Introduction
Hepatocellular carcinoma (HCC), one of the most
common fatal malignancies in many countries in Asia and Africa, is
the leading cause of cancer mortality in China and its incidence is
also increasing in Western countries (1). HCC is complex in etiology and
pathogenesis (2). Risk factors
include aflatoxin, alcohol, hepatitis B virus, hepatitis C virus
and cirrhosis. Different risk factors lead to different molecular
pathways, making the pathogenesis of HCC more complex to interpret
(3,4). Surgical resection provides an
opportunity for cure, but the outcome of surgical HCC patients
remains grave mainly due to frequent tumor recurrence (5–7). Other
therapeutic strategies have a similar shortcoming. Although HCC
prognosis depends on various elements, the biological
characteristics of HCC are important. Thus, in order to aid in the
prediction of prognosis or the selection of a therapeutic target,
more potential markers should be explored.
Arginine is an indispensable amino acid to children,
but one which is semi-essential to adults (8). Since 1930, arginine has been known to
influence the growth of transplantable mice tumors (9). Argininosuccinate synthetase (ASS) is a
key enzyme in the synthesis of arginine from citrulline and is
present in all tissues and culture cells studied. This metabolic
pathway allows cells to synthesize arginine from citrulline, making
this amino acid non-essential for the growth of humans and most
mammalian cells. It is well known that the highest ASS activity is
found in the liver, where the enzyme is involved in the urea cycle
and in the elimination of ammonia (10). Published studies showed HCC to be
auxotrophic for arginine due to the lack of expression of ASS
(11–14). However, the relationship between
reduced levels of ASS expression in HCC tissues and its
clinicopathological significance remains unclear.
The present study aimed to determine the
relationship between the reduced level of ASS expression in HCC
tissues by immunohistochemical detection (IHC) and the
clinicopathological features and post-resectional survival. Our
results suggested that the ASS staining score can be used as an
independent marker for the prognosis of patients presenting with
HCC.
Materials and methods
Patients
Tissue samples were obtained from 71 patients with
HCC who underwent surgical resection in the Union Hospital
Affiliated to Tongji Medical College from 2000 to 2002. The
patients included 59 males and 12 females, with a median age of 49
years (range 33–74). The clinicopathological features of the
patients including gender, age, background liver, viral status,
tumor size, portal vein invasion, histopathological
differentiation, serum α-fetoprotein (AFP) level, TNM staging and
recurrence of HCC are shown in Table
I. The Institutional Ethics Committee approval for the project
was obtained prior to the commencement of the study.
 | Table IRelationship between ASS expression
and clinicopathological features of HCC. |
Table I
Relationship between ASS expression
and clinicopathological features of HCC.
| | ASS expression | |
|---|
| |
| |
|---|
| Variables | Patients | Low | Middle | High | P-valuea |
|---|
| All cases | 71 | 21 | 29 | 21 | |
| Gender | | | | | 0.020 |
| Male | 59 | 14 | 28 | 17 | |
| Female | 12 | 7 | 1 | 4 | |
| Age (years) | | | | | 0.787 |
| <60 | 47 | 14 | 18 | 15 | |
| ≥60 | 24 | 7 | 11 | 6 | |
| Background liver | | | | | 0.047 |
| Normal liver | 3 | 1 | 2 | 0 | |
| Chronic liver | 21 | 10 | 9 | 2 | |
| Cirrhosis | 47 | 10 | 18 | 19 | |
| Viral status | | | | | 0.237 |
| Hepatitis virus
B | 51 | 15 | 20 | 16 | |
| Hepatitis virus
C | 12 | 3 | 6 | 3 | |
| Both hepatitis virus
B and C | 2 | 0 | 0 | 2 | |
| Non-B, Non-C | 6 | 3 | 3 | 0 | |
| Tumor size | | | | | 0.630 |
| <5 cm | 38 | 13 | 15 | 10 | |
| ≥5 cm | 33 | 8 | 14 | 11 | |
| Portal vein
invasion | | | | | <0.001 |
| No | 52 | 21 | 21 | 10 | |
| Yes | 19 | 0 | 8 | 11 | |
| Histopathological
differentiation | | | | | 0.008 |
|
Well-differentiated | 21 | 11 | 6 | 4 | |
| Moderately
differentiated | 26 | 8 | 13 | 5 | |
| Poorly
differentiated | 24 | 2 | 10 | 12 | |
| Serum AFP level | | | | | 0.301 |
| <25 ng/ml | 35 | 13 | 14 | 8 | |
| ≥25 ng/ml | 36 | 8 | 15 | 13 | |
| TNM stage | | | | | <0.001 |
| I–II | 25 | 18 | 6 | 1 | |
| III–IV | 46 | 3 | 23 | 20 | |
| Recurrence | | | | | 0.015 |
| No | 17 | 9 | 7 | 1 | |
| Yes | 54 | 12 | 22 | 20 | |
Immunohistochemical staining
ASS expression was detected immunohistochemically
for paraffin-embedded specimens from 71 patients with HCC. Surgical
specimens were formalin-fixed (10%), paraffin-embedded and
sectioned at 4 μm. For heat-induced epitope retrieval,
deparaffinised sections were soaked in 10 mM citrate buffer (pH
6.0) and treated at 95°C for 30 min in a microwave oven.
Immunohistochemical staining was performed using the avidin-biotin
immunoperoxidase technique. Endogenous peroxidase activity was
first blocked by incubation with 0.3% H2O2 in
methanol for 15 min. Non-specific immunoglobulin binding was then
blocked by incubation with 10% normal goat serum for 10 min.
Sections were incubated at room temperature for 2 h with the
anti-ASS monoclonal antibody (BD Pharmigen) at a 1:50 dilution. The
sections were rinsed and incubated for 30 min with biotinylated
second antibody. After washing, the sections were incubated for 30
min with horseradish peroxidase-conjugated streptavidin and treated
with 3,3′-diaminobenzidine tetrahydrochloride in 0.01%
H2O2 for 10 min. The slides were
counterstained with Meyer’s hematoxylin. As a negative control, the
primary antibody was replaced with normal mouse IgG at an
appropriate dilution.
Staining assessment and scoring
ASS expression levels were classified
semi-quantitatively based on the total combined scores of the
percentage of positively stained tumor cells together with the
staining intensity. A tumor was scored as ‘0’ if <5% of tumor
cells were stained positive, ‘1’ if 5–50% were stained positive and
‘2’ if >50% of the cells were stained positive. The staining
intensity was scored as ‘0’ if no staining of cells occurred or if
there was only weak staining, ‘1’ if there was moderate staining
and ‘2’ in cases of strong staining. The final score of ASS
expression was defined as ‘low ASS expression’ if the sum of the
positivity score and the staining intensity score was 0–1, ‘middle
ASS expression’ if the sum was 2 and ‘high ASS expression’ if the
sum was 3–4. In each case, at least three different tumor areas
were evaluated and the mean of the results was taken as the final
expression score. The scoring procedure was carried out twice by
two independent pathologists who had no knowledge of the clinical
data and corresponding hematoxylin and eosin slides. The
concordance rate between the two primary pathologists was >95%.
In cases of significant disagreement, the slides in question were
reviewed again simultaneously by the original two pathologists,
together with a third pathologist, at a multiheaded microscope, in
order to resolve the divergence of opinion.
Statistical analysis
The Chi-square test was used to show differences of
categorical variables. Patient survival and differences were
determined by the Kaplan-Meier method and log-rank test. Cox
regression (proportional hazard model) was adopted for the
multivariate analysis of prognostic factors. Statistical software
package SPSS 11.5 (SPSS Inc., Chicago, IL) was employed for the
analyses. P<0.05 was defined as statistically significant.
Results
Clinical profiles of the patients
Tissue samples were obtained from 71 patients.
Twenty-one (29.6%) of the tissue samples were histopathologically
well-differentiated, 26 (36.6%) were moderately differentiated and
24 (33.8%) were poorly differentiated. Portal vein invasion of the
tumor cells occurred in 19 (26.8%) of the 71 patients, but not in
the remaining 52 (73.2%) patients (Table I). Up to August 31, 2008 (the census
date), 22 (31%) patients were alive, while 49 (69%) patients had
succumbed to the disease. Follow-up ranged from 3 to 93 months
(median 36 months).
ASS expression in HCC and its
clinicopathological significance
A reduced ASS expression was detected in HCC tissues
obtained from 50 patients (70.4%) in accordance with the
aforementioned criteria. Normal liver tissue as a control exhibited
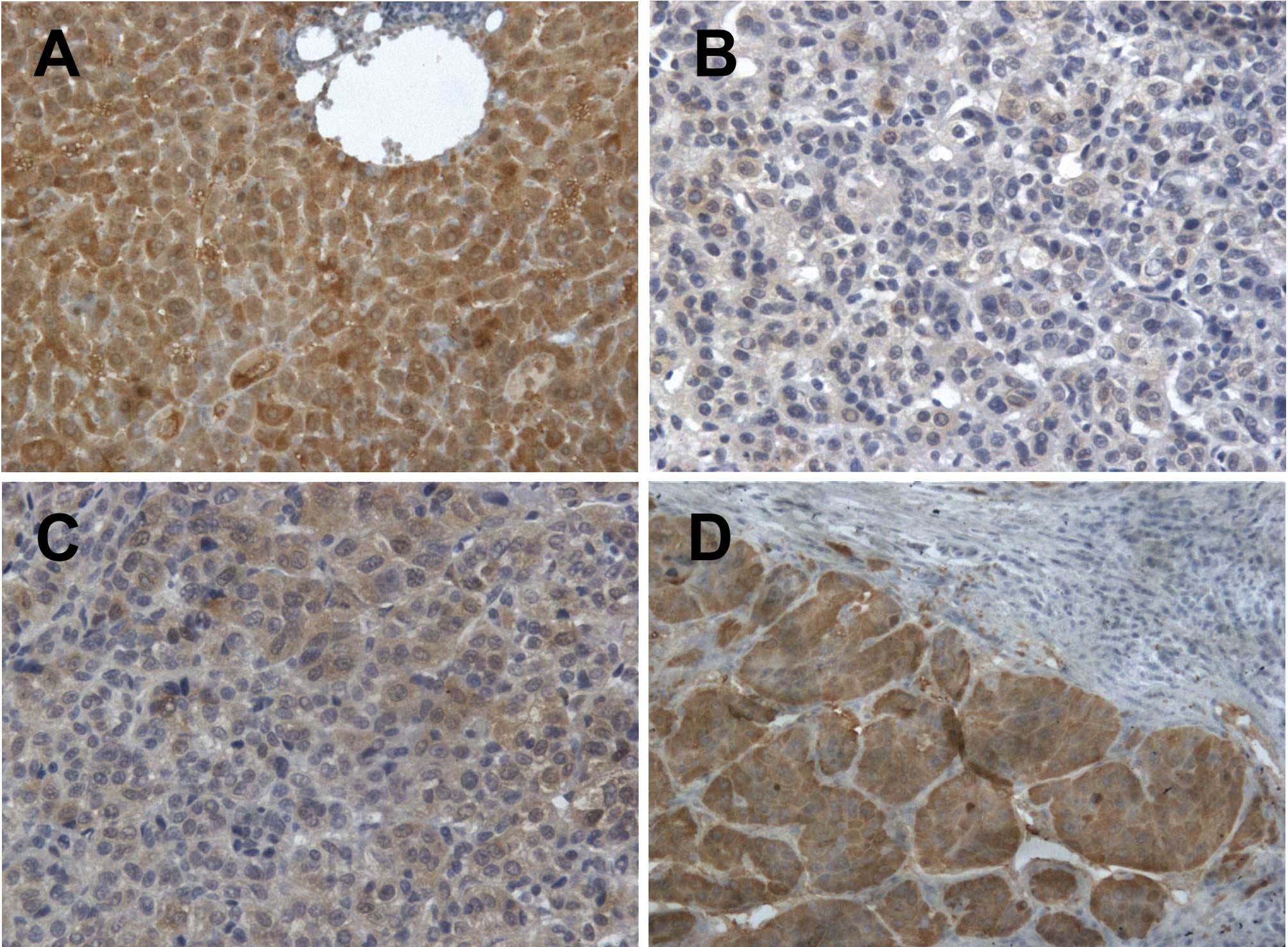
positive staining (Fig. 1A) with
21, 29 and 21 HCC samples exhibiting staining scores of 0, 1 and 2,
respectively (Fig. 1B-D). The
staining scores were significantly associated with gender,
background liver, histopathological differentiation, recurrence,
TNM staging and portal vein invasion of HCC (Table I; P<0.05), but not with age,
viral status, tumor size and serum AFP level of the tumor antigen
marker (Table I; P>0.05).
Factors influencing overall or
disease-free survival of HCC patients after tumor resection
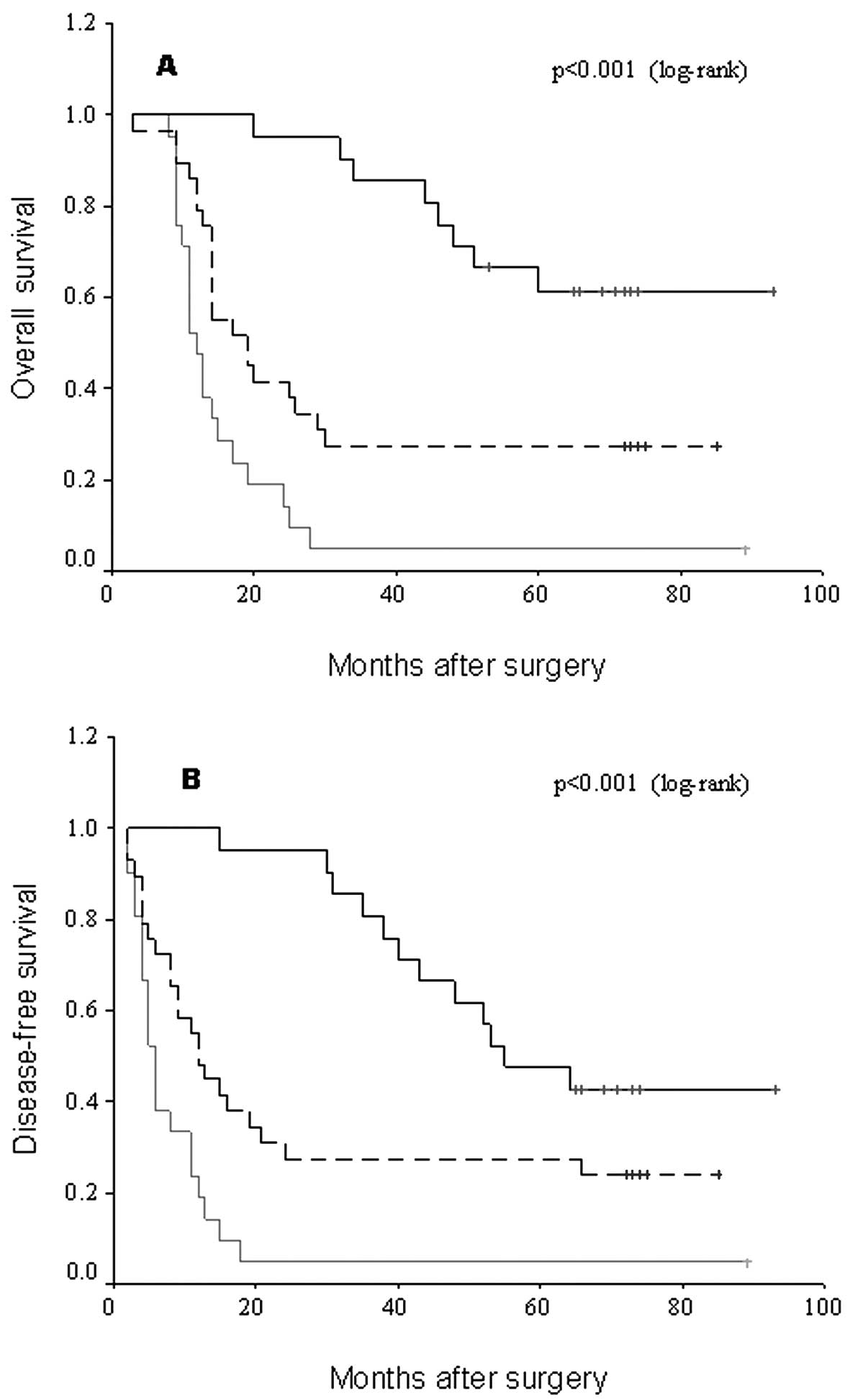
Using the Kaplan-Meier method and log-rank test, HCC
tissues with a higher staining score of ASS were correlated with a
shorter overall or disease-free survival of patients (Table II, Fig.
2A and B; log-rank value 33.00 and 27.72; P<0.001 and
P<0.001, respectively). Survival benefits were found in patients
with earlier TNM staging, a higher histopathological
differentiation grade, absence of portal vein invasion and a better
background liver for overall or disease-free survival (Table II; P<0.05), whereas other
patients showed no predictive values (Table II; P>0.05). The above-mentioned
significant parameters were included in a multivariate Cox
regression analysis which revealed that the ASS staining score (RR,
2.139; 95% CI, 1.283–3.568; P=0.004), portal vein invasion (RR,
3.052; 95% CI, 1.507–6.179; P=0.002) and histopathological
differentiation (RR, 1.401; 95% CI, 0.903–2.172; P=0.004) were
independent prognostic markers for overall survival of patients
with HCC (Table III; P<0.05).
On the other hand, the ASS staining score (RR, 1.880; 95% CI,
1.128–3.133; P=0.015) and portal vein invasion (RR, 2.258; 95% CI,
1.085–4698; P=0.029) were independent prognostic markers for
disease-free survival of patients with HCC (Table III; P<0.05).
 | Table IIUnivariate survival analysis of
overall and disease-free survival in 71 patients with HCC. |
Table II
Univariate survival analysis of
overall and disease-free survival in 71 patients with HCC.
| Variables | No. of cases | OS | DFS |
|---|
| |
|
|
|---|
| | Mean ± SE
(months) | 95% CI | P-valuea | Mean ± SE
(months) | 95% CI | P-valuea |
|---|
| Gender | | | | 0.354 | | | 0.424 |
| Male | 59 | 41±5 | (32–50) | | 34±5 | (25–43) | |
| Female | 12 | 44±8 | (28–59) | | 40±8 | (25–55) | |
| Age (years) | | | | 0.673 | | | 0.920 |
| <60 | 47 | 42±5 | (32–52) | | 37±5 | (26–47) | |
| ≥60 | 24 | 38±6 | (27–50) | | 32±6 | (20–44) | |
| Background
liver | | | | 0.004 | | | 0.002 |
| Normal liver | 3 | 69±4 | (62–77) | | 65±4 | (56–74) | |
| Chronic liver | 21 | 62±8 | (46–78) | | 57±9 | (40–74) | |
| Cirrhosis | 47 | 30±4 | (22–38) | | 23±4 | (16–31) | |
| Viral status | | | | 0.120 | | | 0.273 |
| Hepatitis virus
B | 51 | 39±5 | (30–49) | | 33±5 | (23–42) | |
| Hepatitis virus
C | 12 | 41±10 | (20–61) | | 36±11 | (15–58) | |
| Both hepatitis
virus B and C | 2 | 19±10 | (0–37) | | 9±4 | (2–15) | |
| Non-B, Non-C | 6 | 64±9 | (45–82) | | 55±10 | (37–74) | |
| Tumor size | | | | 0.860 | | | 0.980 |
| <5 cm | 38 | 42±6 | (31–53) | | 35±6 | (24–46) | |
| ≥5 cm | 33 | 42±6 | (30–54) | | 36±6 | (23–48) | |
| Portal vein
invasion | | | | <0.001 | | | <0.001 |
| No | 52 | 54±5 | (44–64) | | 46±5 | (36–56) | |
| Yes | 19 | 12±1 | (10–15) | | 7±1 | (5–10) | |
| Histopathological
differentiation | | | | 0.005 | | | 0.008 |
|
Well-differentiated | 21 | 60±8 | (44–75) | | 50±8 | (35–65) | |
| Moderately
differentiated | 26 | 42±6 | (29–54) | | 37±7 | (24–50) | |
| Poorly
differentiated | 24 | 25±5 | (15–36) | | 19±6 | (8–30) | |
| Serum AFP
level | | | | 0.516 | | | 0.397 |
| <25 ng/ml | 35 | 45±6 | (33–57) | | 39±6 | (27–51) | |
| ≥25 ng/ml | 36 | 38±5 | (27–48) | | 31±6 | (20–42) | |
| TNM stage | | | | <0.001 | | | <0.001 |
| I–II | 25 | 75±6 | (63–87) | | 67±6 | (54–79) | |
| III–IV | 46 | 25±4 | (18–32) | | 19±4 | (12–26) | |
| ASS expression | | | | <0.001 | | | <0.001 |
| Low | 21 | 75±6 | (64–86) | | 66±6 | (54–77) | |
| Middle | 29 | 35±6 | (24–47) | | 31±6 | (19–43) | |
| High | 21 | 17±4 | (10–25) | | 11±4 | (3–19) | |
 | Table IIIMultivariate survival analysis of
overall and disease-free survival in 71 patients with HCC. |
Table III
Multivariate survival analysis of
overall and disease-free survival in 71 patients with HCC.
| Variables | OS | DFS |
|---|
|
|
|
|---|
| RR | 95% CI | P-valuea | RR | 95% CI | P-valuea |
|---|
| TNM stage | 2.246 | (0.834–7.234) | 0.103 | 2.398 | (0.841–6.837) | 0.102 |
| Background
liver | 0.934 | (0.479–1.823) | 0.842 | 1.387 | (0.768–2.507) | 0.278 |
| Portal vein
invasion | 3.052 | (1.507–6.179) | 0.002 | 2.258 | (1.085–4.698) | 0.029 |
| Histopathological
differentiation | 1.401 | (0.903–2.172) | 0.004 | 1.469 | (0.923–2.337) | 0.105 |
| ASS expression | 2.139 | (1.283–3.568) | 0.004 | 1.880 | (1.128–3.133) | 0.015 |
Discussion
ASS is a key enzyme which converts citrulline to
arginine. Tumors which usually express reduced ASS include
hepatocellular carcinoma, melanoma, some mesotheliomas and some
renal cell cancer types (11,14–19).
To the best of our knowledge, this is the first study concerning
ASS expression levels detected by IHC and its prognostic
significance for HCC patients. Our results suggested that ASS is
reductively expressed in a large portion (70.4%) of HCC tissues
with a staining score of 0 or 1. Moreover, the magnitude of
down-regulated expression correlated with better prognosis,
especially for the staining score of 0. A reduced ASS expression
predicts the same clinical value as early TNM staging, rare portal
vein invasion and a reduced possibility of recurrence.
As previously reported, depletion of arginine causes
substantial havoc to cells, in particular tumor cells (13). Due to their rapid proliferation,
tumor cells require amino acids as nutrients. Specifically,
arginine is the first amino acid that is exhausted and depleted
faster than any other nutrient by normal cell metabolism, but
exhibits low recycling efficiency (20). When arginine was depleted, favorable
conditions were anticipated prior to the resumption of normal cell
division. However, tumor cells, with their cell cycle aberrations,
continued to progress with the cell cycle despite the absence of
arginine, leading to a gross imbalance and cell death (20). Based on this fact, a strategy to
degrade arginine was developed to cure ASS (−) tumors because
ASS-expressed normal cells produce arginine from citrulline,
whereas ASS (−) tumor cells cannot. Several enzymes, including
human arginase and arginine deiminase (ADI) which is a microbial
enzyme from mycoplasma spp, are currently in phase II clinical
trials (21–23). These enzymes have a high affinity to
arginine and catalyze the degradation of arginine. It was reported
that ADI activated anti-proliferation on a number of malignant
cells in vitro and anti-tumor effects in vivo
(15). The extent of ADI
anti-proliferation depends on the endogenous activity of ASS, the
rate-limiting enzyme involved in the recycling of citrulline into
arginine. However, arginine depletion can induce ASS expression in
certain melanoma cell lines which can lead to in vitro drug
resistance (13).
A reduced expression of ASS in cancer cells and its
higher expression in the normal liver cells of HCC patients
suggested that only cancer cells are auxotrophic for arginine.
Thus, ADI was able to exert its anti-tumor activity without any
adverse effect on surrounding normal liver cells (16). ASS is the rate-limiting enzyme in
the conversion of citrulline to arginine for ammonia detoxification
through the urea cycle in the liver. A probable explanation for the
loss of ASS activity in HCC is cellular dedifferentiation which may
occur during tumorigenesis. Since dedifferentiation is a regressive
process of cells to a primitive embryonic state, it may be related
to a decrease of ASS expression in the neonatal level of liver.
Thus, the level of ASS remains low during the fetal period in the
liver (16,24). Another explanation involves the
down-regulation of ASS gene expression at the transcriptional level
by its promoter methylation (14).
HCC remains a troublesome malignancy with poor
prognosis, due to its strong drug resistance to conventional cancer
treatments, as well as its frequent metastasis and recurrence. In
spite of extensive research and various therapies, a molecular
marker needs to be found that can be used as a target for the
treatment of HCC. The current study provided a novel candidate,
ASS, as a molecular marker of both the biological behaviors and
surgical outcome of HCC. Measurement by IHC of a reduced ASS
expression was closely correlated with better prognosis and allowed
for a prediction of post-resectional survival.
Taken together, ASS in cancer tissues was
reductively expressed in numerous patients presenting with HCC. ASS
had a direct correlation with cancer differentiation, TNM staging,
portal vein invasion and recurrence, as well as the overall and
disease-free survival of patients with HCC. Therefore, we concluded
that ASS may be a novel marker for predicting prognosis of patients
presenting with HCC, and suggest that ASS be used as a target for
the treatment of HCC. In order to improve therapy for HCC, more
studies need to be conducted in order for ASS expression or
activity in tumor cells to be attenuated.
References
|
1
|
Bosch FX, Ribes J, Cleries R and Diaz M:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farazi PA and DePinho RA: The genetic and
environmental basis of hepatocellular carcinoma. Discov Med.
6:182–186. 2006.PubMed/NCBI
|
|
3
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suriawinata A and Xu R: An update on the
molecular genetics of hepatocellular carcinoma. Semin Liver Dis.
24:77–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S, Mogushi K, Yasen M, Noguchi N,
Kudo A, Kurokawa T, Nakamura N, Inazawa J, Tanaka H and Arii S:
Surgical contribution to recurrence-free survival in patients with
macrovascular-invasion-negative hepatocellular carcinoma. J Am Coll
Surg. 208:368–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamiyama T, Nakanishi K, Yokoo H, Kamachi
H, Tahara M, Suzuki T, Shimamura T, Furukawa H, Matsushita M and
Todo S: Recurrence patterns after hepatectomy of hepatocellular
carcinoma: implication of Milan criteria utilization. Ann Surg
Oncol. 16:1560–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaibori M, Ishizaki M, Saito T, Matsui K,
Kwon AH and Kamiyama Y: Risk factors and outcome of early
recurrence after resection of small hepatocellular carcinomas. Am J
Surg. 198:39–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grabon W: Arginine as a crucial amino acid
in carcinogenesis and tumor growth. Postepy Hig Med Dosw.
60:483–489. 2006.PubMed/NCBI
|
|
9
|
Wu G and Morris SM Jr: Arginine
metabolism: nitric oxide and beyond. J Biochem. 336:1–17. 1998.
|
|
10
|
Husson A, Brasse-Lagnel C, Fairand A,
Renouf S and Lavoinne A: Argininosuccinate synthetase from the urea
cycle to the citrulline-NO cycle. Eur J Biochem. 270:1887–1899.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng
AW, Lo WH and Leung YC: Pegylated recombinant human arginase
(rhArg-peg5,000 mw) inhibits the in vitro and in vivo proliferation
of human hepatocellular carcinoma through arginine depletion.
Cancer Res. 67:309–317. 2007. View Article : Google Scholar
|
|
12
|
Wheatley DN and Campbell E: Arginine
catabolism, liver extracts and cancer. Pathol Oncol Res. 8:18–25.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr
M, Spector S and Savaraj N: Arginine deprivation as a targeted
therapy for cancer. Curr Pharm Des. 14:1049–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dillon BJ, Prieto VG, Curley SA, Ensor CM,
Holtsberg FW, Bomalaski JS and Clark MA: Incidence and distribution
of argininosuccinate synthetase deficiency in human cancers: a
method for identifying cancers sensitive to arginine deprivation.
Cancer. 100:826–833. 2004. View Article : Google Scholar
|
|
15
|
Ensor CM, Holtsberg FW, Bomalaski JS and
Clark MA: Pegylated arginine deiminase (ADI-SS PEG20,000 mw)
inhibits human melanomas and hepatocellular carcinomas in vitro and
in vivo. Cancer Res. 62:5443–5450. 2002.PubMed/NCBI
|
|
16
|
Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH,
Kim JH, Park IS, Yoon DK and Min BH: Renal cell carcinoma does not
express argininosuccinate synthetase and is highly sensitive to
arginine deprivation via arginine deiminase. Int J Cancer.
120:897–905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bowles TL, Kim R, Galante J, Parsons CM,
Virudachalam S, Kung HJ and Bold RJ: Pancreatic cancer cell lines
deficient in argininosuccinate synthetase are sensitive to arginine
deprivation by arginine deiminase. Int J Cancer. 123:1950–1955.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park H, Lee JB, Shim YJ, Shin YJ, Jeong
SY, Oh J, Park GH, Lee KH and Min BH: Arginine deiminase enhances
MCF-7 cell radiosensitivity by inducing changes in the expression
of cell cycle-related proteins. Mol Cell. 25:305–311.
2008.PubMed/NCBI
|
|
19
|
Kim JH, Kim JH, Yu YS, Kim DH, Min BH and
Kim KW: Anti-tumor activity of arginine deiminase via arginine
deprivation in retinoblastoma. Oncol Rep. 18:1373–1377.
2007.PubMed/NCBI
|
|
20
|
Scott L, Lamb J, Smith S and Wheatley DN:
Single amino acid (arginine) deprivation: rapid and selective death
of cultured transformed and malignant cells. Br J Cancer.
83:800–810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ascierto PA, Scala S, Castello G, Daponte
A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci
MT, Ensor CM, Prestayko AW, Holtsberg FW, Bomalaski JS, Clark MA,
Savaraj N, Feun LG and Logan TF: Pegylated arginine deiminase
treatment of patients with metastatic melanoma: results from phase
I and II studies. J Clin Oncol. 23:7660–7668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izzo F, Marra P, Beneduce G, Castello G,
Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski
JS, Clark MA, Ng C and Curley SA: Pegylated arginine deiminase
treatment of patients with unresectable hepatocellular carcinoma:
results from phase I/II studies. J Clin Oncol. 22:1815–1822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takaku H, Matsumoto M, Misawa S and
Miyazaki K: Anti-tumor activity of arginine deiminase from
Mycoplasma argini and its growth-inhibitory mechanism. Jpn J Cancer
Res. 86:840–846. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hurwitz R and Kretchmer N: Development of
arginine-synthesizing enzymes in mouse intestine. Am J Physiol.
251:103–110. 1986.PubMed/NCBI
|