Introduction
New anti-tumor agents are under investigation in
both academic and industrial research laboratories worldwide. Drugs
that modulate the microtubule assembly are of great interest in
anti-cancer therapy, and a number of these drugs are currently
employed in clinical development (1,2).
Several tubulin polymerization inhibitors characterized by an
indole nucleus have been obtained from natural sources or prepared
by semi-synthesis. Arylthioindoles (ATIs) inhibit tubulin assembly
by interacting with the colchicine site on β-tubulin close to its
interface with α-tubulin within the α,β-dimer (3). ATIs proved to be effective inhibitors
of tubulin polymerization and cancer cell growth, with activities
comparable with those of colchicine and combretastatin A-4
(4,5).
In a recent study, we characterized the activity of
the newly synthesized arylthioindole RS 2518 and reported its
ability to bind β-tubulin and accumulate cells in the
G2/M phase of the cell cycle, thus promoting the
so-called mitotic catastrophe (6).
This study aimed to define the destiny of tumor cells after
incubation with a high concentration of RS 2518, i.e., 100 μM vs.
10 μM previously used (6).
Materials and methods
Cell cultures and treatments
Human tumor cells (listed in Table I) and normal fibroblasts were grown
at 37°C in a humidified atmosphere containing 5% CO2 in
DMEM supplemented with 10% FCS, 4 mM glutamine, 2 mM Na pyruvate,
100 U/ml penicillin and 0.1 mg/ml streptomycin (all reagents were
from Celbio, Milan, Italy). Cells were trypsinized when
subconfluent. Cell cultures were treated with the compound RS 2518
or its analogues (stock solution 10 mM in DMSO, kept at room
temperature in the dark) at concentrations ranging from 0.5 to 100
μM for 24 h, followed by either 24- or 48-h recovery. When the
treatment caused the cells to detach, the attached and floating
populations were analyzed separately. As a positive control of
apoptosis, cells were treated with 100 μM etoposide for 24 h.
 | Table IEffect of RS 2518 on the proliferation
of a panel of human tumor cell lines and normal fibroblasts. |
Table I
Effect of RS 2518 on the proliferation
of a panel of human tumor cell lines and normal fibroblasts.
| Cell line | Origin
(carcinoma) | 1 μM RS
2518
24 h | 1 μM RS
2518
24+24 h |
|---|
| HeLa | Uterine cervix | 45.77±0.007 | 33.12±0.004 |
| HCT116 | Colon | 67.97±0.050 | 22.73±0.022 |
| SW613-B3 | Colon | 63.54±0.016 | 63.64±0.041 |
| HT29 | Colon | 60.03±0.016 | 32.53±0.002 |
| PC3 | Prostate | 53.39±0.006 | 35.49±0.027 |
| MCF7 | Breast | 66.38±0.008 | 76.89±0.079 |
| FO46 | Normal
fibroblasts | 88.18±0.010 | 111.72±0.003 |
Cell morphology
Cells grown in T75 flasks were observed under the
microscope Olympus IX71 and photographed with a Nikon DS Chamber
Head DS-5M (Enfield, CT, USA).
Cell proliferation
Cytotoxicity assay
Cells were incubated with the drug, washed with PBS,
trypsinized and pelleted. After the addition of 1 ml of 0.1 M NaOH,
samples were vortexed and heated for 30 min at 50°C. Samples were
then allowed to reach room temperature and kept at 4°C until the
spectrophotometric analysis at 280 nm. Experiments in duplicate
were repeated at least three times.
MTT assay
Cells were seeded in 96-multiwell plates at a
density of 103/100 μl, while fibroblasts were seeded at
a density of 1.5×103/100 μl. Cells were treated 24 h
later with different drug concentrations for 24 h followed by a 24-
or 48-h recovery in a drug-free medium. At the end of the
treatments, 20 μl of CellTiter 96 Non-Radioactive Cell
Proliferation Assay (MTT; Promega, Milan, Italy) were added to each
well. Plates were then incubated for 4 h at 37°C in the dark and
analyzed with a microplate reader (Gio De Vita, Roma, Italy) at 492
nm. Experiments were performed in quadruplicate and repeated three
times.
Cell cycle
To evaluate the distribution of a cellular
population in the phases G1, S and G2/M of
the cycle by flow cytometry, cells were seeded at a density of
5×105/ml in 3 ml of complete medium in Petri dishes (6
cm in diameter). After 24 h, the medium was replaced with 1 ml of
drug-containing medium. At the end of the 24-h treatment, cells
were washed with PBS, trypsinized and recovered by centrifugation
for 5 min at 1500 rpm. The pellet was resuspended with 1 ml of cold
0.9% NaCl and 2 ml of cold 100% ethanol were added drop by drop
(final concentration ~70%). The cellular suspension, kept at −20°C
until the analysis, was stained with propidium iodide (30 μg/ml)
for 30 min. RNA was digested with 2 mg/ml of RNase A for 30 min at
room temperature. Finally, samples were analyzed with a Coulter
Epics flow cytometer (Beckman Coulter, Milan, Italy) using the
software XL. For each sample, at least 10,000 cells were analyzed.
The fluorescence intensity was converted into histograms, and the
percentage of cells in each phase of the cellular cycle was
calculated with the XL2 software. Experiments were repeated at
least three times.
PARP-1 proteolysis
Samples of 2.5×106 cells (fresh or stored
in liquid nitrogen) were resuspended with 100 μl of denaturing
buffer (62.5 mM Tris/HCl pH 6.8, 4 M urea, 10% glycerol and 0.003%
bromophenol blue), supplemented with β-mercaptoethanol (final
concentration 4%). Cells were disrupted by sonication in ice (50 W
twice for 20 sec). Extracts were then heated for 10 min at 65°C and
run on 7.5% denaturing polyacrylamide gel. Protein transfer was
performed at 200 mA for 3 h at 4°C and confirmed by staining the
membrane with red ponceau (Sigma Aldrich). The membrane was
saturated with PTN (PBS containing 10% newborn calf serum and 0.1%
Tween-20) for 1 h at room temperature and incubated overnight at
4°C with the PARP-1-specific MAb C2-10 (diluted 1:1000 in PTN;
Alexis, Vinci Biochem, Italy). After 5 washings in PBS containing
0.1% Tween-20, the membrane was incubated for 30 min with the
anti-mouse secondary antibody 77039, conjugated with horseradish
peroxidase (1:10000 in PTN; Jackson Immunoresearch, Suffolk, UK)
and then washed 5 times for 5 min in PBS. Visualization of the
immunoreactive bands was obtained by a chemoluminescent substrate
(SuperSignal West Pico from Pierce, Celbio). Three independent
experiments were carried out.
Results and Discussion
To define the destiny of cancer cells after
incubation with a high concentration of RS 2518, i.e., 100 μM, we
investigated the effect of the drug on cell metabolism. When
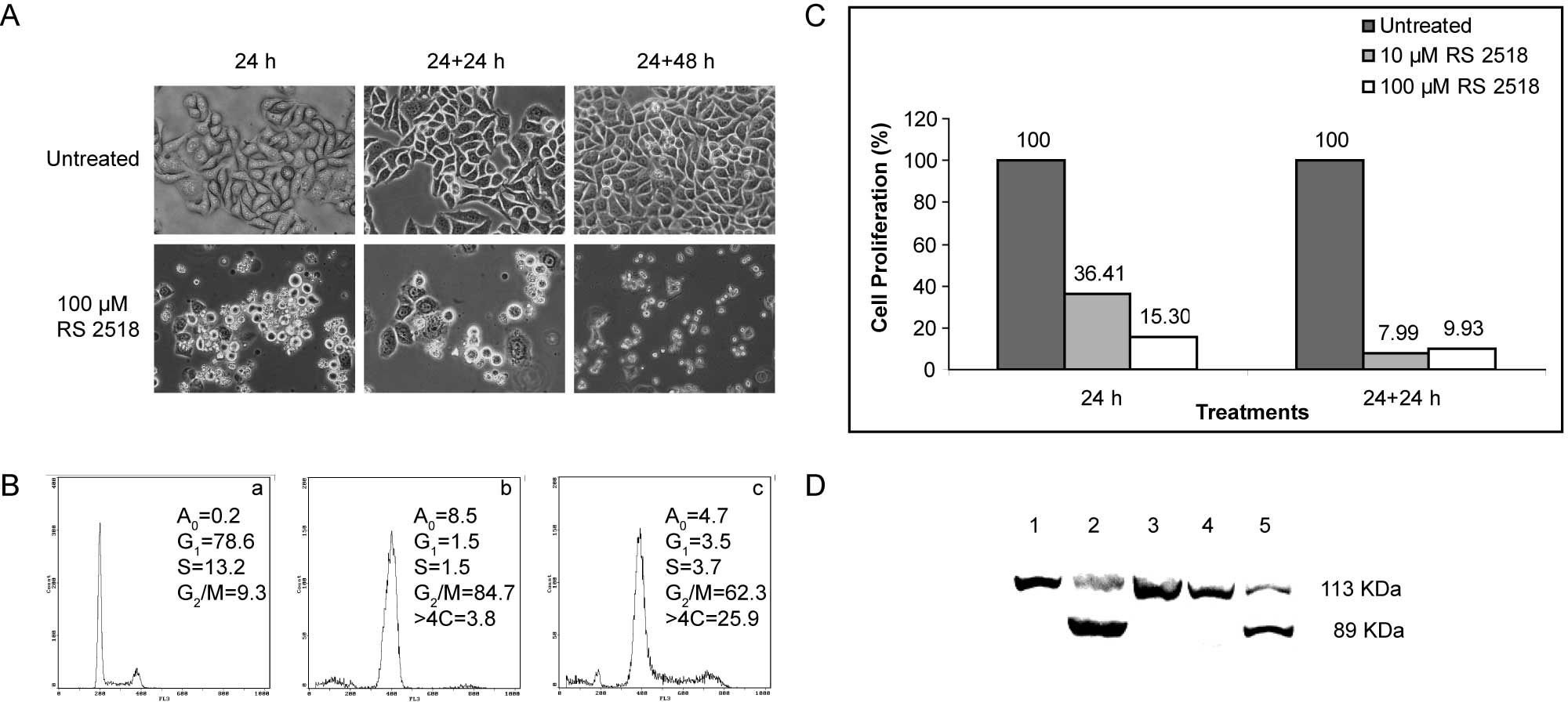
treated with 100 μM RS 2518 for 24 h, HeLa cells showed a reduced
adhesion property, resulting in cells floating in the culture
medium (Fig. 1A). This effect was
not reversed even after a further 24- or 48-h growth in a drug-free
medium (Fig. 1A).
Given that ATIs interfere with tubulin metabolism,
we analyzed cell cycle distribution by flow cytometry. In HeLa
cells treated for 24 h with 100 μM RS 2518, we observed a net
increase of cells accumulated in the G2/M phase
(Fig. 1B), reaching 84.7% at the
end of the treatment and 62.3% after a further 24-h growth in the
absence of the drug. The apparently weaker effect observed after
the recovery time may be explained by the appearance of a cell
fraction with a DNA content of >4C, which corresponds to
multinucleated cells; this population reached 25.9% and possibly
originates from endoreduplication events occurring when cells are
blocked in mitosis.
We investigated the effect of the compound RS 2518
on HeLa cell proliferation. Using the cytotoxicity assay, we found
that RS 2518 considerably affected cell proliferation in a time-
and dose- dependent manner (Fig.
1C). Compared to control cells, samples treated for 24 h with
10 μM RS 2518 showed a residual proliferation of 36.41%, which
further decreased after a 24-h recovery in a drug-free medium
(7.99%). Treatment with the highest concentration (100 μM) affected
cell proliferation even further (Fig.
1C). Notably, we found that the effects observed at the highest
concentration include the activation of caspase-dependent
apoptosis, as evidenced by PARP-1 proteolysis (Fig. 1D), a marker of this process
(7). The apoptotic destiny of
G2/M-blocked cells was already reported for other
tubulin-interacting drugs (8),
e.g., paclitaxel (9) and platycodin
D (10). The ability to induce
apoptosis is extremely important, given that cancer cells are
usually refractory to undergo apoptosis when treated with
chemotherapeutic agents (11).
We then extended the analysis of the effect of RS
2518 to a panel of cancer cell lines (Table I) and to normal human fibroblasts
FO46. The data reported in Table I
refer to a 24-h treatment with 1 μM RS 2518 and revealed that the
compound affected the metabolism of the cancer cell lines involved
(estimated IC50 ~0.5 μM), while it did not interfere with normal
fibroblast proliferation.
Based on these promising data, we evaluated the
effect of six analogues of RS 2518 (namely RS 2439, 2983, 2999,
3154, 3162 and 3273) on the cancer cell lines HeLa and HCT116. The
results obtained (Table II) showed
that HeLa and HCT116 cell proliferation was affected by a 24-h
treatment with 0.5 μM of each compound, with the exception of RS
2439 on HCT116 cells. Of note is that human normal fibroblasts were
insensitive to the drugs.
 | Table IIEffect of RS 2518 analogues on cell
proliferation. |
Table II
Effect of RS 2518 analogues on cell
proliferation.
| Compound | HeLa | HCT116 | Normal
fibroblasts |
|---|
| RS 2439 | 57.07±0.008 | 108.57±0.011 | 89.11±0.011 |
| RS 2983 | 54.72±0.004 | 70.72±0.028 | 93.34±0.007 |
| RS 2999 | 51.67±0.004 | 69.80±0.009 | 87.31±0.005 |
| RS 3154 | 40.77±0.006 | 52.32±0.071 | 83.89±0.005 |
| RS 3162 | 52.37±0.002 | 72.83±0.062 | 94.26±0.026 |
| RS 3273 | 52.59±0.001 | 81.93±0.021 | 93.28±0.004 |
Taken together, these data demonstrate for the first
time that the ATI RS 2518 is able to affect tumor cell
proliferation by promoting a significant accumulation of cells in
G2/M, leading to apoptosis. RS 2518 was thus selected as
a lead compound for further investigation as a potential anti-tumor
drug. Recent data showed that ATIs used in this study are efficient
in inhibiting the proliferation of several tumor cell lines
(12).
Acknowledgements
This study was supported by Fondazione Banca del
Monte di Lombardia (AIS), Istituto Pasteur-Fondazione Cenci
Bolognetti (RS) and Progetti di Ricerca di Università, Sapienza
Università di Roma (RS). G.V. is a PhD student from IUSS, Pavia,
Italy (Dottorato in Scienze Biomolecolari e Biotecnologie) and
G.L.R. was supported by a FIRC fellowship.
References
|
1
|
Pellegrini F and Budman DR: Tubulin
function, action of antitubulin drugs and new drug development.
Cancer Invest. 23:264–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carlson RO: New tubulin targeting agents
currently in clinical development. Expert Opin Investig Drugs.
17:707–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brancale A and Silvestri R: Indole, a core
nucleus for potent inhibitors of tubulin polymerization. Med Res
Rev. 27:209–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Martino G, La Regina G, Coluccia A, et
al: Arylthioindoles, potent inhibitors of tubulin polymerization. J
Med Chem. 47:6120–6123. 2004.
|
|
5
|
De Martino G, Edler MC, La Regina G, et
al: Arylthioindoles, potent inhibitors of tubulin polymerization. 2
Structure activity relationships and molecular modeling studies. J
Med Chem. 49:947–954. 2006.PubMed/NCBI
|
|
6
|
La Regina G, Edler MC, Brancale A, et al:
New arylthioindoles inhibitors of tubulin polymerization. 3
Biological evaluation, SAR and molecular modeling studies. J Med
Chem. 50:2865–2874. 2007.PubMed/NCBI
|
|
7
|
Soldani C and Scovassi AI:
Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update.
Apoptosis. 7:321–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhalla KN: Microtubule-targeted anticancer
agents and apoptosis. Oncogene. 22:9075–9086. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bottone MG, Soldani C, Tognon G, et al:
Multiple effects of paclitaxel are modulated by a high c-myc
amplification level. Exp Cell Res. 290:49–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim MO, Moon DO, Choi YH, Lee JD, Kim ND
and Kim GY: Platycodin D induces mitotic arrest in vitro, leading
to endoreduplication, inhibition of proliferation and apoptosis in
leukemia cells. Int J Cancer. 122:2674–2681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Redmond KM, Wilson TR, Johnston PG and
Longley DB: Resistance mechanisms to cancer chemotherapy. Front
Biosci. 13:5138–5154. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
La Regina G, Sarkar T, Bai R, et al: New
arylthioindoles and related bioisosteres at the sulfur bridging
group 4 Synthesis, tubulin, polymerization, cell growth inhibition,
and molecular modelling studies. J Med Chem. (In press).
|