Introduction
Ganoderma lucidum (Fr.) Karst. (Polyporaceae)
is a medicinal mushroom known to the Japanese as ‘Rei-shi’ or
‘Mannentake’, and to the Chinese as ‘Lingzhi’. Its fruiting bodies
have been used for their medicinal properties in traditional
Chinese medicine for over 2,000 years. The use of this mushroom for
promotion of vitality and as an anti-aging agent is described in
detail in the classic compendium of traditional Chinese medicine
Shen Nung Ben Cao Jin (dated 206 B.C. - 8 A.D.). Furthermore,
Ganoderma lucidum (G. lucidum) was used more recently in
China and other oriental countries for the treatment of debility
and weakness, hypertension, cardiovascular disease, bronchitis,
arthritis, neurasthenia, insomnia, hepatopathy, chronic hepatitis,
nephritis, gastric ulcer, asthma, diabetes, altitude sickness, AIDS
and cancer (1–3). Of particular interest among the
reported biological/pharmacological properties of G. lucidum
are its anti-tumor activities, including cell cycle arrest,
induction of apoptosis, inhibition of motility, anti-angiogenesis
and anti-mutagenesis (3–9). The fruiting bodies and the mycelium of
the mushroom have very similar compositions, but the mycelium
contains several additional nutrients and beneficial components.
Thus, the mycelium is considered to be the ‘essence’ of the
mushroom organism and is consumed as a functional food. The use of
mycelia of Ganoderma as a ‘designer food’ means that culture
techniques for this organism are well established.
A water-soluble extract from the culture medium of
G. lucidum (Rei-shi) mycelia (MAK) after fermentation on a
solid medium containing bagasse contains various types of
substances, such as polysaccharides, proteins, water-soluble lignin
and triterpenes. Previously, we reported that MAK shows preventive
effects on the development of chemical carcinogen-induced aberrant
crypt foci, colon adenomas, colon tumors and pulmonary
adenocarcinomas in rats (10–13).
Furthermore, we reported that MAK has protective effects against
X-irradiation-induced small intestinal injury in mice (14). Many of the adverse effects of
anti-cancer drugs also arise from the ability of these drugs to
inhibit DNA synthesis and cell division in normal cells. Thus, we
hypothesized that MAK is able to protect against small intestinal
injury after chemotherapy. The present study was conducted to
assess the effects of MAK and Agarics blazei on small
intestinal injury after the administration of various types of
anti-cancer drugs.
Materials and methods
Animals
Two hundred and thirty 6-week-old male B6C3F1/Crlj
mice (Charles River Japan, Inc.) were used in the present study.
They were housed five to a polycarbonate cage and kept under
constant conditions of temperature (24±2°C) and relative humidity
(55±10%) with a 12-h light/dark cycle. The animals were maintained
in accordance with the ‘Guidelines for the Care and Use of
Laboratory Animals’ established by the Hiroshima University and fed
a commercial diet (Oriental Yeast Co. Ltd., Tokyo, Japan) alone or
with a 1.25, 2.5 or 5.0% (w/w) supplement of MAK or AGA. Normal tap
water was also provided ad libitum.
Anti-cancer drugs
Anti-cancer drugs were obtained as follows: 5FU
(5-FU injection 250 Kyowa; Kyowa Hakko Co., Ltd., Tokyo, Japan),
UFT (prepared by Taiho Pharmaceutical Co., Ltd., Tokyo, Japan),
CDDP (Randa injection; Nippon Kayaku Co., Ltd., Tokyo, Japan), CPA
(Endoxan; Shionogi Pharmaceutical Co., Ltd., Osaka, Japan) and
Iressa (Iressa tablets 250; AstraZeneca K.K., Osaka, Japan).
G. lucidum mycelia and Agarics
blazei
A water-soluble extract from the culture medium of
MAK was prepared by Noda Shokkin-Kogyo Co., Ltd. (Chiba, Japan). In
brief, MAK were cultured in a solid medium composed mainly of
sugar-cane bagasse for three months. The whole medium containing
the mycelia was then extracted with hot water. The extract was
filtered and spray-dried to obtain MAK. Agarics was
purchased as a commercial powder of Agarics blazei
Murrill.
Autopsy
Mice were sacrificed 3.5 days after injection of the
anti-cancer drugs. Immediately after sacrifice, segments of the
jejunum from the ileum junction (30–40 cm) were removed and fixed
in Carnoy’s solution. The segments were cut into several pieces,
bundled together, embedded in paraffin, sectioned at a thickness of
3 μm and stained with hematoxylin and eosin. To quantify
regenerating crypts, the number of crypts/circumference was
determined in cross-section. In each mouse, the number of
regenerative crypts in 10 gut cross-sections was scored.
Experiment 1
This was a comparative study of the ability of MAK
and AGA to attenuate the small intestinal injury induced by 5FU.
Mice were fed a basal diet (MF) alone or with 1.25, 2.5 or 5.0% of
MAK or AGA beginning one week prior to treatment with the
anti-cancer drug, which was administered intravenously (i.v.) (250
mg/kg) or intraperitoneally (i.p.) (250 or 500 mg/kg). At 3.5 days
after the administration of 5FU, the mice were sacrificed. Body
weight and organ weights of thymus, liver, kidney, spleen and
testis were recorded, and the number of regenerative crypts of the
small intestine was measured as described above.
Experiment 2
This was a study of the protective effect of MAK on
small intestinal injury induced by several anti-cancer drugs. At
one week after initiation of treatment with MAK, the anti-cancer
drugs were administered as follows: CPA was given orally (p.o.; 250
mg/kg), i.p. (200 mg/kg) and subcutaneously (s.c.; 150 mg/kg).
Iressa was p.o. as one dose (2,000 mg/kg) or as two doses (4,000
mg/kg) with a 2-h interval between the doses. Mice were sacrificed
at 3.5 days after the administration of each anti-cancer drug. Body
and organ weights and the number of regenerative crypts were
measured.
Statistical analysis
Statistical significance was determined by Dunnett’s
method for multiple comparisons.
Results
Experiment 1: Comparative study of the
ability of MAK and AGA to attenuate 5FU-induced small intestinal
injury
The administration of 5FU (250 mg/kg, i.v.) resulted
in body weight and organ weight loss in spleen, liver, thymus and
kidney, but not in testis (Table
I). Treatment with MAK and AGA did not affect these weight
losses, except that kidney weight loss was attenuated in the 5FU +
MAK and 5FU + AGA groups, with the exception of 5FU + 5% MAK.
 | Table IBody and organ weights (relative
weights). |
Table I
Body and organ weights (relative
weights).
| Group | BW (g) | Spleen (mg) | Liver (g) | Thymus (mg) | Kidney (g) | Testis (g) |
|---|
| Normal | 23.1±1.1 | 0.090±0.019 | 1.37±0.09 | 0.044±0.009 | 0.39±0.02 | 0.149±0.011 |
| 5% MAK | 23.6±0.6a | 0.095±0.017a | 1.48±0.10a | 0.050±0.009a | 0.41±0.03a | 0.148±0.017 |
| 5% AGA | 24.1±1.6a | 0.111±0.037a | 1.48±0.12a | 0.062±0.008a | 0.41±0.03a | 0.152±0.007 |
| 5FU + MF | 21.1±1.5c | 0.038±0.006c | 1.18±0.16c | 0.028±0.012d | 0.32±0.02d | 0.127±0.019 |
| 5FU + 5% MAK | 21.3±1.2 | 0.042±0.003 | 1.19±0.09 | 0.022±0.004 | 0.34±0.02 | 0.114±0.027 |
| 5FU + 5% AGA | 21.6±0.8 | 0.045±0.004 | 1.26±0.10 | 0.027±0.009 | 0.36±0.03b | 0.134±0.023 |
| 5FU + 2.5% MAK | 21.6±1.2 | 0.042±0.004 | 1.21±0.05 | 0.027±0.005 | 0.36±0.04b | 0.132±0.019 |
| 5FU + 2.5% AGA | 21.4±1.6 | 0.045±0.005 | 1.25±0.17 | 0.014±0.005 | 0.36±0.02b | 0.133±0.018 |
| 5FU + 1.25% MAK | 22.3±0.6 | 0.042±0.002 | 1.22±0.05 | 0.019±0.007 | 0.36±0.01b | 0.127±0.021 |
| 5FU + 1.25% AGA | 22.9±0.5 | 0.047±0.004 | 1.28±0.06 | 0.027±0.012 | 0.39±0.01a | 0.147±0.005 |
Small intestine tissue specimens were prepared and
counted for the number of regenerative crypts (Table II). The administration of 5FU (250
mg/kg, i.v.) significantly decreased the number of regenerative
crypts compared with the normal group (83.7±11.2 vs. 110.2±8.9,
p<0.01). The 5FU + MAK groups showed a significant increase
compared with the 5FU-alone group (p<0.01), whereas treatment
with 5FU + AGA did not affect the number of regenerative crypts
(82.0±9.5 to 87.6±14.9). The number of regenerative crypts
significantly decreased after treatment with 5FU (250 mg/kg, i.p.)
compared with the normal group (89.1±11.0 vs. 100.6±10.5,
p<0.01). Again, the number of regenerative crypts in the 5FU +
MAK groups (106.6±16.6 to 118.1±15.3) was significantly higher than
that in the 5FU-alone group (p<0.01), whereas treatment with 5FU
+ AGA did not affect the number of regenerative crypts (80.4±8.4 to
89.6±11.2). Administration of 5FU (500 mg/kg, i.p.) resulted in a
significant decrease in the number of regenerative crypts compared
with the normal group (67.7±8.2 vs. 100.6±10.5, p<0.01). This
decrease in the number of regenerative crypts was significantly
attenuated in the 5FU + 5% MAK and 5FU + 2.5% MAK groups compared
with the 5FU-alone group (83.4±7.9 and 82.8±7.9, p<0.01),
whereas the 5FU + AGA and 5FU + 1.25% MAK groups showed no such
effect (70.9±8.1 to 73.6±10.7). Small intestinal crypt regeneration
was unaffected by MAK or AGA alone.
 | Table IINumber of regenerative crypts. |
Table II
Number of regenerative crypts.
| Group | 250 mg/kg i.v. | 250 mg/kg i.p. | 500 mg/kg i.p. |
|---|
| Normal | 110.2±8.9a | 100.6±10.5a | 100.6±10.5a |
| 5% MAK | 112.9±12.2 | 100.5±9.2 | 100.5±9.2 |
| 5% AGA | 116.2±13.6 | 103.7±10.0 | 103.7±10.0 |
| 5FU + MF | 83.7±11.2 | 89.1±11.0 | 67.7±8.2 |
| 5FU +5% MAK | 97.0±9.9a | 106.6±16.6a | 83.4±7.9a |
| 5FU + 5% AGA | 82.0±9.5 | 80.4±8.4 | 73.6±10.7 |
| 5FU + 2.5% MAK | 110.3±14.3a | 116.5±14.3a | 82.8±7.9a |
| 5FU + 2.5% AGA | 87.6±14.9 | 89.6±11.2 | 70.9±8.1 |
| 5FU + 1.25%
MAK | 113.6±15.0a | 118.1±15.3a | 71.2±9.5 |
| 5FU + 1.25%
AGA | 85.5±9.9 | 87.3±10.8 | 73.4±7.9 |
Experiment 2: Protective effect of MAK on
small intestinal injury induced by several anti-cancer drugs
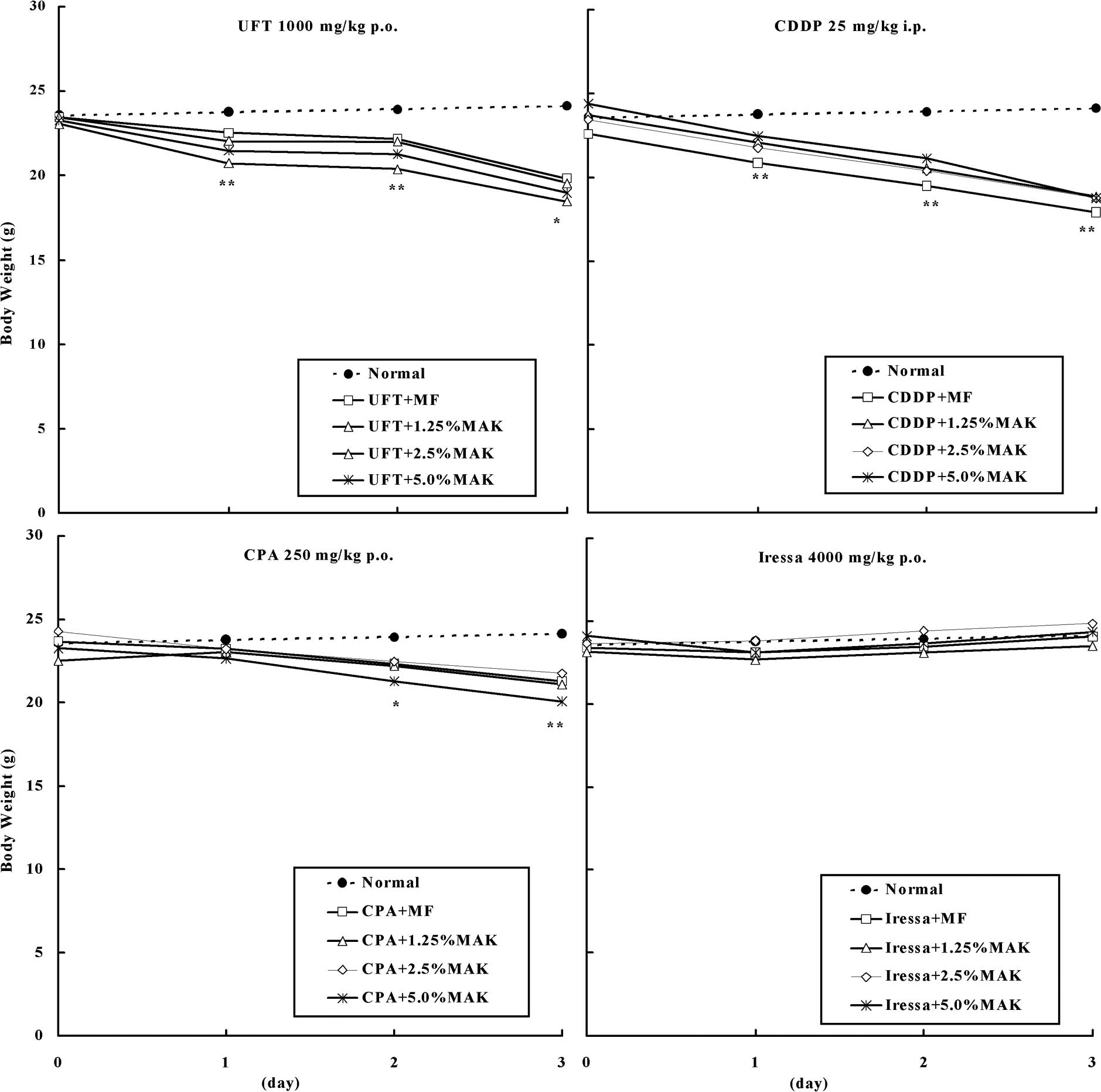
Fig. 1 shows body
weight changes of mice administered with UFT 1,000 mg/kg (p.o.),
CDDP 25 mg/kg (i.p.), CPA 250 mg/kg (p.o.) or Iressa 4,000 mg/kg
(p.o.). The administration of UFT or CDDP decreased the body weight
from the following day, and administration of CPA decreased body
weight after 2 days, whereas the administration of Iressa had no
effect on body weight. Treatment with MAK did not alter the body
weight loss or gain associated with administration of these
anti-cancer drugs.
Tissue specimens of the groups were prepared and
counted to assess the number of regenerative crypts (Table III). Following administration of
UFT (1,000 mg/kg, p.o.), the number of regenerative crypts
significantly decreased compared with the normal group (65.8±8.1
vs. 107.6±10.4, p<0.01). At all dose levels, treatment with MAK
significantly increased the number of regenerative crypts compared
with the UFT-alone group (80.6±11.4 to 102.7±2.5, a 22.5–56.1%
up-regulation compared with UFT + MF, p<0.01). Following
administration of CDDP (12.5 mg/kg, i.p.), the number of
regenerative crypts significantly decreased compared with the
normal group (89.8±11.7 vs. 107.6±10.4, p<0.01). The number of
regenerative crypts in the CDDP + MAK groups showed a significant
increase compared with the CDDP-alone group (117.4±16.7 to
121.7±12.6, a 30.7–35.4% up-regulation compared with CDDP + MF;
p<0.01). The number of regenerative crypts after administration
of CDDP (25.0 mg/kg, i.p.) showed a significant decrease compared
with the normal group (9.5±4.4 vs. 108.0±13.9, p<0.01). The
number of regenerative crypts in the CDDP + MAK groups
significantly increased compared with the CDDP-alone group
(21.9±4.7 to 32.5±3.4, a 130.5–242.1% up-regulation vs. CDDP + MF;
p<0.01). Following administration of CPA (250 mg/kg, p.o.), the
number of regenerative crypts significantly increased compared with
the normal group (120.9±12.1 vs. 110.5±12.6, p<0.01) and this
up-regulation was further increased by treatment with MAK
(136.6±13.8 to 150.0±14.7, a 13.0–24.1% increase vs. CPA + MF,
p<0.01). The number of regenerative crypts after administration
of CPA (200 mg/kg, i.p.) showed a significant increase compared
with the normal group (135.5±13.6 vs. 107.4±9.7, p<0.01) and
again this up-regulation was further increased by treatment with
MAK (145.1±16.3 to 152.2±17.2, a 7.1–12.3% increase vs. CPA + MF;
p<0.01). Following administration of CPA (150 mg/kg, s.c.), the
number of regenerative crypts was similar to that of the normal
group (117.8±14.9 vs. 107.4±9.7), but treatment with MAK caused a
significant up-regulation compared with the CPA-alone group
(130.4±13.8 to 160.4±17.1, a 10.7–36.2% increase vs. CPA + MF;
p<0.01). The number of regenerative crypts after the
administration of Iressa (2,000 mg/kg, p.o.) was similar to that of
the normal group (107.5±11.7 vs. 108.0±13.9), but the Iressa + MAK
group showed a significant up-regulation compared with the
Iressa-alone group (117.8±10.6 to 123.6±14.7, a 9.6–15.0% increase
vs. Iressa + MF; p<0.01). Similarly, the number of regenerative
crypts was unaffected by the administration of Iressa (4,000 mg/kg,
p.o.) (110.0±11.8 vs. 110.5±12.6 in the normal group), but was
significantly up-regulated in the Iressa + MAK group compared with
the Iressa-alone group (132.2±15.5 to 161.5±19.8, a 20.2–46.8%
increase vs. Iressa + MF; p<0.01).
 | Table IIIEffect of MAK on crypt regeneration
after administration of several anti-cancer drugs. |
Table III
Effect of MAK on crypt regeneration
after administration of several anti-cancer drugs.
| Group | No. of regenerative
crypts | Increased rate
(%) |
|---|
| UFT 1,000 mg/kg
p.o. |
| Normal | 107.6±10.4 | |
| UFT + MF | 65.8±8.1 | |
| UFT + 1.25%
MAK | 80.6±11.4a | (22.5)b |
| UFT + 2.5%
MAK | 94.2±11.5a | (43.2) |
| UFT + 5.0%
MAK | 102.7±12.5a | (56.1) |
| CDDP 12.5 mg/kg
i.p. |
| Normal | 107.6±10.4 | |
| CDDP + MF | 89.8±11.7 | |
| CDDP + 1.25%
MAK | 117.4±16.7a | (30.7) |
| CDDP + 2.5%
MAK | 121.7±12.6a | (35.4) |
| CDDP + 5.0%
MAK | 118.4±14.6a | (31.7) |
| CDDP 25 mg/kg
i.p. |
| Normal | 108.0±13.9 | |
| CDDP + MF | 9.5±4.4 | |
| CDDP + 1.25%
MAK | 21.9±4.7a | (130.5) |
| CDDP + 2.5%
MAK | 26.6±5.3a | (180.0) |
| CDDP + 5.0%
MAK | 32.5±3.4a | (242.1) |
| CPA 250 mg/kg
p.o. |
| Normal | 110.5±12.6 | |
| CPA + MF | 120.9±12.1 | |
| CPA + 1.25%
MAK | 136.6±13.8a | (13.0) |
| CPA + 2.5%
MAK | 148.7±15.0a | (23.0) |
| CPA + 5.0%
MAK | 150.0±14.7a | (24.1) |
| CPA 200 mg/kg
i.p. |
| Normal | 107.4±9.7 | |
| CPA + MF | 135.5±13.6 | |
| CPA + 1.25%
MAK | 145.1±16.3a | (7.1) |
| CPA + 2.5%
MAK | 147.0±17.4a | (8.5) |
| CPA + 5.0%
MAK | 152.2±17.2a | (12.3) |
| CPA 150 mg/kg
s.c. |
| Normal | 107.4±9.7 | |
| CPA + MF | 117.8±14.9 | |
| CPA + 1.25%
MAK | 130.4±13.8a | (10.7) |
| CPA + 2.5%
MAK | 150.6±17.0a | (27.8) |
| CPA + 5.0%
MAK | 160.4±17.1a | (36.2) |
| Iressa 2,000 mg/kg
p.o. |
| Normal | 108.0±13.9 | |
| Iressa + MF | 107.5±11.7 | |
| Iressa +1.25%
MAK | 117.8±10.6a | (9.6) |
| Iressa + 2.5%
MAK | 119.9±13.7a | (11.5) |
| Iressa + 5.0%
MAK | 123.6±14.7a | (15.0) |
| Iressa 4,000 mg/kg
p.o. |
| Normal | 110.5±12.6 | |
| Iressa + MF | 110.0±11.8 | |
| Iressa + 1.25%
MAK | 132.2±15.5a | (20.2) |
| Iressa + 2.5%
MAK | 151.5±16.9a | (37.7) |
| Iressa + 5.0%
MAK | 161.5±19.8a | (46.8) |
Histologically, no marked changes such as
desquamation, edema and/or necrosis were noted in the small
intestine following the administration of CPA or Iressa.
Discussion
MAK prevented small intestinal injury by increasing
the number of regenerative crypts, but had no effect on body weight
loss induced by 5FU, UFT and CDDP. Wang et al reported that
a G. lucidum extract ameliorated CDDP-induced nausea,
vomiting and food intake in a concentration-dependent manner in a
rat pica model measuring kaolin intake (15). In a clinical study reported by
Shu-Ru et al, a Chinese medicinal herb complex containing a
G. lucidum extract decreased leucopenia and neutropenia
induced by chemotherapy and/or radiotherapy (16). Nonaka et al reported that an
antlered form of G. lucidum (Rokkaku-reishi) relieved
CPA-induced weight loss, and suggested that G. lucidum is
useful in reducing the adverse effects of anti-cancer drugs
(17). Furthermore, Nonaka et
al reported that G. lucidum inhibited transplanted tumor
growth, elongated life span when administered orally to mice
(18) and showed anti-tumor
activity when administered after tumor inoculation.
Recently, we found that 5FU decreased the formation
of aberrant crypt foci (ACF) induced by azoxymethane, and that
combination with MAK further decreased the number of ACF (19). We suggested that MAK reduces the
gastrointestinal adverse effects of anti-cancer drugs without
attenuating their beneficial anti-tumor activity.
In the present experiment, MAK attenuated small
intestinal damage caused by 5FU, whereas AGA did not. Further
studies are required to elucidate the difference in the effects of
mycelia and fruiting bodies, although we noted earlier that AGA
does not protect against small intestinal damage by X-irradiation.
We conclude that AGA is ineffective for the prevention of acute
small intestinal injury. On the other hand, the present study
showed that MAK is active against small intestinal injury induced
by 5FU, UFT, CPA, Iressa and CDDP.
It is well established that, regardless of the
administration route, the mechanism by which anti-cancer drugs such
as 5FU, UFT and CDDP exert both their therapeutic and toxic effects
is by inhibition of DNA synthesis. Recently, Tong et al
reported that Reishi increased the uptake of BrdU in a mouse spleen
cell cultivation system and promoted cell proliferation (20). In our study, the number of
regenerative crypts increased by administration of CPA or Iressa
plus MAK, but not of MAK alone. Furthermore, we observed no marked
changes such as desquamation, edema and/or necrosis in the small
intestine after administration of CPA or Iressa. Clinically, small
intestinal injury was reported after administration of both CPA and
Iressa (21). The reason that these
two anti-cancer drugs did not affect small intestinal crypts in
this experiment is not clear, but it is conceivable that they had
some effect other than inhibition of DNA synthesis, since crypt
regeneration was accelerated by MAK after administration of these
agents. It is possible that MAK-induced crypt regeneration after
administration of these drugs is a response to a mild injury that
cannot be detected by microscopic observation.
Taken together, the results suggest that Reishi can
immediately promote cell growth to repair acute injury caused by
trauma such as X-irradiation and/or administration of
chemotherapeutic drugs. Recently, Fukatsu et al reported
that 5FU influences gut-associated lymphoid tissue (known as GALT)
by reducing the number of lymphocytes in the small intestinal
intraepithelial space and lamina propria (22). Strober et al reported that
the breakdown of GALT may contribute to chronic inflammatory bowel
disease, such as Crohn’s disease and ulcerative colitis (23). Previously, we found that MAK shows a
preventive action on the DSS-induced mouse model of ulcerative
colitis (unpublished data). In this context, the acceleration of
regenerative crypt growth after administration of 5FU, UFT, CPA and
Iressa suggests that MAK functions through a GALT-mediated
mechanism of action. It is thought that a part of the activity of
Rokkaku-reishi against the adverse effects of CPA may be mediated
by its immunomodulating properties, such as natural killer
activity, interferon-γ production, cytotoxic T-lymphocyte activity
and inhibition of abnormal changes in the interleukin-4 level
(17,18). We also previously reported that MAK
has immunomodulating functions, such as the up-regulation of
phagocytosis and NK activity and increased TNF-α production
(24). Thus, if the administration
of anti-cancer drugs such as CPA and Iressa induces immunological
dysregulation in the GALT, such effects may be reversed by the
immunomodulatory action of MAK.
Edema or cell infiltration of the fore-stomach
and/or renal injury after administration of anti-cancer drugs were
confirmed by the microscopic observations in this experiment,
although MAK appeared to have little effect on these lesions (data
not shown). Conversely, Omori et al reported that Reishi
reduced the renal disorder induced by CDDP (25). However, our study only had 3.5 day
duration and the longer-term effect of MAK on injury to these
organs needs to be established in further studies.
In conclusion, our results suggest that MAK
ameliorates the small intestinal damage induced by cancer
treatments such as radiotherapy and chemotherapy, thereby improving
the quality of life of cancer patients. Further studies of longer
duration are required to assess the value of this preparation in
the treatment of other adverse effects of anti-cancer drugs, such
as organ injury and alopecia.
References
|
1
|
Lin ZB: Pharmacological functions of
Ganoderma lucidum. Modern Research of Ganoderma Lucidum. Lin
ZB: 2nd edition. Beijing Medical University Press; pp. 284–309.
2001
|
|
2
|
Wasser SP and Weis AL: Medicinal
properties of substances occurring in higher basidiomycetes
mushrooms: current perspective. Int J Med Mushrooms. 1:31–62. 1999.
View Article : Google Scholar
|
|
3
|
Shiao MS: Natural products of the
medicinal fungus Ganoderma lucidum: occurrence, biological
activities and pharmacological functions. Chem Rec. 3:172–180.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu H, Ahn NS, Yang X, Lee YS and Kang KS:
Ganoderma lucidum extract induces cell cycle arrest and
apoptosis in MCF-7 human breast cancer cell. Int J Cancer.
102:250–253. 2002. View Article : Google Scholar
|
|
5
|
Sliva D, Labarrere C, Slivova V, Sedlak M,
Lloyd FP Jr and Ho NW: Ganoderma lucidum suppresses motility
of highly invasive breast and prostate cancer cells. Biochem
Biophys Res Commun. 298:603–612. 2002. View Article : Google Scholar
|
|
6
|
Ghafar MA, Golliday E, Bingham J,
Mansukhani MM, Anastasiadis AG and Katz AE: Regression of prostate
cancer following administration of genistein combined
polysaccharide (GCP™), a nutritional supplement: a case report. J
Altern Complement Med. 8:493–497. 2002.PubMed/NCBI
|
|
7
|
Gao Y, Zhou S, Jiang W, Huang M and Dai X:
Effects of ganopoly (a Ganoderma lucidum polysaccharide
extract) on the immune functions in advanced-stage cancer patients.
Immunol Invest. 32:201–215. 2003.PubMed/NCBI
|
|
8
|
Song YS, Kim SH, Sa JH, Jin C, Lim CJ and
Park EH: Anti-angiogenic and inhibitory activity on inducible
nitric oxide production of the mushroom Ganoderma lucidum. J
Ethnopharmacol. 90:17–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lakshmi B, Ajith TA, Sheena N, Gunapalan N
and Janardhanan KK: Antiperoxidative, anti-inflammatory and
antimutagenic activities of ethanol extract of the mycelium of
Ganoderma lucidum occurring in South India. Teratog Carcinog
Mutagen. 1:85–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu H, Uesaka T, Katoh O, Kyo E and
Watanabe H: Prevention of the development of preneoplastic lesions,
aberrant crypt foci, by a water-soluble extract from cultured
medium of Ganoderma lucidum (Rei-shi) mycelia in male F344
rats. Oncol Rep. 8:1341–1345. 2001.PubMed/NCBI
|
|
11
|
Lu H, Kyo E, Uesaka T, Katoh O and
Watanabe H: Prevention of development of
N,N′-dimethylhydrazine-induced colon tumors by a water-soluble
extract from cultured medium of Ganoderma lucidum (Rei-shi)
mycelia in male ICR mice. Int J Mol Med. 9:113–117. 2002.
|
|
12
|
Lu H, Kyo E, Uesaka T, Katoh O and
Watanabe H: A water-soluble extract from cultured medium of
Ganoderma lucidum (Rei-shi) mycelia suppresses
azoxymethane-induction of colon cancers in male F344 rats. Oncol
Rep. 10:375–379. 2003.PubMed/NCBI
|
|
13
|
Kashimoto N, Hayama M, Kamiya K and
Watanabe H: Inhibitory effect of a water-soluble extract from the
culture medium of Ganoderma lucidum (Rei-shi) mycelia on the
development of pulmonary adenocarcinoma induced by N-nitrosobis
(2-hydroxypropyl) amine in Wistar rats. Oncol Rep. 16:1181–1187.
2006.PubMed/NCBI
|
|
14
|
Kubo N, Myojin Y, Shimamoto F, Kashimoto
N, Kyo E, Kamiya K and Watanabe H: Protective effects of a
water-soluble extract from cultured medium of Ganoderma
lucidum (Rei-shi) mycelia and Agaricus blazei Murrill
against X-irradiation in B6C3F1 mice: increased small intestinal
crypt survival and prolongation of average time to animal death.
Int J Mol Med. 15:401–406. 2005.PubMed/NCBI
|
|
15
|
Wang CZ, Basila D, Aung HH, Mehendale SR,
Chang WT, McEntee E, Guan X and Yuan CS: Effects of Ganoderma
lucidum extract on chemotherapy-induced nausea and vomiting in
a rat model. Am J Chin Med. 33:807–815. 2005.
|
|
16
|
Zhuang SR, Chen SL, Tsai JH, Huang CC, Wu
TC, Liu WS, Tseng HC, Lee HS, Huang MC, Shane GT, Yang CH, Shen YC,
Yan YY and Wang CK: Effect of citronellol and the Chinese medical
herb complex on cellular immunity of cancer patients receiving
chemotherapy/radiotherapy. Phytother Res. 23:785–790. 2009.
View Article : Google Scholar
|
|
17
|
Nonaka Y, Ishibashi H, Nakai M, Shibata H,
Kiso Y and Abe S: Soothing effect of Ganoderma lucidum
antlered form on cyclophosphamide-induced adverse reaction. Jpn J
Cancer Chemother (in Japanese). 32:1586–1588. 2005.PubMed/NCBI
|
|
18
|
Nonaka Y, Ishibashi H, Nakai M, Shibata H,
Kiso Y and Abe S: Effects of the antlered form of Ganoderma
lucidum on tumor growth and metastasis in
cyclophosphamide-treated mice. Biosci Biotechnol Biochem.
72:1399–1408. 2008.PubMed/NCBI
|
|
19
|
Kashimoto K, Ishii S, Nishihama T,
Yanagida M, Hayama M and Watanabe H: Effects of a water-soluble
extract of Ganoderma lucidum mycelia (MAK) on ACF and crypt
survival by 5-FU in F344 rats. In: 67th Annual Meeting of the
Japanese Cancer Association Proceedings; pp. 3832008
|
|
20
|
Tong MH, Chien PJ, Chang HH, Tsai MJ and
Sheu F: High processing tolerances of immunomodulatory proteins in
Enoki and Reishi mushrooms. J Agric Food Chem. 56:3160–3166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inomata S, Takahashi H, Nagata M, Yamada
G, Shiratori M, Tanaka H, Satoh M, Saitoh T, Sato T and Abe S:
Acute lung injury as an adverse event of gefitinib. Anticancer
Drugs. 15:461–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukatsu K, Nagayoshi H, Maeshima Y, Ueno
C, Saitoh D and Mochizuki H: Fish oil infusion reverses
5-fluorouracil-induced impairments in mucosal immunity in mice.
Clin Nutr. 27:269–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strober W, Fuss IJ and Blumberg RS: The
immunology of mucosal models of inflammation. Annu Rev Immunol.
20:495–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kashimoto N, Kyo E, Uesaka T, Katoh O and
Watanabe H: Immunomodulation and antitumor activities of a water
soluble extract from cultured medium of Ganoderma lucidum
(Reishi) mycelia. J Jpn Mibyo Sys Assoc (in Japanese). 9:293–296.
2003.
|
|
25
|
Ohmori K, Ito M, Kishi M, Mizutani H,
Katada T and Konishi H: Effect of Reishi mushroom on side effects
induced by CDDP. Biotherapy (in Japanese). 9:95–98. 1995.
|