Introduction
In the 1990s adjuvant 5-fluorouracil (5-FU)-based
chemotherapy combined with radiotherapy in patients with stage II
and III rectal cancer after surgical resection was established as
the new standard of care decreasing the rate of local recurrence
and improving patient survival (1,2). A
significant change in the treatment of rectal cancer occurred with
the introduction of total mesorectal excision (TME) resulting in
decreased local recurrences and improved survival (3). In 1995, when this trial started,
preoperative therapy was applied to downstage tumors in order to
increase sphincter-sparing surgery in distal rectal tumors and to
decrease the overall toxicity.
We conducted a single centre phase II trial in order
to investigate the feasibility and outcome of 5-FU-based
preoperative chemotherapy combined with long-term irradiation
followed by surgical treatment including TME, followed by
postoperative 5-FU-based chemotherapy. The results, after a median
follow-up of approximately three years (38.9 months; range
2.8–108.2 months), are presented and discussed within the context
of the results published in the literature.
Patients and methods
Patients were enrolled between November 1995 and
November 2004. Eligibility criteria included histopathologically
confirmed adenocarcinoma of the rectum [according to the 1987
International Union Against Cancer (UICC) staging system] within 15
cm of the anal verge. The clinical TNM stage was assessed by
clinical examination, rigid proctoscopy, endorectal
ultrasonography, computed tomography scanning and nuclear magnetic
resonance of the abdomen and pelvis. Patients with T3, T4 and with
distal T2 (<4 cm from the anal verge) rectal cancers,
respectively, were included irrespective of their nodal status.
Patients with a single hepatic or lung metastasis were eligible.
Patients were required to have a leukocyte level
>3.0×109/l and a thrombocyte level
>100×109/l, normal liver function with bilirubin
values <2 mg/dl and normal renal function with creatinine values
<1.5 mg/dl. Patients ≤85 years of age and with a performance
status according to Karnofsky of >70% were accepted. Exclusion
criteria were: prior pelvic irradiation, other uncontrolled severe
disease precluding administration of irradiation, pregnancy or lack
of contraception in women with childbearing potential, ileus,
imminent perforation, evidence of fistules in the pelvic region,
distant metastases or number of liver or lung metastases >1,
synchronous or metachronous rectal or colonic tumors, previous
other cancers except non-melanoma skin cancer. Written informed
consent was obtained, and the trial was approved by the medical
ethics committee.
The study was conceived as a non-randomized phase II
trial and was performed at a single centre in Vienna, Austria.
Radiotherapy was delivered with a linear accelerator using 6- to
15-MV photons and a dorsal three-field technique achieving 50.4 Gy
in daily fractions of 1.8 Gy after 3-D planning, 5 days/week. The
planning target volume was designed to include all macroscopically
identified disease and the internal iliac and presacral nodes up to
the superior border L5. The distal border was the bottom of the
obturator foramina or 5 cm below the distal extent of the primary
tumor. The anal canal was irradiated in the case of tumors lying in
the lower third of the rectum.
Preoperative chemotherapy was delivered in three
5-day courses during the 1st, 4th and 7th week after the start of
radiotherapy. 5-FU was administered at a dose of 450
mg/m2 intravenously (i.v.) bolus/day for 5 days and
leucovorin at a dosage of 25 mg/m2 i.v. bolus/day for 5
days. A dose reduction of 5-FU to 75% during radiotherapy was
allowed. In addition, dose reduction was foreseen in case of
toxicity ≥G3 according to the National Cancer Institute of Canada –
Common Toxicity Criteria (4). The
5-FU and leucovorin doses were selected based on studies by Moertel
et al (5) and Poon et
al (6). Three cycles of the
chemotherapy in combination with irradiation were administered to
increase the downstaging effect. Surgery was scheduled after
completion of chemoradiotherapy. TME was intended to be performed
in all patients. Patients with a single hepatic metastasis were
scheduled for additional liver resection, after R0 resection of the
primary tumor. Postoperative chemotherapy started 4 weeks after
surgery and was delivered in three cycles, every 4 weeks at the
same doses that were used preoperatively.
During preoperative treatment, patients were
monitored weekly and during postoperative treatment biweekly for
signs of acute toxic effects. Toxicity was classified according to
the National Cancer Institute of Canada - Common Toxicity Criteria
(NCIC-CTC) (4) and the Radiation
Therapy Oncology Group (RTOG) criteria (7). Toxicity was evaluated over the entire
treatment period comprising chemoradiotherapy, the postoperative
period and the following 3 months while receiving postoperative
adjuvant chemotherapy.
Clinical tumor response was evaluated preoperatively
according to the WHO (World Health Organisation) criteria (8). Patients were reviewed by one reference
radiologist (B.H.). Time to progression was defined as the time
interval from the start of treatment to progression or was censored
at the last patient contact with proven freedom from progression.
Tumor downstaging was defined by a comparison of the clinical
pretreatment TN categories (determined by scanning with computed
tomography, nuclear magnetic resonance or ultrasonography) to the
histopathological TN categories. For this purpose, fresh resection
specimens were transported unopened to the Department of Pathology.
After opening of the rectum, the tumor or fibrotic area was
identified and described macroscopically. Surgical specimens were
fixed in 4% formaldehyde. If no tumor was visible, the suspicious
area was sliced and embedded. If the tumor was visible, a minimum
of four paraffin blocks was processed. For determination of the
residual tumor, samples were taken from the lateral surface of the
specimens as well as from the proximal and distal resection
margins. Lymph nodes were dissected, and step sections were
routinely performed. Histological typing and grading were performed
according to the WHO criteria (9)
and staging according to the UICC (10). The minimum requirement for tumor
diagnosis was the presence of vital tumor cells or cell groups. The
number of lymph nodes examined and involved was determined
microscopically.
Follow-up after completion of therapy was initially
performed at 3 months, after 2 years at 6 months and after 5 years
at 12-month intervals.
The primary aim of this study was to determine the
histopathological response rate of patients who underwent surgery
after chemoradiation. Secondary aims included evaluation of the
feasibility of this preoperative regimen, determination of the
clinical objective response rate after completion of the
chemoradiotherapy preoperatively, evaluation of the rate of local
and distant relapses, and calculation of the disease-free and
overall survival.
To determine the sample size as a minimum
requirement, the number of evaluable patients was set to 62. Such a
sample size allowed for the estimation of confidence intervals for
histopathological complete remissions given an ex ante
expectation of roughly 10% with a precision of ±7.5%. Disease-free
interval and overall survival were estimated using the Kaplan-Meier
method. The log-rank test was used to calculate influence of
gender, age, clinical tumor stage, presence of distant metastasis,
distance of the primary tumor from the anal verge, presence of
sphincter infiltration, response to therapy, total dose of
radiotherapy, dose of chemotherapy, number of cycles of
chemotherapy, histopathological tumor stage, number of lymph nodes
investigated, tumor grade, residual tumor, resection margin, degree
of downstaging of the tumor, requirement of definitive colostomy
and type of surgical procedure on disease-free survival and overall
survival. Statistical analyses were performed using SPSS software
15.0.
Results
Patients
Of the 101 patients enrolled only 75 were eligible.
The reasons for excluding the remaining 26 patients were: T2 and
distance from the anal verge >4 cm (n=5), unknown number of
hepatic metastases or >1 hepatic metastasis (n=7), presence of
simultaneous liver and lung metastases (n=1), presence of ≥2 lung
metastases (n=2), presence of liver and peritoneal metastases
(n=1), synchronous rectal and colonic carcinoma (n=3), metachronous
rectal and colonic carcinoma (n=1), multiple liver and lung
metastases and another malignancy (n=1), presence of a second
malignancy (n=2), TME not performed (n=2) and deviation from the
chemotherapeutic regimen (n=1). Out of the 75 eligible patients, 2
patients withdrew their informed consent, 1 patient relocated and 1
patient was lost to follow-up. Therefore, 71 patients were
considered to be evaluable for the primary endpoint. The baseline
characteristics of these 71 patients are listed in Table I.
 | Table ICharacteristics of the 71 evaluable
patients treated with preoperative chemoradiotherapy followed by
surgical treatment and postoperative chemotherapy. |
Table I
Characteristics of the 71 evaluable
patients treated with preoperative chemoradiotherapy followed by
surgical treatment and postoperative chemotherapy.
| Age (years) |
| Median | 62 |
| Range | 39–84 |
| Gender, no. (%) |
| Male | 43 (60.6) |
| Female | 28 (39.4) |
| Distance of tumor
from anal verge, no. (%) |
| 0–5 cm | 33 (47.8) |
| >5–10 cm | 28 (40.6) |
| >10 cm | 8 (11.6) |
| Not evaluable | 2 |
| cT category, no.
(%) |
| T2 | 6 (8.5) |
| T3 | 50 (70.4) |
| T4 | 15 (21.1) |
| cN category, no.
(%) |
| N0 | 17 (29.3) |
| N1 | 27 (46.6) |
| N2 | 14 (24.1) |
| Not evaluable | 13 |
| cM category, no.
(%) |
| M0 | 63 (88.7) |
| Liver
metastasis | 6 (8.5) |
| Lung metastasis | 2 (2.8) |
Treatment administration
Preoperatively 206 cycles (median 3 cycles) of
5-FU/leucovorin and postoperatively, 149 cycles (median 3 cycles)
were delivered. Seventy-seven percent of the patients received at
least 50 Gy and 3 preoperative cycles of chemotherapy, 87% at least
45 Gy and 3 cycles of preoperative chemotherapy and 94% received at
least 45 Gy and 2 preoperative chemotherapy cycles, respectively.
Due to toxicity in 16/71 (23%) patients, the planned preoperative
chemoradiotherapy (3 cycles of chemotherapy and ≥50 Gy) could not
be administered due to ileus (n=1), diarrhoea (n=7), local reaction
or proctitis (n=4), neutropenia and/or neutropenic fever (n=3) and
mucositis (n=1). Chemotherapy was performed in 54/71 (76%) patients
postoperatively. In 17/71 (24%) patients the planned postoperative
treatment was not administered due to cardiomyopathy G4 (fatal)
(n=1; 1%); reduced performance status (n=1; 1%); eight (11%)
patients requested <6 cycles of treatment; 2 (3%) were lost to
follow-up after surgical treatment; 3 (4%) had a reduction to 3
cycles due to having achieved pT2N0 or pT0pN0, respectively and in
2 (3%) patients chemotherapy was changed to FOLFOX following
surgical treatment comprising curative local and hepatic resection.
In 3 patients, chemotherapy was discontinued due to toxicity (skin
necrosis due to the cytostatic agent, n=1; diarrhoea, n=1; fever
and mucositis, n=1).
Efficacy
The clinical response rate in the evaluable patients
reached 79.7% (51/64) (95% CI, 69.8–89.5; CR, n=3; PR, n=48; NC,
n=11 and PD, n=2). The reasons for non-evaluability were occurrence
of an ileus on day 39 (n=1) and loss of radiographic films (n=6).
Tumor downstaging occurred in 48% of the T-categories (T3, 44% and
T4, 80%), in 53% of the N-categories and in 74% of the UICC stages,
respectively. Patients with downstaged tumors located ≤4 cm from
the anal verge did not undergo more frequent sphincter sparing
operations than patients with non-downstaged distal rectal
carcinomas.
All patients underwent a TME. A histopathologic
complete remission (pCR), ypT0ypN0, was found in 14.1% of the
patients (95% CI, 6.0–22.2; Table
II). Curative liver resection was performed in 4 patients.
 | Table IIPostoperative pathological tumor
stage, type of surgery and completeness of resection of the 71
evaluable patients. |
Table II
Postoperative pathological tumor
stage, type of surgery and completeness of resection of the 71
evaluable patients.
| Lymph nodes, no.
operated | |
| Mean | 17.51 |
| Range | 2–55 |
| Lymph nodes, no.
positive | |
| Mean | 0.79 |
| Range | 0–18 |
| Degree of resection
histopathologically, no. (%) | |
| R0 | 61 (88.4) |
| R1 | 3 (4.3) |
| RX | 5 (7.2) |
| Not evaluable | 2 |
| Distance from tumor
to resection margin, no. (%) | |
| <1 cm | 10 (15.6) |
| ≥1 cm | 54 (84.4) |
| Not evaluable | 7 |
| Histopathological
stage according to UICC, no. (%) | |
| 0 | 9 (12.9) |
| I | 15 (21.4) |
| II | 27 (38.6) |
| III | 15 (21.4) |
| IV | 4 (5.7) |
| Not evaluable | 1 |
| ypT category | |
| ypT0 | 10 (14.1) |
| ypT1 | 1 (1.4) |
| ypT2 | 18 (25.4) |
| ypT3 | 38 (53.5) |
| ypT4 | 4 (5.6) |
| ypN category | |
| ypN0 | 53 (74.6) |
| ypN1 | 15 (21.1) |
| ypN2 | 3 (4.2) |
| ycM category | |
| M0 | 66 (94.3) |
| M1 | 4 (5.7) |
| Not evaluable | 1 |
| ypL facultative
descriptor | |
| ypL0 | 45 (72.6) |
| ypL1 | 17 (27.4) |
| Not evaluable | 9 |
| ypV facultative
descriptor | |
| ypV0 | 58 (95.1) |
| ypV1 | 3 (4.9) |
| Not evaluable | 10 |
| Grade | |
| G1 | 1 (1.5) |
| G2 | 49 (75.4) |
| G3 | 15 (23.1) |
| GX | 6 |
| Sphincter
infiltration | |
| Yes | 3 (4.3) |
| No | 67 (95.7) |
| Not evaluable | 1 |
| Definitive
colostomy | |
| Yes | 18 (25.7) |
| No | 52 (74.3) |
| Not evaluable | 1 |
| Type of
resection | |
| Abdominoperineal
resection | 18 (25.4) |
| Low anterior
resection | 53 (74.6) |
Four (6.1%) patients developed local recurrences
only, and 2 (3%) more patients also had distant metastases. A total
of 13 (19.7%) patients developed distant metastases
exclusively.
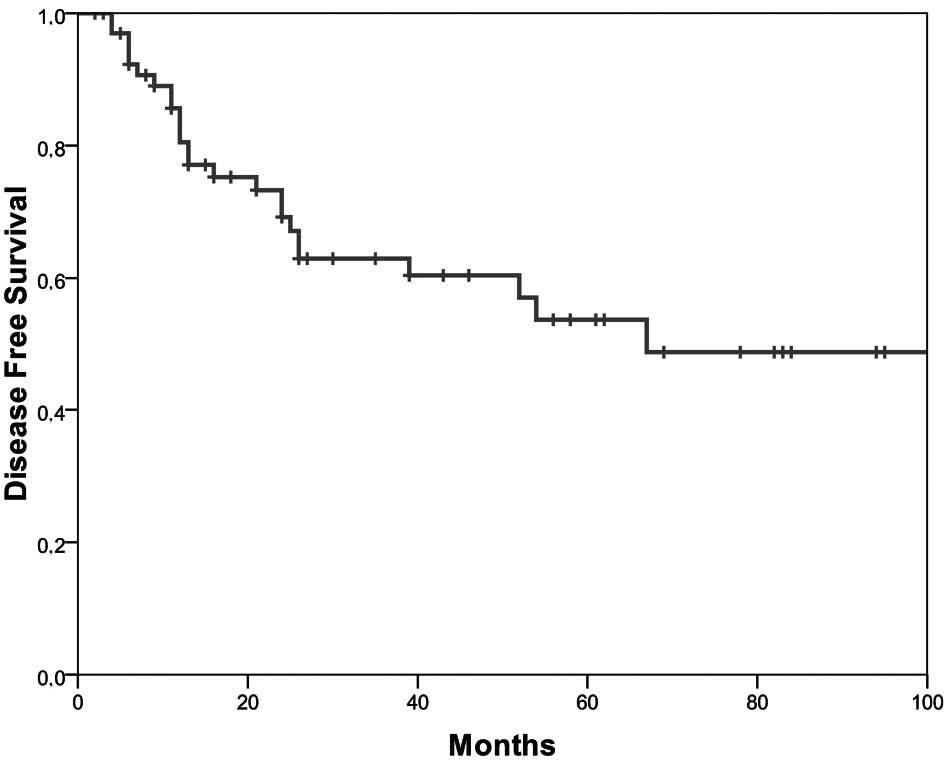
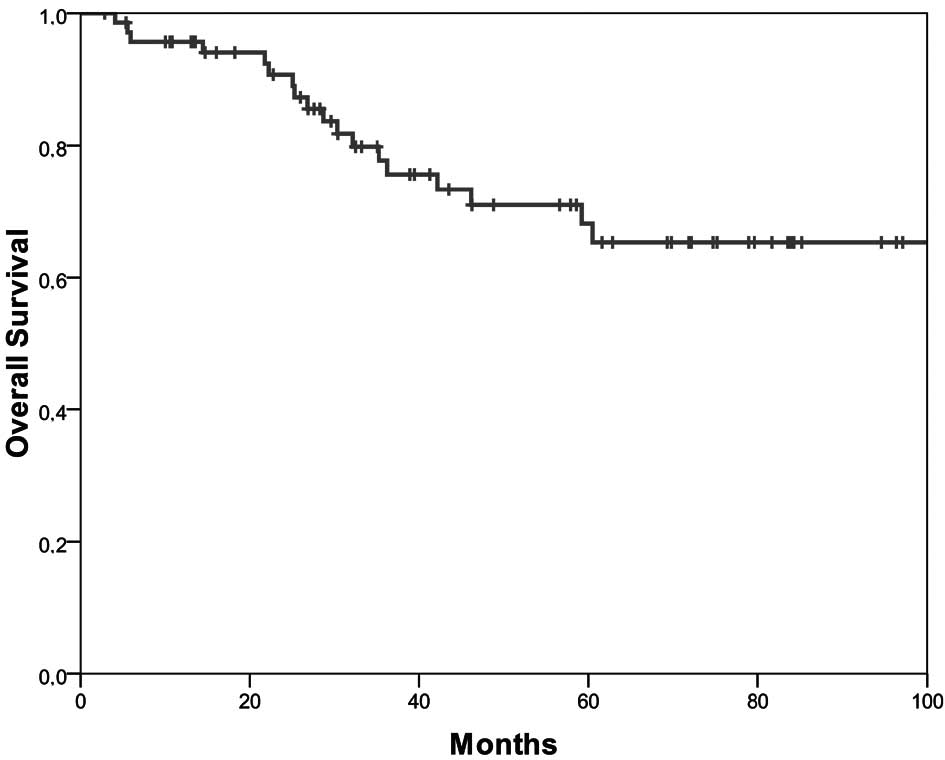
The 5-year disease-free survival was 53.7% (Fig. 1) and the 5-year overall survival was
68% (Fig. 2). Younger patients had
a longer disease-free (p=0.02) and overall survival (p=0.01).
Female patients survived longer (p=0.04). The presence of a
singular liver or lung metastasis influenced the length of both the
disease-free (p=0.008) and overall survival (p=0.02) significantly.
The clinically assessed preoperative N-category after completion of
combined chemoradiotherapy was associated with a significant impact
on survival (p=0.011). Patients with 6 cycles of chemotherapy,
i.e., patients who had received the complete postoperative adjuvant
chemotherapy survived longer (p=0.054).
Side effects
The following severe (grade 3 and 4) haematological
toxicities were observed: leukopenia 27% (15% grade 3, 12% grade
4), neutropenia 17% (8% grade 3, 9% grade 4) and thrombocytopenia
6% (6% grade 4), respectively. The neutrophil nadir was reached
after a median of 43 days (range 0–214). The acute severe
non-haematologic toxicities are listed in Table III. Postoperatively, an
anastomotic leakage with a consecutive abscess occurred in 1
patient, an abscess in the anastomotic region in 1 patient, a
fistula with consecutive abscess in the anastomotic region in 1
patient and a stenotic anastomosis in a fourth patient. A delay in
wound healing was observed in 1 patient and ejaculation dysfunction
in 3 patients.
 | Table IIIMaximal non-haematotoxic adverse
effects/patient graded by NCIC-CTC or RTOG in the 71 evaluable
patients treated with preoperative radiotherapy and chemotherapy
with 5-FU and leucovorin followed by surgical treatment and
postoperative adjuvant chemotherapy for locally advanced and distal
T2 rectal cancer. |
Table III
Maximal non-haematotoxic adverse
effects/patient graded by NCIC-CTC or RTOG in the 71 evaluable
patients treated with preoperative radiotherapy and chemotherapy
with 5-FU and leucovorin followed by surgical treatment and
postoperative adjuvant chemotherapy for locally advanced and distal
T2 rectal cancer.
| NCIC-CTC category
for severity, no. (%) |
|---|
|
|
|---|
| Adverse effect | 3 | 4 |
|---|
| Bowel
obstruction/paralytic bowel | 2 (3) | 1 (1) |
| Diarrhoea | 5 (7) | 7 (10) |
| Stomatitis | 1 (1) | 2 (3) |
| Nausea | 1 (1) | 0 |
| Vomiting | 0 | 1 (1) |
| Infection | 6 (8) | 0 |
| Neutropenic
fever | 2 (3) | 0 |
| Skin necrosis | 0 | 1 (1) |
| Cardiovascular
function | 0 | 1 (1) (fatal) |
| Venous
thromboembolism | 2 (3) | 1 (1) |
| Fainting | 3 (4) | 0 |
| Confusion | 1 (1) | 0 |
| Vaginitis | 2 (6)a | 0 |
| Urinary
incontinence | 1 (1) | 0 |
| Proctitis | 2 (3) | 0 |
| Skin toxicity -
RTOG | 2 (3) | 0 |
Discussion
A pCR was found in 14.1% of our patients thereby
meeting the primary endpoint of our study. It is known from other
studies that the ability to accurately predict the pathologic stage
by clinical staging following preoperative chemotherapy and
concurrent radiation remains suboptimal. Thus, only by ascertaining
a pathologic complete response can a correct result be achieved
(11,12). The high pCR of 14.1% was highly
comparable to the median percentage of 11% (range 8–27%) observed
in other studies administering 5-FU ± leucovorin in combination
with preoperative irradiation in locally advanced rectal cancer
(13–18). Two recently published multicentric
randomized phase III trials treating patients with locally advanced
rectal cancers with preoperative chemoradiation yielded an equally
high pathological complete sterilization rate independent of
whether oxaliplatin was added to 5-FU or not, thus indicating that
5-FU-containing preoperative chemotherapy in combination with
irradiation remains the actual standard of care unless long term
follow-up in the future reveals differences in efficacy (19,20).
Since pCR was shown to be of prognostic significance in independent
investigations, it is speculated whether or not pCR should be used
as a basis for subsequent postoperative adjuvant therapy (15,21,22).
In this single centre trial, high median 5-year
disease-free and 5-year overall survival rates were obtained,
especially when one considers that 11% of our patients had a
singular pulmonary or hepatic metastasis and in view of our
observation that the presence of a singular distant metastasis
influenced the disease-free and overall survival. The length of the
disease-free interval and the overall survival in our study was
nearly identical to that found by Bosset et al (23), although in their study a lower
percentage of T4 tumors was included and patients with distant
metastasis were excluded; both results were also in line with those
of Sauer et al (17). The low local
relapse rate of 6.1% observed in our study was highly comparable to
that found in other studies (17,23),
although we included a four-time higher percentage of T4 tumors
compared to Sauer et al and a two-time higher percentage of T4
tumors than Bosset et al. Apart from T-categories, other
independent prognostic factors such as the TME technique (24), or the skills and training of the
surgeon (25) may influence the
rate of local recurrences in rectal cancer. In addition,
preoperative in contrast to postoperative chemoradiation per se was
confirmed to be an independent prognostic variable for predicting
local recurrences in a randomized controlled study (17). Long-term versus short-term
irradiation continues to be the preferred treatment in our patient
cohort, since it has been shown in distal rectal tumors ≤5 cm from
the anal verge that short-term irradiation fails to reduce the rate
of local relapses (26).
Additionally, the equieffectiveness of short- and long-term
irradiation has only been shown for patients with resectable T3 and
T4 rectal carcinomas (11).
The rate of distant relapses was highly comparable
to that reported by Bosset et al (23) and Sauer et al (17). Using 5-FU plus leucovorin we
achieved a more effective chemotherapy in comparison with other
investigators who used 5-FU alone (6). Furthermore, an association between the
number of cycles of chemotherapy and survival was shown in our
study. The subgroup analysis indicated a survival advantage for the
patients undergoing postoperative treatment. This result may
influence the treatment decision after surgery in daily practice.
Also Bosset et al (23) and Janjan
et al (27) observed a reduction in
mortality in patients who received postoperative chemotherapy.
Presumably, further intensification of chemotherapy by its
combination with newer cytostatic and biologic agents will
substantially change the prognosis of locally advanced rectal
cancer (28–30).
Acute toxicity observed in our trial was high and
highly comparable to other studies (13,17,31).
However, the higher haematologic toxicity observed in our study and
in that of Bosset et al was certainly caused by the intravenous
bolus administration of 5-FU in contrast to the continuous infusion
of 5-FU reported by Sauer et al (32). The high overall severe toxicity
found in our study was mainly attributed to both the high dose of
radiotherapy ≥50 Gy and to the administration of three preoperative
cycles of chemotherapy. If the treatment of our patients had been
terminated at the lower threshold of irradiation of 45 Gy and after
only two cycles of the chemotherapy, preoperative chemoradiation
would have been completed in 94% of our patients. Our theory is in
line with Bosset et al (23) who
completed chemoradiation in 96% of their patients with a dose of 45
Gy of irradiation and two preoperative cycles of chemotherapy.
Therefore, we recommend a dose reduction of irradiation to 45 Gy in
combination with only two cycles of chemotherapy consisting of 5-FU
and leucovorin, followed by four postoperative cycles of this
chemotherapy using the same doses as in our study. The maintenance
of the total number of six cycles of chemotherapy seems important
in light of the longer survival of the patients in our study.
In conclusion, this treatment is very effective and
yields a high pCR rate, a low local relapse rate and long
disease-free and overall survival. The comparable high pCR rate
obtained in studies adding oxaliplatin to 5-FU in comparison to
5-FU monotherapy indicates that the treatment used in our study can
be further used as a standard of care. The proposed preoperative
combined modality treatment consisting of only two preoperative
5-FU-containing cycles of chemotherapy concurrently administered
with 45-Gy irradiation followed by surgery including, TME and
postoperative adjuvant 5-FU-containing chemotherapy of four cycles,
represents an effective and feasible treatment for locally advanced
rectal cancer.
References
|
1
|
Gastrointestinal Tumor Study Group.
Prolongation of the disease-free interval in surgically-treated
rectal carcinoma. N Engl J Med. 312:1465–1472. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Douglass HO Jr, Moertel CG, Mayer RJ, et
al: Survival after postoperative combination treatment of rectal
cancer. N Engl J Med. 315:1294–1295. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapiteijn E, Putter H and van de Velde CJ:
Impact of the introduction and training of total mesorectal
excision on recurrence and survival in rectal cancer in The
Netherlands. Br J Surg. 89:1142–1149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Cancer Institute of Canada
(NCIC). Common toxicity criteria (CTC) grading system (revised
edition). 1994
|
|
5
|
Moertel CG, Fleming TR, Macdonald JS, et
al: Levamisole and fluorouracil for adjuvant therapy of resected
colon carcinoma. N Engl J Med. 322:352–358. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poon MA, O’Connell MJ, Moertel CG, et al:
Biochemical modulation of fluorouracil: evidence of significant
improvement of survival and quality of life in patients with
advanced colorectal carcinoma. J Clin Oncol. 7:1407–1418.
1989.PubMed/NCBI
|
|
7
|
Radiation Therapy Oncology Group. [cited;
Available from: http://www.rtog.org/].
|
|
8
|
Miller AB, Hoogstraten B, Staquet M, et
al: Reporting results of cancer treatment. Cancer. 47:207–214.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Histological typing of intestinal tumours.
WHO. Springer; Berlin: 1989
|
|
10
|
TNM classification of malignant tumours.
4th edition. 2nd revision. UICC. Springer; Berlin: 1992
|
|
11
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, et al: Sphincter preservation following preoperative
radiotherapy for rectal cancer: report of a randomised trial
comparing short-term radiotherapy vs. conventionally fractionated
radiochemotherapy. Radiother Oncol. 72:15–24. 2004. View Article : Google Scholar
|
|
12
|
Minsky BD: Preoperative treatment for
upper cT3 N0 rectal tumors: is it required? ASCO Educational Book.
Govindan R and Curzio J: Alexandria, VA: pp. 204–207. 2009
|
|
13
|
Grann A, Minsky BD, Cohen AM, et al:
Preliminary results of preoperative 5-fluorouracil, low-dose
leucovorin and concurrent radiation therapy for clinically
resectable T3 rectal cancer. Dis Colon Rectum. 40:515–522. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janjan NA, Khoo VS, Abbruzzese J, et al:
Tumor downstaging and sphincter preservation with preoperative
chemoradiation in locally advanced rectal cancer: the M.D. Anderson
Cancer Center experience. Int J Radiat Oncol Biol Phys.
44:1027–1038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaminsky-Forrett MC, Conroy T, Luporsi E,
et al: Prognostic implications of downstaging following
preoperative radiation therapy for operable T3–T4 rectal cancer.
Int J Radiat Oncol Biol Phys. 42:935–941. 1998.PubMed/NCBI
|
|
16
|
Minsky BD, Cohen AM, Kemeny N, et al:
Enhancement of radiation-induced downstaging of rectal cancer by
fluorouracil and high-dose leucovorin chemotherapy. J Clin Oncol.
10:79–84. 1992.PubMed/NCBI
|
|
17
|
Sauer R, Becker H, Hohenberger W, et al:
Preoperative versus postoperative chemoradiotherapy for rectal
cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bosset JF, Calais G, Mineur L, et al:
Enhanced tumorocidal effect of chemotherapy with preoperative
radiotherapy for rectal cancer: preliminary results – EORTC 22921.
J Clin Oncol. 23:5620–5627. 2005.PubMed/NCBI
|
|
19
|
Gerard J, Azria D, Gourgou-Bourgade S, et
al: Randomized multicenter phase III trial comparing two
neoadjuvant chemoradiotherapy (CT-RT) regimens (RT45-Cap versus
RT50-Capox) in patients (pts) with locally advanced rectal cancer
(LACR): results of the ACCORD 12/0405 PRODIGE 2. J Clin Oncol.
27:S7972009.
|
|
20
|
Aschele C, Pinto S, Cordio G, et al:
Preoperative fluorouracil (FU)-based chemoradiation with and
without weekly oxaliplatin in locally advanced rectal cancer:
pathologic response analysis of the Studio Terapia Adjuvante Retto
(STAR)-01 randomized phase III trial. J Clin Oncol.
27:S8042009.
|
|
21
|
Rodel C, Martus P, Papadoupolos T, et al:
Prognostic significance of tumor regression after preoperative
chemoradiotherapy for rectal cancer. J Clin Oncol. 23:8688–8696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valentini V, Coco C, Picciocchi A, et al:
Does downstaging predict improved outcome after preoperative
chemoradiation for extraperitoneal locally advanced rectal cancer?
A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys.
53:664–674. 2002. View Article : Google Scholar
|
|
23
|
Bosset JF, Collette L, Calais G, et al:
Chemotherapy with preoperative radiotherapy in rectal cancer. N
Engl J Med. 355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapiteijn E, Marijnen CA, Nagtegaal ID, et
al: Preoperative radiotherapy combined with total mesorectal
excision for resectable rectal cancer. N Engl J Med. 345:638–646.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quirke P: Training and quality assurance
for rectal cancer: 20 years of data is enough. The Lancet Oncology.
4:695–702. 2003.PubMed/NCBI
|
|
26
|
Peeters KC, Marijnen CA, Nagtegaal ID, et
al: The TME trial after a median follow-up of 6 years: increased
local control but no survival benefit in irradiated patients with
resectable rectal carcinoma. Ann Surg. 246:693–701. 2007.
|
|
27
|
Janjan NA, Abbruzzese J, Pazdur R, et al:
Prognostic implications of response to preoperative infusional
chemoradiation in locally advanced rectal cancer. Radiother Oncol.
51:153–160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willett C, Duda D, Boucher Y, et al: Phase
I/II study of neoadjuvant bevacizumab with radiation therapy and
5-fluorouracil in patients with rectal cancer: initial results.
Proc ASCO. 25:S1732007.
|
|
29
|
Arnold D, Hipp M, Liersch T, et al:
Cetuximab, capecitabine and oxaliplatin (Cet-CapOx) with concurrent
radiotherapy (RT) in advanced rectal cancer (RC): results of a
phase I/II trial. Proc ASCO. 25:S1742007.
|
|
30
|
Nogue M, Salud A, Vicente P, et al:
Addition of bevacizumab to induction plus concomitant
capecitabine-oxaliplatin (XELOX) chemoradiotherapy (CRT) in MRI
poor prognosis locally advanced rectal cancer: avacross study. Proc
ASCO. 4100:S1922009.
|
|
31
|
Bosset JF, Pavy JJ, Hamers HP, et al:
Determination of the optimal dose of 5-fluorouracil when combined
with low-dose D,L-leucovorin and irradiation in rectal cancer:
results of three consecutive phase II studies. EORTC Radiotherapy
Group. Eur J Cancer. 29A:1406–1410. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
The Meta-Analysis Group In Cancer.
Toxicity of fluorouracil in patients with advanced colorectal
cancer: effect of administration schedule and prognostic factors. J
Clin Oncol. 16:3537–3541. 1998.PubMed/NCBI
|