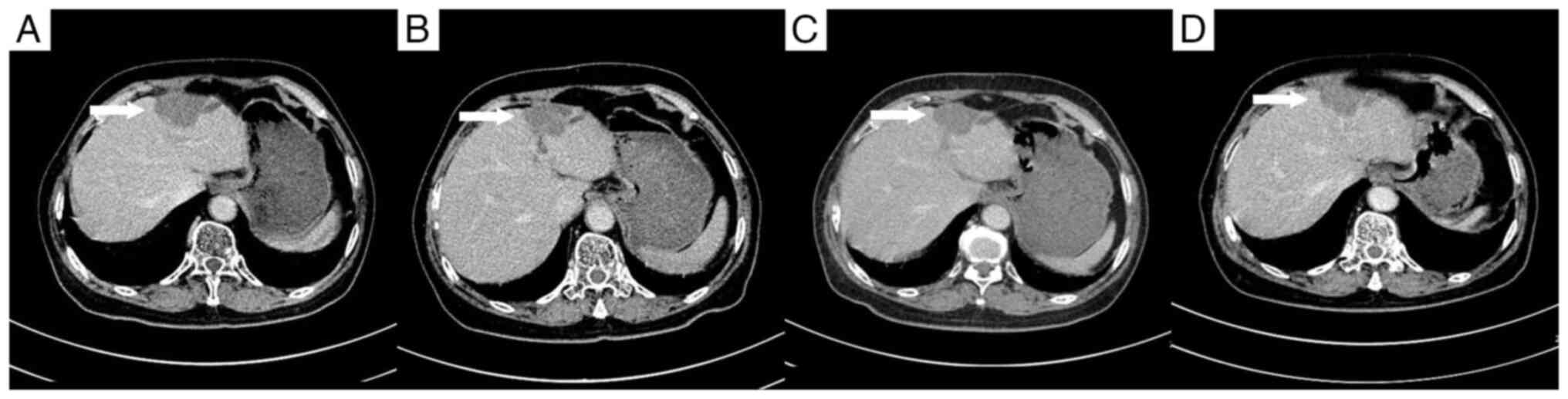
day 1), followed by TACE 8 days later, targeting the metastatic
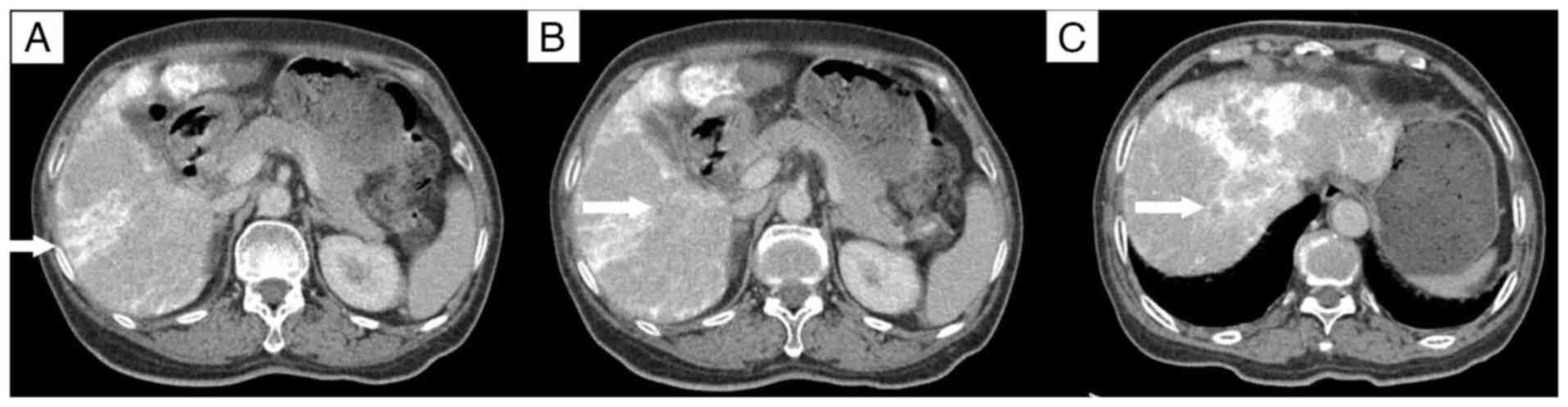
lesions in the right hepatic lobe. A contrast-enhanced CT scan
conducted 10 days post-TACE showed no significant reduction in the
size of the three lesions in the right hepatic lobe compared with
the size in the images taken before the procedure, with persistent
mild enhancement (Fig. 4).
Due to the presence of multiple residual lesions in
the right lobe of the liver, particularly one adjacent to the
portal vein and the surrounding major blood vessels, RFA therapy
was deemed inappropriate. Furthermore, the patient declined further
RFA treatment due to the infection that occurred following the
previous RFA procedure. Therefore, in November 2013, the patient
underwent HIFU ablation for the residual lesions in the right lobe
of the liver. This was followed by three cycles of the XELOX
regimen, starting 8 days later. A follow-up CT scan conducted at 1
month post-HIFU showed complete resolution of the lesions beneath
the liver capsule and adjacent to the portal vein in the right
lobe, with no enhancement observed in the lesion at the apex of the
right lobe (Fig. 5).
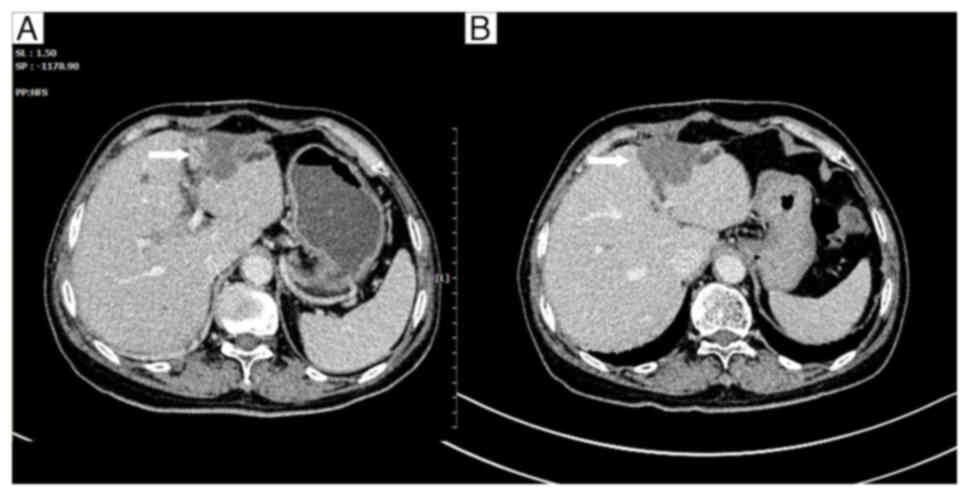
After the patient completed a total of six cycles of
XELOX chemotherapy, a CT scan at the end of January 2014 confirmed
the disappearance of the lesion at the apex of the right lobe
(Fig. 6). A cluster of low-density
masses was observed in the left lobe, with partial enhancement of
some lesions (Fig. 7A).
Approximately 1 month later, HIFU treatment was performed under
general anesthesia for the lesion in the left lobe (the lesion that
had previously been treated with RFA). A subsequent CT scan
performed at the end of May 2014 showed the clustered low-density
mass in the left lobe with no evident enhancement (Fig. 7B).
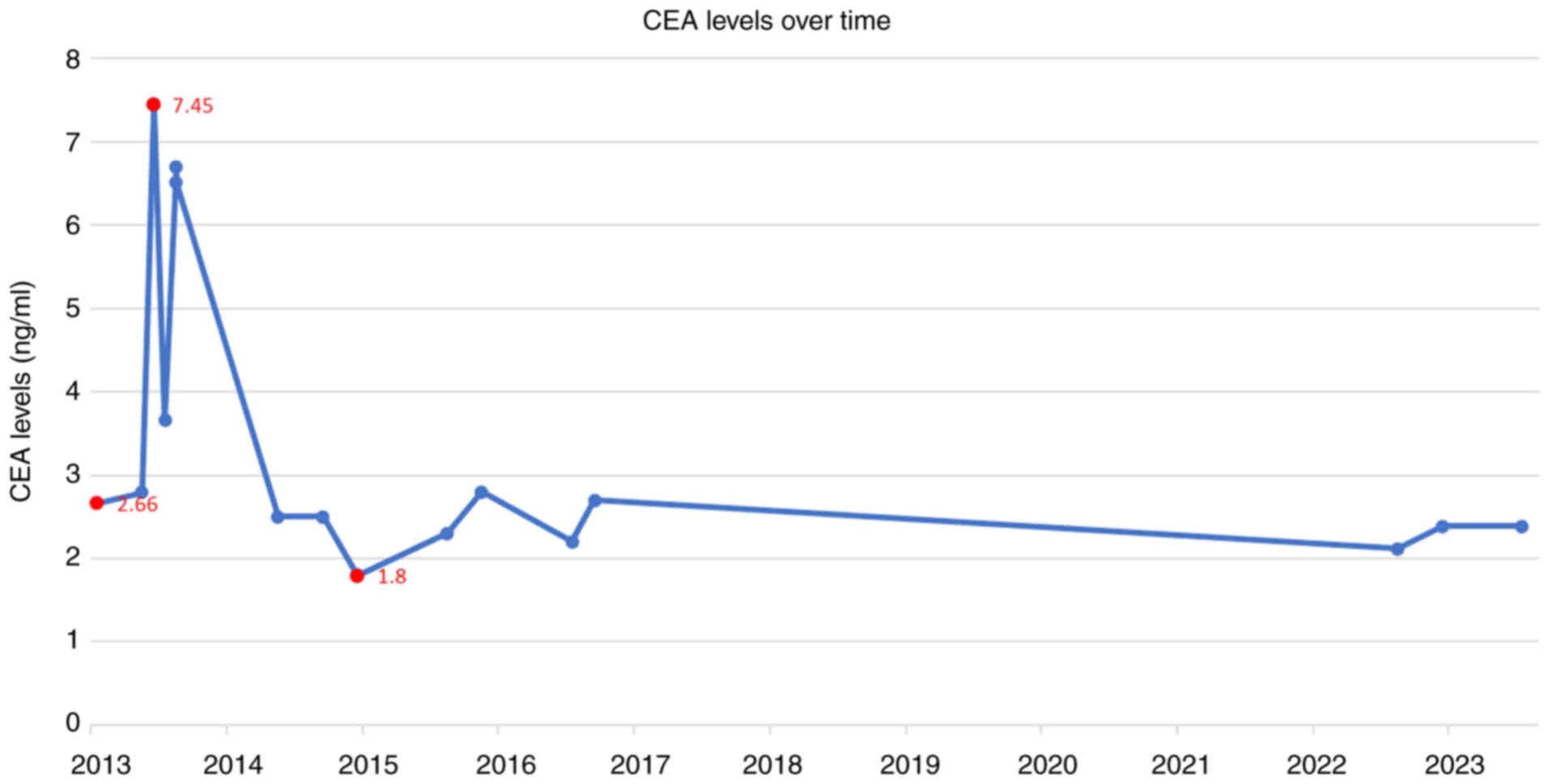
Tumor marker levels, including carcinoembryonic
antigen (CEA), α-fetoprotein (AFP), carbohydrate antigen (CA)12-5,
CA15-3, and CA19-9, were monitored every 1–3 months from June 2013
to February 2014, and every 6–12 months thereafter until July 2015.
An analysis of the timeline of changes in the patient's CEA levels,
alongside the diagnosis and treatment of rectal cancer liver
metastases, revealed that the main lesion was located in the left
lobe of the liver. Before HIFU treatment for the left liver lesion,
CEA levels remained elevated above the normal range. However,
following HIFU treatment, CEA levels returned to a normal level
(Fig. 8). AFP, CA12-5, CA15-3 and
CA19-9 levels remained within the normal range throughout the
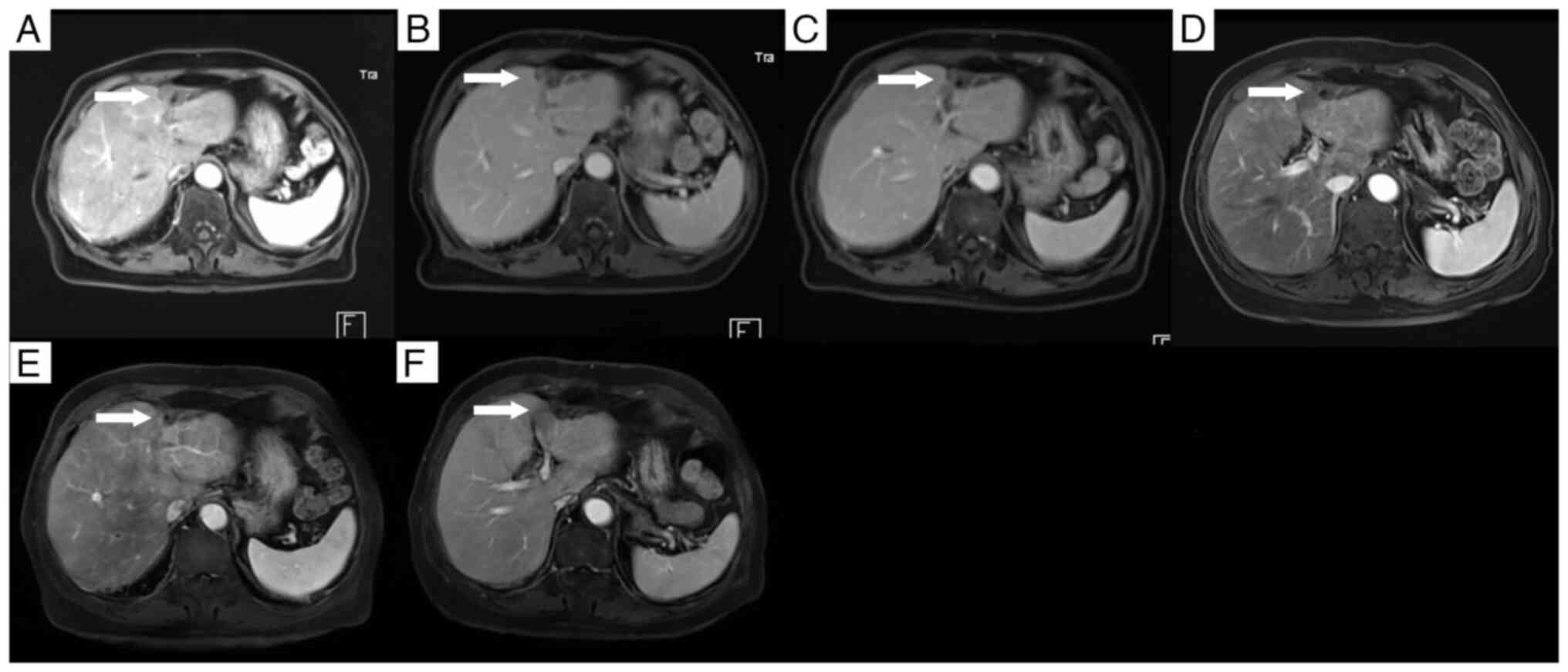
disease course. Contrast-enhanced CT scans over the 2 years
following HIFU therapy showed no evidence of tumor recurrence
(Fig. 9). Beginning in the sixth
year after surgery, annual contrast-enhanced MRI examinations were
performed, facilitated by upgrades in the hospital's imaging
equipment. These MRI studies confirmed the absence of tumor
recurrence in the right hepatic lobe. In the left hepatic lobe,
only residual scar tissue was observed, with no signs of tumor
recurrence (Fig. 10).
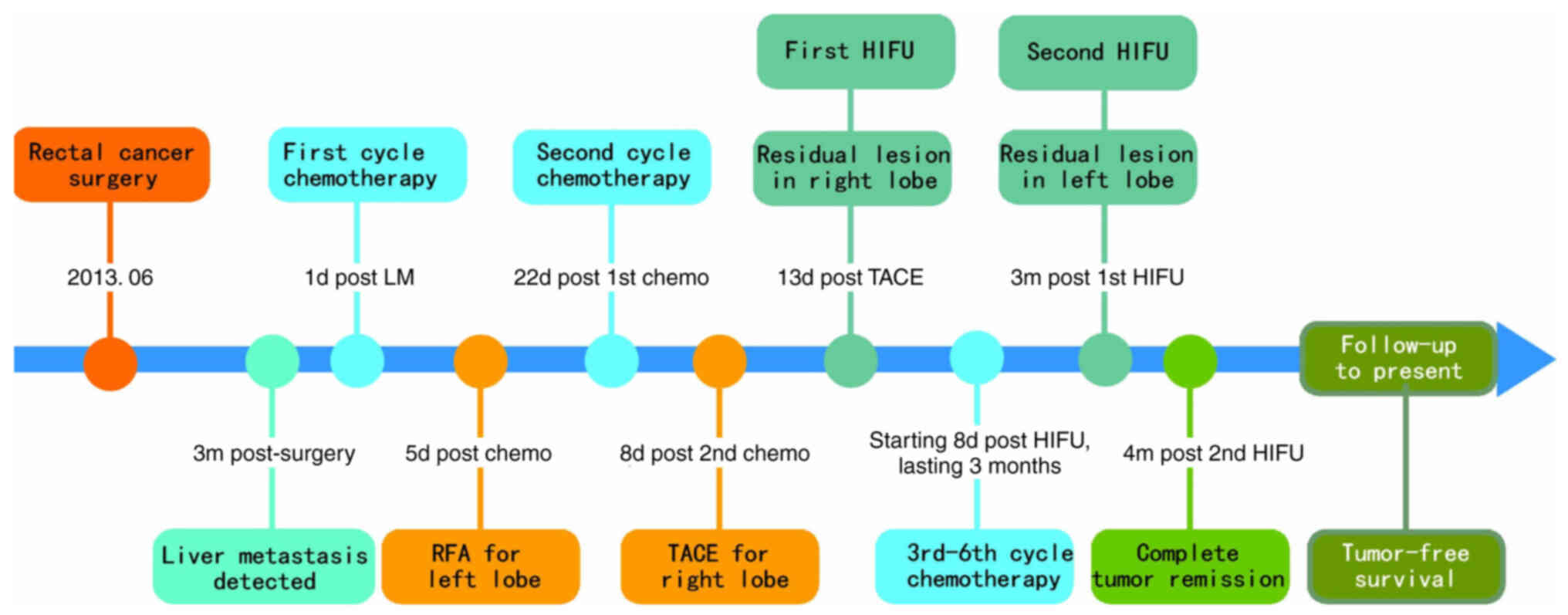
When reviewing the patient's entire treatment course
(Fig. 11), liver metastases were
detected during the 3-month postoperative follow-up after
colorectal cancer surgery. An integrated approach combining
systemic therapy and local treatment was adopted. Early
interventions such as RFA and TACE failed to effectively control
the local lesions. Subsequently, focused ultrasound ablation
achieved excellent local therapeutic outcomes. Over a long-term
follow-up period of 10 years, with annual re-examinations, no tumor
recurrence was observed, indicating a complete cure.
Discussion
Compared with other types of metastatic liver
cancer, CRLM usually presents with a more favorable prognosis,
justifying the exploration of more intensive treatment strategies.
HIFU treatment can improve the penetration of chemotherapy drugs
into tumor tissues by enhancing the local blood supply through
local thermal effects, thereby increasing chemosensitivity. In a
previous single-center retrospective study (15), a total of 523 patients with
unresectable pancreatic ductal adenocarcinoma (PDAC) were
recruited. Of these patients, 347 received HIFU combined with
gemcitabine (GEM), which was administered through either regional
arterial chemotherapy or systemic chemotherapy. The remaining
patients received GEM alone. The median overall survival time for
the patients receiving HIFU combined with GEM therapy was 7.4
months, compared with 6.0 months for those receiving GEM alone
(P=0.002). The 6-month, 10-month, 1-year and 2-year survival rates
for the HIFU + GEM and GEM alone groups were 66.3 vs. 47.5%
(P<0.0001), 31.12 vs. 15.9% (P<0.0001), 21.32 vs. 13.64%
(P=0.033) and 2.89 vs. 2.27% (P=0.78), respectively. These findings
suggested that the combination of HIFU and GEM improves overall
survival compared with standard chemotherapy alone in patients with
PDAC (15). However, the efficacy
of tumor therapy can be hindered by the tumor-specific hypoxic
microenvironment, high osmotic pressure and vascular dysfunction.
The hypoxic environment not only promotes tumor formation and
progression, but also contributes to chemoresistance (16,17).
Considering the unique hypoxic environment inside tumors, a novel
bio-targeted synergistic system comprising genetically engineered
bacteria and multifunctional nanoparticles can overcome the
limitations of HIFU in terms of targeting ability and single-image
monitoring mode. This synergistic approach has shown promise in
improving the efficacy and safety of focused ultrasound ablation
surgery (18). Other studies have
highlighted that local ablation may stimulate immune responses,
which will contribute to tumor control (19,20).
It has been proposed that, by identifying tumor antigen exposure,
thermal ablation of tumors may trigger an ‘in vivo
vaccination’, thereby stimulating the production of antibodies and
promoting the eradication of the local tumor and control of distant
metastasis while establishing antitumor immune memory, a phenomenon
referred to as ‘antitumor induced immunity’ (21). HIFU can be combined with other
interventional treatment methods, such as TACE, to achieve a
synergistic effect. While TACE aims to devascularize tumors by
embolizing their feeding arteries, its efficacy can be limited by
alternate blood supplies or the formation of collateral
circulation, often leading to incomplete necrosis. In such cases,
subsequent HIFU ablation can target these residual lesions,
achieving local tumor control and improving treatment efficacy
(22).
The most common pathological type of colon cancer is
adenocarcinoma, accounting for >90% of all cases.
Adenocarcinomas can be further classified into various subtypes,
including well-differentiated, moderately differentiated and poorly
differentiated adenocarcinomas, among which the well-differentiated
type generally carries the most favorable prognosis (23). In the treatment of colon cancer,
systemic chemotherapy is crucial in addition to surgery. In the
present case, although the pathological type of the patient's colon
cancer was well-differentiated adenocarcinoma, the patient declined
chemotherapy after surgery, which led to the rapid development of
liver metastases. The initial treatment involved RFA for lesions in
the left hepatic lobe and TACE for lesions in the right hepatic
lobe, combined with two cycles of chemotherapy. However, following
these treatments, the patient failed to achieve complete tumor
remission and experienced complications. Subsequently, the patient
underwent HIFU as a local therapy while maintaining chemotherapy
treatment, which ultimately resulted in complete tumor remission.
It is notable that throughout the treatment course, the patient
received a total of six cycles of XELOX chemotherapy, which played
a crucial role in achieving the favorable final outcome. This also
suggests that the combination of HIFU with chemotherapy may produce
synergistic effects, thereby enhancing the efficacy of the
chemotherapy. For irregularly shaped tumors, HIFU enables
three-dimensional conformal complete ablation, offering advantages
over local ablation methods such as RFA or MWA, while also
demonstrating a superior safety profile. In treating multiple small
tumors, HIFU allows for greater precision under real-time
monitoring. For lesions adjacent to the diaphragm or major blood
vessels, HIFU can achieve complete tumor ablation without damaging
these critical structures (24).
With the application of new technologies and AI assistance, HIFU
treatment will become even more precise and efficient in the future
(25,26). The absence of tumor recurrence
during the long-term follow-up in the present case suggests that
HIFU may contribute to stimulating a systemic antitumor immune
response. The successful outcome of this integrated treatment
strategy provides new insights and directions for future cancer
therapy. Nevertheless, more rigorous, prospective and comparative
studies are needed to definitively establish the role of HIFU in
the management of CRLM.
Acknowledgements
Not applicable.
Funding
The present study was supported by the State Key Laboratory of
Ultrasound in Medicine and Engineering, College of Biomedical
Engineering, Chongqing Medical University, Chongqing, China (grant
no. 2023KFKT019).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YY, HZ and YS contributed equally to this work. YY
designed the study, provided recommendations for patient treatment
plans, and wrote the paper. HZ acquired patient imaging and
pathological images, and was responsible for data collection,
organization, and analysis, as well as contributing to the paper
writing. YS performed the HIFU treatment and follow-up of patients,
and participated in paper writing. FY conducted the chemotherapy
and follow-up of patients. DZ and YL assisted in the HIFU treatment
and follow-up of patients. GH is the corresponding author and was
responsible for designing the study, providing recommendations for
patient treatment plans, performing HIFU treatment for patients,
contributing to paper writing and overseeing the overall work of
the research team. All authors have read and approved the final
manuscript. YY, HZ, YS, FY, DZ, YL and GH confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This case report is retrospective in nature does not
harm the interests of the patients and ensures no disclosure of
patient information. The requirement for ethical approval was
waived by the Ethics Committee of Suining Central Hospital
(Suining, China).
Patient consent for publication
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (Ethics Committee of Suining Central Hospital) and
with the Helsinki Declaration. Written informed consent was
obtained from the patient for being included in the study and for
the publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Engstrand J, Nilsson H, Stromberg C, Jonas
E and Freedman J: Colorectal cancer liver metastases - a
population-based study on incidence, management and survival. BMC
Cancer. 18:782018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Zhong X, He X, Hu Z, Huang H, Chen
J, Chen K, Zhao S, Wei P and Li D: Liver metastasis from colorectal
cancer: Pathogenetic development, immune landscape of the tumour
microenvironment and therapeutic approaches. J Exp Clin Cancer Res.
42:1772023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sathe A, Mason K, Grimes SM, Zhou Z, Lau
BT, Bai X, Su A, Tan X, Lee H, Suarez CJ, et al: Colorectal cancer
metastases in the liver establish immunosuppressive spatial
networking between tumor-associated SPP1+ macrophages and
fibroblasts. Clin Cancer Res. 29:244–260. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chinese College of Surgeons, Section of
Gastrointestinal Surgery, Branch of Surgery, Chinese Medical
Association, Section of Colorectal Surgery, Branch of Surgery,
Chinese Medical Association, Colorectal Cancer Professional
Committee, Chinese Anti-Cancer Association and Colorectal Cancer
Professional Committee et al, . China guideline for diagnosis and
comprehensive treatment of colorectal liver metastases (version
2023). Zhonghua Wei Chang Wai Ke Za Zhi. 26:1–15. 2023.(In
Chinese). PubMed/NCBI
|
|
6
|
Cremolini C, Antoniotti C, Stein A,
Bendell J, Gruenberger T, Rossini D, Masi G, Ongaro E, Hurwitz H,
Falcone A, et al: Individual patient data meta-analysis of
FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as
initial therapy of unresectable metastatic colorectal cancer. J
Clin Oncol. Aug 20–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song W, Tiruthani K, Wang Y, Shen L, Hu M,
Dorosheva O, Qiu K, Kinghorn KA, Liu R and Huang L: Trapping of
lipopolysaccharide to promote immunotherapy against colorectal
cancer and attenuate liver metastasis. Adv Mater. 30:e18050072018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weber M, Lam M, Chiesa C, Konijnenberg M,
Cremonesi M, Flamen P, Gnesin S, Bodei L, Kracmerova T, Luster M,
et al: EANM procedure guideline for the treatment of liver cancer
and liver metastases with intra-arterial radioactive compounds. Eur
J Nucl Med Mol Imaging. 49:1682–1699. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mimmo A, Pegoraro F, Rhaiem R, Montalti R,
Donadieu A, Tashkandi A, Al-Sadairi AR, Kianmanesh R and Piardi T:
Microwave ablation for colorectal liver metastases: A systematic
review and pooled oncological analyses. Cancers (Basel).
14:13052022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orsi F and Varano G: Minimal invasive
treatments for liver malignancies. Ultrason Sonochem. 27:659–667.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Maio A, Alfieri G, Mattone M, Ghanouni
P and Napoli A: High-Intensity focused ultrasound surgery for tumor
ablation: A review of current applications. Radiol Imaging Cancer.
6:e2300742024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Padilla F, Foley J, Timbie K, Bullock TNJ
and Sheybani ND: Guidelines for immunological analyses following
focused ultrasound treatment. J Immunother Cancer. 11:e0074552023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheybani ND, Batts AJ, Mathew AS, Thim EA
and Price RJ: Focused ultrasound hyperthermia augments release of
glioma-derived extracellular vesicles with differential
immunomodulatory capacity. Theranostics. 10:7436–7447. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashar H, Singh A, Kishore D, Neel T, More
S, Liu C, Dugat D and Ranjan A: Enabling chemo-immunotherapy with
HIFU in canine cancer patients. Ann Biomed Eng. 52:1859–1872. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ning Z, Xie J, Chen Q, Zhang C, Xu L, Song
L and Meng Z: HIFU is safe, effective, and feasible in pancreatic
cancer patients: A monocentric retrospective study among 523
patients. Onco Targets Ther. 12:1021–1029. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li K, Gong Y, Qiu D, Tang H, Zhang J, Yuan
Z, Huang Y, Qin Y, Ye L and Yang Y: Hyperbaric oxygen facilitates
teniposide-induced cGAS-STING activation to enhance the antitumor
efficacy of PD-1 antibody in HCC. J Immunother Cancer.
10:e0040062022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu XH, Du JX, Zhu D, Ren SZ, Chen K and
Zhu HL: Recent research on methods to improve tumor hypoxia
environment. Oxid Med Cell Longev. 2020:57212582020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Tang Y, Du Y, Lin L, Zhang Z, Ou
X, Chen S, Wang Q and Zou J: Genetically engineered
bacteria-mediated multi-functional nanoparticles for synergistic
tumor-targeting therapy. Acta Biomater. 150:337–352. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mauri G, Nicosia L, Xu Z, Di Pietro S,
Monfardini L, Bonomo G, Varano GM, Prada F, Della Vigna P and Orsi
F: Focused ultrasound: tumour ablation and its potential to enhance
immunological therapy to cancer. Br J Radiol. 91:201706412018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ran LF, Xie XP, Xia JZ, Xie FL, Fan YM and
Wu F: Specific antitumour immunity of HIFU-activated cytotoxic T
lymphocytes after adoptive transfusion in tumour-bearing mice. Int
J Hyperthermia. 32:204–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mauri G, Orsi F and Sconfienza LM:
Systemic effects of local tumor ablation: Oncogenesis and antitumor
induced immunity. Radiology. 283:9232017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You Y, Wang Z, Ran H, Zheng Y, Wang D, Xu
J, Wang Z, Chen Y and Li P: Nanoparticle-enhanced synergistic HIFU
ablation and transarterial chemoembolization for efficient cancer
therapy. Nanoscale. 8:4324–4339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su F, Li J, Zhao X, Wang B, Hu Y, Sun Y
and Ji J: Interpretable tumor differentiation grade and
microsatellite instability recognition in gastric cancer using deep
learning. Lab Invest. 102:641–649. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Landgraf L, Bailis N, Unger M,
Jochimsen TH and Melzer A: Image-guided high-intensity focused
ultrasound, a novel application for interventional nuclear
medicine? J Nucl Med. 62:1181–1188. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luan S, Ji Y, Liu Y, Zhu L, Zhao H, Zhou
H, Li K, Zhu W and Zhu B: AI-powered ultrasonic thermometry for
HIFU therapy in deep organ. Ultrason Sonochem. 111:1071542024.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang K, Li Q, Xu J, Tang MX, Wang Z, Tsui
PH and Zhou X: Frequency-domain robust PCA for real-time monitoring
of HIFU treatment. IEEE Trans Med Imaging. 43:3001–3012. 2024.
View Article : Google Scholar : PubMed/NCBI
|