Introduction
Alleic loss of chromosome 3p is one of the most
frequent and earliest documented events in lung cancer with a wide
range of 3p mutations (12–26), suggesting the presence of multiple
tumor-suppressor genes on 3p (1–3). The
chromosome locus 3p22.3 is a ‘hot spot’ for chromosomal aberrations
and loss of heterozygosity in cancers, including lung cancer
(4,5). Further analysis led to the
identification of the DLC1 gene (6), which was later renamed deleted in
lung and esophageal cancer 1 (DLEC1) on 3p22.3. Loss of
DLEC1 expression has been observed in lung, esophageal,
renal, ovarian and nasopharyngeal carcinoma cell lines and primary
tumors. Moreover, functional analyses strongly suggest that
DLEC1 is a tumor-suppressor gene (6–8).
Promoter hypermethylation has been shown to be responsible for the
silencing of DLEC1 in ovarian cancer and nasopharyngeal
carcinoma (7,8). Furthermore, there have been
methylation analyses reported for hepatocellular carcinoma
(4), gastric (9) and lung cancers (5).
This study investigated whether the promoter
hyper-methylation of DLEC1 plays a role in lung cancer in
Japanese patients, and whether it has any prognostic significance.
The methylation status of the promoter regions of DLEC1 was
investigated using methylation-specific PCR. The findings were
compared to the clinicopathological features of lung cancer.
Patients and methods
Patients
The study group included lung cancer patients who
had undergone surgery at the Nagoya City University Hospital.
Written informed consent was obtained. Approval was granted by the
Institutional Ethics Committee of the Nagoya City University
Graduate School of Medical Sciences. The lung tumors were
classified according to the general criteria for the clinical and
pathological record of lung cancer in Japan (10). Tumor samples were immediately frozen
and stored at −80°C until assayed. The clinical and pathological
characteristics of the 116 lung cancer patients for DLEC1
sequencing analysis follow. Eighty-nine patients (76.7%) were male,
27 were female. Thirty-eight patients (32.8%) had squamous cell
carcinomas; 65, adenocarcinomas and 10 had adenosquamous cell
carcinomas. Eighty-five (73.8%) were smokers and 31 were
non-smokers. The clinical and pathological characteristics of the
lung cancer patients for the methylation analyses are documented in
Tables I, II and III, respectively. The samples from these
patients were previously sequenced for EGFR (11–14).
 | Table IClinicopathological data of 116 lung
cancer patients. |
Table I
Clinicopathological data of 116 lung
cancer patients.
| DLEC1 gene
status |
|---|
|
|
|---|
| Factors | Methylated cases | Unmethylated
cases | p-value |
|---|
| Mean age (years) | 64.5±8.9 | 66.3±13.3 | 0.2211 |
| Stage | | | 0.7028 |
| I | 24 (36.9%) | 21 (41.2%) | |
| II–IV | 41 (63.1%) | 30 (58.8%) | |
| Lymph node
metastasis | | | 0.5734 |
| N0 | 34 (52.3%) | 30 (58.8%) | |
| N+ | 31 (47.7%) | 21 (41.2%) | |
| Smoking | | | 0.2910 |
| Never smoker | 20 (30.8%) | 11 (21.6%) | |
| Smoker | 45 (69.2%) | 40 (79.4%) | |
| EGFR status | | | 0.1657 |
| Wild-type | 49 (75.4%) | 44 (86.3%) | |
| Mutation | 16 (24.6%) | 7 (13.7%) | |
| Pathological
subtypes | | | 0.4269 |
| SCC | 19 (29.2%) | 19 (37.3%) | |
| Non-SCC | 46 (70.8%) | 32 (62.7%) | |
| Age | | | 0.3478 |
| ≤65 | 32 (49.2%) | 20 (39.2%) | |
| >65 | 33 (50.8%) | 31 (60.8%) | |
| Gender | | | 0.9999 |
| Male | 50 (76.9%) | 39 (76.5%) | |
| Female | 15 (23.1%) | 12 (23.5%) | |
 | Table IIClinicopathological data of 221 lung
cancer patients. |
Table II
Clinicopathological data of 221 lung
cancer patients.
| p16 gene
status |
|---|
|
|
|---|
| Factors | Methylated
cases | Unmethylated
cases | p-value |
|---|
| Mean age
(years) | 66.1±8.9 | 64.3±10.9 | 0.4076 |
| Stage | | | 0.2726 |
| I | 39 (43.3%) | 67 (51.5%) | |
| II–IV | 51 (56.7%) | 63 (48.5%) | |
| Lymph node
metastasis | | | 0.1207 |
| N0 | 51 (56.0%) | 87 (66.9%) | |
| N+ | 40 (44.0%) | 43 (33.1%) | |
| Smoking | | | 0.0122 |
| Never smoker | 15 (16.5%) | 41 (31.5%) | |
| Smoker | 76 (83.5%) | 89 (68.5%) | |
| EGFR status | | | 0.0071 |
| Wild-type | 80 (87.9%) | 94 (72.3%) | |
| Mutation | 11 (12.1%) | 36 (27.7%) | |
| Pathological
subtype | | | 0.030 |
| SCC | 49 (53.8%) | 54 (38.6%) | |
| Non-SCC | 42 (46.2%) | 86 (61.4%) | |
| Age | | | 0.2198 |
| ≤65 | 37 (40.7%) | 64 (49.2%) | |
| >65 | 54 (59.3%) | 66 (50.8%) | |
| Gender | | | 0.6141 |
| Male | 74 (81.3%) | 101 (77.7%) | |
| Female | 17 (18.7%) | 29 (22.3%) | |
 | Table IIIClinicopathological data of 118 lung
cancer patients. |
Table III
Clinicopathological data of 118 lung
cancer patients.
| CDH1 gene
status |
|---|
|
|
|---|
| Factors | Methylated
cases | Unmethylated
cases | p-value |
|---|
| Mean age
(years) | 66.6±8.9 | 62.7±11.2 | 0.0491 |
| Stage | | | 0.5669 |
| I | 22 (33.8%) | 21 (39.6%) | |
| II–IV | 43 (66.2%) | 32 (60.4%) | |
| Lymph node
metastasis | | | 0.5780 |
| N0 | 34 (52.3%) | 31 (58.5%) | |
| N+ | 31 (47.7%) | 22 (41.5%) | |
| Smoking | | | 0.4073 |
| Never smoker | 15 (23.1%) | 16 (30.2%) | |
| Smoker | 50 (76.9%) | 37 (69.8%) | |
| EGFR status | | | 0.2594 |
| Wild-type | 54 (83.1%) | 39 (73.6%) | |
| Mutation | 11 (16.9%) | 14 (26.4%) | |
| Pathological
subtypes | | | 0.0514 |
| SCC | 37 (56.9%) | 40 (75.5%) | |
| Non-SCC | 28 (43.1%) | 13 (24.5%) | |
| Age | | | 0.0275 |
| ≤65 | 24 (36.9%) | 29 (54.7%) | |
| >65 | 41 (63.1%) | 24 (43.3%) | |
| Gender | | | 0.5096 |
| Male | 52 (80.0%) | 39 (73.6%) | |
| Female | 13 (20.0%) | 14 (26.4%) | |
Methylation-specific polymerase chain
reaction analysis
DNA was prepared from tissue samples using standard
methods. Bisulfite modification of genomic DNA was performed using
the MethylCode Bisulfite Conversion Kit (Invitrogen, CA, USA).
Briefly, 500 ng of genomic DNA was denatured by incubation with CT
Conversion Reagent at 98°C for 10 min and 68°C for 2.5 h and at 4°C
for several min. Modified DNA was purified by the Spin Column and
then eluted with Elution Buffer.
The primer sequences for the DLEC1 gene for
methylated (M) sequences were: forward, 5-GTTTCGTAGTTCGGTT TCGT C-3
and reverse, 5-CGAAATATCTTAAATACGCA ACG-3 (107 bp). The primer
sequences for the DLEC1 gene for unmethylated (U) sequences
were: forward, 5-TAGTTTT GTAGTTTGGTTTTGTT-3 and reverse,
5-ACAAAATATCT TAAATACACACAACA-3. The primer sequences for the
CDH1 gene for methylated (M) sequences were: forward,
5-GGTGAATTTTTAGTTAATTAGCGGTAC-3 and reverse,
5-CATAACTAACCGAAAACGCCG-3. The primer sequences for the CDH1
gene for unmethylated (U) sequences were: forward,
5-GGTAGGTGAATTTTTAGTTAATTAGTGGTA-3 and reverse,
5-ACCCATAACTAACCAAAAACACCA-3. The primer sequences for the
p16 gene for methylated (M) sequences were: forward,
5-TTATTAGAGGGTGGGGTG GATTGT-3 and reverse, 5-G ACCCCGAACCGCGACCG
TAA-3. The primer sequences for the p16 gene for
unmethylated (U) sequences were: forward, 5-TTATTAGAGGGT
GGGGTGGATTGT-3 and reverse, 5-CAACCCCAAACC ACAACCATA-3. The cycling
conditions were: initial denaturation at 94°C for 5 min, followed
by 40 cycles at 94°C for 45 sec, 65°C (p16, M), 60°C
(p16, U), 58°C (DLEC1, M), 57°C (CDH1) or 55°C
(DLEC1, U) for 45 sec, and 72°C for 45 sec.
Statistical analysis
Statistical analyses were carried out using the
Mann-Whitney U test for unpaired samples and the Wilcoxon
signed-rank test for paired samples. Linear relationships between
variables were determined by means of simple linear regression.
Correlation coefficients were determined by rank correlation using
Spearman’s and the χ2-test. Survival of the lung cancer
patients was examined by the Kaplan-Meier method, and differences
were examined by the log-rank test. Analyses were carried out using
the Stat-View software package (Abacus Concepts Inc., Berkeley, CA,
USA), and differences were considered significant at p<0.05.
Results
DLEC1 gene methylation status in Japanese
lung cancer patients
Methylation-specific PCR showed that the
DLEC1 promoter region was methylated in 65 out of 116
(79.8%) lung cancer types. The methylation status of DLEC1
was not associated with squamous histology (squamous cell carcinoma
29.2% vs. non-squamous cell carcinoma 37.3%; p=0.4269) and smoking
status (never smoker 30.8% vs. smoker 21.6%; p=0.291). In addition,
the DLEC1 methylation status did not correlate with gender
(p=0.9999), age (p=0.3478), lymph node metastasis (p=0.5734) and
pathological stages (I vs. II–IV; p=0.7028). DLEC1
methylation independently existed with EGFR mutations
(p=0.1657).
The p16 promoter region was methylated in 91
out of 221 (78.8%) lung cancer types (Table II). The methylation status was
correlated with squamous histology (p=0.03), smoking status (never
smoker vs. smoker; p=0.0122) and EGFR wild-type (p=0.0071).
However, the p16 methylation status did not correlate with
survival (p=0.6215).
The CDH promoter region was methylated in 65
out of 118 (78.8%) lung cancer types (Table III). A higher frequency of
methylation cases of the CDH1 promoter region was associated
with younger patients (p=0.0491). However, the methylation status
did not correlate with survival (p=0.8011).
DLEC1 gene methylation status and
survival in lung cancer patients
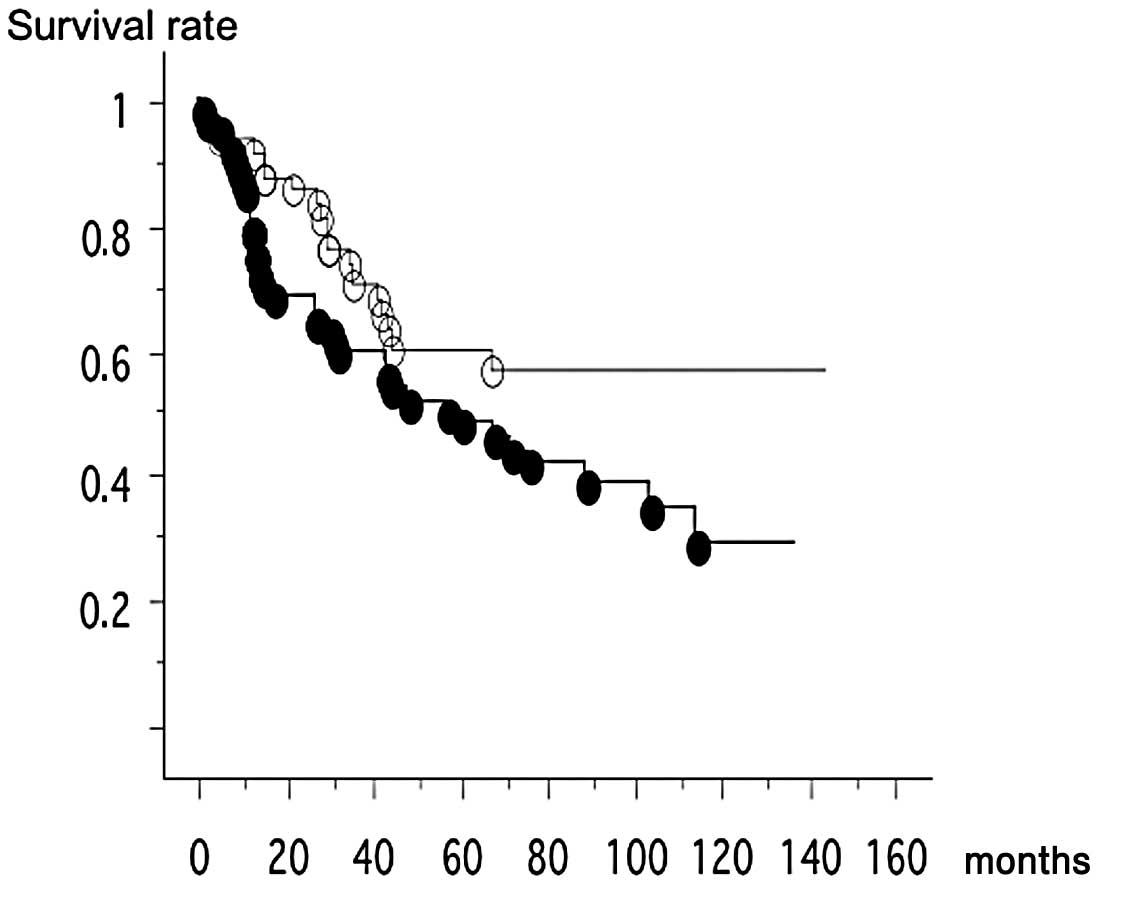
Of the 65 DLEC1 methylated cases, 37 patients
succumbed to the disease, while of the 51 unmethylated cases, 18
patientts succumbed to the disease. Thus, patients with
DLEC1 methylated cancer were significantly associated with
poor survival (log-rank test, p=0.0407) (Fig. 1). In the multivariate analysis,
pathological stage [stage I vs. stage III–IV, p=0.0038, relative
risk, 2.495 (1.342–4.636)] and DLEC1 methylation status were
independent prognostic factors [p=0.0348, relative risk, 1.865
(1.046–3.362)].
Discussion
We demonstrated that the DLEC1 gene was
hypermethylated in Japanese lung cancer patients. We did not find
any correlations between methylation status and gender,
pathological stage and smoking status in Japanese NSCLC patients.
However, the DLEC1 methylation status was correlated with
survival in Japanese lung cancer patients.
DLEC1 is a candidate tumor-suppressor gene
found in multiple cancer types (3–5,7,15,16).
DLEC1 suppresses tumor growth or reduces the invasiveness of
cancer cells. In ovarian cancer cell lines, the number of colonies
formed in DLEC1 transfectants was significantly lower than
that in mock transfectants (8),
which showed that DLEC1 suppressed the growth of ovarian
cancer cells and/or inactivation of CDC2 kinase, thereby blocking
cells at the G2/M phase and preventing tumor development in nude
mice (4,5). The demethylating agent 5-aza caused
the loss of mRNA expression in lung cancer cell lines (16). DLEC1 methylation is
cancer-specific, as it was only rarely detected in matched normal
lung tissue (5). As there is no
antibody available for DLEC1, we were unable to determine
whether methylated tumors show a loss or reduced DLEC1
protein expression. However, a loss of DLEC1 RNA expression
was previously shown in 8 out of 30 primary lung cancer types,
although this loss was not due to gene mutations (6). No correlation between DELC1
methylation and clinical parameters of gastric cancer was found
(17), similar to our
investigation.
It was demonstrated that p16 methylation
occurs more frequently in squamous cell carcinoma (16,18).
These previous results are consistent with our present findings.
Several studies have demonstrated a significant association between
DNA methylation and tobacco smoking (20,21).
Methylation of the p16INK4A gene was induced in rats treated with
tobacco-specific NKK[4-N-methyl-N-nitrosamino-1-3-prydil
1-1-butanone (NNK), polyaromatic hydrocarbon] (20). Lung tumors that were induced in
F344/N rats after exposure to cigarette smoke by inhalation
displayed de novo methylation of p16INK4a (21).
The correlation between CDH1 gene methylation
and survival is controversial. Although Nakata et al
demonstrated a marginal correlation between CDH1 methylation
and survival (p=0.0473) (16), Kim
et al demonstrated that CDH1 methylation itself did
not correlate with either survival or clinicopathological factors
(22).
Preclinically, the majority of ovarian cancer cell
lines siginificantly up-regulated DLEC1 transcripts after histone
deacetylase (HDAC) inhibitor treatment (8). Moreover, exposure to the HDAC
inhibitor PXD101 (belinostat) had varying effects on hepatocellular
carcinoma cell lines (23). Thus,
several HDAC inhibitors were found to exhibit antiproliferative
activity and induce apoptosis in human cancer cells (24). Moreover, the restoration of
tumor-suppressor genes, such as DLEC1, by HDAC inhibitors
may contribute to antitumor effects.
Acknowledgements
The authors would like to thank Mrs. Tomomi Shibata
for the excellent technical assistance. This work was supported by
the Grand-in-Aid for Research, Nagoya City University (2006) and
Grants-in-Aid for Scientific Research, Japan Society for the
Promotion of Science (JSPS) (nos. 21591820 and 21390394).
References
|
1
|
Hung J, Kishimoto Y, Sugio K, et al:
Allele-specific chromosome 3p deletions occur at an early stage in
the pathogenesis of lung carcinoma. JAMA. 273:558–563. 1995.
View Article : Google Scholar
|
|
2
|
Wistuba II, Behrens C, Virmani AK, et al:
High resolution chromosome 3p allelotyping of human lung cancer and
preneoplastic/preinvasive bronchial epithelium reveals multiple,
discontinuous sites of 3p allele loss and three regions of frequent
breakpoints. Cancer Res. 60:1949–1960. 2000.
|
|
3
|
Zaborovsky ER, Lerman MI and Minna JD:
Tumor suppressor genes on chromosome 3p involved in the
pathogenesis of lung and other cancers. Oncogene. 21:6915–6935.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu GH, Salto-Tellez M, Ross JA, et al:
The tumor suppressor gene DLEC1 is frequently silenced by DNA
methylation in hepatocellular carcinoma and induces G1 arrest in
cell cycle. J Hepatol. 48:433–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seng TJ, Currey N, Cooper WA, et al: DLEC1
and MLH1 promoter methylation are associated with poor prognosis in
non-small cell lung carcinoma. Br J Cancer. 99:375–382. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daigo Y, Nishiwaki T, Kawasoe T, Tamari M,
Tsuchiya E and Nakamura Y: Molecular cloning of a candidate tumor
suppressor gene, DLC1, from chromosome 3p. 213. Cancer Res.
59:1966–1972. 1999.PubMed/NCBI
|
|
7
|
Kwong J, Chow LS, Wong WK, et al:
Epigenetic inactivation of deleted in lung and esophageal cancer 1
gene in nasopharyngeal carcinoma. Genes Chromosomes Cancer.
46:171–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwong J, Lee JY, Wong KK, et al: Candidate
tumor-suppressor gene DLEC1 is frequently downregulated by promoter
hypermethylation and histone hypoacetylation in human epithelial
ovarian cancer. Neoplasia. 8:268–278. 2006. View Article : Google Scholar
|
|
9
|
Kang GH, Lee S, Cho NY, et al: DNA
methylation profiles of gastric carcinoma characterized by
quantitative DNA methylation analysis. Lab Invest. 88:161–170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japan Lung Cancer Society. General Rule
for Clinical and Pathological Record of Lung Cancer. 5th edition.
Jpn Lung Cancer Soc. 5. pp. 1–177. 1999
|
|
11
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Endo K, Konishi A, Sasaki H, et al:
Epidermal growth factor receptor gene mutation in non-small cell
lung cancer using highly sensitive and fast TaqMan PCR assay. Lung
Cancer. 50:375–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki H, Shimizu S, Endo K, et al: EGFR
and erbB2 mutation status in Japanese lung cancer patients. Int J
Cancer. 118:180–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki H, Endo K, Konishi A, et al: EGFR
mutation status in Japanese lung cancer patients: genotyping
analysis using LighyCycler. Clin Cancer Res. 11:2924–2929. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith IM, Mithani SK, Liu C, et al: Novel
integrative methods for gene discovery associated with head and
neck squamous cell carcinoma development. Arch Otolaryngol Head
Neck Surg. 135:487–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakata S, Sugio K, Uramoto H, et al: The
methylation status and protein expression of CDH1, p16INK4A, and
fragile histidine triad in nonsmall cell lung carcinoma: epigenetic
silencing, clinical features, and prognostic significance. Cancer.
106:2190–2199. 2006. View Article : Google Scholar
|
|
17
|
Ying J, Poon FF, Yu J, et al: DLEC1 is a
functional 3p22.3 tumor suppressor silenced by promoter CpG
methylation in colon and gastric cancers. Br J Cancer. 100:663–669.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jarmalaite S, Kannio A, Anttila S, et al:
Aberrant p16 promoter methylation in smokers and former smokers
with non-small cell lung cancer. Int J Cancer. 106:913–918. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim DH, Nelson HH, Wiencke JK, et al:
p16(INK4a) and histology-specific methylation of CpG islands by
exposure to tobacco smoke in non-small cell lung cancer. Cancer
Res. 61:3419–3424. 2001.PubMed/NCBI
|
|
20
|
Rom WN, Jay JG, Lee TC, Jiang Y and
Tchou-Wong KM: Molecular and genetic aspects of lung cancer. Am J
Respir Crit Care Med. 161:1355–1367. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swafford DS, Middleton SK, Palmisano WA,
et al: Frequent aberrant methylation of p16INK4a in primary rat
lung tumors. Mol Cell Biol. 17:1366–1374. 1997.PubMed/NCBI
|
|
22
|
Kim DS, Kim MJ, Lee JY, et al: Aberrant
methylation of E-cadherin and H-cadherin genes in non-small cell
lung cancer and its relation to clinicopathologic features. Cancer.
110:2785–2792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma BB, Sung F, Tao Q, et al: The
preclinical activity of the histone deacetylase inhibitor PXD101
(belinostat) in hepatocellular carcinoma cell lines. Invest New
Drugs. Jan 27–2009.(Epub ahead of print).
|
|
24
|
Takai N, Kawamata N, Gui D, Said JW,
Miyakawa I and Koeffler HP: Human ovarian carcinoma cells: histone
deacetylase inhibitors exhibit antiproliferative activity and
potently induce apoptosis. Cancer. 15:2760–2770. 2004. View Article : Google Scholar
|