Introduction
Genital human papillomavirus (HPV) is the most
common sexually transmitted infection. In the 1970s, investigation
on the possible role of HPV in cancer was postulated (1). Recently, it has been established that
HPVs are important in human carcinogenesis. HPV infection can cause
normal cells on infected mucous membranes and the skin to become
abnormal. There are more than 40 HPV genotypes that can infect
genital lesions of women and men in regions such as the vulva,
vagina, cervix, penis, anus and rectum. Low-risk HPV types can
cause genital warts in men and women. High-risk HPV types can cause
cervical cancer and other less common cancers, including vulvar,
vaginal, penile, anal and rectal (2). Although HPV-DNA can be detected in
some penile cancer tissues, the presence of HPV may be insufficient
by itself to establish full malignant transformation, and other
factors must be considered to be important in the carcinogenic
process. The synergistic action of HPV associated with poor
hygienic conditions may explain the epidemiological evidence that
many people are infected with HPV although only a small percentage
progress to malignancy over a period of many years, often several
decades (3,4).
Virchow suggested in the nineteenth century that
chronic inflammation is linked to cancer, but this was not widely
accepted before the discovery of nuclear factor-κB (NF-κB)
(5,6). Many investigators reported a key role
for the transcription factor NF-κB signaling pathway in controlling
the initiation and progression of human cancer. NF-κB and
associated regulatory proteins are activated downstream of numerous
oncoproteins. There is abundant evidence for the activation of
NF-κB-dependent target genes in malignant tumors (7,8). The
proportion of total cancer deaths attributable to infectious agents
is estimated to be 20–25% in developing countries and 7–10% in
industrialized countries. A causal relationship between chronic
infection and cancer has been widely accepted (9). In particular, there is a strong
association between tumor viruses and the development of human
cancers. Both DNA and RNA viruses have been reported to be capable
of causing cancer. Epstain-Barr virus (EBV), HPV, hepatitis B virus
(HBV) and Kaposi’s sarcoma-associated herpes virus (KSHV) are DNA
viruses known to lead to the development of cancers.
Cancer-inducing RNA viruses include hepatitis C virus (HCV) and
human T lymphotropic virus type-1 (HTLV-1). EBV is associated with
Burkitt’s lymphoma, nasopharyngeal carcinoma, Hodgkin’s lymphoma
and non-Hodgkin’s lymphoma. HBV and HCV viruses are associated with
hepatocellular carcinoma, HTLV-1 with adult T-cell
leukemia/lymphoma and HPV with cervical, penile, anal,
oropharyngeal and skin cancers (2).
In the present study, the procedure of in
situ hybridization (ISH) was used to determine the tissue
localization of HPV-DNA. Polymerase chain reaction (PCR) was used
for the detection of HPV genotypes in penile cancer. This study
aimed to determine the prevalence of HPV in penile cancer and the
subsequent overexpression of NF-κB.
Materials and methods
Tissue specimens
Thirty-four surgical specimens of penile tissue,
obtained at Nagasaki University Hospital and Japanese Red Cross
Nagasaki Hospital, Japan from 1972 to 2006, were used in this
study. The specimens were fixed in 10% formalin and embedded in
paraffin for histochemical, ISH and HPV-DNA sequence studies.
Histological analysis was performed using tissue sections (3.5 μm)
stained with hematoxylin and eosin. Penile cancer was classfied as
i) keratinizing squamous cell carcinoma, ii) non-keratinizing
squamous cell carcinoma and iii) verrucous carcinoma. Non-cancer
penile tissue was classified as i) Bowen’s disease, ii) condyloma
and iii) balanitis. Parallel sections were prepared for ISH and
HPV-DNA sequences.
In situ hybridization
Paraffin-embedded tissue specimens were cut into
3.5-μm sections and collected on silane-coated glass slides. The
ISH detection kit (Dako Co., Carpinteria, CA, USA) was used for the
detection of HPV-DNA. Wide spectrum HPV-DNA probe (Dako),
ISH-positive control probe, ISH-negative control probe and
HPV-positive control slides were examined.
The steps involved in the ISH procedure using the
HPV detection kit are: After hybridization with the probes, an
alkaline phosphatase-conjugated antibody against digoxigenin was
applied to the sections. The localization of HPV-DNA was detected
using NBT/BCIP substrate and observed under a light microscope.
DNA isolation from paraffin-embedded
tissue
DNA was isolated from sections of formalin-fixed,
paraffin-embedded tissue by using the DNA isolator PS kit (Wako
Pure Chemicals Industries, Ltd., Osaka, Japan), according to the
manufacturer’s instructions. Briefly, two 5-μm sections were cut
with a new blade and collected in a 1.5-ml tube, deparaffinized in
xylene, washed with 70% ethanol and digested with protease. The DNA
was precipitated with isopropanol and washed with 70% ethanol. The
dried DNA was dissolved in 20 ml of TE buffer. A DNA aliquot of 2
ml was used for PCR.
General primer-mediated HPV-PCR and
sequencing
General primer-mediated HPV-PCR was undertaken using
SPF10 primers, which can detect at least 43 different HPV
genotypes, as previously described (10). The PCR products were sequenced for
the detection and typing of HPV-DNA. Using the primers
5′-CTTGACCAGCCTCTCTCATGC-3′ and 5′-TGCAGTCTTAGACCCCACCC-3′, PCR
amplification of the C-reaction (CRP) gene was carried out in a
separate reaction. This resulted in a 1,000-bp product using the
SPF10 primers.
PCR and subsequent sequencing were carried out for
the detection and typing of HPV-DNA (10). Samples were subjected to PCR using
PureTaq Ready-to-Go PCR Beads (Amersham Biosciences Corp.,
Piscataway, NJ, USA) with 5-mM SPF primers located in the L1 open
reading frame of the HPV gene. The PCR amplifications were carried
out in a thermal cycler (Applied Biosystems, Foster, CA, USA) under
the following conditions: 94°C for 9 min; 45 cycles of 30 sec at
94°C, 45 sec at 45°C and 45 sec at 72°C and a final extension of 5
min at 72°C. The amplified products were separated on a 3% agarose
21 gel (Wako) and detected with ethidium bromide. The 65-bp
fragment of SPF was purified using a Qiaex II Gel Extraction Kit
(Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s
instructions, subsequently cloned into the pGEM-T Easy Vector
System (Promega Corp., Madison, WI, USA) and transformed into
Escherichia DH5a competent cells (Promega). Plasmids were
isolated from several independent colonies by using a Gene Elute
Plasmid Miniprep Kit (Sigma-Aldrich Japan, Tokyo, Japan). The
Genetic Analyzer (Applied Biosystems) was used for DNA sequencing.
The sequences of 22-bp interprimer regions in the PCR products were
compared with the GenBank database using the BLAST program.
Immunohistochemistry for NF-κB
Sections (3.5-μm) were placed on silane-coated glass
slides. The slides were deparaffinized to remove embedded medium
and then dehydrated. Slides were boiled in 0.01 mol/l citrate
buffer, pH 7.0, at 98°C for 40 min for antigen retrieval, and then
cooled at room temperature for 30 min. After the slides were rinsed
in 0.01 mol/l phosphate-buffered saline (PBS), pH 7.4, the
endogenous peroxidase activity was blocked with 3%
H2O2 and abolute methanol for 10 min. The
tissue sections were covered with a 1:50 dilution of mouse
monoclonal anti-human NF-κB antibody (Cell Signaling Technology
Inc., Beverly, MA, USA) or control serum at 37°C for 3 h. After
being washed with PBS, the sections were covered with EnVision
(Dako) at 37°C for 40 min, and rinsed in PBS. Antigenic sites on
sections were demonstrated by reacting the sections with a mixture
of 0.05% 33-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl
buffer and 0.01% hydrogen peroxide for 10 min. The sections were
then counterstained with methyl green for 10 min, dehydrated in
ethanol, cleared in xylene and mounted.
Results
Clinicopathological findings
Penile specimens were diagnosed histologically as
penile cancer (16 cases), Bowen’s disease (3 cases), condyloma (10
cases) and balanitis (5 cases). Sixteen cases of penile cancer were
classified into 10 cases of keratinizing squamous cell carcinoma, 4
cases of non-keratinizing carcinoma and 2 cases of verrucous
carcinoma. The age of penile cancer patients ranged from 38 to 77
years (mean 64 years).
Detection of HPV-DNA and typing
Table I shows the
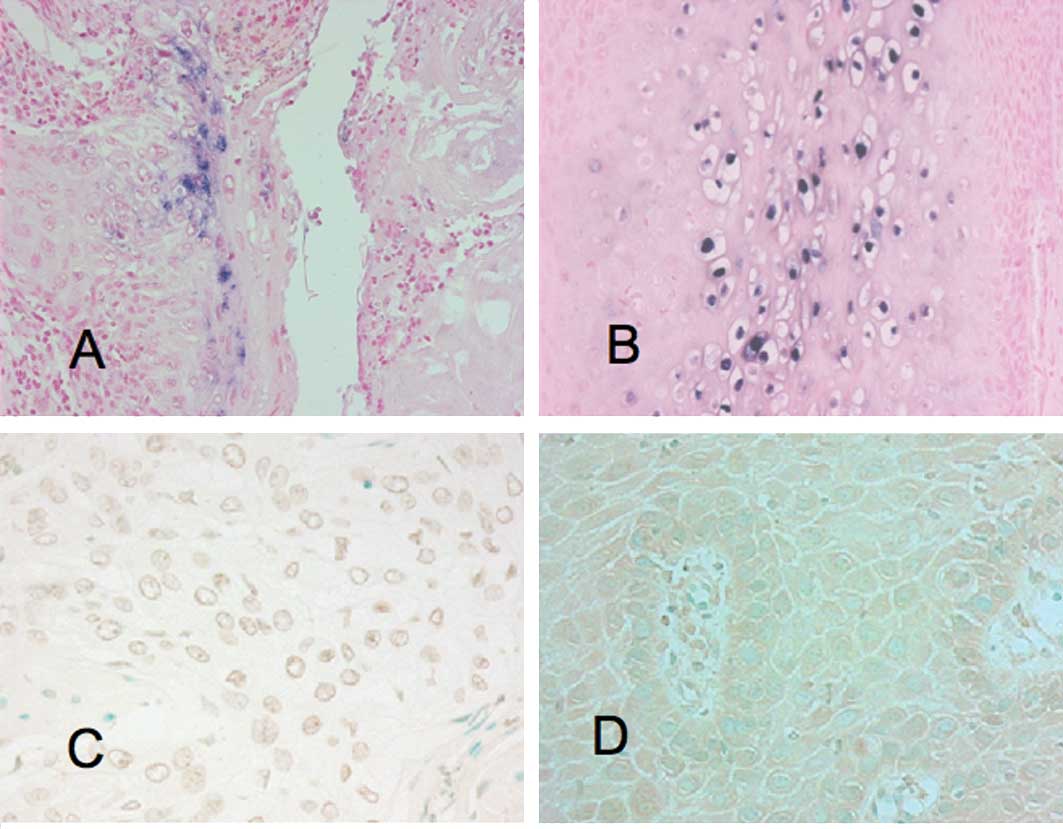
detection of HPV in cancer and non-cancer cases by ISH (Fig. 1) and PCR methods. A prevalence of
80% HPV-positive specimens was found in the cancer cases by PCR.
The PCR method gave higher positive rates than the ISH method in
both the cancer and non-cancer cases. Of the 16 cancer cases, 12
(75%) showed HPV-positivity. In contrast, of the 18 non-cancer
cases, 11 (61.1%) showed HPV-positivity. The results of HPV
genotype detection in the different histological categories are
summarized in Table II. Among the
12 HPV-positive penile cancers there were 5 single and 7 multiple
HPV infection cases. The single HPV-22 infection was the most
prevalent (25%), and single and multiple HPV-22 infections (83.3%)
were detected in all cancer HPV-positive cases. The single HPV-11
infection was the most prevalent (45.5%), and single and multiple
HPV-11 infections (81.8%) were detected in all non-cancer
HPV-positive cases.
 | Table IHPV-DNA detection by ISH and
SPF-PCR. |
Table I
HPV-DNA detection by ISH and
SPF-PCR.
| | HPV-positive for
ISH | HPV-positive for
PCR |
|---|
| |
|
|
|---|
| Diagnosis | No. | No. | % | No. | % |
|---|
| Keratinizing SCC | 10 | 4 | 40.0 | 8 | 80.0 |
| Non-keratinizing
SCC | 4 | 1 | 25.0 | 3 | 75.0 |
| Verrucous
carcinoma | 2 | 1 | 50.0 | 1 | 50.0 |
| All cancer cases | 16 | 6 | 37.5 | 12 | 75.0 |
| Bowen’s disease | 3 | 0 | 0.0 | 2 | 66.7 |
| Condyloma | 10 | 3 | 30.0 | 9 | 90.0 |
| Balanitis | 5 | 0 | 0.0 | 1 | 20.0 |
| All non-cancer
cases | 18 | 3 | 16.7 | 11 | 61.1 |
 | Table IIHPV-DNA genotype detection in penile
cancer and non-cancer cases. |
Table II
HPV-DNA genotype detection in penile
cancer and non-cancer cases.
| | HPV-positive | Single-HPV-6 | Single-HPV-11 | Single-HPV-22 | Single-HPV-68 | Double-HPV-6,-11 |
Double-HPV-11,-22 |
Double-HPV-11,-70 |
Double-HPV-22,-56 |
Double-HPV-22,-68 |
Triple-HPV-6,-11,-22 |
Triple-HPV-18,-22,-56 |
Triple-HPV-22,-56,-68 |
Triple-HPV-45,-52,-96 |
|---|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Diagnosis | No. | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|---|
| Keratinizing
SCC | 10 | 8 | 80.0 | | | | | 3 | 30.0 | | | | | 1 | 10.0 | | | 1 | 10.0 | | | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | | |
| Non-keratinizing
SCC | 4 | 3 | 75.0 | | | | | | | 1 | 25.5 | | | | | | | 1 | 25.0 | 1 | 25.0 | | | | | | | | |
| Verrucous
carcinoma | 2 | 1 | 50.0 | 1 | 50.0 | | | | | | | | | | | | | | | | | | | | | | | | |
| All cancer
cases | 16 | 12 | 75.0 | 1 | 6.3 | 0 | 0.0 | 3 | 18.8 | 1 | 6.3 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 12.5 | 1 | 6.3 | 1 | 6.3 | 1 | 6.3 | 1 | 6.3 | 0 | 0.0 |
| Bowen’s
disease | 3 | 2 | 66.7 | | | | | | | | | | | | | | | | | | | 1 | 33.3 | | | | | 1 | 33.3 |
| Condyloma | 10 | 9 | 90.0 | | | 5 | 50.0 | 1 | 10.0 | | | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | | | | | | | | | | | | |
| Balanitis | 5 | 1 | 20.0 | | | | | 1 | 20.0 | | | | | | | | | | | | | | | | | | | | |
| All non-cancer
cases | 18 | 11 | 61.1 | 0 | 0.0 | 5 | 27.8 | 2 | 11.1 | 0 | 0.0 | 1 | 5.6 | 1 | 5.6 | 1 | 5.6 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 |
Immunohistochemistry for NF-κB
Immunohistochemical analyses for NF-κB were
performed for all of the specimens (Fig. 1). The PCR method showed that of the
24 HPV-positive cases, 15 (62.5%) were NF-κB-positive in the
nucleus and 8 (33.3%) were NF-κB-positive in the cytoplasm.
Moreover, NF-κB was detected in 16 (66.7%) cases in the nucleus
and/or cytoplasm. Of the 10 HPV-negative cases, 6 (60%) were
NF-κB-positive in the nucleus, 3 (30%) were NF-κB-positive in the
cytoplasm and 7 (70%) were NF-κB-positive in the nucleus and/or
cytoplasm (Table III). The ISH
method showed that of the 9 HPV-positive cases, 8 (88.9%) were
NF-κB-positive in the nucleus and 2 (22.2%) were NF-κB-positive in
the cytoplasm. Additionally, NF-κB was detected in all 9 (100%)
cases in the nucleus and/or the cytoplasm. Of the 25 HPV-negative
cases, 14 (56%) were NF-κB-positive in the nucleus, 7 (28%) were
NF-κB-positive in the cytoplasm and 15 (60%) were NF-κB-positive in
the nucleus and/or the cytoplasm (Table IV).
 | Table IIIHPV-DNA detected by PCR and
correlation with NF-κB. |
Table III
HPV-DNA detected by PCR and
correlation with NF-κB.
| HPV by PCR | NF-κB in
nucleus | NF-κB in
cytoplasm | NF-κB in nucleus
and cytoplasm |
|---|
|
|
|
|
|
|---|
| Diagnosis | No. | % | No. | % | No. | % | No. | % |
|---|
| HPV-positive
cases | 24 | 70.6 | 15 | 62.5 | 8 | 33.3 | 16 | 66.7 |
| Keratinizing
SCC | 8 | 23.5 | 7 | 87.5 | 3 | 37.5 | 7 | 87.5 |
| Non-keratinizing
SCC | 3 | 8.8 | 2 | 66.7 | 0 | 0.0 | 2 | 66.7 |
| Verrucous
carcinoma | 1 | 2.9 | 1 | 100.0 | 0 | 0.0 | 1 | 100.0 |
| Bowen’s
disease | 2 | 5.9 | 2 | 100.0 | 2 | 100.0 | 2 | 100.0 |
| Condyloma | 9 | 26.5 | 3 | 33.3 | 3 | 33.3 | 4 | 44.4 |
| Balanitis | 1 | 2.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV-negative
cases | 10 | 29.4 | 6 | 60.0 | 3 | 30.0 | 7 | 70.0 |
| Keratinizing
SCC | 2 | 5.9 | 1 | 50.0 | 0 | 0.0 | 1 | 50.0 |
| Non-keratinizing
SCC | 1 | 2.9 | 0 | 0.0 | 1 | 100.0 | 1 | 100.0 |
| Verrucous
carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s
disease | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Condyloma | 1 | 2.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Balanitis | 4 | 11.8 | 3 | 75.0 | 0 | 0.0 | 3 | 75.0 |
| Total | 34 | 100.0 | 20 | 58.8 | 11 | 32.4 | 23 | 67.6 |
 | Table IVHPV-DNA detected by ISH and
correlation with NF-κB. |
Table IV
HPV-DNA detected by ISH and
correlation with NF-κB.
| HPV by ISH | NF-κB in
nucleus | NF-κB in
cytoplasm | NF-κB in nucleus
and cytoplasm |
|---|
|
|
|
|
|
|---|
| Diagnosis | No. | % | No. | % | No. | % | No. | % |
|---|
| HPV-positive
cases | 9 | 26.5 | 8 | 88.9 | 2 | 22.2 | 9 | 100.0 |
| Keratinizing
SCC | 4 | 11.8 | 4 | 100.0 | 1 | 0.0 | 4 | 100.0 |
| Non-keratinizing
SCC | 1 | 2.9 | 1 | 100.0 | 0 | 0.0 | 1 | 100.0 |
| Verrucous
carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s
disease | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 100.0 |
| Condyloma | 3 | 8.8 | 2 | 66.7 | 0 | 0.0 | 3 | 22.2 |
| Balanitis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV-negative
cases | 25 | 73.5 | 14 | 56.0 | 7 | 28.0 | 15 | 60.0 |
| Keratinizing
SCC | 6 | 17.6 | 4 | 66.7 | 1 | 16.7 | 4 | 66.7 |
| Non-keratinizing
SCC | 3 | 8.8 | 1 | 66.7 | 1 | 66.7 | 2 | 66.7 |
| Verrucous
carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s
disease | 3 | 8.8 | 3 | 100.0 | 3 | 100.0 | 3 | 100.0 |
| Condyloma | 7 | 20.6 | 2 | 28.6 | 1 | 13.0 | 2 | 28.6 |
| Balanitis | 5 | 14.7 | 3 | 60.0 | 0 | 0.0 | 3 | 60.0 |
| Total | 34 | 100.0 | 22 | 64.7 | 9 | 26.5 | 24 | 70.6 |
Discussion
Human papillomavirus (HPV) infection is associated
with a broad spectrum of benign and malignant neoplastic epithelial
changes. The association of HPV with neoplastic transformation has
been extensively investigated in lesions of the uterine cervix, and
the role of HPV in malignant transformation of the cervical
epithelium has been well established. Although several
epidemiological, clinical and pathological studies have indicated
that this virus is sexually transmitted, detailed information on
the association of HPV with penile cancer is less clearly
understood than that with cervical cancer. The virus can affect the
squamous epithelium of the male genitalia in a similar way to the
female genital tract. The prevalence of HPV in tumor tissue has
been reported to vary considerably (2). The frequency of HPV-positive penile
cancer (75%) reported in this study is similar to that reported by
Picconi et al (71%) (11)
and Senba et al (79%) (12).
Higher rates were reported by Sarkar et al (82%) (13) and Tornesello et al (83%)
(14). Lower rates were reported by
Senba et al (68.2%) (15),
Cupp et al (66%) (16),
Tornesello et al (46%) (17), Rubin et al (42%) (18) and Cubilla et al (31%)
(19).
HPVs can be classified into two groups depending on
whether they cause benign or malignant tumors. The high-risk group
includes HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -54, -56,
-58, -59, -66, -68 and -69, and the low-risk group includes HPV-6,
-11, -26, -30, -34, -40, -42, -43, -44, -53, -55, -57, -61, -62,
-64, -67, -70, -71, -73, 74, -79, -81, -82, -83 and -84 (20). It is generally accepted that the
malignant transformation of penile epidermis cells is associated
with infection by high-risk HPV genotypes. Previous studies have
shown that among the high-risk HPV genotypes, HPV-16 and -18 are
ascendant types and are correlated with penile cancer, constituting
13–60 and 3–19% of infections, respectively (11,16,18,21).
The low-risk genotypes are almost regularly encountered in benign
lesions, and the predominance of HPV-6 and -11, which are ascendant
types, is correlated with benign tumors. Notably, in this series,
the high-risk HPV-18 was detected in only 1 case of penile cancer
as part of a multiple infection with HPV-22 and -56. HPV-16
infections were not identified. The most prevalent genotype was the
HPV-22 found in 83.3% of the penile cancer cases. In addition,
HPV-11 was found in 81.8% of the non-cancer cases. Thus, in
Japanese penile cancer patients, the HPV genotypes differed from
those of patients from other countries.
Published data from recent studies suggest that
NF-κB activation is a key component in inflammation-based cancer
progression. This transcription factor is indispensable for the
malignant progression of transformed cells associated with various
inflammatory cells and a network of signaling molecules. The
expression and function of numerous cytokines, chemokines, growth
factors and survival factors are NF-κB-dependent. NF-κB activation
has been implicated in a variety of processes related to
transformation and oncogenesis (7,8,22).
NF-κB activation is important in the early stages of damaged
DNA-induced carcinogenesis. Increased NF-κB activity is associated
with many types of cancers including those associated with viral
infections. NF-κB activity is modulated for many different viruses,
such as human immunodeficiency virus 1, human T-lymphotropic virus
1, Epstein-Barr virus, hepatitis B virus, adenoviruses and HPVs
(23–28). NF-κB-dependent proliferation of
cells and protection from apoptosis are likely to have significant
effects on the oncogenesis associated with HPV infections. In this
study, for the HPV-positive cases detected by ISH, NF-κB was
detected in the nucleus and/or cytoplasm in 100% of the samples.
However, NF-κB was detected in the nucleus and/or cytoplasm in only
60% of the HPV-negative cases. It is known that the PCR method,
which amplifies the virus genome, is more sensitive than the ISH
method. HPV-DNA was detected in 37.5 and 75% of cases of penile
cancer, using ISH and PCR, respectively. These results suggest that
ISH is a valid method for detecting HPV infection from cases that
present with a high level of HPV infection. Moreover, a high level
of HPV infection leads to a more active NF-κB. HPV oncoproteins E6
and E7 are essential factors for HPV oncogenesis. E7 and E6 react
with the tumor-suppressor genes p53 and pRb in host cell protein,
resulting in induced cellular immortalization, transformation and
carcinogenesis, due to their interference with cell cycle and
apoptosis control. E7- and E6-induced genetic instability leads to
the activation of oncogenes and inactivation of tumor-suppressor
genes (25). A fraction of the E7
protein is found in association with the IκB kinase complex and
attenuates induced kinase activity of IκB kinase α (IKKα) and IKKβ,
thus resulting in impaired IκBα phosphorylation and degradation.
While E7 obviates IKK activation in the cytoplasm, the E6 protein
reduces NF-κB p65-dependent transcriptional activity within the
nucleus. It has been suggested that the HPV oncogene-mediated
suppression of NF-κB activity contributes to HPV escape from the
immune system (24). Therefore, HPV
E6 and E7 oncoproteins are important regulatory proteins inside
host cells and are associated with the transcriptional activity of
NF-κB.
References
|
1
|
Zur Hausen H: Papillomaviruses in the
causation of human cancers – a brief historical account. Virology.
44:219–224. 2009.
|
|
2
|
Senba M, Mori N and Wada A: Oncogenesis of
and the link between inflammation and cancer due to human
papillomavirus (HPV) infectionand the development of vaccine
control strategies. Cancer Res J. 2:307–338. 2009.
|
|
3
|
Smith JS, Herrero R, Bosetti C, et al:
Herpes simplex virus-2 as a human papillomavirus cofactor in the
etiology of invasive cervical cancer. J Natl Cancer Inst.
94:1604–1613. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith JS, Munoz N, Herrero R, et al:
Evidence for Chlamydia trachomatis as a human papillomavirus
cofactor in the etiology of invasive cervical cancer in Brazil and
the Philippines. J Infect Dis. 185:324–331. 2002.
|
|
5
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karin M and Greten FR: NF-κB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
8
|
Karin M: Nuclear factor-κB in cancer
development and progression. Nature. 441:431–436. 2006.
|
|
9
|
Schottenfeld D and Beebe-Dimmer J: Chronic
inflammation: a common and important factor in the pathogenesis of
neoplasia. CA Cancer J Clin. 56:69–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kleter B, Doorn LJ, Schegget J, et al:
Novel short-fragment PCR assay for highly sensitive broad-spectrum
detection of anogenital human papillomaviruses. Am J Pathol.
135:1731–1739. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Picconi MA, Ejian AM, Distefano AL, et al:
Human papillomavirus (HPV) DNA in penile carcinomas in Argentina:
analysis of primary tumors and lymph nodes. J Med Virol. 61:65–69.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senba M, Kumatori A, Fujita S, et al: The
prevalence of human papilomavirus genotypes in penile cancers from
northern Thailand. J Med Virol. 78:1341–1346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarkar FH, Miles BJ, Plieth DH and
Crissman JD: Detection of human papillomavirus in squamous neoplasm
of the penis. J Urol. 147:389–392. 1992.PubMed/NCBI
|
|
14
|
Tornesello ML, Buonaguro FM, Beth-Giraldo
E, Kyalwazi SK and Giraldo G: Human papillomavirus (HPV) DNA in
penile carcinomas and two cell lines from high-incidence area for
genital cancers in Africa. Int J Cancer. 51:587–592. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senba M, Buziba N, Mori N, Wada A, Irie S
and Toriyama K: Detection of human papillomavirus and cellular
regulators p16INK4a, p53, and NF-κB in penile cancer
cases in Kenya. Acta Virol. 53:43–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cupp MR, Malek RS, Goellner JR, Smith TF
and Espy MJ: The detection of human papillomavirus deoxyribonucleic
acid in intraepithelial, in situ, verrucous and invasive carcinoma
of the penis. J Urol. 154:1024–1029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tornesello ML, Duraturo ML, Losito S, et
al: Human papillomavirus genotypes and HPV 16 variants in penile
carcinoma. Int J Cancer. 122:132–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubin MA, Kleter B, Zhou M, et al:
Detection and typing of human papillomavirus DNA in penile
carcinoma: evidence for multiple independent pathways of penile
carcinogenesis. Am J Pathol. 159:1211–1218. 2001. View Article : Google Scholar
|
|
19
|
Cubilla AL, Reuter VE, Gregoire L, et al:
Basaloid squamous cell carcinoma: a distinctive human papilloma
virus-related penile neoplasm. A report of 20 cases. Am J Surg
Pathol. 22:755–761. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gross G and Pfister H: Role of human
papillomavirus in penile cancer, penile intraepithelial squamous
cell neoplasia and in genital warts. Med Microbiol Immunol.
193:35–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gregoire L, Cubilla AL, Reuter VE, Haas GP
and Lancaster WD: Preferential association of human papillomavirus
with high-grade histological variants of penile invasive squamous
cell carcinoma. J Natl Cancer Inst. 87:1705–1709. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiriakidis S, Andreakos E, Monaco C,
Foxwell B, Feldmann M and Paleolog E: VEGF expression in human
macrophages is NF-κB-dependent: studies using adenoviruses
expressing the endogenous NF-κB inhibitor IκBα and kinase-defective
form of the IκB kinase 2. J Cell Sci. 116:665–674. 2003.
|
|
23
|
Nees M, Geoghegan JM, Hyman T, Frank S,
Miller L and Woodworth CD: Papillomavirus type 16 oncogenes
downregulate expression of interferon-responsive genes and
upregulate proliferation-associated and NF-κB-responsive genes in
cervical keratinocytes. J Virol. 75:4283–4296. 2001.PubMed/NCBI
|
|
24
|
Spitkovsky D, Hehner SP, Hofmann TG,
Moller A and Schmitz ML: The human papillomavirus oncoprotein E7
attenuates NF-κB activation by targeting IκB kinase complex. J Biol
Chem. 277:25576–25582. 2002.PubMed/NCBI
|
|
25
|
Havard L, Delvenne P, Frare P, Boniver J
and Giannini SL: Differential production of cytokines and
activation of NF-κB in HPV transformed keratinocytes. Virology.
298:271–285. 2002.
|
|
26
|
Havard L, Rahmouni S, Boniver J and
Delvenne P: High levels of p105 (NFκB1) and p100 (NFκB2) proteins
in HPV 16-transformed keratinocytes: role of E6 and E7
oncoproteins. Virology. 331:357–366. 2005.
|
|
27
|
Mishra A, Bharti AC, Varghese P, Saluja D
and Das BC: Differential expression and activation of NF-κB family
proteins during oral carcinogenesis: role of high risk human
papillomavirus infection. Int J Cancer. 119:2840–2850. 2006.
|
|
28
|
James MA, Lee JH and Klingelhutz AJ: Human
papillomavirus type 16 E6 activates NF-κB, induces cIAP-2
expression and protects against apoptosis in a PDZ binding
motif-dependent manner. J Virol. 80:5301–5307. 2006.
|