Introduction
Colorectal carcinoma (CRC) is one of the most common
malignancies worldwide. Each year in the United States and Canada,
CRC is diagnosed in >160,000 people and ~65,000 succumb to the
disease, accounting for at least 10% of all cancer deaths. The
lifetime risk of developing CRC is 1 in 17, affecting men and women
alike, with 90% of cases occurring after the age of 50 years. Of
note is that the incidence of CRC has increased substantially in
Asia during the past few decades (1).
Neutrophil gelatinase-associated lipocalin (NGAL) is
a 25-kDa secreted protein of the lipocalin superfamily (2). An elevated NGAL expression is observed
in human cancers such as breast, pancreatic, ovarian and
cholesteatoma as well as esophageal squamous cell carcinoma
(3–7). Our previous studies demonstrated that
the over-expression of NGAL plays an important role in the
malignant transformation of human immortalized esophageal
epithelial cells and it is involved in the invasion of esophageal
squamous cell carcinoma cells (8–10).
Others have reported very high expression levels of NGAL in colonic
epithelium in areas of inflammation, with a weak expression
occasionally seen in some epithelial cells of normal colon
(11). However, Lee et al
found that the ectopic expression of NGAL in colon cancer cells had
little effect on their growth and viability (12). Conflicting results have been
reported from various laboratories. Consequently, the clinical
importance of NGAL expression in CRC remains unsettled.
Lipocalins are characterized by multiple molecular
recognition properties, including the ability to bind to cell
surface receptors (13). Several
studies found that NGAL binds iron and delivers the latter to cells
(14–16). Devireddy et al isolated a
specific cell-surface receptor (24p3R/NGALR) for lipocalin 24p3, a
highly conserved murine homolog of NGAL, and demonstrated that the
expression of this receptor conferred on cells the ability to take
up iron or undergo apoptosis depending on the state of 24p3,
independent of the cell type (17).
However, the expression patterns and specific features of NGALR in
CRC are still unknown. Therefore, this study aimed to investigate
the expression of NGAL and NGALR in CRC specimens, and determine
any relationship between the expression of these proteins and
tumour progression.
Materials and methods
Cases and clinical parameters
This study was approved by the ethics committee of
the Medical College of Shantou University. Written informed consent
to use resected samples for research was obtained from patients
undergoing surgery.
For this retrospective study, archival
formalin-fixed, paraffin-embedded specimens from 102 primary CRC
patients were obtained from the Pathology Department of the Medical
College of Shantou University, collected between 1992 and 2006.
The patients were 53 males and 49 females (median
age 57 years, range 20–81). All patients except one were deceased
at the end of follow-up.
Immunohistochemical staining and
scoring
Briefly, each tissue section was de-paraffinised,
rehydrated and then incubated with fresh 3% hydrogen peroxide for
10 min. After PBS rinse, antigen retrieval from the tissue was
carried out by autoclaving in 0.01 M citrate buffer (pH 6.0) at
120°C for 3 min. Two drops (100 μl) or enough to completely cover
tissue of the primary antibody were applied to each section and
incubated in a moist chamber for 30 min. After the PBS rinse, the
tissue sections were incubated for 10 min at room temperature with
HRP polymer conjugate. Subsequently, they were stained with 0.003%
3,3-diaminobenzidine tetrahydrochloride and 0.005% hydrogen
peroxide in 0.05 M Tris•HCl (pH 7.2), counterstained with Mayer’s
hematoxylin, dehydrated and mounted.
Negative controls were prepared by substituting PBS
for primary antibody. The polyclonal rabbit NGALR antibody was
raised using a C-terminal NGALR peptide (CDHVPLLATPNAL) as the
immunogen and then affinity-purified on a peptide-coupled Sepharose
column (Beijing Biosynthesis Biotechnology Co., Ltd). This antibody
has been selected for its ability to recognize human NGALR. Rat
anti-human NGAL antibody (1:50 dilution, R&D Systems, USA),
rabbit anti-human ferritin antibody (1:2000 dilution, Sigma, USA),
mouse anti-Ki-67 nuclear antigen (1:100 dilution, Golden Bridge
International) and NGALR (1:10 dilution) were used.
The SuperPicTure Polymer Detection and Liquid DAB
Substrate kits (Zymed, Carlsbad, CA, USA) were used to carry out
immunohistochemical staining. Positive samples were defined as
those showing brown signals in the cell cytoplasm or nucleus. When
>5% of cells in a given specimen were positively stained, it was
defined as a positive case.
Statistical analysis
The association between NGAL or NGALR expression and
clinicopathological features of patients was analysed by the
Chi-square or Fisher’s exact probability test. The concordance of
NGAL and NGALR, NGAL and ferritin, or NGALR and Ki67 expression was
determined by the κ test. The significance of NGAL or NGALR
expression levels to patient survival was examined using the
Kaplan-Meier method and log-rank test. Statistical analyses were
performed using SPSS for Windows (Version 13.0). The accepted level
of significance was P<0.05.
Results
Overexpression of NGAL is significantly
associated with tumour invasion in CRC
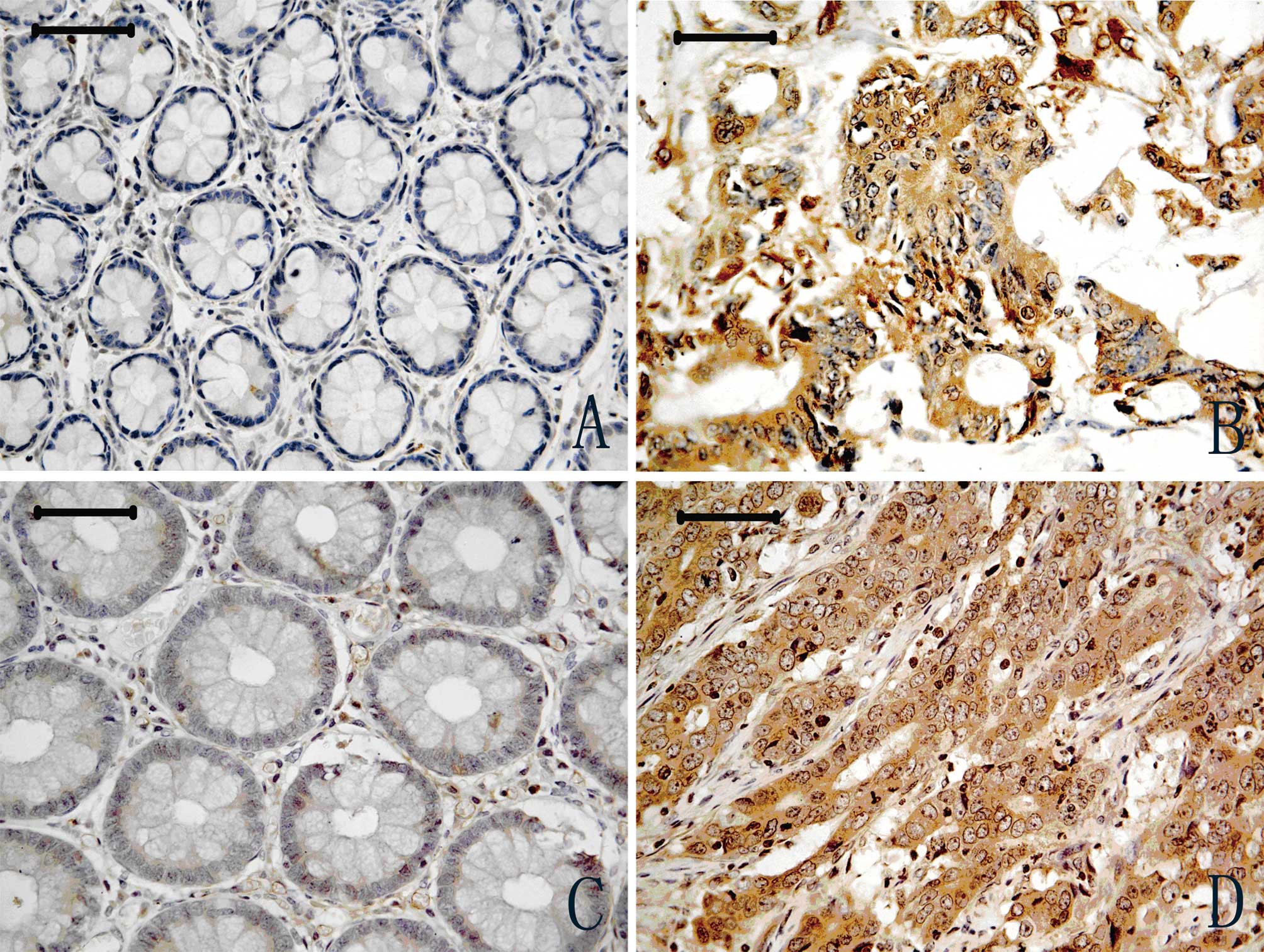
Representative results of NGAL immunostaining are
shown in Fig. 1. Cytoplasm
positivity for NGAL staining was observed in tumour tissues
(Fig. 1B), and a weak expression
was observed in adjacent normal colon tissues (Fig. 1A). A total of 102 CRC cases were
included in the final analysis. NGAL expression was significantly
increased in CRC (29/102, 28.4%) compared with adjacent normal
tissues (0/81, 0.0%) (P<0.001). The percentage of NGAL
positivity was significantly associated with CRC invasion
(P<0.01), and NGAL expression appeared to intensify with the
development of the cancer invasion. No significant association was
observed between NGAL expression and cell differentiation, although
the P-value was borderline (P=0.057). NGAL expression was not
significantly associated with age, gender, cell differentiation,
lymph node metastasis or Tumor, Node and Metastasis (TNM) stages
(P>0.05; Table I) in patients
with CRC. No significant association between NGAL expression and
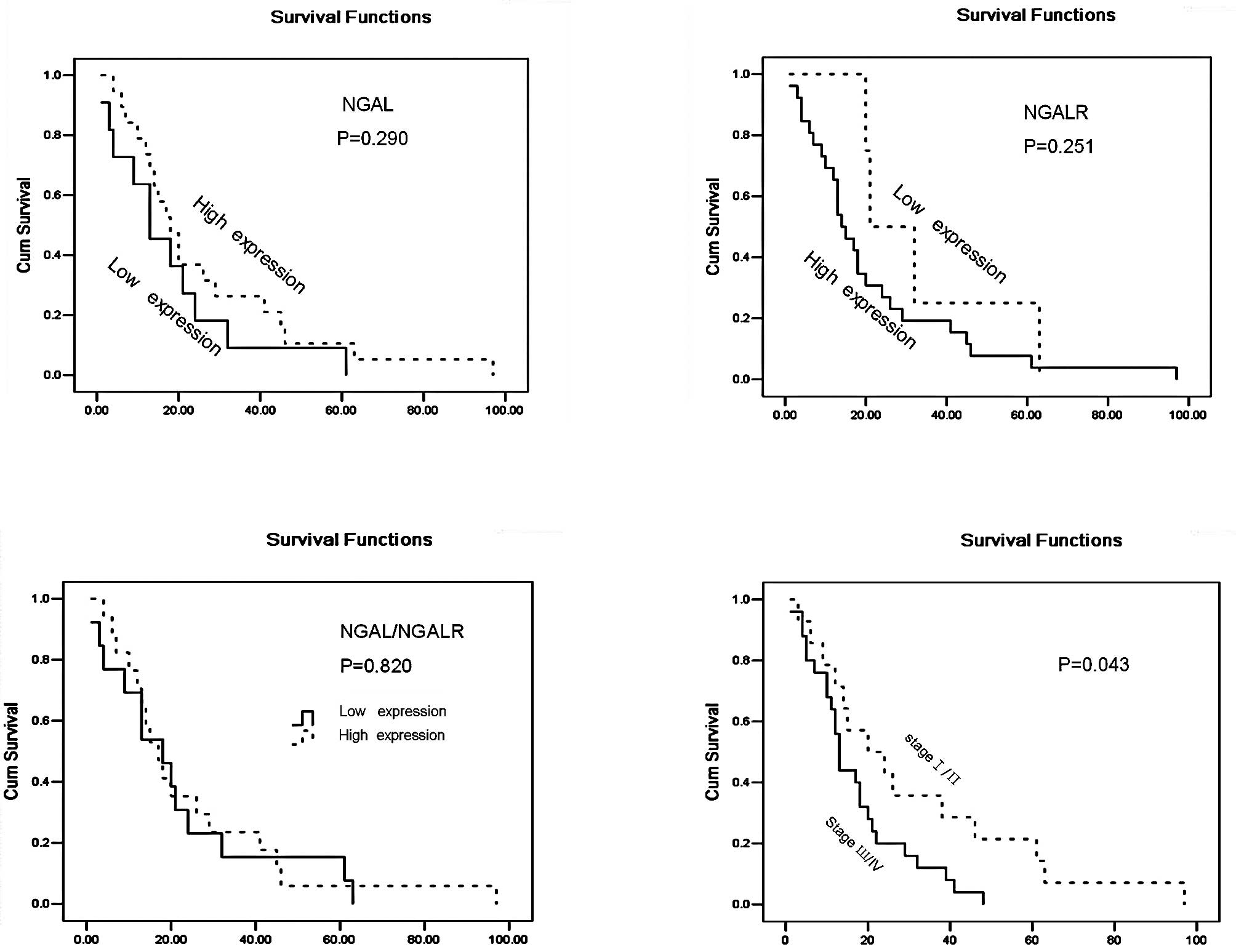
patient survival was noted (P=0.290) (Fig. 3).
 | Table IRelationship between
clinicopathological features and NGALR and/or NGAL positivity. |
Table I
Relationship between
clinicopathological features and NGALR and/or NGAL positivity.
| Parameters | NGALR | NGAL | NGAL/NGALR |
|---|
|
|
|
|
|---|
| % positive rate | P-value | % positive rate | P-value | % positive
ratea | P-value |
|---|
| Mean age, years
(range) |
| <57 (46) | 39.1 (18/46) | 0.587 | 30.4 (14/46) | 0.684 | 19.6 (9/46) | 0.825 |
| ≥57 (56) | 33.9 (19/56) | | 26.8 (15/56) | | 17.9 (10/56) | |
| Gender |
| Male | 30.2 (16/53) | 0.184 | 22.4 (11/49) | 0.198 | 17.0 (9/53) | 0.675 |
| Female | 42.9 (21/49) | | 34.0 (18/53) | | 20.4 (10/49) | |
| Regional lymph
node |
| N0 | 28.8 (15/52) | 0.112 | 25.0 (13/52) | 0.593 | 13.5 (7/52) | 0.172 |
| N1 | 44.0 (22/50) | | 32.0 (16/50) | | 24.0 (12/50) | |
| Primary tumour |
| T1/T2 | 10.5 (2/19) | 0.018 | 10.5 (2/19) | 0. 026 | 5.3 (1/19) | 0.049 |
| T3 | 38.3 (23/60) | | 26.6 (16/60) | | 16.7 (10/60) | |
| T4 | 52.2 (12/23) | | 47.8 (11/23) | | 34.8 (8/23) | |
| Histopathology |
|
Well-differentiated | 22.7 (5/22) | 0.193 | 9.1 (2/22) | 0.057 | 0 (0/22) | 0.004 |
| Moderately
differentiated | 38.0 (27/71) | | 32.4 (23/71) | | 21.3 (15/71) | |
| Poorly
differentiated | 55.6 (5/9) | | 44.4 (4/9) | | 44.4 (4/9) | |
| TNM stages |
| I/Пa | 23.7 (9/38) | 0.042 | 23.7 (9/38) | 0.413 | 7.9 (3/38) | 0.032 |
| Пb/Ш/IV | 43.6 (28/64) | | 31.3 (20/64) | | 25.0 (16/64) | |
| Colorectal
cancer | 36.3 (37/102) | 0.000 | 28.4 (29/102) | 0.000 | | |
| Normal mucosa | 8.6 (8/93) | | 0 (0/81) | | | |
Overexpression of NGALR is significantly
associated with tumour invasion and TNM stages in CRC
NGALR-positive staining was observed in the
cytoplasm of CRC tumour cells (Fig.
1D), whereas weak positive staining was observed in a
restricted intracellular area close to the cell membrane in
adjacent normal tissues (Fig. 1C).
The number of CRC specimens staining positive for NGALR (37/102,
36.6%) was significantly higher than that for normal glandular
organ (8/93, 8.6%) (P<0.01). NGALR expression was strongly
associated with deep cancer invasion and with later TNM stages
(P<0.05 and P<0.05). No significant association was found
between NGALR expression and gender, age or lymph node metastasis.
A significant association was found between tumour TNM stages and
patient survival (P=0.043; Fig. 3).
However, there was no significant association between NGALR
expression levels and patient survival (Fig. 3).
NGAL and NGALR co-expression is
correlated with deeper invasion and tumour progression in patients
with CRC
A positive correlation between NGAL and NGALR
protein expression was observed in the 102 CRC cases (r=0.432;
P<0.01). We therefore analyzed the relationship between NGAL and
NGALR expression in these cases and the clinicopathological
factors. The cases in which NGAL and NGALR were both positive
(NGAL/NGALR co-expression) were stratified into group I (n=19,
18.6%), the positive group, while the rest of the cases were put
into group П (n=83, 71.4%), the negative group. Statistical
analysis showed that the two groups exhibited differences in
invasion (P<0.05), cell differentiation (P<0.01) and TNM
stages (P<0.05). No significant associations with age, gender
and patient survival were found for either group (Table I; Fig.
3).
NGAL and NGALR expression is associated
with iron transport in cancer cells and increased cell
proliferation
To explore whether a high expression of NGAL and
NGALR is associated with iron transport and cell proliferation, we
measured the expression level of ferritin and Ki67 in CRC cells by
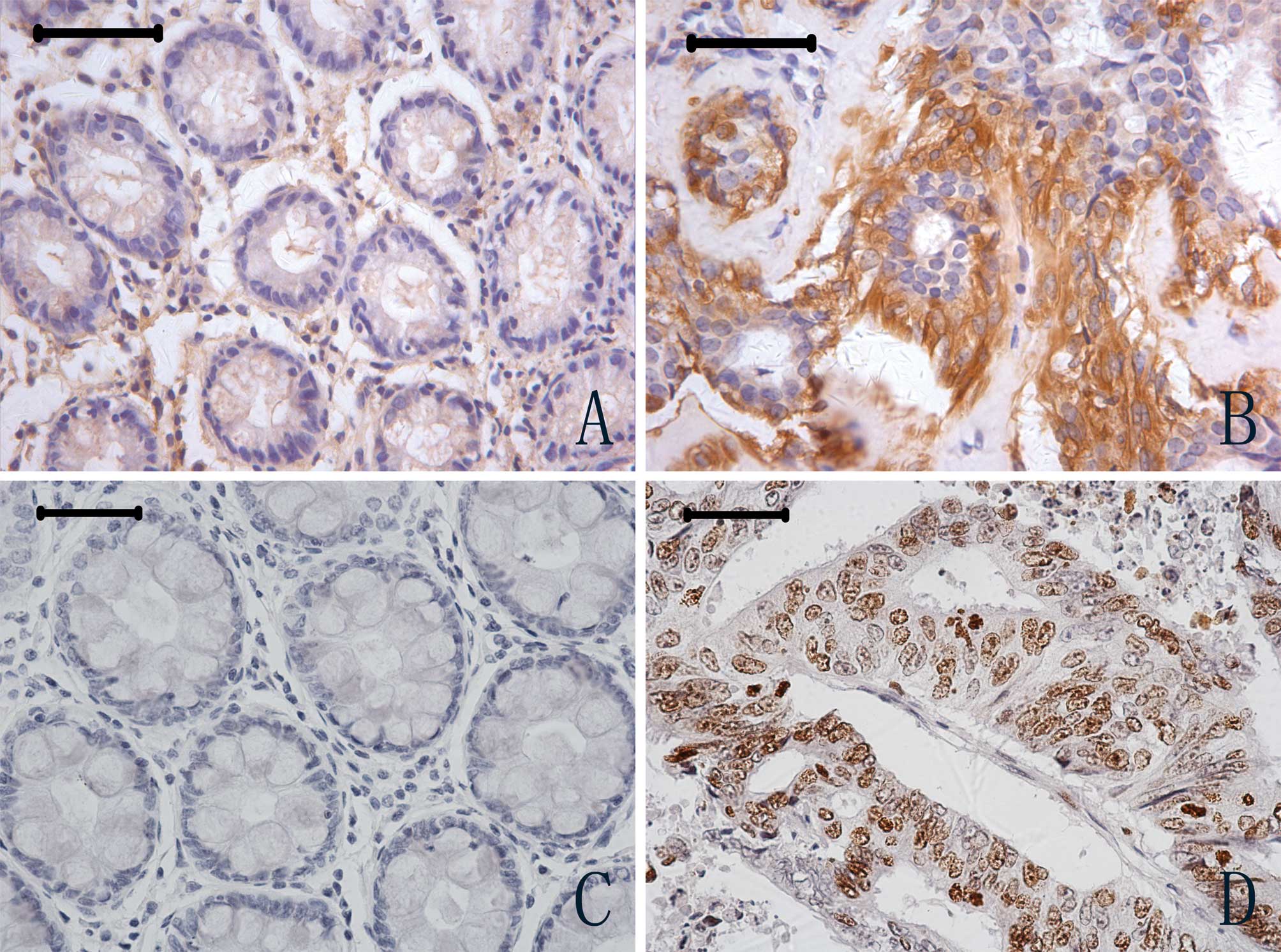
immunohistochemistry. Ferritin-positive staining was observed in
the cytoplasm of tumour and normal cells (Figs. 2A and B). However, of the 102 tumor
cases examined, ferritin expression was positive in 40 (39.2%),
whereas of the 57 normal specimens examined, ferritin expression
was positive in only 2 cases (3.51%); a statistically significant
difference (P<0.01). Expression of Ki67 was observed in the
nucleus of CRC tumour and normal cells (Figs. 2C and D). Of the 102 tumour
specimens examined, Ki67 expression was positive in 54 cases
(52.9%), compared to 7 positive specimens in 44 normal cases
(15.9%); again a significant difference in expression levels
(P<0.01).
As for the relationships among the proteins
themselves, there were significant positive associations between
the expression of NGAL and ferritin (r=0.374, P<0.001), as well
as NGALR and Ki67 (r=0.228, P<0.05). An analysis of the
relationship between NGAL/NGALR co-expression and the expression of
ferritin or Ki67 revealed significantly positive correlations for
NGAL/NGALR co-expression with ferritin (r=0.349, P<0.001) and
Ki67 (r=0.205; P<0.05) in CRC.
Discussion
NGAL, a member of the lipocalin family, was
originally discovered as a protein stored in specific granules of
the human neutrophil (2). Our group
reported that the over-expression of NGAL is associated with poor
differentiation of oesophageal squamous cell carcinoma (3). In the present study, we found that the
expression of NGAL is significantly increased in CRC tissues, and
that NGAL/NGALR co-expression is associated with poor cellular
differentiation.
A series of studies suggested that NGAL is a novel
iron transporter with functions distinct from those of transferrin
(16–18). Moreover, a specific cell-surface
receptor of 24p3/NGAL (24p3R/NGALR) was cloned (17). Over-expression of 24p3R/NGALR in
cells induces binding and the uptake of 24p3/NGAL, which results in
specific biological responses: iron-loaded 24p3/NGAL increases the
intracellular iron concentration without promoting apoptosis, while
iron-lacking 24p3/NGAL decreases intracellular iron levels, which
induces expression of the proapoptotic protein Bim, leading to
apoptosis (17). In the present
study, NGALR was observed to be significantly up-regulated in CRC
tissues. Furthermore, the co-expression of NGAL and NGALR is
associated with CRC development, as is the expression of ferritin
and Ki67. A correlation analysis revealed significant positive
correlations for NGAL and NGALR, NGAL and ferritin, as well as
NGALR and Ki67. These data suggest that NGAL and NGALR participate
in intracellular iron transport/accumulation and contribute to the
poor differentiation of tumour cells. Results of a large
prospective epidemiological study suggest that iron results in an
increased risk for CRC (19). High
dietary iron promotes the production of reactive oxygen species,
which activates the activator protein 1 and nuclear factor κB
signal transduction pathways, leading to transcription of the genes
involved in cell growth regulation (20–24).
We assume that NGAL and NGALR play an important role in CRC through
an increase of the iron content of cells, possibly activating some
iron-sensitive gene(s) involved in tumour infiltration.
Our previous work showed that NGAL expression is
significantly correlated with the depth of tumour invasion in
oesophageal squamous cell carcinoma, a pathological process
accompanied by over-expression of the NGAL/MMP-9 (matrix
metalloproteinase 9) complex (4).
MMP-9 is a proteolytic enzyme that degrades the extracellular
matrix leading to connective tissue remodelling during normal
biological processes and tumour invasion. NGAL directly associates
with MMP-9, protecting it from degradation and resulting in
increased MMP-9 activity (14,25,26).
Interestingly, we found that the levels of NGAL, NGALR and
NGAL/NGALR are associated with CRC invasion. A role for NGAL in
breast cancer invasion has been suggested following the observation
that the NGAL-overexpressing human breast cancer cell line MCF-7
exhibits an elevated tumour cell proliferative fraction (27).
Several examples of ligand-receptor interactions
involved in tumour progression are known. Relaxin binds to LGR7
(relaxin receptor) and activates signalling cascades, leading to
changes in tumour cell proliferation and altered motility (28). Receptor activator of nuclear factor
κB is expressed on prostate cancer cells and promotes invasion in a
receptor activator of nuclear factor κB ligand-dependent manner
(29). Koshiba et al
reported that the SDF-1/CXCR4 receptor-ligand system may be
involved in the progression of pancreatic cancer, participating in
tumour cell migration and angiogenesis (30). In light of these discoveries, we
speculate that the NGAL/NGALR interaction is a key regulator of
tumour growth and invasion in two distinct pathways: one regulating
genes which promote cancer invasion, and the other activating
iron-related genes also involved in cancer invasion. However,
whether the aberrant expression of NGAL and NGALR in these pathways
leads to an enhanced invasion of CRC needs to be investigated.
This study is the first to investigate expression of
the NGALR protein in a large series of CRC, and to reveal a role of
NGAL and NGALR in tumour invasion and cell differentiation in CRC.
The most important findings are: i) expression of NGAL, NGALR,
ferritin and Ki67 is elevated in CRC; and ii) over-expression of
NGAL and NGALR is associated with cell differentiation and tumour
invasion in CRC. Thus, our findings suggest that NGAL and NGALR are
involved in the transformation and progression of CRC. Therefore,
NGALR may be a novel target for the treatment of CRC.
Acknowledgements
We are very grateful to Professor Ming-Yao Wu and
technicians Qiao-Shan Li and Rui-Ming Zheng from the Pathology
Department of the Medical College of Shantou University for the
specimens. Funding was provided by grants from the National High
Technology Research and Development Program of China (No.
2006AA02A403), the National Natural Science Foundation of China
(No. 30672376, No. 30772485), the Specialized Research Fund for the
Doctoral Program of Higher Education of China (No. 20050560002 and
No. 20050560003), and the Guangdong Scientific Fund for Key Items
(No. 37788, No. 5104541 and No. 7118419).
References
|
1
|
Sung JJ, Lau JY, Goh KL and Leung WK:
Increasing incidence of CRC in Asia: implications for screening.
Lancet Oncol. 6:871–876. 2005. View Article : Google Scholar
|
|
2
|
Kjeldsen L, Johnsen AH, Sengeløv H and
Borregaard N: Isolation and primary structure of NGAL, a novel
protein associated with human neutrophil gelatinase. J Biol Chem.
268:10425–10432. 1993.PubMed/NCBI
|
|
3
|
Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang
Z, Zhang X, Niu Y, Shen Z, Shen J, Wu X and Li E: Upregulation of
neutrophil gelatinase-associated lipocalin in oesophageal squamous
cell carcinoma: significant correlation with cell differentiation
and tumour invasion. J Clin Pathol. 60:555–561. 2007. View Article : Google Scholar
|
|
4
|
Stoesz SP, Friedl A, Haag JD, Lindstrom
MJ, Clark GM and Gould MN: Heterogeneous expression of the
lipocalin NGAL in primary breast cancers. Int J Cancer. 79:565–572.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furutani M, Arii S, Mizumoto M, Kato M and
Imamura M: Identification of a neutrophil gelatinase-associated
lipocalin mRNA in human pancreatic cancers using a modified signal
sequence trap method. Cancer Lett. 122:209–214. 1998. View Article : Google Scholar
|
|
6
|
Bartsch S and Tschesche H: Cloning and
expression of human neutrophil lipocalin cDNA derived from bone
marrow and ovarian cancer cells. FEBS Lett. 357:255–259. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woo HJ, Park JC, Bae CH, Song SY, Lee HM
and Kim YD: Up-regulation of neutrophil gelatinase-associated
lipocalin in cholesteatoma. Acta Otolaryngol. 21:1–6. 2008.
|
|
8
|
Xu LY, Li EM, Xiong HQ, Shen ZY and Cai
WJ: Study of neutrophil gelatinase-associated lipocalin (NGAL) gene
overexpression in the progress of malignant transformation of human
immortalized esophageal epithelial cell. Prog Biochem Biophys.
28:839–843. 2001.
|
|
9
|
Li EM, Xu LY, Cai WJ, Xiong HQ, Shen ZY
and Zeng Y: Functions of neutrophil gelatinase-associated lipocalin
in the esophageal carcinoma cell line SHEEC. Acta Biochim Biophys
Sin. 35:247–254. 2003.PubMed/NCBI
|
|
10
|
Lin JL, Xu LY, Li EM, Cai WJ, Niu YD, Fang
KY, Xiong HQ, Shen ZY and Zeng Y: Antisense blocking of NGAL gene
expression affects the microfilament cytoskeleton in SHEEC
esophageal cancer cells. Prog Biochem Biophys. 31:409–415.
2004.
|
|
11
|
Nielsen BS, Borregaard N, Bundgaard JR,
Timshel S, Sehested M and Kjeldsen L: Induction of NGAL synthesis
in epithelial cells of human colorectal neoplasia and inflammatory
bowel diseases. Gut. 38:414–420. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y
and Kim JS: Ectopic expression of neutrophil gelatinase-associated
lipocalin suppresses the invasion and liver metastasis of colon
cancer cells. Int J Cancer. 118:2490–2497. 2006. View Article : Google Scholar
|
|
13
|
Flower DR: Beyond the superfamily: the
lipocalin receptors. Biochim Biophys Acta. 1482:327–336. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goetz DH, Holmes MA, Borregaard N, Bluhm
ME, Raymond KN and Strong RK: The neutrophil lipocalin NGAL is a
bacteriostatic agent that interferes with siderophore-mediated iron
acquisition. Mol Cell. 10:1033–1043. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Mori K, Li JY and Barasch J: Iron,
lipocalin, and kidney epithelia. Am J Physiol Renal Physiol.
285:F9–F18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Goetz D, Li J-Y, Wang W, Mori K,
Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R and Barasch
J: An iron delivery pathway mediated by a lipocalin. Mol Cell.
10:1045–1056. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devireddy LR, Gazin C, Zhu X and Green MR:
A cell-surface receptor for lipocalin 24p3 selectively mediates
apoptosis and iron uptake. Cell. 123:1293–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori K, Lee HT, Rapoport D, Drexler IR,
Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J,
Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C,
D’Agati V, Devarajan P and Barasch J: Endocytic delivery of
lipocalin-siderophore-iron complex rescues the kidney from
ischemia-reperfusion injury. J Clin Invest. 115:610–621. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wurzelmann JI, Silver A, Schreinemachers
DM, Sandler RS and Everson RB: Iron intake and the risk of
colorectal cancer. Cancer Epidemiol Biomarkers Prev. 5:503–507.
1996.PubMed/NCBI
|
|
20
|
Stone WL, Krishnan K, Campbell SE, Qui M,
Whaley SG and Yang H: Tocopherols and the treatment of colon
cancer. Ann N Y Acad Sci. 1031:223–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawa T, Akaike T, Kida K, Fukushima Y,
Takagi K and Maeda H: Lipid peroxyl radicals from oxidized oils and
heme-iron: implication of a high-fat diet in colon carcinogenesis.
Cancer Epidemiol Biomarkers Prev. 7:1007–1012. 1998.PubMed/NCBI
|
|
22
|
Lund EK, Fairweather-Tait SJ, Wharf SG and
Johnson IT: Chronic exposure to high levels of dietary iron
fortification increases lipid peroxidation in the mucosa of the rat
large intestine. J Nutr. 131:2928–2931. 2001.PubMed/NCBI
|
|
23
|
Kuratko CN: Decrease of manganese
superoxide dismutase activity in rats fed high levels of iron
during colon carcinogenesis. Food Chem Toxicol. 36:819–824. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA: The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil
gelatinase-associated lipocalin (NGAL). J Biol Chem.
276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tschesche H, Zölzer V, Triebel S and
Bartsch S: The human neutrophil lipocalin supports the allosteric
activation of matrix metalloproteinases. Eur J Biochem.
268:1918–1928. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernández CA, Yan L, Louis G, Yang J,
Kutok JL and Moses MA: The matrix metalloproteinase-9/neutrophil
gelatinase-associated lipocalin complex plays a role in breast
tumor growth and is present in the urine of breast cancer patients.
Clin Cancer Res. 11:5390–5395. 2005.
|
|
28
|
Klonisch T, Bialek J, Radestock Y,
Hoang-Vu C and Hombach-Klonisch S: Relaxin-like ligand-receptor
systems are autocrine/paracrine effectors in tumor cells and
modulate cancer progression and tissue invasiveness. Adv Exp Med
Biol. 612:104–118. 2007. View Article : Google Scholar
|
|
29
|
Armstrong AP, Miller RE, Jones JC, Zhang
J, Keller ET and Dougall WC: RANKL acts directly on RANK-expressing
prostate tumor cells and mediates migration and expression of tumor
metastasis genes. Prostate. 68:92–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koshiba T, Hosotani R, Miyamoto Y, Ida J,
Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii
N and Imamura M: Expression of stromal cell-derived factor 1 and
CXCR4 ligand receptor system in pancreatic cancer: a possible role
for tumor progression. Clin Cancer Res. 6:3530–3535.
2000.PubMed/NCBI
|