Introduction
Apoptosis resistance and altered metabolism are
common cancer cell traits. In a murine lymphoma model, selection
for oxidative stress resistance results in concomitant resistance
to apoptosis and the acquisition of a more-aggressive tumor
phenotype. WEHI7.2 thymic lymphoma cells transfected with catalase
(CAT2, CAT38) or selected for resistance to hydrogen peroxide
(200R) demonstrate resistance to glucocorticoid-induced apoptosis
(1,2), one component of the CHOP lymphoma
chemotherapy regimen. The oxidative stress-resistant cells are also
resistant to the cyclophosphamide, doxorubicin and vincristine
components of CHOP (Tome et al, unpublished data).
Catalase-overexpressing cells exhibit increased tumor growth
compared to WEHI7.2 parental cells in a mouse xenograft model
(2). Additionally, the oxidative
stress-resistant cells demonstrate an altered metabolic profile,
including the ability to generate ATP from alternative carbon
sources such as glutamine (3,4). When
treated with glucocorticoids, the oxidative stress-resistant cells
are better able to maintain ATP levels as compared to WEHI7.2 cells
(3). Experiments with an uncoupler
of mitochondrial respiration showed that the CAT38 and 200R cells
produce more ATP from mitochondria than the WEHI7.2 cells (4).
Mitochondria are central to cellular metabolism and
the decision to undergo apoptosis (5). Thus, changes in critical mitochondrial
proteins may explain the more-aggressive tumor phenotype of the
resistant lymphoma variants. In particular, cytochrome c
plays an integral role as an electron carrier in the mitochondrial
respiratory chain, and the release of this protein from the
mitochondrial intermembrane space results in commitment to death
via apoptosis (5). In a previous
study, we showed that cytochrome c release is delayed in the
resistant variants (1), and that
mitochondria isolated from these cells demonstrate an intrinsic
resistance to cytochrome c release (Wilkinson et al,
unpublished data). The present study examined whether alterations
in cytochrome c protein levels correlate with the previously
characterized more-aggressive tumor phenotype of the resistant
cells. To test the potential clinical relevance of these findings,
gene expression data from lymphoma specimens were analyzed.
Materials and methods
Mitochondrial protein levels in cell
lines
WEHI7.2 murine thymic lymphoma cells and variants
were maintained as previously described (1,2).
Mitochondria were isolated as previously described (2), with the exception that the
mitochondrial isolation buffer was prepared in the same manner as
Wang et al (6). SDS-PAGE and
immunoblotting were performed using standard protocols. The
antibodies used were: adenylate kinase-2 (AK-2) (Abcam, Cambridge,
MA, USA); cytochrome c (BD Biosciences, San Jose, CA, USA);
HSP60 (Assay Designs, Ann Arbor, MI, USA); anti-rabbit-HRP (Cell
Signaling Technology, Beverly, MA, USA) and anti-mouse-HRP (Pierce,
Rockford, IL, USA).
Gene expression profiling data
The gene expression profiling (GEP) data sets used
in this analysis were generated by the Leukemia/Lymphoma Molecular
Profiling Project (LLMPP) research group (7–9) and
are publicly available from http://llmpp.nih.gov/. Diagnostic specimens were
collected from 240 de novo diffuse large B-cell lymphoma
(DLBCL) (7), 92 mantle cell
lymphoma (MCL) (9) and 191
follicular lymphoma (FL) patients (8). Patients subsequently received
multi-agent chemotherapy and were monitored to assess treatment
outcome. GEP was performed using the Lymphochip microarray for
DLBCL and MCL and the Affymetrix U133A and U133B microarrays for
FL.
Genes of interest were subjected to expressed
sequence tag validation and statistical analysis as previously
described (10). For cytochrome
c analyses, patients were categorized by increasing
expression, separated into upper and lower expression groups and
Kaplan-Meier plots were generated to compare survival between
groups. To ensure that the number of patients studied would be
similar among the lymphoma types, we compared the top and bottom
10% of patients in the larger DLBCL and FL data sets, as well as
the top and bottom 20% of patients in the smaller MCL data set.
Statistical tests were performed using WinSTAT for Microsoft Excel
(Microsoft, Redmond, WA, USA).
Results
Increased mitochondrial cytochrome c
protein expression in a drug-resistant lymphoma model
Cytochrome c protein levels were increased in
mitochondria from CAT2, CAT38 and 200R cells relative to WEHI7.2
(Fig. 1A). Increased protein levels
were also noted for AK-2, another mitochondrial intermembrane space
protein (Fig. 1A). This protein
supports optimal ATP synthesis by equilibrating adenylate pools
(11). The increased levels of
cytochrome c and AK-2 found in mitochondria from the
oxidative stress-resistant lymphoma cells are proportionate to the
degree of apoptotic resistance measured previously in intact cells
(2). No difference in expression of
the mitochondrial matrix protein HSP60 was found in the cell
variants. Thus, the changes observed in cytochrome c and
AK-2 content cannot be explained by increased mitochondrial mass.
These data suggest that the resistant variants have an increased
mitochondrial respiratory capacity.
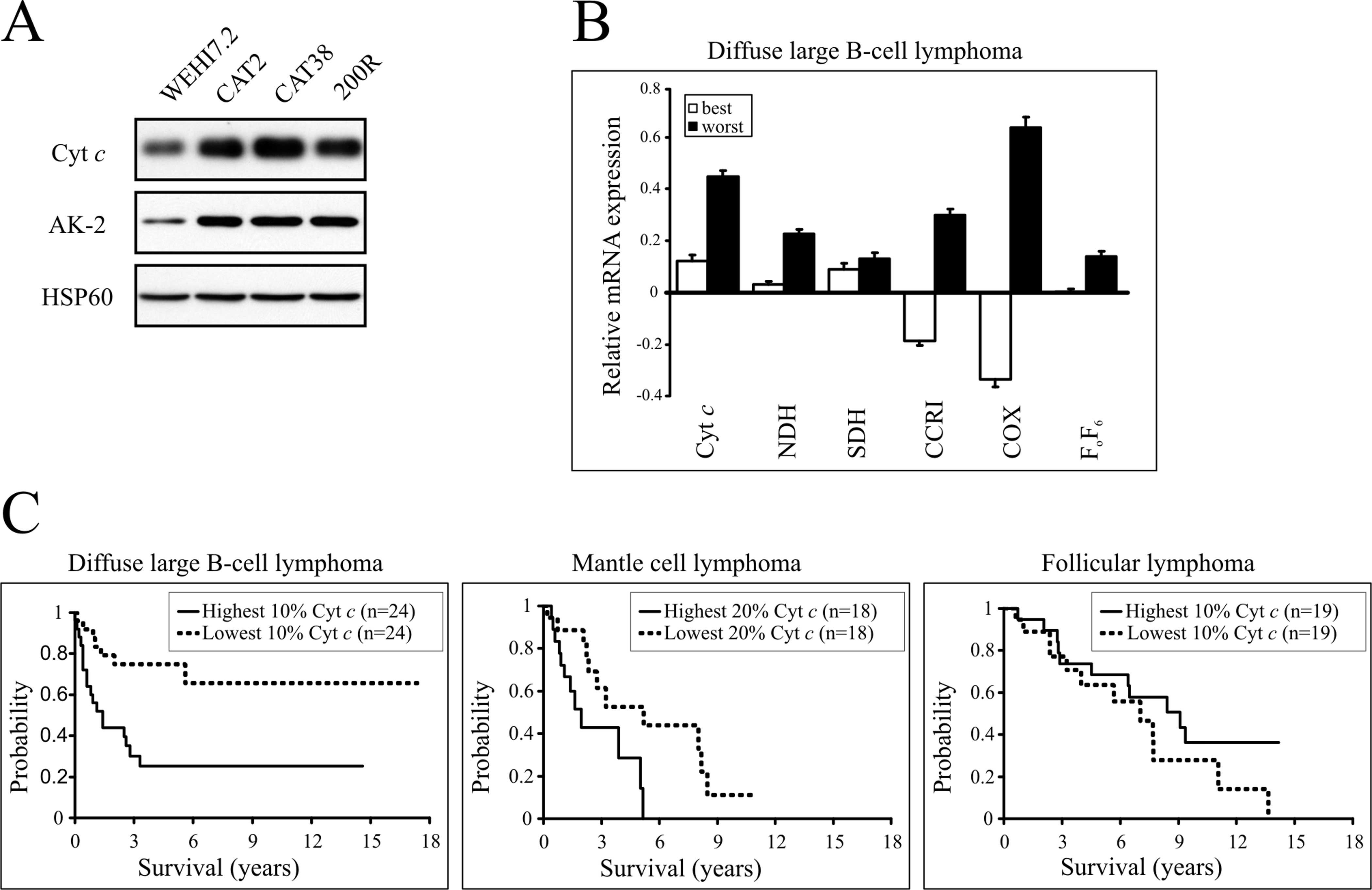 | Figure 1Increased expression of cytochrome
c in lymphoma. (A) Mitochondrial expression of cytochrome
c, AK-2 and HSP60 proteins. Immunoblots of mitochondrial
protein (50 μg) from WEHI7.2 parental, CAT2, CAT38 and 200R cells
were probed with antibodies to cytochrome c, AK-2 and HSP60.
Representative immunoblots are shown. (B) Mean mRNA expression of
oxidative phosphorylation proteins in diffuse large B-cell lymphoma
patients with the worst prognosis vs. those with the best
prognosis. Bars represent the mean relative mRNA expression on a
log2 scale, for the 10% of patients with the best (white bars) and
worst (dark bars) outcome predictor score, plus or minus the
standard error of the mean. (C) Overall survival of patients
separated by cytochrome c expression in diffuse large
B-cell, mantle cell and follicular lymphoma. Kaplan-Meier plots
were used to compare patient survival with the highest cytochrome
c expression (solid line) with that of patients with the
lowest cytochrome c expression (dashed line). The percentage
of patients used for analysis is indicated on each graph. Cyt
c, cytochrome c; NDH, NADH dehydrogenase; SDH,
succinate dehydrogenase; CCRI, cytochrome c reductase I;
COX, cytochrome c oxidase; FOF6,
ATPase FOF6. |
Correlation of increased oxidative
phosphorylation (OXPHOS) proteins with poor prognosis in diffuse
large B-cell lymphoma
Based on the characteristics of the lymphoma
variants observed in this and a previous study (3), an increased expression of cytochrome
c and other genes involved in OXPHOS is expected to
correlate with a poor response to chemotherapy in lymphoma
patients. To test this, we analyzed a GEP data set from DLBCL
patient samples. A previous study used this GEP data set to develop
an outcome predictor score (OPS) that accurately predicted patient
survival following chemotherapy (7). Ten genes coding for proteins involved
in OXPHOS, including cytochrome c, were included on the
LLMPP microarray (Table I). We
first identified cytochrome c as significantly correlated
with OPS since an increased expression was associated with poor
patient prognosis (p<0.05; Table
I and Fig. 1B). Additionally,
the increased expression of NADH dehydrogenase, succinate
dehydrogenase, cytochrome c reductase I, cytochrome c
oxidase and ATPase FOF6 were significantly
correlated with poor patient prognosis by OPS in DLBCL (p<0.05;
Table I and Fig. 1B). AK-2 expression was not
significantly correlated (at p≤0.05) with OPS. Notably, pyruvate
dehydrogenase kinase 3 also showed significant correlation with
OPS. Expression of this gene was significantly lower (p≤0.05) in
the 10% of patients with the worst outcome. The kinase that it
encodes functions to suppress the conversion of pyruvate to
acetyl-CoA by pyruvate dehydrogenase (PDH). Other studies have
shown that the fate of pyruvate can determine tumor metabolism
pathways, with increased PDH stimulating mitochondrial respiration
(12). Thus, the decreased
expression of PDH kinase in DLBCL patients with the worst prognosis
supports the increased activity of PDH, increased production of
acetyl-CoA substrate for entry into the tricarboxylic acid cycle,
and ultimately, the increased generation of mitochondrial ATP.
 | Table ICorrelation of mitochondrial protein
gene expression in diffuse large B-cell lymphoma patient tumor
samples with prognosisa. |
Table I
Correlation of mitochondrial protein
gene expression in diffuse large B-cell lymphoma patient tumor
samples with prognosisa.
| | | Statistical
significance |
|---|
| | |
|
|---|
| Gene | 10% best
prognosisb
Mean | 10% worst
prognosisb
Mean | t-test; best 10% vs.
worst 10%
P-value | Regression all
patients
P-value |
|---|
| Cytochrome
c | 0.1230 | 0.4475 | 0.0498c | 0.0001c |
| NADH
dehydrogenase | 0.0315 | 0.2270 | 0.0486c | 0.0010c |
| Succinate
dehydrogenase | 0.0899 | 0.1310 | 0.7941 | 0.0531c |
| Cytochrome c
reductase I | −0.1857 | 0.2987 | 0.0016c | <0.0001c |
| Cytochrome c
reductase II | 0.2167 | 0.2987 | 0.5866 | 0.7159 |
| Cytochrome c
reductase bp | 0.0367 | 0.0739 | 0.7155 | 0.6744 |
| Cytochrome c
oxidase | −0.3338 | 0.6360 | 0.0003c | <0.0001c |
| ATPase
FOF6 | 0.0022 | 0.1405 | 0.2291 | 0.0132c |
| ATPase
F1β | 0.1261 | 0.1604 | 0.8237 | 0.0872 |
| ATPase
FOg | 0.1425 | 0.1512 | 0.9531 | 0.3684 |
Correlation of increased cytochrome c
expression with poor survival in aggressive lymphoma
Given the results in our cell culture model and the
correlation with survival following chemotherapy in DLBCL, we
predicted that cytochrome c levels would correlate with
overall survival in lymphoma patients. We tested this using GEP
data for three non-Hodgkin lymphomas: DLBCL, MCL and FL. In the
aggressive DLBCL, survival was significantly worse (p<0.05) in
the 10% of patients with the highest cytochrome c expression
compared to those with the lowest expression (Fig. 1C). Similarly, for MCL, another
aggressive B-cell lymphoma, survival was significantly worse
(p<0.05) in the 20% of patients with the highest cytochrome
c expression compared to those with the lowest expression
(Fig. 1C). For FL, an indolent
B-cell lymphoma, the outcome was different. Cytochrome c
expression did not correlate with overall survival in FL (Fig. 1C).
Discussion
This study places mitochondria in the crossroads of
successful adaptation to oxidative stress and carcinogenesis. The
mitochondrial changes observed in our lymphoma model extend
previous findings of metabolic flexibility in the oxidative
stress-resistant cells (3,4). Our attempts to use continuous ethidium
bromide treatment as a technique to deplete mitochondrial DNA
(13) with the WEHI7.2 cells were
unsuccessful (data not shown). The inability to create WEHI7.2
rho0 cells lacking functional OXPHOS suggests that
reliance on mitochondrial respiration is critical for lymphoma
cells. This complements the observed increase in cytochrome
c and AK-2 levels, as well as an increased ability to
maintain mitochondrial ATP generation (3), in the resistant variants. A novel
conclusion from the present study, building on our previous
biochemical analyses (3,4), is that oxidative stress may provide
selective pressure for the concurrent development of apoptosis
resistance and the metabolic adaptations seen in malignant cells.
Since lymphoma can arise in the context of chronic infection and
inflammation, our investigations may have implications for both
current and novel therapies.
Increasing the mitochondrial respiratory proteins
allows the oxidative stress resistant variants to be metabolic
opportunists. Studies in HeLa cells show that growth in
galactose/glutamine instead of glucose leads to increased
dependence on OXPHOS (14). This
biochemical change occurs via increased respiratory chain protein
levels without increasing the mitochondrial mass. Thus, an increase
in respiratory chain proteins, such as cytochrome c, may
indicate enhanced OXPHOS capability and account for metabolic
flexibility in the aggressive variants.
Our analyses show an increased expression of
cytochrome c and other mitochondrial respiration proteins in
aggressive lymphomas from patients with the worst prognosis,
suggesting that results from the lymphoma cell model are clinically
relevant. The up-regulation of respiratory chain proteins in the
DLBCL patients with the worst prognosis is consistent with an
ability to use alternative energy substrates such as glutamine.
Some studies suggest that tumor cells suppress OXPHOS as a
protective mechanism to limit the production of endogenous reactive
oxygen species (12). However, in
our oxidative stress-resistant model and in tumors arising in the
context of chronic inflammation, the ability to tolerate increased
oxidative stress may allow them to take advantage of increased
mitochondrial respiration.
We have shown that an increased cytochrome c
expression is correlated with decreased survival in the aggressive
DLBCL and MCL. The up-regulation of alternate pathways for ATP
production would allow cancer cells to be metabolic opportunists in
a tumor microenvironment where specific nutrients are limiting.
This could provide both a growth advantage during tumor
development, as well as protection from apoptosis-inducing
chemotherapy. A correlation between cytochrome c expression
and overall survival was not observed in the indolent FL,
suggesting that for a less-aggressive cancer, increased OXPHOS
capability would be less critical. Additionally, the majority of
follicular lymphoma cases are characterized by overexpression of
BCL2 (8), and it is possible
that this prominent survival advantage overshadows metabolic
changes.
Our results suggest that an increased ability to use
mitochondria to produce ATP contributes to tumor development. The
WEHI7.2 variants with the mitochondrial alterations are resistant
to multiple lymphoma chemotherapeutics (Tome et al,
unpublished data), suggesting that these mitochondrial
characteristics also contribute to chemoresistance in the clinic.
Targeting such mitochondrial alterations with novel
chemotherapeutics may improve clinical outcome for these aggressive
lymphomas.
Acknowledgements
Funding was from NCI CA71768 (M.M.B), NCI-T32
CA09213 (S.T.W. and H.L.T.), HHMI 52003749 (D.B.F.J.), and ARCS
Foundation, Phoenix Chapter (H.L.T.).
References
|
1
|
Tome ME and Briehl MM: Thymocytes selected
for resistance to hydrogen peroxide show altered antioxidant enzyme
profiles and resistance to dexamethasone-induced apoptosis. Cell
Death Differ. 8:953–961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tome ME, Baker AF, Powis G, Payne CM and
Briehl MM: Catalase-overexpressing thymocytes are resistant to
glucocorticoid-induced apoptosis and exhibit increased net tumor
growth. Cancer Res. 61:2766–2773. 2001.PubMed/NCBI
|
|
3
|
Tome ME, Lutz NW and Briehl MM:
Overexpression of catalase or Bcl-2 alters glucose and energy
metabolism concomitant with dexamethasone resistance. Biochim
Biophys Acta. 1693:57–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tome ME, Briehl MM and Lutz NW: Increasing
the antioxidant defense in WEHI7.2 cells results in a more
tumor-like metabolic profile. Int J Mol Med. 15:497–501.
2005.PubMed/NCBI
|
|
5
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang GQ, Gastman BR, Wieckowski E,
Goldstein LA, Rabinovitz A, Yin XM and Rabinowich H:
Apoptosis-resistant mitochondria in T cells selected for resistance
to Fas signaling. J Biol Chem. 276:3610–3619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosenwald A, Wright G, Chan WC, et al: The
use of molecular profiling to predict survival after chemotherapy
for diffuse large-B-cell lymphoma. N Engl J Med. 346:1937–1947.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dave SS, Wright G, Tan B, et al:
Prediction of survival in follicular lymphoma based on molecular
features of tumor-infiltrating immune cells. N Engl J Med.
351:2159–2169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenwald A, Wright G, Wiestner A, et al:
The proliferation gene expression signature is a quantitative
integrator of oncogenic events that predicts survival in mantle
cell lymphoma. Cancer Cell. 3:185–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tome ME, Johnson DB, Rimsza LM, Roberts
RA, Grogan TM, Miller TP, Oberley LW and Briehl MM: A redox
signature score identifies diffuse large B-cell lymphoma patients
with a poor prognosis. Blood. 106:3594–3601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igamberdiev AU and Kleczkowski LA:
Equilibration of adenylates in the mitochondrial intermembrane
space maintains respiration and regulates cytosolic metabolism. J
Exp Bot. 57:2133–2141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frezza C and Gottlieb E: Mitochondria in
cancer: not just innocent bystanders. Semin Cancer Biol. 19:4–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
King MP and Attardi G: Isolation of human
cell lines lacking mitochondrial DNA. Methods Enzymol. 264:304–313.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rossignol R, Gilkerson R, Aggeler R,
Yamagata K, Remington SJ and Capaldi RA: Energy substrate modulates
mitochondrial structure and oxidative capacity in cancer cells.
Cancer Res. 64:985–993. 2004. View Article : Google Scholar : PubMed/NCBI
|















