Introduction
Lung cancer remains the leading cause of
cancer-associated mortalities globally, with 2.48 million novel
cases and 1.81 million mortalities, recorded in 2022. A model
projection released by the International Agency for Research on
Cancer under the World Health Organization indicates that new cases
of lung cancer will increase by 41% by the year 2050, primarily
driven by population aging in China and India. Notably, the
nationwide low-dose computed tomography (CT) screening initiative
in China has increased the number of cases of early-stage diagnosis
by ~12% since 2023 (1–3).
Epidemiological data from 19 countries has revealed
that non-small cell lung cancer (NSCLC) accounts for 90.3% of all
cases of lung cancer (4). Although
surgical resection remains the main curative approach for
early-stage and resectable locally advanced NSCLC, postoperative
5-year survival rates remain suboptimal: These have been reported
to be 68% for stage IB and 36% for stage IIIA disease, respectively
(5). Although traditional adjuvant
chemotherapy has been reported to modestly reduce the risk of
recurrence, its clinical benefits appear to have reached a plateau,
since only a 5% improvement in 5-year survival rates has been
recorded (6), with additional
limitations including chemotherapy-associated toxicity and the
absence of validated predictive biomarkers. Notably, 30–55% of
patients experience locoregional recurrence or distant metastasis
postoperatively (7), underscoring
the key need for effective eradication of minimal residual disease
(MRD) and the activation of systemic immune surveillance to improve
outcomes.
The advent of immune checkpoint inhibitors (ICIs)
targeting programmed cell death protein-1 (PD-1)/programmed
death-ligand 1 (PD-L1) has revolutionized treatment paradigms for
advanced NSCLC (8). Key phase III
trials, including KEYNOTE-024 and CheckMate 227 (9,10),
have demonstrated notable overall survival (OS) benefits with ICI
monotherapy or combination regimens in metastatic settings. These
breakthroughs have prompted an investigation of immunotherapy in
different perioperative settings, aiming to remodel the tumor
immune microenvironment through neoadjuvant or adjuvant approaches
to achieve synergistic locoregional-systemic control.
Early exploratory studies, such as the LCMC3 and
CheckMate 159 studies (11,12), were able to establish the
feasibility of neoadjuvant immunotherapy. The landmark CheckMate
816 trial first demonstrated in a phase III setting that the use of
the checkpoint inhibitor nivolumab plus chemotherapy led to a
marked improvement in both the pathological complete response (pCR)
rates and event-free survival (EFS) rates compared with
chemotherapy alone in resectable NSCLC, marking the transition of
perioperative immunotherapy from the conceptual stage to clinical
practice (13).
Subsequent trials, including the KEYNOTE-671
(14) and AEGEAN (15) trials, further advanced combination
strategies, achieving pCR rates >30% and demonstrating durable
survival benefits with postoperative adjuvant immunotherapy.
Similar progress in adjuvant immunotherapy has emerged from the
IMpower010 trial, where treatment with atezolizumab led to an
improved 3-year disease-free survival (DFS) rate (increased to 60%)
compared with chemotherapy in PD-L1+ (≥1%) patients with
stage II–IIIA cancer (16), whereas
the KEYNOTE-091 trial established a DFS benefit for pembrolizumab
in PD-L1-unselected cases. These findings have been incorporated
into major guidelines, including those of the National
Comprehensive Cancer Network (NCCN) and the International
Association for the Study of Lung Cancer (IASLC) (17–19).
Nevertheless, several challenges persist in this
rapidly evolving field. First, the selection of biomarkers remains
contentious: The CheckMate 816 trial revealed marked pCR rate
disparities comparing between the PD-L1 ≥1% and PD-L1 <1%
subgroups (13), whereas the
KEYNOTE-091 trial revealed PD-L1-independent DFS benefits.
Secondly, safety optimization of combination strategies warrants
urgent attention, as exemplified by the 32% grade 3–4 adverse event
(AE) rate with dual immunotherapy that was observed in the
CheckMate 77T trial (20).
Furthermore, the limited efficacy of ICIs noted in epidermal growth
factor receptor (EGFR)/anaplastic lymphoma kinase (ALK)-altered
NSCLC needs to be followed up with mechanistic studies (21). Lastly, long-term follow-up data are
required to fully characterize the impact of immune-associated AEs
(irAEs) on perioperative outcomes (22).
Currently, the paradigm of perioperative management
is expanding toward integrated systemic approaches that target not
only tumor cells, but also the microenvironment of the host.
Increasing evidence has highlighted the pivotal role of systemic
inflammation and metabolic dysregulation in modulating treatment
efficacy; for example, an elevated neutrophil-to-eosinophil ratio
was reported to be associated with impaired OS [hazard ratio
(HR)=1.74] and progression-free survival (PFS; HR=1.53) rates
across malignancies, reflecting pro-tumorigenic inflammation that
may undermine immunotherapeutic responses (23). In addition, metabolic syndrome,
which is characterized by chronic inflammation and insulin
resistance, has been reported to increase cancer risk via adipokine
dysregulation and oxidative stress, thereby suggesting that
metabolic interventions may potentially act synergistically with
ICIs (24).
Concurrently, novel biomarkers are refining patient
stratification. The cachexia index, which integrates skeletal
muscle mass, albumin and neutrophil-associated lymphocytes,
predicts survival rates (OS, HR=2.03) independently of tumor stage,
thereby offering a multidimensional assessment of the
nutritional-inflammation status (25). Despite these emerging tools;
however, PD-L1, as a biomarker, retains its clinical relevance in
cases of advanced NSCLC: A high expression (≥50%) of PD-L1 remains
predictive of notably increased ICI responsiveness, warranting a
further exploration of its dynamic changes in perioperative
settings (26). These insights
align with other therapeutic advances that have recently been made;
for example, meta-analyses have confirmed that adjuvant PD-1/PD-L1
inhibitors reduce the recurrence-free survival (RFS) risk across
solid tumors (HR=0.72), thereby supporting their broader
application beyond PD-L1 selection (27).
Future research efforts should prioritize precision
medicine through the application of multi-omics approaches [for
example, single-cell sequencing and spatial transcriptomics
(28)] to decipher dynamic
tumor-immune microenvironment interactions, to develop circulating
tumor DNA (ctDNA)-based MRD monitoring systems and to explore novel
therapeutic combinations [for example, immunotherapy with targeted
agents (29) or epigenetic
modulators (30)] to overcome drug
resistance. Real-world evidence will be key to the validation of
treatment paradigms in special populations. Subsequently, the
present review will systematically examine recent advances that
have been made in perioperative immunotherapy through four
dimensions, namely clinical applications, combination strategies,
biomarker development and future challenges, with the aim of
providing information for evidence-based clinical decision-making
in the future.
Literature identification strategy
The present narrative review has compiled evidence
from pivotal clinical trials published between January 1, 2007 and
August 31, 2025, using the search terms ‘perioperative
immunotherapy’, ‘neoadjuvant/adjuvant’, ‘lung cancer’ and ‘clinical
trial’. Key sources included the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science
(https://www.webofscience.com) databases,
proceedings from major oncology conferences (for example, those
organized by American Society of Clinical Oncology (ASCO) (31–33)
and European Society for Medical Oncology (ESMO) (34) and IASLC (35,36)
and American Association of Cancer Research (AACR) (37) and the Clinical Trials website
(clinicaltrials.gov) (for ongoing
trials). Phase III randomized controlled trials (for example,
CheckMate816 and KEYNOTE-671) were prioritized, along with
practice-changing phase II studies that have informed recent
guideline updates (for example, the updates made to version 7.2025;
NCCN (18) and Neoadjuvant and
Adjuvant Treatments for Early-Stage Resectable NSCLC Consensus
Recommendations From IASLC (19).
In addition, emphasis was placed on perioperative outcomes
(regarding parameters such as the pCR and the EFS/OS rate),
biomarker correlates (PD-L1 and ctDNA) and the safety profiles of
immunotherapy combinations.
Theoretical basis and mechanism of
perioperative immunotherapy
The cancer-immunity cycle, a fundamental biological
process governing immune-mediated tumor recognition and
eradication, comprises seven sequential steps, namely tumor antigen
release, antigen presentation, T-cell activation, T-cell
trafficking to tumor sites, tumor-infiltrating T-cell recognition
of cancer antigens, tumor cell killing and the establishment of
immunological memory. ICIs primarily function by counteracting
immunosuppressive signals within the tumor microenvironment (TME),
thereby reactivating antitumor immune responses (Fig. 1) (38–44).
However, the seven steps in the cancer-immunity cycle of lung
cancer tumors are notably specific and different perioperative
immunotherapy approaches work through a range of different
mechanisms (Table I) (45–50).
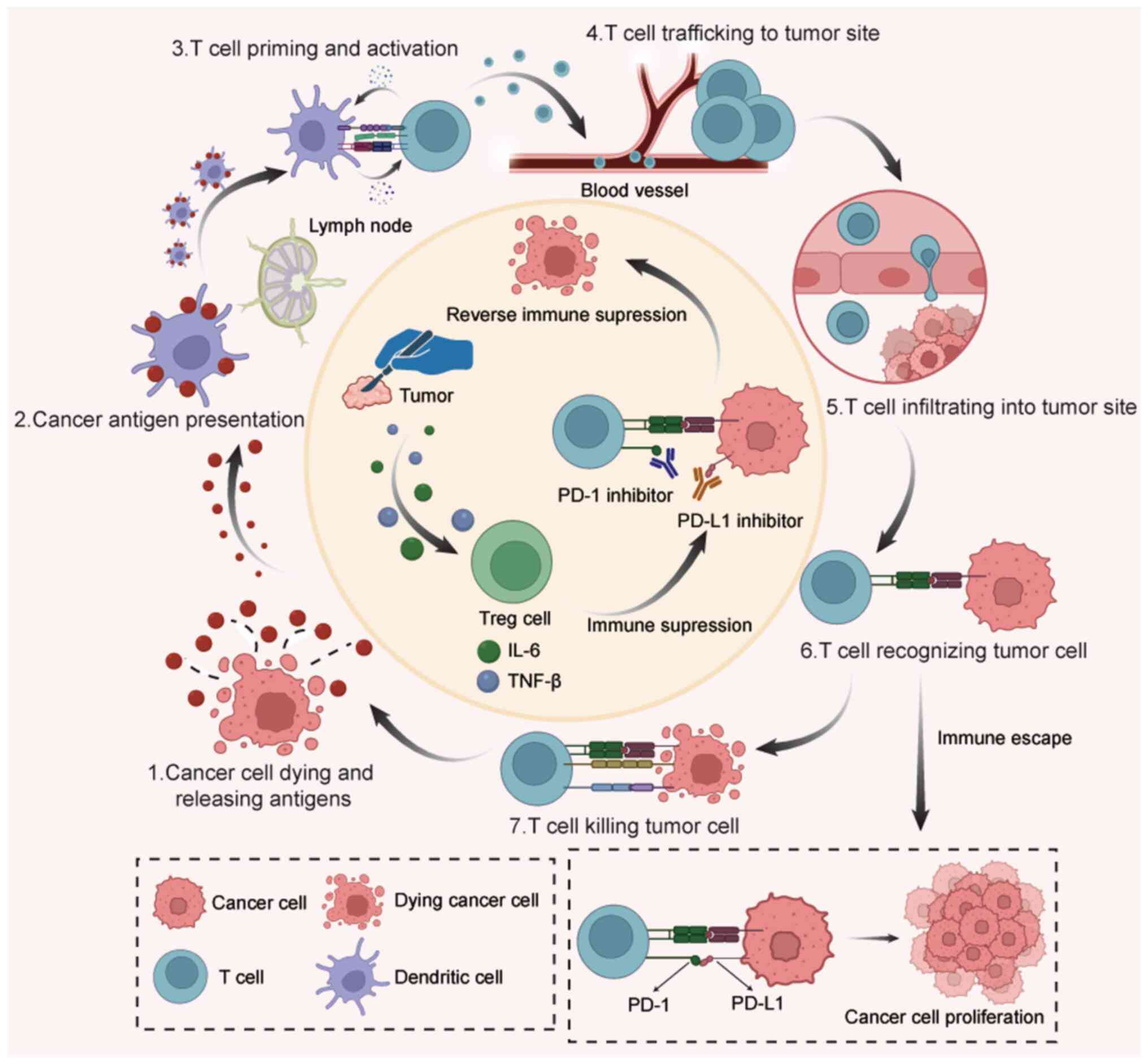 | Figure 1.Mechanism of tumor immune cycle. 1)
When tumor cells die, they release tumor antigens, which are then
captured by DCs in the lymph nodes. 2) Antigen presentation, where
dendritic cells process and present tumor antigens to naïve T
cells. 3) Priming and activation of naïve T cells: The naïve T
cells receive an antigenic signal (from dendritic cells) and a
co-stimulatory signal (e.g., CD28/B7), subsequently differentiating
into effector T cells. 4) After CD40L on the T-cell surface binds
to CD40 on the DC surface, this ‘reverse signaling’ potently acts
back upon the DC. This further upregulates the expression of
co-stimulatory molecules such as B7 on the dendritic cell, thereby
establishing a positive feedback loop for T cell activation. 5)
Migration of activated T cells: Effector T cells exit the lymph
node, enter the bloodstream and traffic towards the tumor site
guided by chemokine signals. 6) Under the influence of chemokines,
T cells first undergo firm adhesion to the vascular endothelium.
They then deform and transmigrate through the junctions between
endothelial cells to enter the tumor immune microenvironment. 7)
Process of T-cell recognition of tumor cells, wherein effector T
cells identify tumor-specific antigens presented on the major
histocompatibility complex molecules of the tumor cells through
their T cell receptors. 8) Cytotoxic killing of tumor cells by T
cells. Effector T cells induce cancer cell apoptosis either by
secreting cytotoxic mediators like perforin and granzymes, or by
activating the death receptor pathway such as Fas/FasL. 9) Lysis of
tumor cells that underwent apoptosis and the subsequent release of
new tumor antigens, indicating the initiation of a renewed
antitumor immunity cycle. 10) Surgery can lead to the formation of
an immunosuppressive microenvironment. The physical tissue damage
from surgical incision causes the release of Damage-Associated
Molecular Patterns (DAMPs); these signals recruit Tregs to the
surgical site and, in some cases, drive the conversion of naïve
CD4+ T cells into newly induced Tregs. This process establishes a
state of local immunosuppression that impedes the antitumor
immunity cycle. 11) Mechanism by which immunotherapy reverses
immune suppression. The co-inhibitory signaling pathway formed by
PD-1 and its primary ligand PD-L1 (B7-H1) impairs the proliferation
and cytokine production of effector T cells. PD-1 inhibitors
(anti-PD-1 antibodies) bind with high affinity to the PD-1 receptor
on T cells, whereas PD-L1 inhibitors (anti-PD-L1 antibodies) bind
to the PD-L1 ligand on tumor cells. This intervention physically
blocks the PD-1/PD-L1 interaction and subsequent downstream
signaling activation. Consequently, the co-stimulatory signals
received by T cells are fully transmitted and TCR signal
transduction is restored to normal. 12) Immunotherapy, primarily
utilizing PD-1/PD-L1 inhibitors, reverses the localized
immunosuppression previously caused by surgery. This enables T
cells to resume their cytotoxic function against tumor cells via
the cancer-immunity cycle, underscoring the critical importance of
perioperative immunotherapy in lung cancer. 13) Immune evasion by
tumor cells, whereby they avoid immune attack through various
immunosuppressive mechanisms (such as PD-L1 overexpression and
infiltration of regulatory T cells) or via antigen loss. 14)
Proliferative phase following tumor immune escape. Specifically,
PD-L1 on tumor cells binds to PD-1 on T cells, delivering an
inhibitory signal. This fosters an immunosuppressive
microenvironment that microenvironment that allows tumor cells to
evade T cell-mediated killing, leading to their uncontrolled
expansion uncontrolled expansion. PD-1, programmed cell death
protein-1; PD-L1, programmed death ligand 1; DC, dendritic cell;
Treg, regulatory T cell. |
 | Table I.Lung cancer-specific mechanism in the
cancer-immunity cycle and perioperative immunotherapy measure. |
Table I.
Lung cancer-specific mechanism in the
cancer-immunity cycle and perioperative immunotherapy measure.
| Method | Lung
cancer-specific mechanism | Perioperative
immunotherapy measure | (Refs.) |
|---|
| Antigen
release | In >30% of lung
adenocarcinomas, NSCLC-derived TGF-β/IL-10 suppresses antigen
release, restricting neoantigen availability | Neoadjuvant
immunotherapy combined with chemotherapy forced the release of
HMGB1/ATP by chemically destroying the structure of tumor cells and
inhibited the mitochondrial dysfunction caused by TGF-β signaling
and compensatory STK11 mutation, thus breaking through the release
barrier of lung cancer-specific antigens | (45,46) |
| Antigen
presentation | Dendritic cell
priming efficiency is reduced in >40% of NSCLC cases due to
impaired antigen presentation caused by HLA class I
downregulation | Neoadjuvant
immunotherapy triggers immuno therapy triggers IFN-γ release from
activated CD8+ T cells. This cytokine then upregulates
HLA-I expression on lung cancer cells via the JAK-STAT pathway,
thereby reversing the antigen presentation defect | (45,47) |
| T-cell
activation | Antitumor immunity
is often compromised by elevated Tregs and MDSCs in the lung tumor
microenvironment, particularly in EGFR-mutant lung cancer | During the
perioperative period, the combination of PD-1 and CTLA-4 inhibitors
promotes T-cell activation via two parallel actions: Blocking the
CTLA-4/CD80 axis to reduce Treg-mediated suppression and reversing
the impairment of dendritic cell cross-presentation commonly
associated with EGFR mutations | (48,49) |
| T-cell
transport | T-cell transport
into lung tumors is hindered by VEGF-mediated dysfunctional
vasculature and dysregulation of the CXCL12/CXCR4 axis in lung
cancer | To restore
efficient T-cell transport to tumors, perioperative immunotherapy
combined with anti-VEGF therapy normalizes the aberrant lung cancer
vasculature by inhibiting VEGF signaling and concurrently
counteracts the dysregulated CXCL12/CXCR4 chemotaxis axis | (46,47) |
| T-cell
infiltration | Lung
cancer-associated fibroblasts synthesize and secrete large amounts
of type III collagen, which encircles tumor cell clusters to form a
dense, enveloping structure that acts as a physical barrier,
thereby preventing CD8+ T-cell infiltration | Perioperative
immunotherapy or operative immunotherapy combined with a TGF-β
inhibitor blocked the TGF-β/SMAD pathway, suppressing collagen
synthesis and cross-linking in cancer-associated fibroblasts,
weakening the physical barrier of the tumor and ultimately
restoring CD8+ T-cell infiltration | (48,49) |
| Tumor
identification | Lung cancer cells
activate the Wnt/β-catenin signaling pathway, whose activation
inhibits the transcription factor NLRC5 (key for MHC-I), leading to
the downregulation of specific antigen presentation on the cell
surface | Perioperative
immunotherapy or operative immunotherapy combined with a CTLA-4
inhibitor blocks the CTLA-4/B7 signaling pathway in lymph nodes.
This action liberates naïve T cells from Treg-mediated suppression,
enabling their activation and clonal expansion against diverse
tumor antigens. Consequently, this broadens the TCR repertoire and
enhances the immune system's capacity to recognize a broader
spectrum of tumor antigens. | (45,49) |
| Killing of lung
cancer cells | The cytotoxic
function of T cells against lung cancer is suppressed through
Fas-L-mediated induction of T cell exhaustion and concomitant
inhibition of granzyme B. | Perioperative
immunotherapy combined with an IL-15 agonist enhances the
tumor-killing efficacy of CD8+ T cells against lung cancer by
activating the JAK3/STAT5 pathway. This activation rescues
Fas-L-mediated T cell depletion and augments granzyme B
expression. | (46,48,50) |
Clinical practice and evidence of
perioperative immunotherapy
Exploration of neoadjuvant monotherapy
immunotherapy
The exploration of ICIs in neoadjuvant therapy began
with early single-agent clinical studies, aimed at evaluating their
potential to induce pathological remission and assessing their
safety. Although neoadjuvant immunotherapy combined with
chemotherapy has become the predominant strategy, research on
neoadjuvant monotherapy immunotherapy continues to provide key
insights into the biological effects of immunotherapy and patient
selection.
In the CheckMate 159 trial (12), the major pathological response (MPR)
rate of nivolumab monotherapy was reported to reach 45% and
patients with a high tumor mutational burden (TMB) exhibited
notable pathological remission. Furthermore, the 5-year RFS risk
for PD-L1+ patients was reduced by 64% (HR=0.36),
whereas the MPR rate for PD-L1− patients was 30%,
demonstrating that PD-L1− patients could also derive
certain benefit from the therapy. Furthermore, the LCMC3 study
(11) further validated the
feasibility of neoadjuvant immunotherapy monotherapy. The MPR rate
of atezolizumab monotherapy was reported to be 20%, although this
increased significantly to 33% in the PD-L1 ≥50% subgroup compared
with only 11% in the tumor proportion score (TPS) <1% group
(P=0.01). The MPR rate in the TMB ≥16 mutant/Mb subgroup was also
33%, whereas it was only 13% in the low TMB group (P=0.12),
highlighting the synergistic screening potential of dual markers.
Furthermore, compared with traditional neoadjuvant chemotherapy
studies that had an MPR rate of 19% and a treatment-related AE
(TRAE) rate of 57%, (51), the MPR
rate of neoadjuvant monotherapy immunotherapy increased to 20–45%,
with markedly reduced toxicity. The incidence of grade 3 TRAEs was
11–23%, which was notably lower than the 60% incidence of TRAEs
observed in neoadjuvant chemotherapy. Notably, the surgical delay
rate in the LCMC3 study was extremely low. Similarly, in the
NEOSTAR monotherapy group (52),
the MPR rate was 22%, while the pCR rate was 9%, both markedly
higher compared with those of patients receiving traditional
chemotherapy. However, there remained a gap in the efficacy of
monotherapy compared with the dual immunotherapy combination: For
example, the MPR rate of the nivolumab plus ipilimumab combination
increased to 38%, whereas that of the monotherapy group was only
22% and the dual immunotherapy combination also led to the
induction of higher levels of effector T cell infiltration, with a
50% increase in CD8+ T cells compared with neoadjuvant
immunotherapy monotherapy (P=0.033) (34,52–54).
In general, the pCR of the neoadjuvant monotherapy
immunotherapy was reported to be low (generally of the order of
<10-15%). Furthermore, the pCR was notably lower compared with
that of immunotherapy combined with chemotherapy; for example, the
MPR in the CheckMate 816 trial was reported to be 24%. It should be
noted that the majority of the single-agent studies had small
sample sizes and limited representativeness (Table II).
 | Table II.Clinical trials of neoadjuvant ICIs
for lung cancer. |
Table II.
Clinical trials of neoadjuvant ICIs
for lung cancer.
| First author,
year | Trial (NCT
Identifier) | Phase | Stage | No. of
patients | ICI | Primary
endpoint | pCR rate, % | MPR rate, % | Survival
outcomes | (Refs.) |
|---|
| Chaft et al,
2022 | LCMC3
(NCT02927301) | II | IB-IIIB | 181 | Atezolizumab | MPR | 6.80 | 20.40 | DFS rate, 72%; OS
rate, 82% | (11) |
| Forde et al,
2021 | CheckMate 159
(NCT02259621) | II | I–IIIA | 21 | Nivolumab | Safety | 10.00 | 45.00 | RFS rate, 60%; OS
rate, 80% | (12) |
| Forde et al,
2022 | CheckMate-816
(NCT02998528) | III | IB-IIIA | 358 | Nivolumab | EFS, pCR | 24.0 vs. 2.20 | 36.9 vs. 8.90 | EFS rate, 57 vs.
43%; OS rate, 78 vs. 64%; mOS, NR vs.73.7month | (13) |
| Besse et al,
2020 | PRINCEPS
(NCT02994576) | II | IA-IIIA | 30 | Atezolizumab | The rate of
patients without major toxicities or morbidities | 0 | 14.00 | NA | (34) |
| Zhu et al,
2022 | LungMate 002
(unregistered) | II | II–III | 50 | Toripalimab | MPR | 27.80 | 55.60 | NA | (57) |
| Wislez et
al, 2022 | IONFSCO
(NCT03030131) | II | IB-IIIA | 46 | Durvalumab | The complete
surgical resection rate | 7.00 | 19.00 | DFS rate, 78.3%; OS
rate, 89.1% | (58) |
| Lei et al,
2023 | TD-FOREKNOW
(NCT04338620) | II | IIIA or IIIB | 88 | Camrelizumab | pCR | 32.60 vs. 8.90 | 65.10 vs.
15.60 | EFS rate, 76.9 vs.
67.6%; DFS rate, 78.4 vs.71.7 % | (59) |
| Liu et al,
2023 | CTONG 1804
(NCT04015778) | II | IIA or IIIB | 48 | Nivolumab | MPR | 25 | 50 | NA | (60) |
| Shao et al,
2023 | neoSCORE
(NCT04459611) | II | IB-IIIA | 60 | Sintilimab | MPR | 19.2 vs. 24.1 | 26.90 vs.
41.40 | NR | (61) |
| Zhang et al,
2024 | neoSCORE II
(NCT05429463) | III | IIA-IIIB | 250 | Sintilimab | MPR | Ongoing | Ongoing | Ongoing | (62) |
| NA | LungMate-009
(NCT04728724) | II | III | 100 | Sintilimab | MPR | Ongoing | Ongoing | Ongoing | (63) |
Advances in neoadjuvant immunotherapy
combined with chemotherapy
At present, a combination of ICIs and chemotherapy
has become the standard regimen for the neoadjuvant treatment of
resectable NSCLC (55). This
strategy works by enhancing tumor antigen release via
chemotherapeutic-induced immunogenic cell death (ICD) and
synergistically activating systemic antitumor immune responses with
ICIs, thereby causing marked improvements in pathological remission
rates and long-term survival.
CheckMate 816 trial
Global studies have demonstrated that the pCR rate
of nivolumab in combined chemotherapy was 24%, markedly higher
compared with the 2.2% reported for the chemotherapy control group
and the MPR rate was 34%, also much higher compared with the MPR
rate of 12% recorded for the chemotherapy control group. Of note,
the pCR rate of Chinese patients was as high as 25% (13,56).
Furthermore, in the LungMate 002 study (50), the pCR rate for the neoadjuvant
toripalimab plus chemotherapy subgroup was reported to be as high
as 27.8% and the MPR rate was as high as 55.6%. Patients with high
baseline expression of chitinase 3-like protein 1 (CHI3L1)
[according to the immunohistochemical (IHC) score] exhibited
significantly improved pCR and survival outcomes (for OS, HR=0.17
and P=0.017), demonstrating that CHI3L1 can serve as an independent
predictive marker. By contrast, the pCR rate for neoadjuvant
immunotherapy alone was only 10–15%, whereas the pCR improvement
following neoadjuvant immunotherapy combined with chemotherapy was
notable (the combination therapy group achieved 27.8% pCR in
LungMate 002, while the pCR rate in the combination therapy group
was 24% in CheckMate 816), highlighting the enhanced efficacy of
the combined regimen.
Regarding surgical feasibility, in the CheckMate 816
study, the surgery rate for the immunotherapy combined with
chemotherapy group was reported to be 83.2% (compared with 75.4%
for the chemotherapy alone group) and more minimally invasive
surgeries were performed. Furthermore, in the LungMate 002 study,
65.8% of the 76% of the patients who were potentially resectable in
the neoadjuvant immunotherapy combined with chemotherapy group were
converted into operable patients, achieving a total R0 resection
rate of 100% (where ‘R0 resection’ refers to a surgical outcome
where all the cancerous tissue is removed). Additionally, the
administration of neoadjuvant immunotherapy combined with
chemotherapy achieved a lymph node downstaging rate of 63.9%,
suggesting that this regimen led to a marked improvement in
surgical feasibility via the reduction of tumor size and
downstaging.
In terms of safety, the incidence of grade 3–4 TRAEs
in the neoadjuvant immunotherapy combined with chemotherapy group
in the CheckMate 816 study was reported to be 33.5%, a percentage
that was similar to the reported percentage of 36.9% in the
chemotherapy alone group, and neoadjuvant immunotherapy combined
with chemotherapy did not lead to increases in the rates of
surgical complications or delays. In the LungMate 002 study, the
incidence of grade 3–4 TRAEs in neoadjuvant immunotherapy combined
with chemotherapy was reported to be 34%. Overall, compared with
monotherapy immunotherapy, the chemotoxicity of combined
chemotherapy was reported to be slightly higher, although its
safety may have been well controlled through standardized
management and this toxicity did not markedly affect surgical
safety. By contrast, monotherapy immunotherapy may lead to a higher
risk of tumor progression due to insufficient efficacy. Subgroup
analysis revealed that the pCR rate (partial response to curative
therapy) for patients with PD-L1 levels ≥1% in the CheckMate 816
trial was 32.6%, markedly higher compared with the pCR rate of
16.7% observed in the subgroup with PD-L1 levels <1%. In
summary, the administration of immunotherapy combined with
chemotherapy causes the release of tumor antigens, thereby
enhancing immune responses and compensating for the insufficient
immune response rate in neoadjuvant monotherapy, which provides a
focus in the field of neoadjuvant immunotherapy with more potential
benefits than drawbacks (Table II)
(11–13,34,57–63).
Synergistic effect of immunotherapy
combined with radiotherapy (RT)
The combined use of RT and ICIs is based on the
principle that RT induces ICD in tumor cells, leading to the
release of damage-associated molecular patterns, which, in turn,
enhance the antigen-presenting ability of dendritic cells (DCs).
ICIs subsequently further alleviate systemic immunosuppression,
creating an ‘in situ vaccine’ effect (64).
Key studies have validated the clinical potential of
this strategy. In the SQUAT trial (65), the MPR rate of the neoadjuvant
durvalumab combined with concurrent chemoradiotherapy (50 Gy) was
reported to be 63%, which was notably higher compared with the MPR
rate of 36.8% reported in the CheckMate 816 trial for neoadjuvant
immunotherapy plus chemotherapy. Furthermore, the pCR rate in the
SQUAT trial was 23%, similar to the 24% pCR rate noted in the
CheckMate 816 trial, indicating that neoadjuvant immunotherapy
combined with chemotherapy led to a notable enhancement in the
extent of pathological remission (65). In terms of long-term survival,
compared with traditional RT, the objective response rate (ORR) for
the KEYNOTE-799 regimen with concurrent chemoradiation therapy
(cCRT) plus pembrolizumab was 70.5–70.6%, whereas the ORR for the
NICOLAS regimen with cCRT plus nivolumab was 73.4%. Both regimens
were noted to have higher ORRs compared with the 35–55% range
observed in traditional chemoradiotherapy (66,67).
In terms of safety, the SQUAT trial reported a 48%
rate of grade 3–4 AEs (including only 1 case of fatality that was
associated with the treatment), which was higher compared with that
of chemotherapy alone or monotherapy immunotherapy. However, the
toxicity profile was reported to be similar to that of historical
Neoadjuvant Chemoradiotherapy data (68). Furthermore, the most notable AE
arising from neoadjuvant immunotherapy combined with RT was shown
to be radiation pneumonitis. The incidence of grade 3 or higher
pneumonia in the KEYNOTE-799 and NICOLAS combination regimens was
6.9% and up to 10%, respectively. By contrast, the incidence of
grade 3 or higher radiation pneumonitis in traditional
chemoradiotherapy is typically reported to be between 7 and 20%
(69). This suggests that the
addition of RT did not exceed the safety threshold. In terms of
surgical feasibility, all patients in the SQUAT trial completed
their surgeries on schedule, with no reports of surgery delays due
to RT.
Regarding the expression of PD-L1, in the
KEYNOTE-799 study, the ORR for patients with PD-L1 TPS <1% in
cohorts A and B was reported to be 66.7 and 71.4%, respectively,
whereas for those patients with PD-L1 ≥1% in cohorts A and B, the
ORR was 75.8 and 72.5%, respectively. This demonstrates that there
is minimal difference in efficacy among patients with different
expression levels of PD-L1. Although the SQUAT trial did not
subdivide the PD-L1 groups, the overall MPR rate was still as high
as 63%, indicating its broad-spectrum efficacy. However, although
the SQUAT trial demonstrated a high MPR rate of 63% for neoadjuvant
chemoradiotherapy combined with immunotherapy, the 2-year PFS rate
was only 43% and no notable improvements were observed in terms of
patient survival. This may suggest that, although RT enhances local
immune activation, it may not effectively eliminate MRD.
Additionally, the SQUAT trial did not report distant recurrence
rates, suggesting that RT might either induce T-cell exhaustion or
upregulate PD-L1, thereby weakening the systemic effects of
durvalumab and potentially affecting the long-term prognosis of
patients.
Postoperative adjuvant
immunotherapy
The results from IMpower010 (16), a randomized, multicenter, open-label
phase 3 trial, demonstrated that postoperative adjuvant
chemotherapy followed by sequential atezolizumab treatment led to a
notable reduction in the recurrence risk in the PD-L1 ≥50% subgroup
(DFS, HR=0.48; OS, HR=0.47). Compared with the Best Supportive Care
(BSC) group, which was reported to have a 5-year DFS rate of only
67.5%, the 5-year DFS rate for the adjuvant atezolizumab treatment
group increased to 84.8% and distant metastasis was well
controlled. The distant metastasis recurrence rate in the adjuvant
atezolizumab treatment group was reported to be 6.8% compared with
12.5% in the BSC group, with particularly good control exerted over
brain metastasis recurrence. Furthermore, in another study,
KEYNOTE-091 (17), treatment
postoperatively with the adjuvant pembrolizumab demonstrated DFS
benefits in the overall population (HR=0.76), although this did not
reach significance in the PD-L1 ≥50% subgroup (HR=0.82).
In terms of safety, the incidence of grade 3–4 irAEs
in the IMpower010 study was only 11%, compared with an incidence of
25% for cisplatin-associated grade 3–4 nausea/vomiting when
traditional adjuvant therapy was administered. This suggests that
adjuvant immunotherapy is both safer and more tolerable compared
with traditional adjuvant chemotherapy. In the KEYNOTE-091 trial,
although the incidence of grade 3–4 irAEs was higher (at 34%) among
patients receiving postoperative adjuvant immunotherapy, only 4
cases were reported to be associated with myocarditis or pneumonia.
This indicated that fatal irAEs are rare and that the majority of
irAEs are manageable, rendering them easier to manage compared with
the long-term toxicities associated with chemotherapy, such as
kidney damage (70) and hearing
loss (71). Notably, in both the
IMpower010 and the KEYNOTE-091 trials, administering sequential
immunotherapy following chemotherapy did not lead to any notable
increase in cumulative toxicity, supporting the synergistic model
of chemotherapy clearing the immunosuppressive microenvironment
combined with immunomodulation (16,17).
In addition to the aforementioned postoperative adjuvant
immunotherapy, clinical trials of postoperative adjuvant
immunotherapy in combination with other regimens, such as
INTerpath-002 and SKYSCRAPER-15, have gradually revealed their
application prospects (Table III)
(16,17,31,32,35–37,72–75).
 | Table III.Clinical trials of adjuvant ICIs for
lung cancer. |
Table III.
Clinical trials of adjuvant ICIs for
lung cancer.
| First author,
year | Trial (NCT
identifier) | Phase | Stage | No. of
patients | ICI | Primary
endpoint | Survival
outcomes | (Refs.) |
|---|
| Felip et al,
2021 | IMpower010
(NCT02486718) | III | IB-IIIA | 1,005 | Atezolizumab | DFS | Stage II–IIIA
(PD-L1 ≥1%) mDFS, NE vs. 35.3 months; Stage II–IIIA (all) mDFS,
42.3 vs. 35.3 months; Stage IB-IIIA (all) mDFS, NE vs. 37.2
months | (16) |
| O'Brien et
al, 2022 | KEYNOTE-091
(NCT02504372) | III | IB-IIIA | 1,177 | Pembrolizumab | DFS | Stage IB-IIIA
(PD-L1 ≥50%) mDFS, 67.0 vs. 47.6 months; Stage IB-IIIA (all) mDFS,
53.8 vs. 43.0 months | (17) |
| Spicer et
al, 2024 | INTerpath-002
(NCT06077760) | III | II, IIIA and
IIIB | 868 | V940 +
pembrolizumab | DFS | Ongoing | (72) |
| Garon et al,
2024 | CANOPY-A
(NCT03447769) | III | II–IIIB | 1,382 | Canakinumab | DFS | mDFS, 35.0 vs.
29.7months | (73) |
| Goss et al,
2024 | ADJUVANT BR.31
(NCT02273375) | III | IB-IIIA | 1,415 | Durvalumab | DFS | NA | (37) |
| Zhu et al,
2023 | DUBHE-L-304
(NCT05487391) | III | II–IIIB | 632 | QL1706 | DFS | Ongoing | (31) |
| Chaft et al,
2017 | ANVIL
(NCT02595944) | III | IBf-IIIA | 903 | Nivolumab | DFS and OS | Ongoing | (74) |
| Calvo et al,
2021 | NADIM-ADJUVANT
(NCT04564157) | III | IB-IIIA | 210 | Nivolumab | DFS | Ongoing | (32) |
| NA | SKYSCRAPER-15
(NCT06267001) | III | IIB-IIIB | 1,150 | Tiragolumab +
Atezolizumab | DFS | Ongoing | (75) |
| Peters et
al, 2021 | MERMAID-1
(NCT04385368) | III | II–III | 89 | Durvalumab | DFS | Ongoing | (35) |
| Spigel et
al, 2021 | MERMAID-2
(NCT04642469) | III | II–III | 30 | Durvalumab | DFS | Ongoing | (36) |
Adjuvant immunotherapy during the
perioperative period
The ‘sandwich model’ of perioperative immunotherapy
for lung cancer comprises a comprehensive strategy that includes
preoperative neoadjuvant immunotherapy combined with chemotherapy,
surgical resection and postoperative adjuvant immunotherapy to
consolidate treatment. This approach aims to activate the immune
system preoperatively to shrink tumors and to reduce the stage of
cancer, to achieve radical resection during surgery and to
continuously eliminate MRD postoperatively.
In the NEOTORCH trial (a randomized, double-blind,
placebo-controlled phase 3 trial), the triple therapy group
achieved a pCR rate of 24.8%, whereas in the RATIONALE-315 trial
(another randomized, double-blind, placebo-controlled phase 3
trial), this rate increased to 56% (76,77).
By contrast, in the LCMC3 study, the pCR rate for patients
receiving only immunotherapy as neoadjuvant treatment was only 6%
(11), and in the CheckMate 816
trial (13), the pCR rate for
patients receiving combined immunotherapy and chemotherapy as
neoadjuvant treatment was only 24%. Furthermore, in the KEYNOTE-671
study (14), the MPR rate for
patients treated with the triple therapy model reached 63.8%, in
the RATIONALE-315 trial it was 56% and in the NEOTORCH trial it was
48.5%; by contrast, in the CheckMate 816 study, the MPR rate for
patients receiving combined immunotherapy and chemotherapy as
neoadjuvant treatment was only 24%, and in the NEOSTAR study, the
MPR rate for the single-agent neoadjuvant immunotherapy group was
only 22%. This clearly demonstrates that, in terms of pathological
remission, the triple therapy model is markedly more effective
compared with monotherapy neoadjuvant treatment (13,52,76,77).
Additionally, from the perspective of surgical
feasibility, the surgical resection rate for patients treated with
the triple therapy model in the KEYNOTE-671 trial was as high as
93%; in the CheckMate 77T study, it was 89%; in the RATIONALE-315
study, it was 91%; whereas in the CheckMate 816 trial, the surgical
resection rate for patients receiving combined immunotherapy and
chemotherapy as neoadjuvant treatment was only 83.2%. According to
the subgroup analysis of CheckMate 816 trial, similarly to the
results in the single neoadjuvant setting, the sandwich model also
demonstrated a more notable benefit for patients with PD-L1 levels
of ≥1%. The EFS HRs were determined to be 0.52 and 0.58 in the
CheckMate 77T and KEYNOTE-671 trials, respectively. However, in the
subgroup with PD-L1 levels <1%, the HRs increased to 0.73 and
0.65, suggesting limited benefits for patients with PD-L1 levels
<1%. Nevertheless, the RATIONALE-315 study demonstrated that
patients with PD-L1 levels <1% still obtained certain benefit
(EFS, HR=0.57). This benefit was primarily due to the unique
mechanism of action of tislelizumab, whereby antibody-dependent
cell-mediated phagocytosis is reduced, thereby overcoming the
limitations of low PD-L1 expression and providing treatment
opportunities for these patients.
Notably, tislelizumab has been underutilized in
perioperative immunotherapy studies for lung cancer, with potential
for enhanced research on this ICI in later stages. Furthermore,
compared with the IMpower010 study, in which a simple adjuvant
therapy was administered, the survival benefits of the sandwich
model were more pronounced. In the NEOTORCH study, the EFS HR for
patients treated with the sandwich model was 0.40; in the
KEYNOTE-671 study, it was 0.59; and in the RATIONALE-315 study, it
was 0.56. By contrast, in studies of adjuvant immunotherapy alone,
the DFS HR for all patients receiving postoperative adjuvant
immunotherapy in the IMpower010 study was 0.79 and in the
KEYNOTE-091 study, the overall DFS HR was 0.76. Although the
primary endpoints of the sandwich model study and the adjuvant
postoperative treatment study differed, the EFS parameter includes
a broader range of events, such as local recurrence, distant
metastasis, second primary cancer and treatment-associated
mortality, rendering it more stringent compared with DFS, which
only includes recurrence/mortality. However, the sandwich model was
still reported to have an improved HR, demonstrating its ability to
comprehensively control tumor progression.
It should be noted that, although the sandwich model
exhibited a notably increased efficacy compared with simple
adjuvant and neoadjuvant immunotherapy, it may face greater
challenges in terms of safety management. In the tislelizumab group
of the RATIONALE-315 trial, the incidence of irAEs was reported to
be 40%, a percentage that was markedly higher than the incidence
rate of 18% for irAEs in the chemotherapy group. Furthermore, the
incidence of grade 3 or higher neutropenia in the tislelizumab
group was as high as 61% and these patients required close
monitoring and management. Additionally, administering long-term
adjuvant immunotherapy may increase the burden on patients. For
example, in the NEOTORCH study, the sandwich model treatment
regimen included treatment with 13 cycles of toripalimab. By
contrast, the treatment may be prematurely discontinued for certain
patients due to toxicity. For example, in the CheckMate 77T study,
35% of the patients did not complete the course of postoperative
adjuvant therapy due to intolerance to treatment-associated
toxicity (14,20,76,77).
In conclusion, perioperative immunotherapy, which
involves tumor shrinkage prior to surgery and continuous
immunosuppression following surgery, markedly outperforms
preoperative or postoperative adjuvant therapy in terms of
pathological remission and survival benefits. It is suitable for
most high-risk patients who are able to tolerate toxicity,
particularly in the cases of borderline resectable patients, to
improve surgical outcomes. For patients with PD-L1 levels ≥1%, it
is recommended to prioritize dual immunotherapy or combination
chemotherapy. These two treatment regimens were reported to achieve
marked pathological remission in the NEOSTAR and RATIONALE-315
studies. Even for the PD-L1− patients, the sandwich
model maintained its superiority compared with postoperative
adjuvant therapy; for example, in the CheckMate 77T trial, the EFS
HR for patients in the PD-L1 <1 group was only 0.65 (Table IV) (12,15,20,33,76–83).
 | Table IV.Clinical trials of ‘sandwich’
combination regimens for lung cancer. |
Table IV.
Clinical trials of ‘sandwich’
combination regimens for lung cancer.
|
|
|
|
|
|
|
| pCR rate, % | MPR rate, % |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author,
year | Trial (NCT
identifier) | Phase | Stage | No. of
patients | ICI | Primary
endpoint | EG | CG | EG | CG | Survival
outcomes | (Refs.) |
|---|
| Spicer et
al, 2024 | KEYNOTE-671
(NCT03425643) | III | II–IIIB | 797 | Pembrolizumab | EFS, OS | 18.1 | 4.0 | 30.2 | 11.0 | mEFS, 47.2 vs. 18.3
months; OS rate, 71.3 vs. 64.0% | (14) |
| Sorscher et
al, 2024 | CheckMate-77 T
(NCT04025879) | III | IIA-IIIB | 461 | Nivolumab | EFS | 25.3 | 4.7 | 35.4 | 12.1 | mEFS, NR vs. 18.4
months | (20) |
| Yue et al,
2025 | RATIONALE-315
(NCT04379635) | III | II–IIIA | 453 | Tislelizumab | MPR, EFS | 40.7 | 5.7 | 56.2 | 15.0 | mEFS, NE vs.
NE | (76) |
| Lu et al,
2024 | Neotorch
(NCT04158440) | III | II–III | 501 | Toripalimab | MPR, EFS | 24.8 | 1.0 | 48.5 | 8.4 | mEFS, NE vs. 15.1
months; OS rate, 81.2 vs. 74.3% | (77) |
| Provencio et
al, 2022 | NADIM
(NCT03081689) | II | IIIA | 46 | Nivolumab | PFS | 63.0 | – | 83.0 | – | PFS rate, 77.1 %;
OS rate, 81.9 % | (78) |
| Provencio et
al, 2023 | NADIMII
(NCT03838159) | II | IIIA-IIIB | 86 | Nivolumab | pCR | 36.8 | 6.9 | 52.6 | 13.8 | PFS rate, 67.2 vs.
40.9%; OS rate, 85.0 vs. 63.6% | (79) |
| Heymach et
al, 2023 | AEGEAN
(NCT03800134) | III | IIA-IIIB | 802 | Durvalumab | pCR, EFS | 17.2 | 4.3 | 33.3 | 12.3 | mEFS, NR vs. 25.9
months | (15) |
| Yue et al,
2025 | RATIONALE-315
(NCT04379635) | III | II–IIIA | 453 | Tislelizumab | MPR, EFS | 40.7 | 5.7 | 56.2 | 15.0 | mEFS, NE vs.
NE | (76) |
| NA | ORIENT-99
(NCT05116462) | III | IIB-IIIB | 800 | Sindilizumab | pCR, EFS | Ongoing | Ongoing | Ongoing | (80) |
| NA | KN035-CN-017
(NCT06123754) | III | IIIA-IIIB (N2) | 390 | Envafolimab | MPR, EFS | Ongoing | Ongoing | Ongoing | (81) |
| Romero Román et
al, 2021 | IMpower-030
(NCT03456063) | III | II–IIIB | 451 | Atezolizumab | EFS | Ongoing | Ongoing | Ongoing | (82) |
| Cascone et
al, 2022 | NeoCOAST-2
(NCT05061550) | II | II–IIIB | 490 | Arm 1, oleclumab +
Durvalumab; Arm 2, monalizumab + durvalumab; Arm 3, Volrustomig;
Arm 4, Dato-DXd + durvalumab; Arm 5, AZD0171 + durvalumab | pCR, AEs, SAEs | Ongoing | Ongoing | Ongoing | (33) |
| Yan et al,
2023 | SHR-1316-111-303
(NCT04316364) | III | II–IIIB | 537 | SHR-1316 | MPR, EFS | Ongoing | Ongoing | Ongoing | (83) |
As discussed previously, employing the sandwich
therapy regimen leads to a notable improvement in pathological
response rates and survival outcomes, although it also presents
greater safety challenges compared with administering monotherapy
with neoadjuvant immunotherapy. First, the sandwich therapy regimen
is associated with markedly higher rates of TRAEs, particularly
irAEs, compared with monotherapy. Furthermore, its extended
treatment duration markedly increases the risks of cumulative
toxicity, leading to its discontinuation during the adjuvant
immunotherapy phase, thereby imposing heavier financial burdens on
patients. The current consensus recommends neoadjuvant
immunotherapy combined with chemotherapy for all patients with
operable stage II–III lung cancer. However, whether postoperative
immunotherapy should be upgraded to sandwich therapy remains a
subject of ongoing debate (19,55,84).
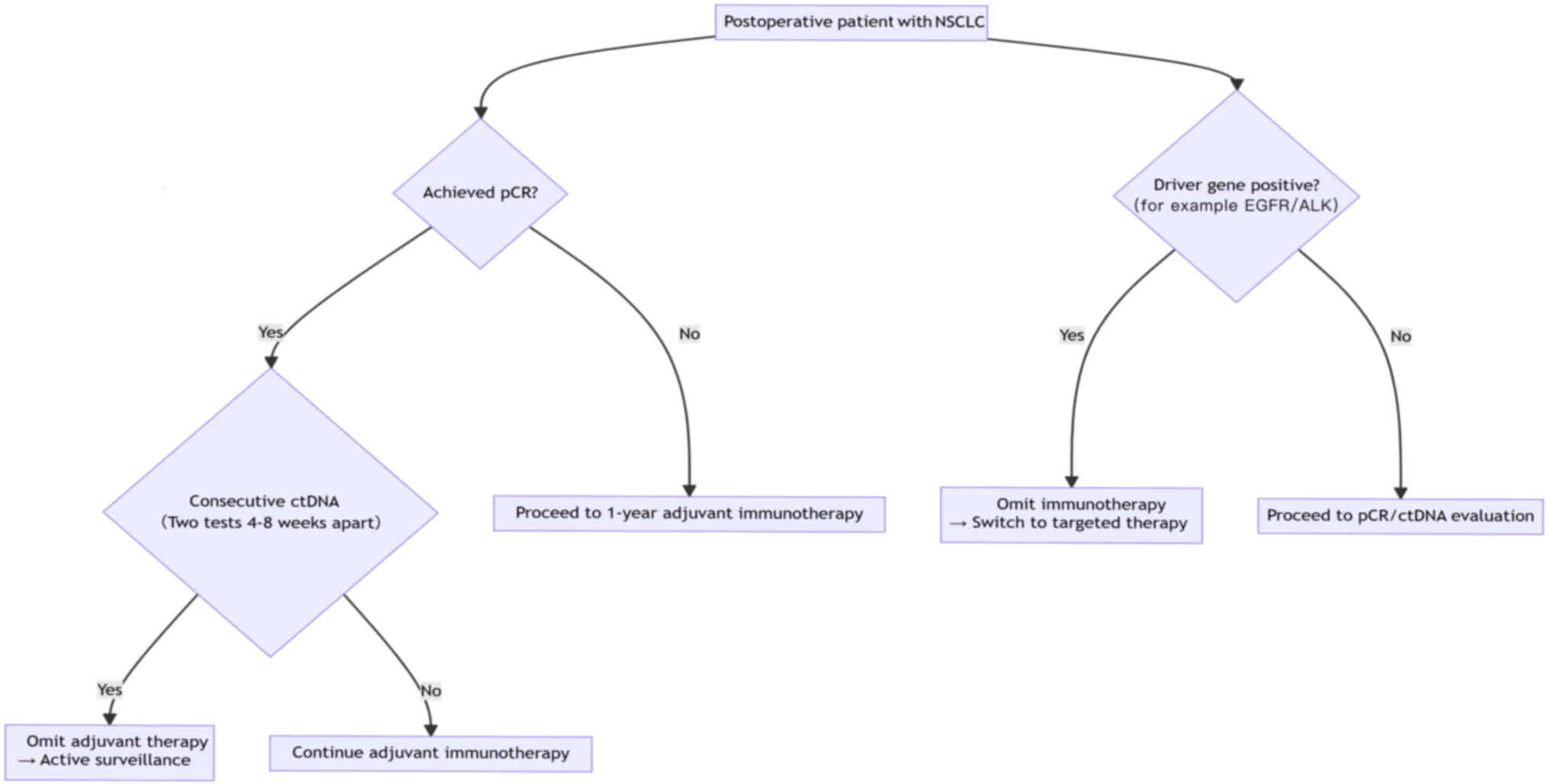
Therefore, the findings of recent studies on perioperative
immunotherapy for lung cancer have led to the development of the
clinical decision pathway for omission of adjuvant immunotherapy in
NSCLC (Fig. 2). The core criteria
for pathogenesis-guided adjuvant immunotherapy are pCR with
postoperative sustained ctDNA negativity or switching to targeted
therapy in driver gene-cases. High-risk patients (namely, those
with stage III lung cancer, non-pCR patients and those with high
expression of PD-L1) should still complete standardized 1-year
adjuvant therapy; however, it must be emphasized that, even in
terms of the pCR, patients with squamous cell carcinoma still face
a 5-year recurrence risk >30%. Therefore, maintaining full
adjuvant therapy is recommended. The current strategy faces
challenges due to limitations in pCR assessment (at present, ~8% of
micro-metastases are missed) and variations in ctDNA detection
sensitivity (currently, the false negative rate is in the range
5–10%). Thus, decision-making regarding exemption requires
multidisciplinary team consultation (84–88).
Biomarkers
Biological markers perform a key role in
perioperative immunotherapy, which is aimed at identifying
potential beneficiaries, optimizing treatment decisions and
predicting the risk of drug resistance. Currently, the most
extensively studied markers are PD-L1 expression, TMB and dynamic
monitoring of ctDNAs. However, their clinical application faces
numerous challenges. Emerging biomarkers, such as Gene Expression
Omnibus (GEO) profiles and tumor-infiltrating lymphocytes (TILs),
are beginning to gain prominence.
Traditional biomarkers
In perioperative immunotherapy for lung cancer,
PD-L1, ctDNAs and TMB each have unique value as biomarkers,
although each of them has its limitations. PD-L1 expression is the
most widely used biomarker. The CheckMate 816 study demonstrated
that patients with PD-L1 levels of ≥1% had a pCR rate of 32.6%. The
KEYNOTE-671 study further confirmed that patients with high PD-L1
expression (TPS ≥50%) received more notable survival benefits.
Specifically, in patients treated with the sandwich model, those
with low PD-L1 expression (TPS <50%) had an EFS HR of 0.61,
whereas those with high PD-L1 expression had an EFS HR of only 0.41
(13,14). However, in the NEOTORCH study, no
clear association was identified between EFS benefit and PD-L1
expression. Although the EFS HR values for the subgroup with PD-L1
levels ≥50% and the sandwich model treatment subgroup with a PD-L1
level <50% were 0.31 and 0.35 respectively, neither of these
subgroups reached the level of statistical significance, suggesting
that PD-L1 expression levels may not serve as an independent
predictor of efficacy (77).
Furthermore, the spatiotemporal heterogeneity of PD-L1 limits its
predictive power, with temporal heterogeneity indicating that PD-L1
expression may vary dynamically with treatment. For example,
preoperative neoadjuvant therapy may alter the TME, leading to
changes such as TILs and the release of inflammatory factors. This
can result in the measured postoperative PD-L1 expression levels in
specimens being inconsistent with the baseline levels of patients,
thereby reducing the predictive value of PD-L1. The spatial
heterogeneity is evident in notable differences in PD-L1 expression
being reported among different regions within the same tumor or
between the primary lesion and metastatic sites. Therefore, biopsy
specimens may only reflect local expression status, rather than the
overall tumor characteristics (89).
As biomarkers, ctDNAs have performed particularly
well with respect to MRD monitoring. The CheckMate 816 subgroup
analysis revealed that patients who tested positive for ctDNA
post-surgery had an EFS event rate of 72.1% within 12 months,
compared with an EFS event rate of only 14.3% for those who tested
negative. Furthermore, the exploratory analysis of the KEYNOTE-671
study indicated a positive association between ctDNA clearance and
the pCR rate following neoadjuvant immunotherapy. In the ctDNA
clearance group where ctDNA was undetectable post-treatment, the
pCR rate reached 46.8%, whereas in the non-clearance group, where
ctDNA remained detectable post-treatment, the pCR rate was reported
to be only 7.7% (11,12). Therefore, the ‘TNMB staging’ model,
combining ctDNA and TNM staging, was proposed in the MEDAL study,
in which the results reported that the model was capable of
improving the accuracy of recurrence prediction by 28% (90).
TMB is a surrogate indicator of tumor neoantigen
load. The CheckMate 227 study demonstrated that, among patients
with high TMB (≥10 mut/Mb), those treated with nivolumab plus
ipilimumab achieved a median PFS of 7.2 months, whereas it was only
5.5 months in those with low TMB (<10 mut/Mb; HR=0.58). This
finding suggests that TMB may serve as a potential predictor of
short-term efficacy for immune-combination therapy (10). However, several perioperative
studies lack a standardized TMB testing standard (10,11).
Whole exome sequencing, which covers ~30,000 genes, does provide a
high-precision assessment of TMB, although it is costly and
complex, making it difficult to implement the method widely. By
contrast, TMB may be inferred from lower-cost targeted panel
sequencing, which covers limited genomic regions (typically 1–2
Mb), using the application of fitting algorithms. However,
different panel designs can lead to biased results; for example,
panels with smaller coverage areas may underestimate TMB values.
Furthermore, the TMB thresholds have been reported to vary across
different detection platforms; for example, the
FoundationOne®CDx platform (Foundation Medicine) detects
tissue TMB (tTMB) with a preset threshold of 10, 13 or 16 mut/Mb
for tTMB, whereas a preset threshold of 20 mut/Mb is used for blood
TMB (bTMB). Although all methods are able to distinguish between
high and low TMB levels, inconsistent thresholds have been reported
to result in varying clinical benefits. Currently, whole-exome
sequencing (WES) and large-panel sequencing are the most commonly
used methods for measuring TMB. However, a bridging study involving
patients with NSCLC found that 199 missense mutations detected by
WES corresponded to a TMB of 10 mut/Mb measured using the
FoundationOne CDx (F1 CDx) panel. Although the overall consistency
between the two methods reached 84, ~16% of patients may still be
misclassified as having low or high TMB due to platform
differences. Furthermore, in Cohort C of the BFAST trial,
atezolizumab was compared with chemotherapy as first-line therapy
for patients with unresectable, advanced-stage NSCLC characterized
NSCLC characterized by a bTMB cutoff of ≥10 mut/Mb. Interestingly,
in an exploratory analysis where bTMB was evaluated using the F1L
CDx assay with an equivalent cutoff of bTMB ≥13.60 mut/Mb,
atezolizumab significantly improved PFS compared with chemotherapy.
These examples indicate that even within the same population,
differences in threshold settings across platforms may result in
some patients being unable to be accurately matched with the most
suitable treatment regimen (91,92).
Other emerging markers
Gene expression profiling (GEP) analysis comprises
an exploration of immune-associated gene expression patterns in the
TME using high-throughput sequencing technology, including the
analysis of biological markers such as the interferon-γ (IFN-γ)
signaling pathway, T cell activation-associated genes [including
CD8A and granzyme B (GZMB)] and immunosuppressive molecules [such
as TGF-β and indoleamine 2,3-dioxygenase 1 (IDO1)] (93). In the LungMate 002 study, a receiver
operating characteristic curve was constructed by comparing the IHC
scores of baseline CHI3L1 with the post-treatment pathological
remission status. The area under the curve was calculated to be
0.732, indicating that the IHC score of CHI3L1 in baseline tumor
samples can predict the treatment response. Patients with high
CHI3L1 expression were reported to have an OS HR of 0.17 and a PFS
HR of 0.29, suggesting that patients with high CHI3L1 expression
had improved survival outcomes. Even in patients with
PD-L1− expression, those with high CHI3L1 expression
still derived a notable benefit from perioperative immunotherapy,
suggesting that CHI3L1 was able to effectively address the
prediction ‘blind spot’ of PD-L1. This is partly because CHI3L1 may
influence matrix remodeling by regulating immune-suppressive cells
in the TME, thereby enhancing the synergistic effect of
chemotherapy combined with immunotherapy.
Furthermore, PD-L1 only reflects the expression of
immune checkpoints on the surface of tumor cells and does not fully
capture the overall immune activity in the TME. Additionally, in
the LungMate 002 study, a high expression of human leukocyte
antigen (HLA)-DR was observed in the response group. HLA-DR, a
class II major histocompatibility complex (MHC) class II cell
surface receptor, performs a key role in antigen presentation and
in the activation of CD4+ T cells. In this study, a
regimen of toripalimab combined with chemotherapy caused the
release of tumor antigens by administering chemotherapy, which,
when combined with PD-1 inhibitors to block immune checkpoints, led
to a synergistic enhancement of HLA-DR-mediated antigen
presentation, forming an immune positive feedback loop.
Furthermore, it was reported that CHI3L1 is positively associated
with HLA-DR expression, suggesting that CHI3L1 may indirectly
promote HLA-DR-mediated antigen presentation by regulating tumor
matrix remodeling (57). In
addition, in the NEOSTAR dual immunotherapy group, a notable
increase in GZMB upregulation was observed. This upregulation of
GZMB was reported to be associated with the expansion of effector
memory T cells (TEM) and tissue-resident memory T cells (TRM)
within tumors, suggesting that the upregulation of GZMB may enhance
T-cell function. Furthermore, patients with high GZMB expression
had a median viable tumor percentage of only ~9% compared with the
percentage of 50% observed in the single-agent immunotherapy group.
This suggested that GZMB-mediated T-cell toxicity is a key driver
of pathological remission. Additionally, in the dual immunotherapy
group, the mRNA levels of C-X-C motif chemokine ligand (CXCL) 9 and
CXCL10 were markedly elevated. In this study, IHC analysis was also
performed, which confirmed that the protein expression levels of
CXCL9 and CXCL10 in the tumor tissues were higher compared with
those of the dual immunotherapy group, particularly in patients
with pathological remission. This may have been due to ipilimumab
blocking the inhibitory signals of regulatory T cells (Tregs),
thereby enhancing the antigen-presenting capability of the DCs and
promoting the secretion of CXCL9/10 (67).
TILs are lymphocytes that migrate from the blood
into tumor tissues, where they serve as a key component of the TME.
These cells have a dual regulatory role in immune response through
their recognition of tumor antigens, which is key to the
development, progression and treatment of tumors. TILs primarily
include CD8+ T cells, CD4+ T cells, Tregs and
natural killer (NK) cells. In the CheckMate 159 study, it was
observed that, following immunotherapy, the number of
neoantigen-specific T cells in both tumor tissues and peripheral
blood were markedly increased. These increases were particularly
pronounced in patients who had achieved pCR, suggesting that
neoantigen-specific T cells may directly contribute to tumor
clearance. Additionally, in the NEOSTAR study, the combined
immunotherapy group exhibited a marked increase in the density of
CD8+ T cells infiltrating the tumor compared with the
monotherapy immunotherapy group. The proportions of TRM and TEM
were also increased, whereas the levels of Tregs and
myeloid-derived suppressor cells (MDSCs), which are
immunosuppressive cells, were decreased. Furthermore, in the LCMC3
study, it was reported that the density of Tregs and MDSCs in the
TME was negatively associated with the pathological response;
specifically, the enrichment of MDSCs was reported to be notably
associated with a low MPR rate for atezolizumab. In addition, it
was reported that immunoglobulin-like transcript 2 T cells were
also negatively associated with the MPR. The LCMC3 study also
demonstrated that the proportions of NK group 2 member D and
non-T/NK cells, including γδ T cells or congenital lymphocytes, in
baseline peripheral blood were positively associated with the MPR
(11,12) (Table
V) (32,36,75,79).
 | Table V.Predictive and prognostic biomarkers
in perioperative immunotherapy for NSCLC. |
Table V.
Predictive and prognostic biomarkers
in perioperative immunotherapy for NSCLC.
| Biomarker | Detection method
positive criteria | pCR/MPR
prediction | Survival
association | Clinical
utility | Limitations | (Refs.) |
|---|
| Inflammatory gene
signature | RNA-seq | pCR AUC=0.72 | OS HR=0.39 | 1. It fills the
prediction gap for patients with PD-L1 TPS <1% | 1. It requires
bioinformatics analysis and is difficult for clinical laboratories
to carry out | (32) |
|
|
|
|
| 2. Patients with
low expression of the inflammatory gene signature. Response may
require combination immunomodulators to enhance the
inflammatory | 2. There are no
large trials to confirm the clinical value of inflammatory gene
signatures |
|
| ctDNA
clearance | Tumor-informed
ctDNA clearance rate NGS ≥90% | pCR PPV= 92% | OS HR=0.33 | 1. It is emerging
MRD monitoring standards | 1.
Preoperative/postoperative paired testing is required to increase
the burden on patients | (36,75) |
|
|
|
|
| 2. It is an early
predictor of the efficacy of perioperative immunotherapy |
|
|
|
|
|
|
| 2. High detection
costs |
|
|
|
|
|
|
| 3. Patients with
undetected ctDNA may require enhanced adjuvant therapy |
|
|
| TILs density
(CD8+) | Multiplex IHC TILs
≥50 per HPF | MPR OR=4.2 | EFS HR=0.44 | 1. Patients with
high TILs density had an improved response to immunotherapy among
different pathologists | 1. There are
differences in the evaluation of TILs density | (36,75) |
|
|
|
|
| 2. Patients with
low TILs density may require combination of Immunotherapy and
chemotherapy | 2. Need for
multiple biopsies, low patient acceptance |
|
| HLA genotype | NGS (HLA-I/II
type)- | HLA-A*02:0; pCR
OR=3.1 | HLA-I
heterozygosity missing DFS HR=0.51 | 1. It can be used
as a personalized prediction for Asian patients | 1. Predictive value
is ethnically different | (36) |
|
|
|
|
| 2. It differences
may be the reason for different immune responses in different
patients | 2. HLA typing
requires special laboratory resources |
|
| TMB | WES/NGS TMB ≥10
mut/Mb | pCR AUC=0.59 | Limited data | 1. It fills the
prediction gap for patients with PD-L1 TPS <1% | 1. High detection
costs | (36) |
|
|
|
|
| 2. Patients with
high TMB may benefit from combination of immunotherapy and
chemotherapy | 2. Different
platforms have different testing standards |
|
|
|
|
|
| 3. Weak predictive
power |
|
|
| PD-L1 TPS | IHC PD-L1
TPS≥1% | pCR OR=2.1 (95% Cl,
1.6–2.8) | DFS HR=0.62 | 1. It is NCCN level
1 evidence | 1. Time
heterogeneity | (36,79) |
|
|
|
|
| 2. It can guide the
choice of immunotherapy monotherapy or immunotherapy combined with
chemotherapy | 2. Spatial
heterogeneity |
|
In addition to the aforementioned GEP analyses and
TILs, the NEOSTAR study also demonstrated that the abundance of
Ruminococcus and Akkermansia in the gut microbiota is
notably associated with the MPR of dual immunotherapy. This finding
suggests that specific bacterial populations may enhance antitumor
effects by modulating systemic immune responses (45), indicating that these specific
bacterial populations may have notable potential as biomarkers for
perioperative immunotherapy.
Application model of biomarkers in
perioperative immunotherapy for lung cancer
Current evidence suggests that integrated biomarker
models are able to enhance prediction accuracy (94). Therefore, our research group
developed a dynamic monitoring model for perioperative
immunotherapy in lung cancer that works through integrating
mainstream non-treatment biomarkers.
Neoadjuvant
First, ctDNA clearance rates were assessed in
patients with advanced unresectable lung cancer after one cycle of
neoadjuvant immunotherapy. Those achieving clearance continued with
the original treatment regimen, whereas those without clearance
either received an additional cycle of neoadjuvant immunotherapy or
switched to immunotherapy combined with chemotherapy (95).
Surgery
During the 4 weeks preceding surgery, changes in the
density of the TILs were continuously monitored. Patients with
increases in the number of CD8+ TILs of ≥30% were
scheduled for surgery as planned, whereas those with less marked
increases received either adjuvant RT or targeted therapy. Due to
the spatial heterogeneity in tumor biomarker expression (Table VI), intraoperative multi-region
sampling (core tumor area and peripheral zone) combined with
frozen-section pathology and IHC analysis was performed to stratify
patients for risk assessment and guide adjuvant therapy decisions.
High-risk patients (who were core MRD+ with peripheral
zone immunosuppression) were recommended adjuvant immunotherapy
combined with chemotherapy to enhance the clearance of residual
lesions in the core area. By contrast, low-risk patients (who were
core MRD− with peripheral zone immune activation) were
advised to receive adjuvant immunotherapy alone in order to reduce
chemotherapy toxicity (96).
 | Table VI.Microenvironmental, biomarker and
clinical differences in tumor core zone and tumor edge zone. |
Table VI.
Microenvironmental, biomarker and
clinical differences in tumor core zone and tumor edge zone.
| Region | TME
characteristics | Differences in
biomarkers | Clinical
implications |
|---|
| Tumor core
zone | Tumors are
characterized by high cellular density, sparse vasculature and
limited oxygen supply | High PD-L1
expression is predominantly found on tumor cells, concurrent with a
low density of CD8+ TILs in the TME | Immune inhibitory
microenvironment has poor response to immunotherapy |
| Tumor edge
zone | Tumors are
characterized by low cellular density, a well-vascularized network
and ample oxygen supply. | High PD-L1
expression is predominantly found on immune cells, concurrent with
a high density of CD8+ TILs in the TME | Immune activation
microenvironment has a good response to immunotherapy |
Adjuvant
Thoracic drainage fluid ctDNA testing was performed
within 24 h postoperatively. If patients were reported to be
positive (tumor fraction ≥0.01%), early adjuvant therapy was
initiated within 72 h. Additionally, TME spatial mapping was used
to evaluate postoperative margins. If the number of CD8+
TILs were <100 cells/high-power field at the invasive margins,
high-risk areas were marked to guide postoperative RT target
design. At 4 weeks post-surgery, plasma ctDNA levels could be used
to assess the MRD status. If two consecutive tests exhibit negative
results, the adjuvant immunotherapy cycle may be shortened from the
standard 16 cycles to 8 cycles. Lastly, patients should undergo
regular soluble PD-L1 (sPD-L1) level testing every 3 months. If the
sPD-L1 level should increase by 2 times or more compared with
baseline values, the adjuvant therapy should be switched to a
combination of immunotherapy and anti-angiogenesis treatment [since
the increase in the sPD-L1 level is notably associated with
increased levels of VEGF in the TME (97)].
Progress in immunotherapy combined with
other treatments
The combined strategy of perioperative immunotherapy
should balance efficacy and safety, particularly considering the
impact of treatment on surgical feasibility, postoperative recovery
and long-term survival. The following section combines the latest
clinical studies to explore the potential and challenges of
different combined modes in perioperative therapy. Immunotherapy
with dual checkpoint inhibitors, via simultaneously blocking
multiple immune suppression pathways, including the PD-1/PD-L1 and
cytotoxic T-lymphocyte associated protein 4 (CTLA-4) pathways,
enhances the antitumor immune response, thereby helping to overcome
drug resistance to a certain extent. This strategy has been
validated in each of the Checkmate 227, Checkmate 568 and Checkmate
817 trials for its efficacy and safety in advanced NSCLC (10,98–100)
and its application in perioperative care is currently being
actively explored.
In the NEOpredict-Lung study (101), patients who received nivolumab
plus relatlimab as neoadjuvant therapy had an MPR rate of 30% and a
pCR rate of 17%. Patients in the PD-L1 ≥50% subgroup demonstrated
both a further pathological remission and a lower proportion of
residual tumor cells. By contrast, in the CheckMate 816 study, the
MPR rate for patients receiving neoadjuvant nivolumab plus
chemotherapy was only 35.4% and the pCR rate was 25.3% (13), demonstrating that the dual
immunotherapy regimen was able to achieve similar pathological
remission levels without chemotherapy. In the EAST ENERGY trial
(89), the dual immunotherapy
regimen demonstrated even more notable advantages, with an MPR rate
of 50% and a pCR rate of 25% for patients receiving neoadjuvant
pembrolizumab plus ramucirumab. Additionally, the dual
immunotherapy regimen also demonstrated promising results in terms
of surgical feasibility. In the NEOpredict-Lung study, patients who
received nivolumab plus relatlimab as neoadjuvant therapy achieved
a 100% surgical completion rate and a 95% R0 resection rate, with
no delays due to treatment toxicity. By contrast, in the CheckMate
77T study, the surgical completion rate for patients receiving
neoadjuvant nivolumab combined with chemotherapy was only 77.7% and
certain patients required adjustments to their surgical plans due
to bone marrow suppression.
Regarding the safety of the dual immunotherapy
regimen, in the NEOpredict-Lung trial, patients receiving nivolumab
plus relatlimab neoadjuvant therapy were reported to have only a
13% incidence of TRAEs of grade 3 or higher and experienced no
chemotherapy-associated toxicities (such as neutropenia).
Additionally, irAEs were mainly associated with thyroid
dysfunction, with a 40% incidence rate in the dual immunotherapy
group and rare serious events, such as pneumonia, which occurred in
<2% of cases. By contrast, in the CheckMate 77T study, the
incidence of TRAEs of grade 3 or higher was as high as 32.5%,
accompanied by notable chemotherapy-associated toxicities,
including a 10.1% incidence of neutropenia. Although the dual
immunotherapy regimen was associated with higher levels of
pathological remission in the PD-L1 level ≥50% group, it still
provided notable benefits to patients with PD-L1 levels <1%; for
example, in the NEOpredict-Lung study, the MPR rate for patients
with PD-L1 levels <1% was 16.7%. Overall, the dual immunotherapy
regimen avoids the risks of bone marrow suppression and
gastrointestinal toxicity associated with chemotherapy, reduces the
risk of postoperative infections and delayed recovery and is
particularly suitable for elderly or poorly tolerated patients.
Furthermore, the EAST ENERGY study demonstrated that
blocking ramucirumab with VEGFR-2 antagonists led to a reshaping of
the TME, an enhancement of CD8+ T-cell infiltration and
a prolongation of the duration of the immune response. However, the
double immunization regimen still needs to be investigated in phase
III trials for the purpose of directly comparing the double
immunization regimen with the combination of chemotherapy and
immunotherapy to further clarify its advantages (10,102).
At present, although no separate studies have been
published on perioperative immunotherapy combined with targeted
therapy for lung cancer, a potential synergistic antitumor
mechanism exists between immunotherapy and targeted therapy. The
MARIPOSA-2 study (103) evaluated
the efficacy of the EGFR-mesenchymal/epithelial transition factor
(MET) bispecific antibody amivantamab in combination with
chemotherapy and lazertinib in patients with advanced EGFR-mutated
NSCLC. The results demonstrated that the median PFS in the combined
treatment group reached 8.3 months, the median intracranial PFS was
12.8 months and the ORR was 63%. This data demonstrates that
amivantamab combined with targeted therapy and chemotherapy has
notable efficacy in treating advanced EGFR-mutated NSCLC,
suggesting the translational potential of immunotherapy combined
with targeted therapy in perioperative lung cancer treatment. In
particular, the enhanced intracranial activity of lazertinib may
prevent occult brain metastasis progression during surgery; in
addition, lazertinib inhibits the EGFR signaling pathway, thereby
reducing the levels of immune-suppressive cytokines such as IL-6
and VEGF, which has the effects of improving the TME and enhancing
the synergistic effect with immunotherapy. Furthermore, the
CheckMate 370 (104) and TATTON
(105) studies also explored the
potential of immunotherapy in combination with targeted therapy in
advanced NSCLC and the results obtained from these studies also
have the potential to be applied to perioperative immunotherapy in
lung cancer in the future.
Furthermore, novel immunomodulators target
non-PD-1/PD-L1 immune checkpoints to modulate key signaling
pathways in the TME, thereby offering a novel direction for
perioperative immunotherapy. The NeoTRACK study (106) explored the use of atezolizumab in
combination with the novel T cell immunoreceptor with Ig and ITIM
domains (TIGIT) inhibitor, tiragolimab, during perioperative care.
Tiragolimab is a novel monoclonal antibody that targets the TIGIT
receptor, which functions as an inhibitory immune checkpoint
receptor primarily expressed on the surface of activated T and NK
cells. By binding to the ligand CD155, which is highly expressed on
the surface of tumor and antigen-presenting cells, TIGIT transmits
inhibitory signals, leading to T-cell exhaustion and NK cell
dysfunction. However, a synergistic inhibitory effect also exists
between TIGIT and the PD-1/PD-L1 pathway. Blocking TIGIT helps
relieve the dual inhibition of T cells and NK cells, thereby
enhancing the antitumor immune response. In models of PD-1/PD-L1
inhibitor resistance, TIGIT inhibition has been reported to restore
the function of exhausted T cells, thereby overcoming immune
escape. This research is currently ongoing (106) and may potentially lead to
applications in perioperative immunotherapy for lung cancer in the
future.
Resistance
Although perioperative immunotherapy for lung cancer
has been reported to be effective and safe in the aforementioned
clinical trials, long-term resistance to immunotherapy remains a
notable concern, particularly for patients requiring postoperative
adjuvant immunotherapy. Generally, the mechanisms of immunotherapy
resistance can be categorized into primary and secondary modes of
resistance. The core of primary drug resistance lies in the ‘cold’
nature of the TME, characterized by insufficient immune cell
infiltration. This is primarily due to disordered tumor vascular
structures and defects in the secretion of chemokines such as
CXCL9/10, which prevent CD8+ T cells from effectively
infiltrating the tumor. Additionally, mutations or epigenetic
silencing in genes associated with antigen presentation, such as
β-2 microglobulin and transporter associated with antigen
processing 1/2, result in the absence of MHC class I molecule
expression, thereby creating a ‘blind spot’ for immune recognition
and leading to an immunosuppressive state against the tumor cells.
Furthermore, this immunosuppressive state is exacerbated by the
infiltration of immune-suppressing cells, such as Tregs and MDSCs.
These cells create an inhibitory TME by secreting cytokines such as
IL-10 and TGF-β and they continue to support tumor cell survival
through abnormal activation of the Wnt/β-catenin or PI3K/AKT
signaling pathways (107–109). If the tumor evades the initial
immune attack, secondary drug resistance will gradually develop.
Initially, drug-resistant subclones of the tumor achieve immune
escape through mutations in driver genes, such as the EGFR T790M
mutation and MET amplification or through epigenetic remodeling
that leads to the loss of neoantigens due to DNA hypermethylation.
Furthermore, tumor cells enhance glycolysis via the Warburg effect,
leading to lactic acid accumulation in the TME, which directly
inhibits T-cell function. Additionally, abnormal tryptophan
metabolism mediated by IDO1 depletes key amino acids in the local
TME, which further disrupts the antitumor immune response.
Epigenetic regulation either alters the accessibility of
immune-associated genes through chromatin modifications that are
mediated via histone deacetylases or dynamically regulates the
expression of immune checkpoints such as PD-L1 through non-coding
RNA networks, including circular RNA/N6-methyladenosine
modifications, thereby forming a multi-dimensional drug resistance
barrier. These mechanisms are interlinked, ultimately leading to a
decline in the clinical response to immunotherapy (Fig. 3) (110–112).
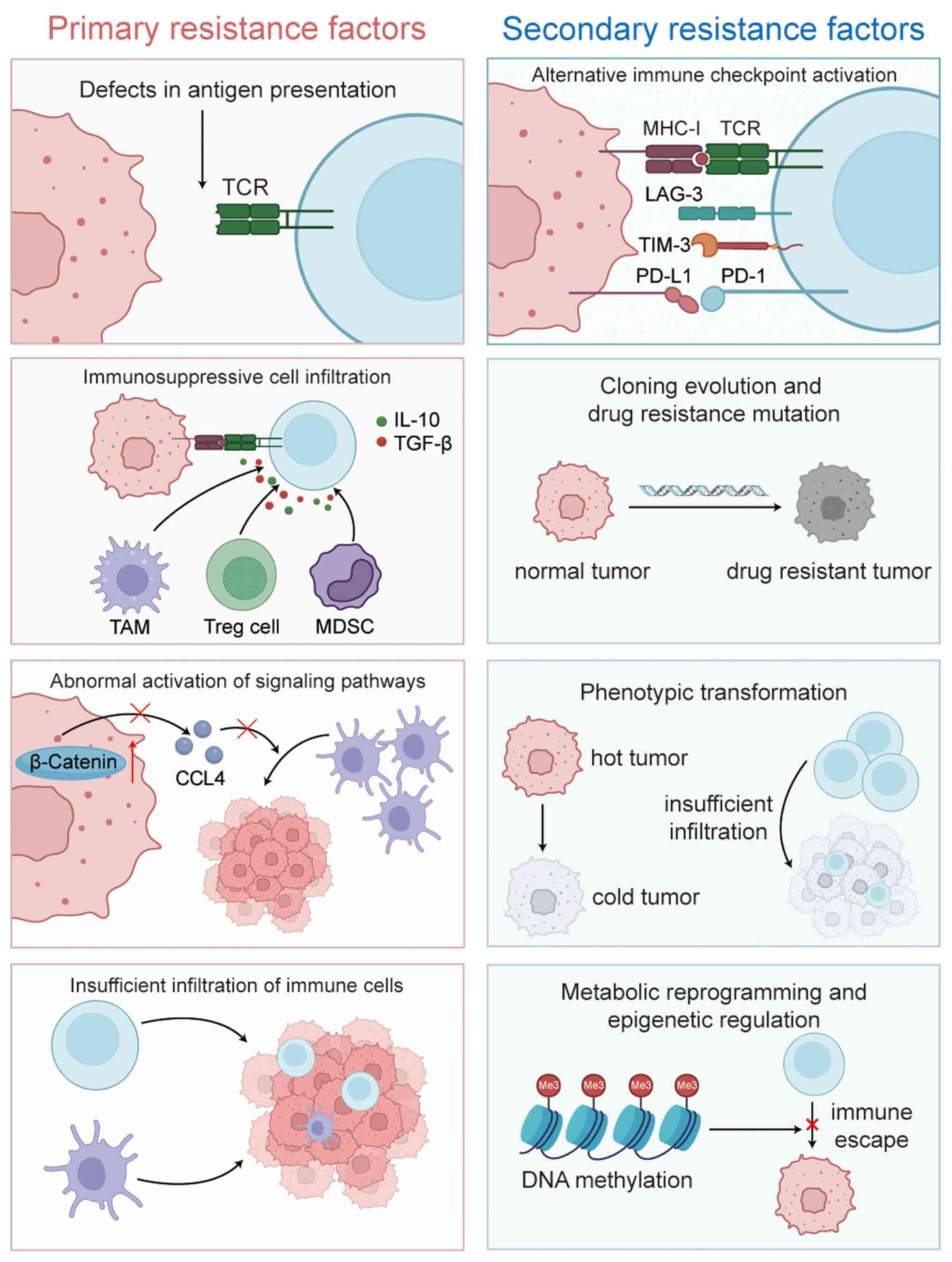 | Figure 3.Mechanism of tumor immune resistance.
TCR, T cell receptor; TAM, tumor-associated macrophages; Treg,
regulatory T cells; MDSC, myeloid-derived suppressor cells; PD-L1,
programmed death ligand 1; PD-1, programmed cell death protein-1;
LAG-3, lymphocyte-activation gene 3; TIM-3, T-cell immunoglobulin
and mucin-domain containing-3; MHC-I, major histocompatibility
complex class I; IL-10, interleukin-10; TGF-β, transforming growth
factor-β; CCL4, C-C motif chemokine ligand 4. |
Offering neoadjuvant dual immunotherapy for lung
cancer may be a viable approach to overcome drug resistance in the
future. The NEOSTAR study demonstrated that, in patients treated
with neoadjuvant nivolumab plus ipilimumab, tumor tissue samples
that were analyzed using multi-color immunofluorescence technology
exhibited a marked reduction in Treg cell infiltration. By
contrast, the density of Tregs in the neoadjuvant nivolumab
monotherapy group did not markedly change either prior to or after
treatment. These findings suggest that this neoadjuvant dual
immunotherapy approach effectively leads to an improvement in the
immune rejection state, possibly due to the high expression of
CTLA-4 on the surface of Treg cells. A combination of ipilimumab
and other CTLA-4 inhibitors may effectively deplete Treg cells,
thereby improving drug resistance (113). Furthermore, since the C-C motif
chemokine ligand 2-CC chemokine receptor 2 (CCL2-CCR2) signaling
axis is key to the functional specialization of MDSCs, clinical
studies have reported that CCR2 antagonists are able to reduce
infiltration of the MDSCs (114–116). At present, the LCMC3 study is
being followed up with novel research aimed at validating the use
of CCR2 antagonists in combination with PD-1 inhibitors for the
perioperative treatment of lung cancer (114). Additionally, lymphocyte activation
gene 3 (LAG-3), which is highly expressed in exhausted T cells,
binds to the T cell receptor-CD3 complex, leading to an inhibition
of T-cell activation by disrupting the interaction between
co-receptors and the Src kinase family member Lck, thereby
negatively regulating immune function. Clinical trials have
demonstrated that a combination of the LAG-3 inhibitor relatlimab
and the checkpoint inhibitor nivolumab is able to reverse T-cell
exhaustion (117–119). The team responsible for the
NEOSTAR study aims to further explore such combination therapies to
improve drug resistance (120).
Conclusions and perspectives
In conclusion, perioperative immunotherapy has
fundamentally reshaped the therapeutic paradigm for resectable
NSCLC. The present systematic analysis has revealed that multimodal
strategies, particularly the ‘sandwich approach’ (namely,
neoadjuvant chemoimmunotherapy to surgery to adjuvant
immunotherapy), demonstrate notably increased pathological response
parameters (pCR, 24.8–56 vs. ≤24% in the neoadjuvant-only patient
cohort) and survival benefits (EFS HR, 0.40–0.59 vs. 0.76–0.79 in
the adjuvant-only group). Notably, the present review has
established two key advancements: i) By integrating the current
traditional biomarkers and emerging biomarkers, a dynamic
monitoring model has been proposed to overcome the spatial and
temporal heterogeneity of PD-L1 and a biomarker-guided
perioperative immunotherapy regimen was also implemented to achieve
truly personalized treatment for patients; and ii) a clinical
decision pathway for the omission of adjuvant immunotherapy in
NSCLC: The sandwich protocol increases irAEs (≥3 irAEs:
chemotherapy group, 40 vs. 18%) and we propose a decision process
to determine whether adjuvant immunotherapy should be exempted
following neoadjuvant immunotherapy combined with chemotherapy.
Three main topics are involved here; first,
concerning biomarker optimization, PD-L1 thresholds remain
contentious (for example, the NEOTORCH trial demonstrated no EFS
association with PD-L1), whereas TMB standardization is impeded by
platform variability (for example, by comparing the
FoundationOne®CDx tTMB with bTMB thresholds). Second, as
far as oncogenic driver limitations are concerned, ICIs exhibit
minimal efficacy in EGFR/ALK-altered NSCLC, thereby necessitating
the development of novel combination therapies. Third, regarding
long-term irAE impacts, immune-associated sequelae (for example,
radiation pneumonitis in 10% of the patients in the NICOLAS trial)
require extended follow-up to define surgical/quality-of-life
trade-offs.
Four key areas require further exploration in terms
of the goal of working toward precision perioperative therapy. The
first future direction for research is dynamic biomarker
integration, whereby multi-omics models (spatial transcriptomics
plus ctDNA) will be developed to track real-time immune remodeling.
The second area is novel combination strategies; for example,
studying target immunosuppressive niches with TGF-β/IDO1 inhibitors
and strengthening antigen presentation via the use of HLA-DR
agonists (as validated in the LungMate 002 trial responders).
Thirdly, future studies should investigate personalized toxicity
mitigation, as in the case of target GEO signatures (for example,
IFN-γ pathway genes) to predict irAE susceptibility. Lastly,
real-world validation studies are necessary in order to establish
registries for special populations (for example,
elderly/oligometastatic populations) and to refine clinical
guidelines (such as the NCCN/ESMO guidelines).
Acknowledgements
Not applicable.
Funding
The present review was supported by the Science and Technology
Department of Yunnan Province, China (grant nos. 202401AY070001-147
and 202503AP140033); Kunming Medical University (grant nos.
QL-JBGS-14 and 2025S270).
Availability of data and materials
Not applicable.
Authors' contributions
GL, XM and HL conceived the present review. XM
reviewed the literature and drafted the manuscript. HL provided
financial support. XM, LL, HZ and ZL reviewed the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Jemal A, Sung H, Kelly K, Soerjomataram I
and Bray F: The Cancer Atlas, Fourth Edition. American Cancer
Society; Atlanta (GA), USA: pp. 1–5. 2025
|
|
3
|
Oncology Society of Chinese Medical
Association, . Chinese Medical Association guideline for clinical
diagnosis and treatment of lung cancer (2024 edition). Zhonghua Yi
Xue Za Zhi. 104:3175–3213. 2024.(In Chinese). PubMed/NCBI
|
|
4
|
Luo G, Zhang Y, Rumgay H, Morgan E,
Langselius O, Vignat J, Colombet M and Bray F: Estimated worldwide
variation and trends in incidence of lung cancer by histological
subtype in 2022 and over time: A population-based study. Lancet
Respir Med. 13:348–363. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE Collaborative Group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anile M, Diso D, Mantovani S, Patella M,
Russo E, Carillo C, Pecoraro Y, Onorati I, De Giacomo T, Rendina EA
and Venuta F: Uniportal video assisted thoracoscopic lobectomy:
Going directly from open surgery to a single port approach. J
Thorac Dis. 6 (Suppl 6):S641–S643. 2014.PubMed/NCBI
|
|
8
|
Liu L, Yan Y, Wang Y, Li Z, Yang L, Yu K
and Zhao Z: Comparative efficacy and safety of first-line
PD-1/PD-L1 inhibitors in immunotherapy for non-small cell lung
cancer: A network meta-analysis. Oncol Lett. 29:1572025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-Positive
Non-Small-Cell Lung Cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hellmann MD, Paz-Ares L, Bernabe Caro R,
Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A,
Lupinacci L, de la Mora Jimenez E, et al: Nivolumab plus Ipilimumab
in Advanced Non-Small-Cell Lung Cancer. N Engl J Med.
381:2020–2031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaft JE, Oezkan F, Kris MG, Bunn PA,
Wistuba II, Kwiatkowski DJ, Owen DH, Tang Y, Johnson BE, Lee JM, et
al: Neoadjuvant atezolizumab for resectable non-small cell lung
cancer: An open-label, single-arm phase II trial. Nat Med.
28:2155–2161. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spicer JD, Garassino MC, Wakelee H,
Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, et
al: Neoadjuvant pembrolizumab plus chemotherapy followed by
adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone
in patients with early-stage non-small-cell lung cancer
(KEYNOTE-671): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet. 404:1240–1252. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heymach JV, Harpole D, Mitsudomi T, Taube
JM, Galffy G, Hochmair M, Winder T, Zukov R, Garbaos G, Gao S, et
al: Perioperative durvalumab for resectable Non-small-cell lung
cancer. N Engl J Med. 389:1672–1684. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: IMpower010 Investigators. Adjuvant atezolizumab
after adjuvant chemotherapy in resected stage IB-IIIA
non-small-cell lung cancer (IMpower010): A randomised, multicentre,
open-label, phase 3 trial. Lancet. 398:1344–1357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Brien M, Paz-Ares L, Marreaud S, Dafni
U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling
M, et al: Pembrolizumab versus placebo as adjuvant therapy for
completely resected stage IB-IIIA non-small-cell lung cancer
(PEARLS/KEYNOTE-091): An interim analysis of a randomised,
triple-blind, phase 3 trial. Lancet Oncol. 23:1274–1286. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riely GJ, Wood DE, Aisner DL, Loo BW Jr,
Axtell AL, Bauman JR, Bharat A, Chang JY, Desai A, Dilling TJ, et
al: NCCN Guidelines® insights: Non-small cell lung
cancer, version 7.2025. J Natl Compr Canc Netw. 23:354–362. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spicer JD, Cascone T, Wynes MW, Ahn MJ,
Dacic S, Felip E, Forde PM, Higgins KA, Kris MG, Mitsudomi T, et
al: Neoadjuvant and adjuvant treatments for early stage resectable
NSCLC: Consensus recommendations from the international association
for the study of lung cancer. J Thorac Oncol. 19:1373–1414. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorscher S: Perioperative nivolumab in
resectable lung cancer. N Engl J Med. 391:5732024.PubMed/NCBI
|
|
21
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in Non-small cell lung
cancer: A Retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and Meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahin TK, Ayasun R, Rizzo A and Guven DC:
Prognostic value of Neutrophil-to-Eosinophil ratio (NER) in cancer:
A systematic review and Meta-analysis. Cancers (Basel).
16:36892024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vitale E, Rizzo A, Santa K and Jirillo E:
Associations between ‘Cancer Risk’, ‘Inflammation’ and ‘Metabolic
Syndrome’: A scoping review. Biology (Basel). 13:3522024.PubMed/NCBI
|
|
25
|
Bas O, Sahin TK, Karahan L, Rizzo A and
Guven DC: Prognostic significance of the cachexia index (CXI) in
patients with cancer: A systematic review and meta-analysis. Clin
Nutr ESPEN. 68:240–247. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rizzo A, Dall'Olio FG, Altimari A, Giunchi
F and Ardizzoni A: Role of PD-L1 assessment in advanced NSCLC: Does
it still matter? Anticancer Drugs. 32:1084–1085. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rizzo A, Mollica V, Marchetti A, Nuvola G,
Rosellini M, Tassinari E, Molina-Cerrillo J, Myint ZW, Buchler T,
Monteiro FSM, et al: Adjuvant PD-1 and PD-L1 inhibitors and
Relapse-free survival in cancer patients: The MOUSEION-04 study.
Cancers (Basel). 14:41422022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Zuani M, Xue H, Park JS, Dentro SC,
Seferbekova Z, Tessier J, Curras-Alonso S, Hadjipanayis A,
Athanasiadis EI, Gerstung M, et al: Single-cell and spatial
transcriptomics analysis of non-small cell lung cancer. Nat Commun.
15:43882024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amilo D, Izuchukwu C, Sadri K, Yao HR,
Hincal E and Shehu Y: A fractional-order model for optimizing
combination therapy in heterogeneous lung cancer: Integrating
immunotherapy and targeted therapy to minimize side effects. Sci
Rep. 14:184842024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munteanu R, Tomuleasa C, Iuga CA, Gulei D
and Ciuleanu TE: Exploring therapeutic avenues in lung cancer: The
epigenetic perspective. Cancers (Basel). 15:53942023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Y, Jiang T, Fang L, Zhang T, Kang X
and Zhou C: A randomized, doubleb linded, multicenter phase 3 study
of platinum-based chemotherapy with or without QL1706 as adjuvant
therapy in completely resected stage II–IIIb NSCLC. J Clin Oncol.
41:TPS86062023. View Article : Google Scholar
|
|
32
|
Calvo V, Domine M, Sullivan I,
Gonzalez-Larriba J, Ortega A, Bernabé R, Sala M, Campos B, De
Castro J, Martín-Martorell P, et al: A phase III clinical trial of
adjuvant chemotherapy versus chemoimmunotherapy for stage IB-IIIA
completely resected non-small cell lung cancer (NSCLC) patients
Nadim-adjuvant: New adjuvant trial of chemotherapy versus
chemoimmunotherapy. J Clin Oncol. 39:TPS85812021. View Article : Google Scholar
|
|
33
|
Cascone T, Spira A, Campelo R, Kim D,
Hamid O, Soo-Hoo Y, Kumar R, Grenga I and Forde P: Abstract CT124:
NeoCOAST-2: A randomized, open-label, phase 2 study of neoadjuvant
durvalumab plus novel immunotherapies and chemotherapy (CT)
followed by adjuvant durvalumab plus novel agents, in patients with
resectable non-small-cell lung cancer (NSCLC). Cancer Res.
82:CT1242022. View Article : Google Scholar
|
|
34
|
Besse B, Adam J, Cozic N, Chaput-Gras N,
Planchard D, Mezquita L, Masip J, Lavaud P, Naltet C, Gazzah A, et
al: Neoadjuvant atezolizumab (A) for resectable non-small cell lung
cancer (NSCLC): Results from the phase II PRINCEPS trial. Ann
Oncol. 31 (Suppl):S794–S795. 2020. View Article : Google Scholar
|
|
35
|
Peters S, Spigel D, Ahn M, Tsuboi M, Chaft
J, Harpole D, Goss G, Barlesi F, Abbosh C, Poole L, et al: P03.03
MERMAID-1: A Phase III study of adjuvant durvalumab plus
chemotherapy in resected NSCLC patients with MRD Post-surgery. J
Thorac Oncol. 16 (Suppl):S258–S259. 2021. View Article : Google Scholar
|
|
36
|
Spigel D, Peters S, Ahn M, Tsuboi M, Chaft
J, Harpole D, Barlesi F, Abbosh C, Mann H, May R, et al: MERMAID-2:
Phase III study of durvalumab in patients with resected, stage
II–III NSCLC who become MRD after curative-intent therapy. J Thorac
Oncol. 16 (Suppl):S745–S746. 2021. View Article : Google Scholar
|
|
37
|
Goss G, Darling GE, Westeel V, Nakagawa K,
Massuti Sureda B, Perrone F, McLachlan S-A, Kang JH, Wu Y-L,
Dingemans A-M.C..et al: LBA48 CCTG BR.31: A global, double-blind
placebo-controlled, randomized phase III study of adjuvant
durvalumab in completely resected non-small cell lung cancer
(NSCLC). Ann Oncol. 35 (Suppl):S12382024. View Article : Google Scholar
|
|
38
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Starzer AM, Preusser M and Berghoff AS:
Immune Escape mechanisms and therapeutic approaches in cancer: The
cancer-immunity cycle. Ther Adv Med Oncol.
14:175883592210962192022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mellman I, Chen DS, Powles T and Turley
SJ: The cancer-immunity cycle: Indication, genotype, and
immunotype. Immunity. 56:2188–2205. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chu NQ, Tan KS, Dycoco J, Adusumilli PS,
Bains MS, Bott MJ, Downey RJ, Gray KD, Huang J, Isbell JM, et al:
Determinants of successful minimally invasive surgery for
resectable non-small cell lung cancer after neoadjuvant therapy. J
Thorac Cardiovasc Surg. 169:753–762.e6. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyoshi T and Tsuboi M: Postoperative
adjuvant therapy with molecularly targeted agents for non-small
cell lung cancer. Int J Clin Oncol. 30:210–214. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim HK, Joung JG, Choi YL, Lee SH, Park
BJ, Choi YS, Ryu D, Nam JY, Lee MS, Park WY, et al: Earlier-phased
cancer immunity cycle strongly influences cancer immunity in
operable Never-smoker lung adenocarcinoma. iScience. 23:1013862020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karasaki T, Nagayama K, Kuwano H, Nitadori
JI, Sato M, Anraku M, Hosoi A, Matsushita H, Morishita Y,
Kashiwabara K, et al: An immunogram for the Cancer-immunity cycle:
Towards personalized immunotherapy of lung cancer. J Thorac Oncol.
12:791–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pio R, Ajona D, Ortiz-Espinosa S,
Mantovani A and Lambris JD: Complementing the Cancer-immunity
cycle. Front Immunol. 10:7742019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Pu H, Zhang X, Guo X, Li G and Zhang
M: Resistance to PD-1/PD-L1 immune checkpoint blockade in advanced
non-small cell lung cancer. Crit Rev Oncol Hematol. 209:1046832025.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang MY, Jiang XM, Wang BL, Sun Y and Lu
JJ: Combination therapy with PD-1/PD-L1 blockade in non-small cell
lung cancer: Strategies and mechanisms. Pharmacol Ther.
219:1076942021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vansteenkiste J, Wauters E, Park K,
Rittmeyer A, Sandler A and Spira A: Prospects and progress of
atezolizumab in non-small cell lung cancer. Expert Opin Biol Ther.
17:781–789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang B, Xiao H, Pu X, Zhou C, Yang D, Li
X, Wang W and Xiao Q: A real-world comparison between neoadjuvant
chemoimmunotherapy and chemotherapy alone for resectable non-small
cell lung cancer. Cancer Med. 12:274–286. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rosner S, Reuss JE, Zahurak M, Zhang J,
Zeng Z, Taube J, Anagnostou V, Smith KN, Riemer J, Illei PB, et al:
Five-year clinical outcomes after neoadjuvant nivolumab in
resectable Non-small cell lung cancer. Clin Cancer Res. 29:705–710.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang X, Yin R and Xu L: Neoadjuvant PD-1
blockade in resectable lung cancer. N Engl J Med.
379:e142018.PubMed/NCBI
|
|
55
|
Kim SS, Cooke DT, Kidane B, Tapias LF,
Lazar JF, Awori Hayanga JW, Patel JD, Neal JW, Abazeed ME, Willers
H, et al: The society of thoracic surgeons expert consensus on the
multidisciplinary management and resectability of locally advanced
Non-small cell lung cancer. Ann Thorac Surg. 119:16–33. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang C, Chen KN, Chen Q, Wu L, Wang Q, Li
X, Ying K, Wang W, Zhao J, Liu L, et al: Neoadjuvant nivolumab plus
chemotherapy versus chemotherapy for resectable NSCLC:
Subpopulation analysis of Chinese patients in CheckMate 816. ESMO
Open. 8:1020402023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu X, Sun L, Song N, He W, Xie B, Hu J,
Zhang J, Yang J, Dai J, Bian D, et al: Safety and effectiveness of
neoadjuvant PD-1 inhibitor (toripalimab) plus chemotherapy in stage
II–III NSCLC (LungMate 002): An open-label, single-arm, phase 2
trial. BMC Med. 20:4932022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wislez M, Mazieres J, Lavole A, Zalcman G,
Carre O, Egenod T, Caliandro R, Dubos-Arvis C, Jeannin G, Molinier
O, et al: Neoadjuvant durvalumab for resectable non-small-cell lung
cancer (NSCLC): Results from a multicenter study (IFCT-1601
IONESCO). J Immunother Cancer. 10:e0056362022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian
F, Liu H, Gu Z, Huang L, Lu Q, et al: Neoadjuvant camrelizumab plus
Platinum-based chemotherapy vs Chemotherapy alone for Chinese
patients with resectable stage IIIA or IIIB (T3N2) Non-small cell
lung cancer: The TD-FOREKNOW randomized clinical trial. JAMA Oncol.
9:1348–1355. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu SY, Dong S, Yang XN, Liao RQ, Jiang
BY, Wang Q, Ben XS, Qiao GB, Lin JT, Yan HH, et al: Neoadjuvant
nivolumab with or without platinum-doublet chemotherapy based on
PD-L1 expression in resectable NSCLC (CTONG1804): A multicenter
open-label phase II study. Signal Transduct Target Ther. 8:4422023.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shao M, Yao J, Wang Y, Zhao L, Li B, Li L,
Wu Z, Chen Z, Fan J and Qiu F: Two vs three cycles of neoadjuvant
sintilimab plus chemotherapy for resectable non-small-cell lung
cancer: NeoSCORE trial. Signal Transduct Target Ther. 8:1462023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang X, Shao M, Yao J, Zhao L, Li L, Chen
M, Zhang Y, Liu H, Chen Z, Li B, et al: NeoSCORE II: Three vs four
cycles of neoadjuvant sintilimab + chemotherapy for squamous
non-small-cell lung cancer. Future Oncol. 20:121–129. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
National Library of Medicine, .
Neoadjuvant Sintilimab or Combined With Chemotherapy for Stage III
Driven Gene Mutation Negative Non-small Cell Lung Cancer.
https://clinicaltrials.gov/study/NCT04728724August
8–2025
|
|
64
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hamada A, Soh J, Hata A, Nakamatsu K,
Shimokawa M, Yatabe Y, Suzuki J, Tsuboi M, Horinouchi H, Sakairi Y,
et al: Neoadjuvant concurrent Chemo-Immuno-radiation therapy
followed by surgery and adjuvant immunotherapy for resectable stage
III N2 NSCLC: Primary results from the SQUAT trial (WJOG 12119L). J
Thorac Oncol. 20:1098–1107. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jabbour SK, Lee KH, Frost N, Breder V,
Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A,
Houghton B, et al: Pembrolizumab plus concurrent chemoradiation
therapy in patients with unresectable, locally advanced, stage III
Non-small cell lung cancer: The phase 2 KEYNOTE-799 Nonrandomized
Trial. JAMA Oncol. 7:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Peters S, Felip E, Dafni U, Belka C,
Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, et
al: Safety evaluation of nivolumab added concurrently to
radiotherapy in a standard first line chemo-radiotherapy regimen in
stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung
Cancer. 133:83–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xu YP, Li B, Xu XL and Mao WM: Is there a
survival benefit in patients with stage IIIA (N2) Non-small cell
lung cancer receiving neoadjuvant chemotherapy and/or radiotherapy
prior to surgical resection: A systematic review and Meta-analysis.
Medicine (Baltimore). 94:e8792015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vokes EE, Herndon JE II, Kelley MJ,
Cicchetti MG, Ramnath N, Neill H, Atkins JN, Watson DM, Akerley W,
Green MR, et al: Induction chemotherapy followed by
chemoradiotherapy compared with chemoradiotherapy alone for
regionally advanced unresectable stage III Non-small-cell lung
cancer: Cancer and Leukemia Group B. J Clin Oncol. 25:1698–704.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hino A, Muto S, Shimada Y, Hori S, Isotani
S, Nagata M and Horie S: Impact of Cisplatin-induced acute kidney
injury on Long-term renal function in patients with solid tumors.
Clin Exp Nephrol. 27:506–518. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kessler L, Koo C, Richter CP and Tan X:
Hearing loss during chemotherapy: Prevalence, mechanisms, and
protection. Am J Cancer Res. 14:4597–4632. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Spicer J, Nair S, Khattak A, Lee J, Brown
M, Meehan R, Shariati N, Deng X, Samkari A and Chaft J: The phase 3
INTerpath-002 study design: Individualized neoantigen therapy (INT)
V940 (mRNA-4157) plus pembrolizumab vs placebo plus pembrolizumab
for resected early-stage non-small-cell lung cancer (NSCLC). Cancer
Res. 84:CT281. 2024. View Article : Google Scholar
|
|
73
|
Garon EB, Lu S, Goto Y, De Marchi P,
Paz-Ares L, Spigel DR, Thomas M, Yang JC, Ardizzoni A, Barlesi F,
et al: Canakinumab as adjuvant therapy in patients with completely
resected Non-Small-cell lung cancer: Results from the CANOPY-A
Double-blind, randomized clinical trial. J Clin Oncol. 42:180–191.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chaft J, Dahlberg S, Gerber D, Oxnard G,
Malik S, Simone C, Edelman M, Heymach J, Rudin C and Ramalingam S:
Adjuvant nivolumab in resected lung cancers (ANVIL): The newest
study in the ALCHEMIST platform. J Clin Oncol. 4:352017.
|
|
75
|
National Library of Medicine, . A Study of
Tiragolumab Plus Atezolizumab Compared With Placebo Plus
Atezolizumab in Participants With Completely Resected Non-small
Cell Lung Cancer Who Have Received Adjuvant Platinum-based
Chemotherapy (SKYSCRAPER-15). https://clinicaltrials.gov/study/NCT06267001August
8–2025
|
|
76
|
Yue D, Wang W, Liu H, Chen Q, Chen C, Liu
L, Zhang P, Zhao G, Yang F, Han G, et al: RATIONALE-315
investigators. Perioperative tislelizumab plus neoadjuvant
chemotherapy for patients with resectable non-small-cell lung
cancer (RATIONALE-315): An interim analysis of a randomised
clinical trial. Lancet Respir Med. 13:119–129. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu S, Zhang W, Wu L, Wang W, Zhang P, Fang
W, Xing W, Chen Q, Yang L, Mei J, et al: Perioperative toripalimab
plus chemotherapy for patients with resectable Non-small cell lung
cancer: The neotorch randomized clinical trial. JAMA. 331:201–211.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Provencio M, Serna-Blasco R, Nadal E, Insa
A, García-Campelo MR, Casal Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, et al: Overall survival and
biomarker analysis of neoadjuvant nivolumab plus chemotherapy in
operable stage IIIA Non-Small-Cell lung cancer (NADIM phase II
trial). J Clin Oncol. 40:2924–2933. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Provencio M, Nadal E, González-Larriba JL,
Martínez-Martí A, Bernabé R, Bosch-Barrera J, Casal-Rubio J, Calvo
V, Insa A, Ponce S, et al: Perioperative nivolumab and chemotherapy
in Stage III Non-Small-Cell lung cancer. N Engl J Med. 389:504–513.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
National Library of Medicine, .
Neoadjuvant and Adjuvant Therapy Studies of Sindilizumab in
Resectable Lung Cancer. https://clinicaltrials.gov/study/NCT05116462August
8–2025
|
|
81
|
National Library of Medicine, . Phase III
Study of Envafolimab Versus Placebo Plus Chemotherapy in Resectable
Stage III NSCLC. https://clinicaltrials.gov/study/NCT06123754August
8–2025
|
|
82
|
Romero Román A, Campo-Cañaveral de la Cruz
JL, Macía I, Escobar Campuzano I, Figueroa Almánzar S, Delgado Roel
M, Gálvez Muñoz C, García Fontán EM, Muguruza Trueba I, Romero
Vielva L, et al: Outcomes of surgical resection after neoadjuvant
chemoimmunotherapy in locally advanced stage IIIA non-small-cell
lung cancer. Eur J Cardiothorac Surg. 60:81–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yan W, Zhong WZ, Liu YH, Chen Q, Xing W,
Zhang Q, Liu L, Ge D, Chen K, Yang F, et al: Adebrelimab (SHR-1316)
in combination with chemotherapy as perioperative treatment in
patients with resectable Stage II to III NSCLCs: An Open-label,
multicenter, phase 1b trial. J Thorac Oncol. 18:194–203. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Expert group consensus on standardized
perioperative immunotherapy for non-small cell lung cancer, .
Consensus expert group on standardized perioperative immunotherapy
for Non-small cell lung cancer: Consensus expert opinion on
standardized perioperative immunotherapy for Non-small cell lung
cancer. China J Clin Oncol. 51:1–14. 2024.
|
|
85
|
Provencio M, Nadal E, Insa A, García
Campelo R, Casal J, Dómine M, Massuti B, Majem M, Rodríguez-Abreu
D, Martínez-Martí A, et al: Perioperative chemotherapy and
nivolumab in non-small-cell lung cancer (NADIM): 5-year clinical
outcomes from a multicentre, single-arm, phase 2 trial. Lancet
Oncol. 25:1453–1464. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
The Multidisciplinary Diagnosis and
Treatment (MDT) Committee of the Chinese Anti-Cancer Association,
the Small Cell Lung Cancer Expert Committee of the China Society of
Clinical Oncology (CSCO), the Tumor Radiotherapy Committee of the
Chinese Anti-Cancer Association, . Expert consensus on radiotherapy
combined with immunotherapy for inoperable lung cancer (2024
Edition). J Cancer Prevention Control. 31:1223–1239. 2024.
|
|
87
|
Uprety D, Roden AC and Peters S: Adjuvant
Immunotherapy should be used in patients with Non-Small cell
carcinoma with a pathologic complete response to neoadjuvant
immunotherapy. J Thorac Oncol. 20:34–38. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Phillips WJ, Camidge DR and Wheatley-Price
P: Adjuvant immunotherapy should not be used in patients with a
pathologic complete response to neoadjuvant chemoimmunotherapy. J
Thorac Oncol. 20:30–33. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sun T, Chen Z, Wei K and Tang H: Research
progress on predictive biomarkers of immunotherapy efficacy in
Non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 27:459–465.
2024.(In Chinese). PubMed/NCBI
|
|
90
|
Chen K, Yang F, Shen H, Wang C, Li X,
Chervova O, Wu S, Qiu F, Peng D, Zhu X, et al: Individualized
tumor-informed circulating tumor DNA analysis for postoperative
monitoring of non-small cell lung cancer. Cancer Cell.
41:1749–1762.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Peters S, Oliner KS, L'Hernault A,
Ratcliffe M, Madison H, Lai Z, Stewart R, Mann H, Lowery C, Garon
EB, et al: Durvalumab with or without tremelimumab in combination
with chemotherapy in first-line metastatic non-small-cell lung
cancer: Outcomes by tumor mutational burden in POSEIDON. ESMO Open.
10:1050582025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Budczies J, Kazdal D, Menzel M, Beck S,
Kluck K, Altbürger C, Schwab C, Allgäuer M, Ahadova A, Kloor M, et
al: Tumour mutational burden: Clinical utility, challenges and
emerging improvements. Nat Rev Clin Oncol. 21:725–742. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu F, Fan J, He Y, Xiong A, Yu J, Li Y,
Zhang Y, Zhao W, Zhou F, Li W, et al: Single-cell profiling of
tumor heterogeneity and the microenvironment in advanced non-small
cell lung cancer. Nat Commun. 12:25402021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Long Y, Jin R and Li H: Mechanistic
insights into the immune biomarker of perioperative immune
checkpoint inhibitors for non-small cell lung cancer. Transl Lung
Cancer Res. 14:2821–2841. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ohara S, Suda K and Tsutani Y: Utility and
future perspectives of circulating tumor DNA analysis in Non-Small
cell lung cancer patients in the era of perioperative
Chemo-immunotherapy. Cells. 14:13122025. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Derraze Y, Hashemi SMS, Ulas EB, Ziesemer
KA, Lissenberg-Witte B, Radonic T and Bahce I: Evaluating the
significance of combining PD-L1 and TILs as biomarkers in non-small
cell lung cancer patients treated with immunotherapy: A systematic
review. BJC Rep. 3:652025. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Costantini A, Takam Kamga P,
Pons-Tostivint E, Fradin D, Emile JF and Giroux-Leprieur E: Soluble
PD-L1 (sPD-L1) as a biomarker of durable response and survival in
patients with advanced non-small cell lung cancer (NSCLC) treated
with first-line immune checkpoint inhibitors (ICIs). Cancer Immunol
Immunother. 74:2942025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ready N, Hellmann MD, Awad MM, Otterson
GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M,
et al: First-line nivolumab plus ipilimumab in advanced
Non-Small-Cell lung cancer (CheckMate 568): Outcomes by programmed
death ligand 1 and tumor mutational burden as biomarkers. J Clin
Oncol. 37:992–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ready NE, Audigier-Valette C, Goldman JW,
Felip E, Ciuleanu TE, Rosario García Campelo M, Jao K, Barlesi F,
Bordenave S, Rijavec E, et al: First-line nivolumab plus ipilimumab
for metastatic non-small cell lung cancer, including patients with
ECOG performance status 2 and other special populations: CheckMate
817. J Immunother Cancer. 11:e0061272023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Paz-Ares LG, Ciuleanu TE, Pluzanski A, Lee
JS, Gainor JF, Otterson GA, Audigier-Valette C, Ready N, Schenker
M, Linardou H, et al: Safety of First-Line Nivolumab Plus
Ipilimumab in Patients With Metastatic NSCLC: A Pooled Analysis of
CheckMate 227, CheckMate 568, and CheckMate 817. J Thorac Oncol.
8:79–92. 2023. View Article : Google Scholar
|
|
101
|
Schuler M, Cuppens K, Plönes T, Wiesweg M,
Du Pont B, Hegedus B, Köster J, Mairinger F, Darwiche K, Paschen A,
et al: Neoadjuvant nivolumab with or without relatlimab in
resectable non-small-cell lung cancer: A randomized phase 2 trial.
Nat Med. 30:1602–1611. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Aokage K, Koyama S, Kumagai S, Nomura K,
Shimada Y, Yoh K, Wakabayashi M, Fukutani M, Furuya H, Miyoshi T,
et al: Efficacy, safety, and influence on the tumor
microenvironment of neoadjuvant pembrolizumab plus ramucirumab for
PD-L1-Positive NSCLC: A Phase II Trial (EAST ENERGY). Clin Cancer
Res. 30:5584–5592. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Passaro A, Wang J, Wang Y, Lee SH, Melosky
B, Shih JY, Wang J, Azuma K, Juan-Vidal O, Cobo M, et al:
MARIPOSA-2 Investigators. Amivantamab plus chemotherapy with and
without lazertinib in EGFR-mutant advanced NSCLC after disease
progression on osimertinib: Primary results from the phase III
MARIPOSA-2 study. Ann Oncol. 35:77–90. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Spigel DR, Reynolds C, Waterhouse D, Garon
EB, Chandler J, Babu S, Thurmes P, Spira A, Jotte R, Zhu J, et al:
Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus
Crizotinib for the First-line treatment of anaplastic lymphoma
kinase translocation-positive advanced Non-small cell lung cancer
(CheckMate 370). J Thorac Oncol. 13:682–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hartmaier RJ, Markovets AA, Ahn MJ,
Sequist LV, Han JY, Cho BC, Yu HA, Kim SW, Yang JC, Lee JS, et al:
Osimertinib+ savolitinib to overcome acquired MET-mediated
resistance in epidermal growth factor Receptor-Mutated,
MET-Amplified Non-small cell lung cancer: TATTON. Cancer Discov.
13:98–113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Roesch RM, Schnorbach J, Klotz LV, Griffo
R, Thomas M, Stenzinger A, Christopoulos P, Allgaeuer M, Schneider
M, Schuler M, et al: NeoTRACK trial: Neo adjuvant T i R agolumab, A
tezolizumab and C hemotherapy-dissection of IO-efficacy in NSCLC by
longitudinal trac K ing-protocol of a non-randomised, open-label,
single-arm, phase II study. BMJ Open. 15:e0966172025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Alsaafeen BH, Ali BR and Elkord E:
Resistance mechanisms to immune checkpoint inhibitors: Updated
insights. Mol Cancer. 24:202025. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kalbasi A and Ribas A: Tumour-intrinsic
resistance to immune checkpoint blockade. Nat Rev Immunol.
20:25–39. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Said SS and Ibrahim WN: Cancer resistance
to immunotherapy: Comprehensive insights with future perspectives.
Pharmaceutics. 15:11432023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kluger H, Barrett JC, Gainor JF, Hamid O,
Hurwitz M, LaVallee T, Moss RA, Zappasodi R, Sullivan RJ, Tawbi H,
et al: Society for immunotherapy of cancer (SITC) consensus
definitions for resistance to combinations of immune checkpoint
inhibitors. J Immunother Cancer. 11:e0059212023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cabanos HF and Hata AN: Emerging insights
into targeted Therapy-tolerant persister cells in cancer. Cancers
(Basel). 13:26662021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wei SC, Levine JH, Cogdill AP, Zhao Y,
Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and
Allison JP: Distinct cellular mechanisms underlie Anti-CTLA-4 and
Anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xu M, Wang Y, Xia R, Wei Y and Wei X: Role
of the CCL2-CCR2 signalling axis in cancer: Mechanisms and
therapeutic targeting. Cell Prolif. 54:e131152021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pienta KJ, Machiels JP, Schrijvers D,
Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA,
Takimoto C, et al: Phase 2 study of carlumab (CNTO 888), a human
monoclonal antibody against CC-chemokine ligand 2 (CCL2), in
metastatic Castration-resistant prostate cancer. Invest New Drugs.
31:760–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Noel M, O'Reilly EM, Wolpin BM, Ryan DP,
Bullock AJ, Britten CD, Linehan DC, Belt BA, Gamelin EC, Ganguly B,
et al: Phase 1b study of a small molecule antagonist of human
chemokine (C-C motif) receptor 2 (PF-04136309) in combination with
nab-paclitaxel/gemcitabine in first-line treatment of metastatic
pancreatic ductal adenocarcinoma. Invest New Drugs. 38:800–811.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Overman MJ, Gelsomino F, Aglietta M, Wong
M, Limon Miron ML, Leonard G, García-Alfonso P, Hill AG, Cubillo
Gracian A, Van Cutsem E, et al: Nivolumab plus relatlimab in
patients with previously treated microsatellite
instability-high/mismatch repair-deficient metastatic colorectal
cancer: The phase II CheckMate 142 study. J Immunother Cancer.
12:e0086892024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kelly RJ, Landon BV, Zaidi AH, Singh D,
Canzoniero JV, Balan A, Hales RK, Voong KR, Battafarano RJ, Jobe
BA, et al: Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor
relatlimab in resectable esophageal/gastroesophageal junction
cancer: A phase Ib trial and ctDNA analyses. Nat Med. 30:1023–1034.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gopal AK, Armand P, Neelapu SS, Bartlett
NL, Spurgeon SE, Kuruvilla J, Savage KJ, Leonard JP, Gelb AB, Ahmed
N, et al: Nivolumab plus relatlimab for patients with relapsed or
progressed B-cell malignancies in RELATIVITY-022. Blood Adv.
9:3383–3394. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Aggarwal V, Workman CJ and Vignali DAA:
LAG-3 as the third checkpoint inhibitor. Nat Immunol. 24:1415–1422.
2023. View Article : Google Scholar : PubMed/NCBI
|