Introduction
Surgery is the mainstay of treatment in colorectal
cancer (CRC). However, during the past decade the role of
chemotherapy has expanded considerably. Previously dominated by
bolus injections of 5-fluorouracil (5-FU), chemotherapy has made
considerable progress in CRC by biochemical modulation of the 5-FU
effect by leucovorin (LV) (1), the
development of infused 5-FU/LV regimens (2,3) and
the introduction of two new cytotoxic drugs, irinotecan and
oxaliplatin (4–6). Particularly, 5-FU has played a central
role in the treatment of CRC, and many attempts have been made to
improve its efficacy and potentiate its action over the last 50
years.
One approach was the biochemical modulation with
compounds such as methotrexate (7)
and LV (8). Another approach was
the attempt to influence the mode of administration on the
mechanism of action and pharmacology of 5-FU. Many studies have
compared the protracted venous infusion method, also known as
continuous infusion, to the bolus infusion of 5-FU alone or in
combination with LV (9). Since
publication of the meta-analysis, several other randomized trials
have been performed to compare infusional 5-FU to bolus 5-FU
regimens. A number of infusional 5-FU regimens that were developed
have been useful in the treatment of patients with CRC. De Gramont
et al developed a regimen that contained a 400
mg/m2 5-FU bolus, 200 mg/m2/2 h LV, followed
by 600 mg/m2 5-FU over 22 h on days 1 and 2 per week. A
randomized trial demonstrated that this regimen was associated with
significantly higher response rates and a longer time to
progression compared with bolus 5-FU/LV (10). Furthermore, the clinical effect of
chemotherapy for CRC has made rapid progress with the development
of the combination regimens, 5-FU/LV/CPT-11 and
5-FU/LV/oxaliplatin. In these triplet regimens, infusional 5-FU,
derived from the De Gramont regimen, showed a better outcome than
bolus 5-FU (11). The De
Gramont-based regimen of combining a bolus and infusional
administration of 5-FU, has currently taken on a central role in
CRC chemotherapy, especially in triplet regimens.
On the other hand, our attempt was to modify the
pharmacology of 5-FU. We reported the efficacy of pharmacokinetic
modulating chemotherapy (PMC), which is based on the concept that
the benefit of a continuous intravenous 5-FU infusion can be
potentiated by low-dose oral uracil/tegafur (UFT). PMC consists of
the continuous infusion of 5-FU over 24 h for 1 day a week at 600
mg/m2/day, and an oral dose of UFT, a 5-FU derivative at
400 mg/day for 5–7 days per week, repeated every week (12–14).
Interestingly, PMC resulted in low recurrence and a high-survival
rate even in p53 mutant colorectal cancer, which is generally
chemoresistant (12). Moreover, we
experimentally revealed the potential mechanism of PMC efficacy in
CRC (15). In PMC, the infusion of
higher amounts of 5-FU once a week in combination with lower
amounts of 5-FU taken orally resulted in two different cytotoxic
effects, depending on the dose: G1-S arrest and apoptosis with
higher 5-FU concentrations, and G2-M arrest and mitotic catastrophe
with lower 5-FU concentrations. Thus, PMC is characteristic in that
efficacy is obtained from a new type of infusional 5-FU, and
potentiation of 5-FU via cell-cycle regulation may play an
additional role in multi-drug regimens as well as FOLFIRI or
FOLFOX. Theoretically, it is also conceivable that LV plus PMC
constitute an ideal 5-FU/LV treatment, since LV is a reduced folate
that is easily metabolized to an essential cofactor in the
inhibition of thymidylate synthase by FdUMP, an active form of 5-FU
(16). On the basis of these
results, we therefore conducted a phase II study to assess the
efficacy and safety of LV plus PMC as a front-line chemotherapy in
patients with metastatic CRC.
Patients and methods
Patient eligibility
Patients with metastatic CRC were eligible for
enrollment in the study. Other eligibility criteria were:
histologically or cytologically confirmed advanced CRC or
postoperative recurrent cancer with metastasis to other organs
(liver, lung and lymph nodes); at least one measurable lesion to
Response Evaluation Criteria in Solid Tumours (RECIST) criteria
(17); no prior chemotherapy
(patients receiving postoperative chemotherapy with oral
fluoropyrimidines or 5-FU/LV were acceptable if recurrence occurred
at least 24 weeks after the completion of such therapy) or
radiotherapy; age between 20 and 79 years; an Eastern Cooperative
Oncology Group performance status of 0–2; a life expectancy >12
weeks from the start of treatment; acceptable major organ function
(white blood cell count between 4,000 and 12,000 mm3,
platelet count >100,000 mm3, haemoglobin >9.5
g/dl, serum AST/ALT <2.5 times the institutional upper limit of
normal (ULN), serum total bilirubin <1.5 times the ULN, serum
creatinine < ULN and normal electrocardiogram). Written informed
consent was required and the study was approved by the ethics
boards of the participating centres.
Chemotherapy schedule
Patients received PMC, which consists of continuous
infusion of 5-FU over 24 h for 1 day/week at 600
mg/m2/day and an oral dose of UFT at 400 mg/day for 5
days/week, repeated every week. On days 1, 8 and 15, l-LV (250
mg/m2) was administered as a 2-h infusion with PMC. One
treatment course consists of 3 cycles of l-LV plus PMC, followed by
2 cycles of PMC alone. Treatment courses were repeated every 5
weeks until evidence of disease progression, unacceptable toxicity
or patient refusal. Patients then received second-line chemotherapy
based on the preference of their attending physician.
Treatment criteria
Prior to receiving treatment on days 8, 15, 22 and
29, each patient was screened to ensure that the white blood cell
count was >3,000 mm3; neutrophil count was >1,500
mm3; platelet count was >100,000 mm3;
temperature was <38°C and that no detectable infection nor
diarrhoea, or other toxicities more than grade 2, assessed
according to the National Cancer Institute Common Toxicity Criteria
(NCI-CTC) version 2 were apparent (18). The scheduled dose was not
administered when any of the criteria described above was not
fulfilled. Treatment was suspended until the patient recovered.
However, if administration criteria were not fulfilled for 5 weeks,
the patient was removed from the study.
Dose modification criteria
Patients were checked for toxicity during each cycle
and the doses of 5-FU were reduced according to the dose
modification criteria. 5-FU was reduced by 25% of the previous dose
in the case of more than grade 3 haematologic (leukopenia,
neutropenia and thrombocytopenia) and non-haematologic toxicity
(excluding nausea, vomiting, anorexia and alopecia). If performance
status was >3 after chemotherapy was administered, the patient
was removed from the study.
Endpoints and evaluation criteria
The primary object of the study was response rate
(RR), with toxicity and overall survival (OS) being secondary.
Responses were classified according to RECIST criteria (18). Tumour measurement was independently
reviewed by a radiologist, who was blinded from the tumour
assessments carried out by the investigators. Patients who received
at least one treatment course were considered assessable for
response and toxicity. OS was calculated from the first day of the
treatment to the date patients succumbed to the disease or last
follow-up. Toxicity was monitored according to the NCI-CTC version
2.
Sample size
The study comprised a meta-analysis of 6 randomized
trials involving 1,219 patients with CRC compared to bolus and
infusional 5-FU. The response rate was significantly higher with
infusional 5-FU (22 vs. 14%) (1).
Our PMC revealed the response rate of <30% in our experiences,
while 5-FU/LV achieved response rates of 20–30% in patients
receiving initial chemotherapy (1,8).
Accordingly, 30% was considered to be the expected response rate
and ±15% as the 95% confidence interval. Thus, the required number
of patients was estimated to be 35 patients. Therefore, the target
number of patients was set at 37 to allow for some exclusions from
analysis.
Results
Patient characteristics
From April 2002 to April 2005, 37 patients were
enrolled. The baseline characteristics are listed in Table I. The median age was 63 years
(range, 38–77). Patients had a histologically proven adenocarcinoma
of the colon and rectum. Nineteen (51.4%) had liver metastasis and
6 (16.2%) had lung metastasis. Three (8.1%) patients had both liver
and lung metastasis. Six (16.2%) had peritoneal metastasis
including ovarian. Three (8.1%) had lymph node metastasis and 2
(5.4%) had local recurrence. The patients had metastatic disease at
the time of study entry.
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| No. of patients | 37 |
| Gender |
| Male | 21 |
| Female | 16 |
| Median age
(range) | 65 (38–77) |
| PS |
| 0 | 27 |
| 1 | 10 |
| Prior treatment |
| None | 12 |
| Surgery | 25 |
| Tumour |
| Primary | 25 |
| Recurrent | 12 |
| Histology |
|
Well-differentiated | 10 |
| Moderately
differentiated | 13 |
| Poorly
differentiated | 2 |
| Mucinous | 2 |
| Sites of
metastasis |
| Liver | 19 (51.4%) |
| Lung | 6 (16.2%) |
| Peritoneal
dissemination | 6 (16.2%) |
| Lymph nodes | 3 (8.1%) |
| Local | 2 (5.4%) |
Treatment and drug delivery
In total, 595 cycles were administered with a median
of 15 cycles per patient (range, 3–48 cycles). The delivered
relative dose intensities were 91% for 5-FU.
Tumour response and survival
Thirty-five patients were assessable for tumour
response. The objective response rate was 31.4% (95% CI,
23.3–38.7%). There was complete response (CR) in 2 patients,
partial response (PR) in 9, stable disease (SD) in 19 and
progressive disease (PD) in 5 patients (according to RECIST). The
tumour stabilization rate (including SD) was 85.7% (95% CI,
73.6–96.5%).
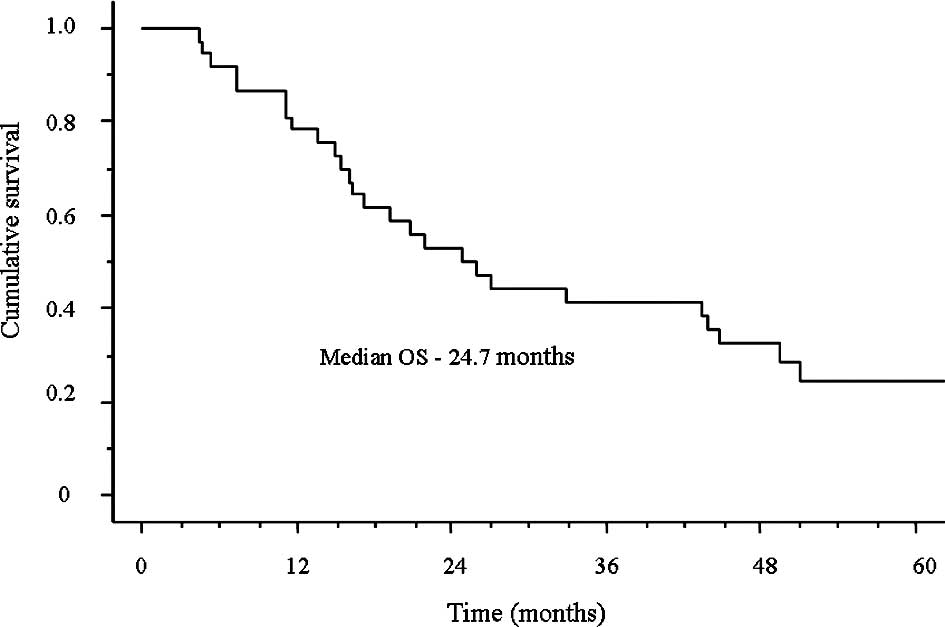
The median survival time (MST) was 24.7 months and
the median follow-up time was 21.9 months (range, 4.5–84.6;
Fig. 1). Furthermore, the 1-year
survival rate was 78.3% and the 2-year survival rate was 52.8%.
Toxicity
Patients were assessable for safety. Toxic effects
observed during the study are listed in Table II. The most common toxic effects
were neutropenia and hand-foot skin reaction. Eight patients
(21.6%) experienced neutropenia during their course of therapy,
although more than grade 3 neutropenia was detected in only 1
patient (2.7%). Nine patients (24.3%) experienced hand-foot skin
reactions, of which 7 had grade 1, while 2 had grade 2. There was
no treatment-related death or life-threatening toxicity at grades
3–4. Grade 3 nausea, diarrhoea and oesophagitis occurred in 1
patient each (2.7%). Only 1 patient was admitted to the emergency
room for paralysis of the lower body due to cervical
metastasis.
 | Table IIToxic profile. |
Table II
Toxic profile.
| Toxicity | Grade | ≥Grade 3 | Total |
|---|
|
| | |
|---|
| 1 | 2 | 3 | 4 | | |
|---|
| Haematological |
| Neutropenia | 6 | 1 | 1 | 0 | 1 (2.7%) | 8 (21.6%) |
|
Non-haematological |
| Hand-foot skin
reaction | 7 | 2 | 0 | 0 | 0 (0.0%) | 9 (24.3%) |
| Fatigue | 1 | 2 | 0 | 0 | 0 (0.0%) | 3 (8.1%) |
| Anorexia | 6 | 0 | 0 | 0 | 0 (0.0%) | 6 (16.2%) |
| Nausea | 0 | 0 | 1 | 0 | 1 (2.7%) | 1 (2.7%) |
| Vomiting | 1 | 0 | 0 | 0 | 0 (0.0%) | 1 (2.7%) |
| Diarrhoea | 3 | 3 | 1 | 0 | 1 (2.7%) | 7 (18.9%) |
| Oesophagitis | 0 | 0 | 1 | 0 | 1 (2.7%) | 1 (2.7%) |
Discussion
The pharmacokinetics of 5-FU are influenced by the
dose and schedule of administration. Particularly, a number of
infusional 5-FU regimens have been developed that are useful in the
treatment of patients with CRC. In general, these regimens have a
more favourable toxicity profile compared with bolus 5-FU/LV.
However, the principal mechanism of action of 5-FU with various
clinical schedules has yet to be clearly defined. Both 5-FU
concentration and duration of exposure influence the mechanism of
cytotoxicity. Short-term, high-concentration exposures are thought
to favour RNA-directed 5-FU toxicity, whereas DNA-directed effects
are felt to be more prominent with longer exposures to lower drug
concentration (19–21). These pathways are not mutually
exclusive, and more than one mechanism of action may contribute to
cytotoxicity. Therefore, bolus and infusional 5-FU represents
another strategy to improve outcomes by potentially allowing more
than one cytotoxic mechanism to occur (10,21).
This may support recent standard intravenous 5-FU regimens
consisting of the bolus and infusional 5-FU in combination with LV,
derived from the De Gramont regimen.
We attempted to produce another effective
chemotherapy using a 5-FU-based combination, since the Japanese
national insurance did not allow the use of LV in the treatment of
CRC until 1999. Kusunoki et al designed a regimen of PMC in
1989, involving continuous the intravenous. infusion of 5-FU for 24
h/week and oral administration of UFT twice a day for 5–7 days/week
(12,13), based on experiments using rat models
by Fujii et al (22). UFT is
a combination of tegafur, a prodrug of 5-FU, and uracil at a molar
ratio of 1:4. Dihydropyrimidine dehydrogenase, a key enzyme that
degrades 5-FU into therapeutically inactive metabolites, catalyzes
the reduction of 60–90% of administered 5-FU, and its catalytic
activity correlates with the rate of 5-FU clearance. Uracil
inhibits hepatic dihydropyrimidine dehydrogenase, and thus enhances
the plasma 5-FU level and antitumour activity of 5-FU (23). Our PMC regimen has improved the
prognosis of patients with CRC over the past 10 years (12–15,24).
PMC showed an improved prognosis of irradiated rectal cancer with
p53 overexpression (12). This
result suggested that the antitumour property of 5-FU is enhanced
by rectal tumours with a loss of the p53-related apoptotic pathway.
In addition, an in vitro study showed that efficacy of the
PMC regimen is based on targeting at least two different phases of
the cell cycle, regardless of the status of the p53 gene. Surgical
specimens after PMC suggested the coexistence of mitotic
catastrophe and apoptosis in these specimens (15).
The current study identified the efficacy and safety
of LV plus PMC as a front-line chemotherapy in patients with
metastatic CRC. Although the response rate of LV plus PMC appeared
to be similar to conventional 5-FU/LV, a high tumour stabilization
rate was obtained (85.7%) and the OS was 24.7 months. OS in excess
of 20 months may be the result of treatments following LV plus PMC.
Nineteen of the 37 patients (51.4%) received LV plus PMC, combined
with CPT-11 as the second-line chemotherapy. Since Japanese
national insurance only allowed the use of oxaliplatin in the
treatment of CRC in 2005, we also used the FOLFOX regimen as
second- or third-line chemotherapy. Furthermore, we attempted
secondary surgery or radiofrequency thermal ablation to remove
metastases in 4 patients who obtained tumour stabilization after
second-line chemotherapy. Thus, many factors, as well as LV plus
PMC, affect the survival of patients with CRC. However, it is
conceivable that LV augments the original PMC characteristics with
an enhanced efficacy from a new type of infusional 5-FU and
potentiation of 5-FU via cell cycle regulation.
In a recent comparative study, we revealed that the
use of chemotherapeutic agents or regimens against CRC differed
among countries (25). Regional
characteristics as to the administration of standard 5-FU were
actually identified as factors favouring the use of infused 5-FU/LV
in the EU, and oral fluoropyrimidines in Japan. The study concluded
that an increasing mutual understanding of regional characteristics
along with global evidence could result in standardized treatments
suited for regional characteristics, thereby prolonging patient
survival. This suggests that the conventional 5-FU/LV regimen is
open to further improvement. In addition, recent reports also
suggested that 5-FU pharmacokinetics remain an important factor for
prognosis in patients with CRC, even after progression of modern
chemotherapy (26,27). Therefore, we anticipate that the De
Gramont-based regimen is not necessarily going to be the standard
5-FU/LV regimen in the future. The concept of pharmacokinetic
modulation for either the PMC or De Gramont regimen resemble each
other, and our study suggested that the optimal administration of
5-FU/LV has yet to be defined.
In conclusion, we identified the usefulness of a new
type of infusional 5-FU combined with LV for the treatment of CRC.
The combination of PMC and LV could be a baseline 5-FU/LV with an
acceptable rate of toxicity in the first-line treatment of advanced
CRC.
Acknowledgements
This study was conducted by the Mie Colorectal
Cancer Chemotherapy Study Group. Study investigators: Yoshiyuki Ito
and Toshio Kato (Toyama Hospital, Mie, Japan); Toru Masuda and Eiki
Ojima (Mie Prefecture General Medical Centre, Mie, Japan); Kazuo
Matsumoto (Shingu Municipal Medical Centre, Wakayama, Japan);
Tatsushi Kitagawa and Koichi Matsumoto (Yokkaichi Social Insurance
Hospital, Mie, Japan); Tsuneki Kinoshita and Keiji Iriyama (Kuwana
Municipal Hospital, Mie, Japan); Hisashi Urata and Kenji Takeuchi
(Nabari Municipal Hospital, Mie, Japan).
References
|
1
|
Meta-analysis Group in Cancer. Efficacy of
intravenous continuous infusion of fluorouracil compared with bolus
administration in advanced colorectal cancer. J Clin Oncol.
16:301–308. 1998.PubMed/NCBI
|
|
2
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer. Irinotecan Study Group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douillard JY, Cunningham D, Roth AD, et
al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: a multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Gramont A, Figer A, Seymour M, et al:
Leucovorin and fluorouracil with or without oxaliplatin as
first-line treatment in advanced colorectal cancer. J Clin Oncol.
18:2938–2947. 2000.
|
|
5
|
Giacchetti S, Perpoint B, Zidani R, et al:
Phase III multicenter randomized trial of oxaliplatin added to
chronomodulated fluorouracil-leucovorin as first-line treatment of
metastatic colorectal cancer. J Clin Oncol. 18:136–147. 2000.
|
|
6
|
Wagner JS, Adson MA and van Heerden JA:
The natural history of hepatic metastases from colorectal cancer: a
comparison with resective treatment. Ann Surg. 199:502–508. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Advanced Colorectal Cancer Meta-Analysis
Project. Modulation of fluorouracil by leucovorin in patients with
advanced colorectal cancer: evidence in terms of response rate.
Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol.
10:896–903. 1992.PubMed/NCBI
|
|
8
|
Thirion P, Michiels S, Pignon JP, et al:
Modulation of fluorouracil by leucovorin in patients with advanced
colorectal cancer: an updated meta-analysis. J Clin Oncol.
22:3766–3775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hansen RM, Quebbeman E and Anderson T:
5-Fluorouracil by protracted venous infusion. A review of current
progress. Oncology. 46:245–250. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeGramont A, Bosset JF, Milan C, et al:
Randomized trial comparing monthly low-dose leucovorin and
fluorouracil bolus with bimonthly high-dose leucovorin and
fluorouracil bolus plus continuous infusion for advanced colorectal
cancer: a French intergroup study. J Clin Oncol. 15:808–815.
1997.
|
|
11
|
Goldberg RM, Sargent DJ, Morton RF, et al:
A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar
|
|
12
|
Kusunoki M, Yanagi H, Kotera H, Noda M and
Yamamura T: Effects of pharmacokinetic modulating chemotherapy
using oral UFT and continuous venous 5FU infusion on the prognosis
of irradiated rectal carcinomas with p53 overexpression. Int J
Oncol. 13:653–657. 1998.
|
|
13
|
Kusunoki M, Yanagi H, Noda M and Yamamura
T: The usefulness of pharmacokinetic modulating chemotherapy (UFT
plus 5FU) in the treatment of unresectable colorectal carcinomas.
Oncol Rep. 6:547–552. 1998.PubMed/NCBI
|
|
14
|
Kusunoki M, Yanagi H, Noda M, Yoshikawa R
and Yamamura T: Results of pharmacokinetic modulating chemotherapy
in combination with hepatic arterial 5-fluorouracil infusion and
oral UFT after resection of hepatic colorectal metastases. Cancer.
15:1228–1235. 2000. View Article : Google Scholar
|
|
15
|
Yoshikawa R, Kusunoki M, Yanagi H, et al:
Dual antitumor effects of 5-fluorouracil on the cell cycle in
colorectal carcinoma cells: a novel target mechanism concept for
pharmacokinetic modulating chemotherapy. Cancer Res. 1:1029–1037.
2001.
|
|
16
|
Kubota T, Fujita S, Kodaira S, et al:
Antitumor activity of fluoropyrimidines and thymidylate synthetase
inhibition. Jpn J Cancer Res. 82:476–482. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
NCI Common Toxicity Criteria Version 2.0.
Bethesda: National Cancer Institute, Division of Cancer Treatment;
1999
|
|
19
|
Aschele C, Sobrero A, Faderan MA and
Bertino JR: Novel mechanism(s) of resistance to 5-fluorouracil in
human colon cancer (HCT-8) sublines following exposure to two
different clinically relevant dose schedules. Cancer Res.
52:1855–1864. 1992.PubMed/NCBI
|
|
20
|
Sobrero AF, Aschele C, Guglielmi AP, et
al: Synergism and lack of cross-resistance between short-term and
continuous exposure to fluorouracil in human colon adenocarcinoma
cells. J Natl Cancer Inst. 85:1937–1944. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobrero AF, Aschele C and Bertino JR:
Fluorouracil in colorectal cancer - a tale of two drugs:
implications for biochemical modulation. J Clin Oncol. 15:368–381.
1997.PubMed/NCBI
|
|
22
|
Fujii S, Ikenaka K, Fukushima M and
Shirasaka T: Effect of uracil and its derivatives on antitumor
activity of 5-fluorouracil and
1-(2-tetrahydrofuryl)-5-fluorouracil. Gann. 69:763–772.
1978.PubMed/NCBI
|
|
23
|
Fujii S, Kitano S, Ikenaka K and Shirasaka
T: Effect of co-administration of uracil or cytosine on the
anti-tumor activity of clinical doses of
1-(2-tetrahydrofuryl)-5-fluorouracil and level of 5-fluorouracil in
rodents. Gann. 70:209–214. 1979.PubMed/NCBI
|
|
24
|
Inoue Y, Miki C, Hiro J, et al: Improved
survival using multi-modality therapy in patients with lung
metastases from colorectal cancer: A preliminary study. Oncol Rep.
14:1571–1576. 2005.PubMed/NCBI
|
|
25
|
Inoue Y, Toiyama Y, Tanaka K, Miki C and
Kusunoki M: A comprehensive comparative study on the
characteristics of colorectal cancer chemotherapy. Jpn J Clin
Oncol. 39:367–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamelin E, Delva R, Jacob J, et al:
Individual fluorouracil dose adjustment based on pharmacokinetic
follow-up compared with conventional dosage: results of a
multicenter randomized trial of patients with metastatic colorectal
cancer. J Clin Oncol. 26:2099–2105. 2008. View Article : Google Scholar
|
|
27
|
Di Paolo A, Lencioni M, Amatori F, et al:
5-fluorouracil pharmacokinetics predicts disease-free survival in
patients administered adjuvant chemotherapy for colorectal cancer.
Clin Cancer Res. 14:2749–2755. 2008.PubMed/NCBI
|