Introduction
Liver metastases are frequently inoperative and
significantly affect the prognoses of patients with cancer. To
develop novel strategies for early diagnostics and curative
therapies, a further understanding of tumor cell adaptation to the
hepatic microenvironment is required.
NOD/SCID/IL-2Rγcnull (NOG) mice exhibit strongly
immunodeficient phenotypes due to the lack of T, B and NK cells and
impairments in certain innate immune responses, allowing for the
construction of liver metastasis models with stable reproducibility
of the experimental outcome (1,2).
Through liver metastasis models that employed NOG mice, a highly
liver-metastatic human pancreatic cancer cell subline, derived from
a poorly liver-metastatic parental line, was previously established
and examined. Moreover, a key molecular regulator of liver
metastasis was identified (2).
Colorectal cancer types commonly metastasize to the
liver although some patients present operable liver metastases, in
contrast to patients with pancreatic cancer types (3). The similarities or differences in the
biology of liver metastases between colorectal and pancreatic
cancer types remain to be elucidated. The present study established
and examined a highly liver-metastatic human colorectal cancer cell
subline through experimental procedures analogous to our previous
study (2). Notably, the
ecto-5′-nucleotidase CD73 (also known as NT5E), which catalyzes the
conversion of purine 5′ mononucleotides to nucleosides, was poorly
expressed in the established SW48LM2 cell subline compared to the
parental SW48 cell line. Consistent with a reduced CD73 expression,
results of the metabolomic analysis showed an altered metabolism of
purine nucleotides. In contrast to a number of studies showing a
positive association of CD73 expression with metastatic phenotypes,
these results suggest that a reduction in CD73 expression in tumor
cells is involved in the mechanisms by which liver metastases are
formed.
Materials and methods
Cells
The human colorectal cancer cell line SW48 was
purchased from the American Type Culture Collection (Manassas, VA,
USA). The SW48LM2 cell subline was established as described below.
SW48 and SW48LM2 were maintained in Leibovitz’s L-15 (Sigma)
supplemented with antibiotics and 10% fetal bovine serum (HyClone,
UT, USA). Prior to being transferred to the mice, the cell lines
were incubated in a 100% air (37°C) incubator and passaged on
reaching 80% confluence. To culture SW48 and SW48LM2 cells for 7
days under hypoxia, the AnaeroPack Anaero cultivation system with
AnaeroPack MicroAero (Mitsubishi Gas Chemical, Japan) was used,
providing 8–9% O2 conditions.
Establishment of a highly
liver-metastatic cell subline from the parental SW48
The in vivo experiments were performed in
accordance with institutional guidelines and were approved by the
Animal Experimentation Committee of the Tokai University, the Keio
University and the Central Institute for Experimental Animals
(CIEA). NOG mice were bred and used at the age of 7–9 weeks. Liver
metastases were experimentally induced by intrasplenic transfer of
human cancer cells as described in a previous study (4). NOG mice were euthanized 6 weeks after
the intrasplenic transfer of 1×105 SW48 cells, which
metastasize poorly to the liver (5). The livers were removed from the mice
and macroscopically observed. Hepatic metastatic foci with
diameters >2 mm were collected and cut into 1-mm3
cubes. Foci were dispersed into single cells in a solution of
trypsin-EDTA (IBL Co., Ltd., Japan). Contamination with
mouse-derived cells was examined by PCR using the primers: forward,
5′-TGTAG GTACTAACACTGGCTCGTGTGACAA-3′ and reverse,
5′-GGTGTTGAAGGTCTCAAACATGATCTGTA-3′. PCR with this set of primers
can amplify both human and mouse β-actin genes as 247- and 273-bp
DNA fragments, respectively. To examine for contamination with
other human cell lines, short tandem repeat analysis was carried
out using the markers: D5S818, D13S317, D7S820 and DS16S539. Primer
information is available at the UniSTS database on the NCBI website
(http://www.ncbi.nlm.njh.gov/). To
confirm the ability to form liver metastases, the cells derived
from the metastatic foci, i.e., SW48LM2 cells, were cultured and
intrasplenically transferred at 1×104 cells to a new
cohort of NOG mice, followed by macroscopic observation of livers
removed 6 weeks after the transfer.
Subcutaneous tumor formation
For subcutaneous tumor formation, 1×104
SW48 or SW48LM2 cells suspended in 0.2 ml of serum-free medium were
subcutaneously transferred to NOG mice. Palpable tumors were
measured weekly, and the mice were sacrificed for histopathological
examination 6 weeks after the transfer.
Evaluation of morphological invasiveness
of subcutaneous tumors by fractal dimension analysis
For quantitative evaluation of the invasiveness
based on the geometry of tumor tissues, fractal dimension analysis
was applied to the histological findings as reported in a previous
study (6). Mice were sacrificed 2
weeks after the subcutaneous transfer, followed by harvesting of
the formed tumors. Paraffin-embedded samples were sectioned at 5
μm. Immunostaining of the sections with the mouse monoclonal
anti-multi-cytokeratin (clone AE1/AE3; Leica Microsystems, Japan)
was performed on the Bond Max system (Leica Microsystems). The
photographic images of the immunostained sections were prepared by
the Imager M1 (Carl Zeiss Microimaging, Japan) and then converted
to the gray-scaled images using Photoshop CS3 (Adobe Systems Inc.,
San Jose, CA, USA). The fractal dimension analysis was carried out
using the software ImageJ version 1.33 (http://rsb.info.nih.gov/ij/).
Assay of in vitro invasion of cancer
cells
Comparison of the invasiveness of SW48 and SW48LM2
cells in vitro was carried out using the BD BioCoat Matrigel
invasion chamber (BD Biosciences, Bedford, MA, USA) as recommended
by the manufacturer.
Assay of spheroid formation of cancer
cells
The spheroid culture was carried out using the
Sumilon Celltight Spheroid 96U (Sumitomo Bakelite, Japan). The
viable cell numbers were analyzed using the Cell Counting Kit-8
(Dojindo Laboratories, Japan) as recommended by the
manufacturer.
Real-time RT-PCR assay
Total RNA was extracted from collected cells using
the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to
cDNA synthesis with the PrimeScript RT reagent kit (Takara Bio
Inc., Japan). The reactions were prepared with the obtained cDNA
and the SYBR Premix Ex Taq II. Gene-specific primers were designed
by the Perfect Real Time support system (Takara Bio Inc.) and
subjected to the real-time PCR assay using the Thermal Cycler Dice
Real Time system (Takara Bio Inc.). The examined molecules were:
VEGF-A, VEGF189, TSP1, TSP2, MMP2, MMP7, E-cadherin, S100A4, CA9,
CA12, HIF1a, HIF2a, NME1, GLUT1, GLUT3, CYGB, MYC, MCT4, SCO2,
COX4-1, COX4-2, TLR3, TLR4, TLR5, TLR7, TLR9, MCL1, LDHA, LDHB,
ALDH1L2, ALDH3A1, TFAM, UCP2, TWIST, Slug, Snail, ZEB1, ZEB2,
vimentin, CD73, CD47, NT5M, NT5C1A, NT5C1B and NT5C2.
Metabolomic analysis using capillary
electrophoresis-mass spectrometry (CE-MS)
Extracts from the pellets of cultured cells were
measured by the Agilent CE Capillary Electrophoresis system
equipped with an air pressure pump, an Agilent 1100 series MSD mass
spectrometer and an Agilent 1100 series isocratic high performance
liquid chromatography pump, a G1603A Agilent CE-MS adaptor kit and
G1607A Agilent CE-MS sprayer kit (Agilent Technologies) as
previously described (7).
Flow cytometric analysis
Cells were stained with PE conjugated mouse
anti-human CD73 (clone AD2; BD Biosciences, San Diego, CA, USA).
The flow cytometric analysis was performed using MoFlo (Beckman
Coulter, Miami, FL, USA).
Statistical analysis
Statistical analysis was carried out using the
two-sample t-test or Fisher’s exact test. Values of p<0.05 were
considered to be statistically significant.
Results
Establishment of a highly
liver-metastatic human colorectal cancer cell subline
Poorly formed metastatic foci were noted in the
liver following the intrasplenic transfer of SW48 cells to NOG mice
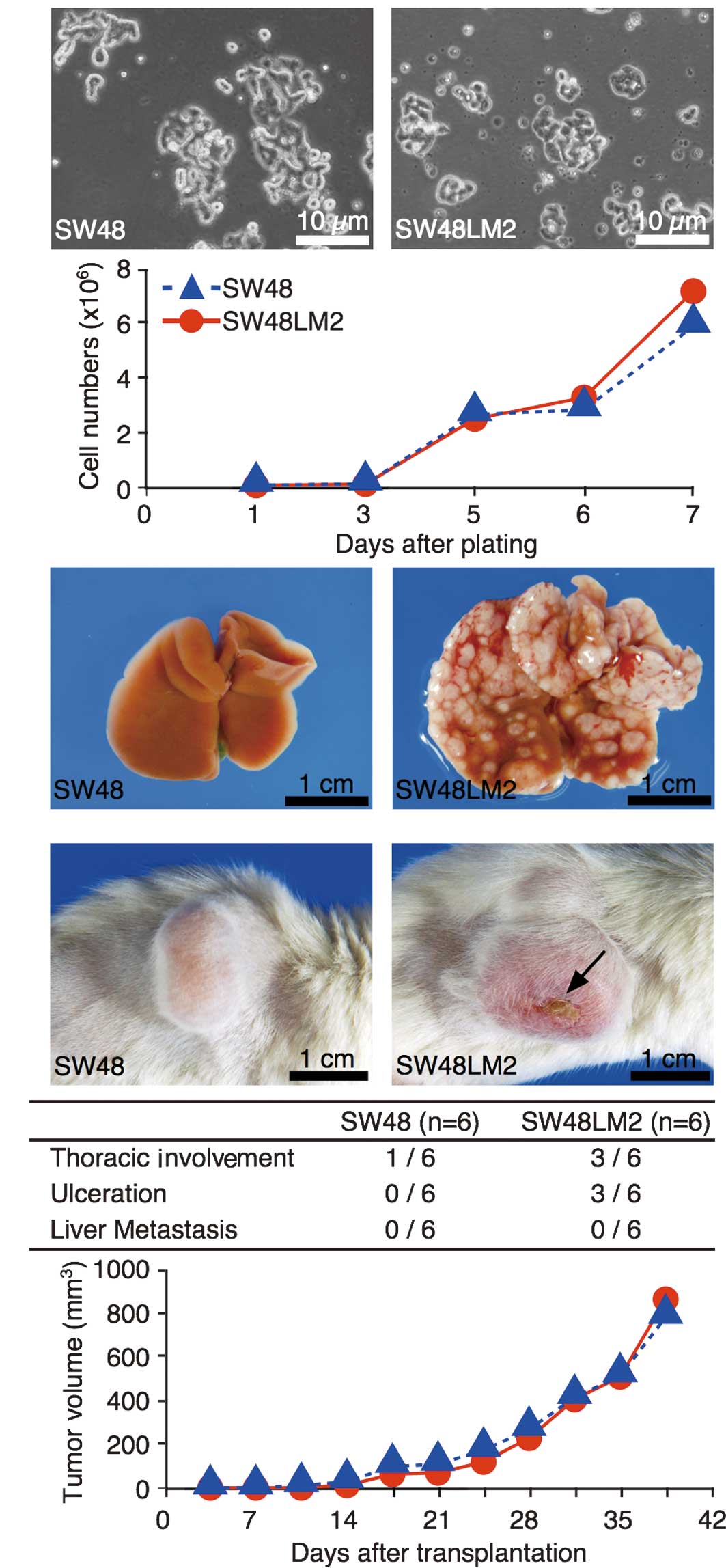
(Fig. 1A). Cells were isolated from
the visible foci and were then subjected to the short-term culture.
The intrasplenic transfer of the cultured cells to NOG mice led to
the rapid formation of hepatic metastatic foci (Fig. 1A). This established cell subline was
termed SW48LM2. The growth under monolayer culture conditions was
similar between the SW48 and SW48LM2 cells (Fig. 1A).
Comparison of subcutaneous tumor
formation and in vitro spheroid formation in the SW48 and SW48LM2
cells
The growth features of the subcutaneous tumors were
similar between the SW48 and SW48LM2 cells (Fig. 1B). In the subcutaneous transfer, no
metastases were macroscopically observed in the liver or other
organs, except thoracic involvement and skin ulcerations. The
latter metastases tended to occur more frequently in the
subcutaneous transfer of SW48LM2 cells, although the differences
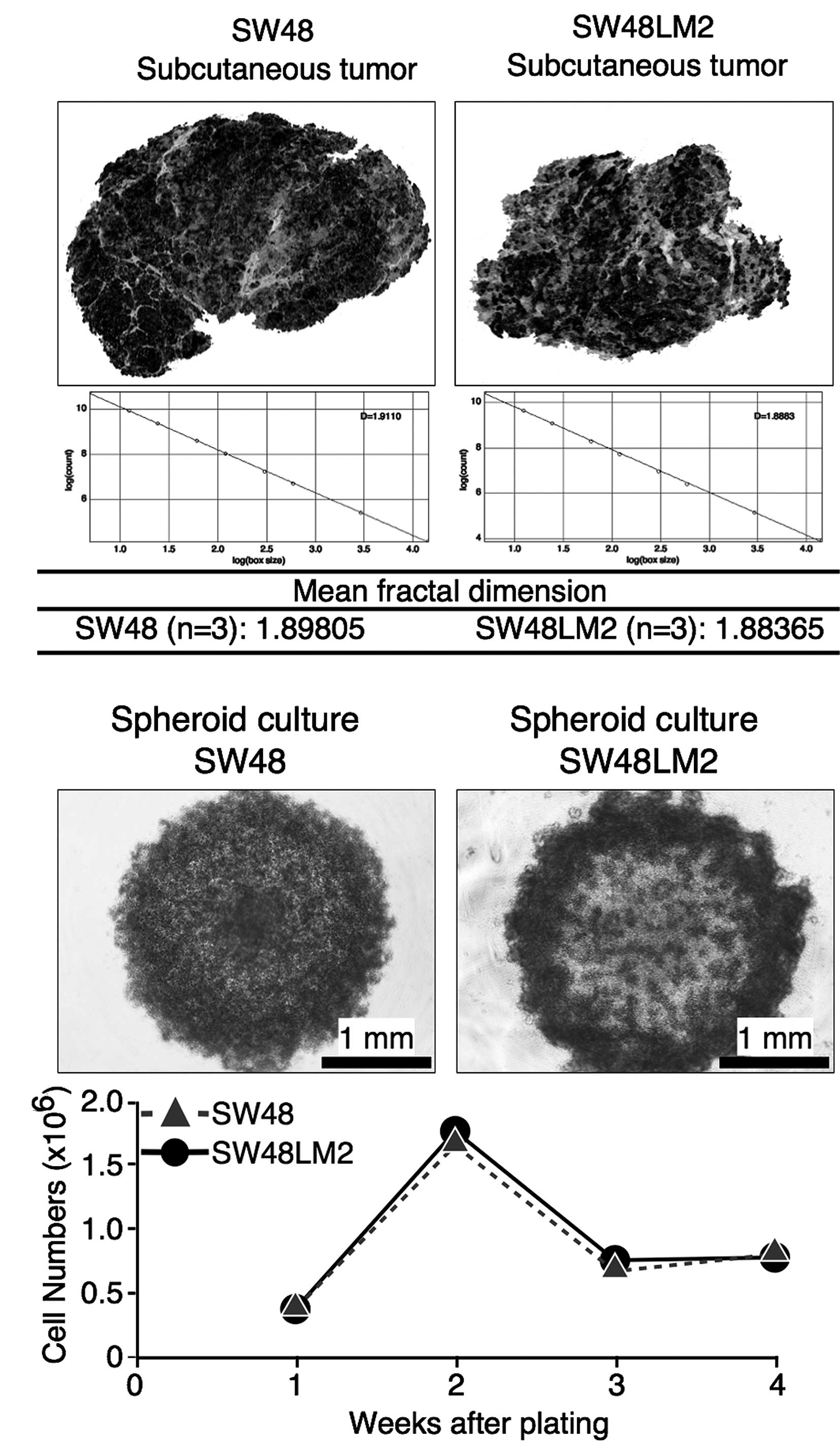
were not statistically significant (Fig. 1B). For evaluation of the
invasiveness of the SW48LM2 cells based on tumor shape profiles,
subcutaneous tumors, formed 2 weeks after the transfer, were
subjected to fractal dimension analysis. The mean fractal
dimensions showed that the two cell lines were similar in
morphological invasiveness (Fig.
2A). In addition, the in vitro invasion assay detected
no significant differences between SW48 and SW48LM2 cells (data not
shown). Similar changes in the viable cell numbers were observed
throughout the time period of the in vitro spheroid
formation between the SW48 and SW48LM2 cells, whereas the spheroids
were morphologically different (Fig.
2B). As internal hypoxia acutely affects spheroid formation via
tumor cells, such morphological differences appeared to reflect the
distinct features of the cells with respect to hypoxic adaptation
(8). We therefore carried out the
analysis of monolayer-cultured cells under normoxia and
hypoxia.
Profiles of gene expression potentially
associated with tumor progression in the SW48 and SW48LM2
cells
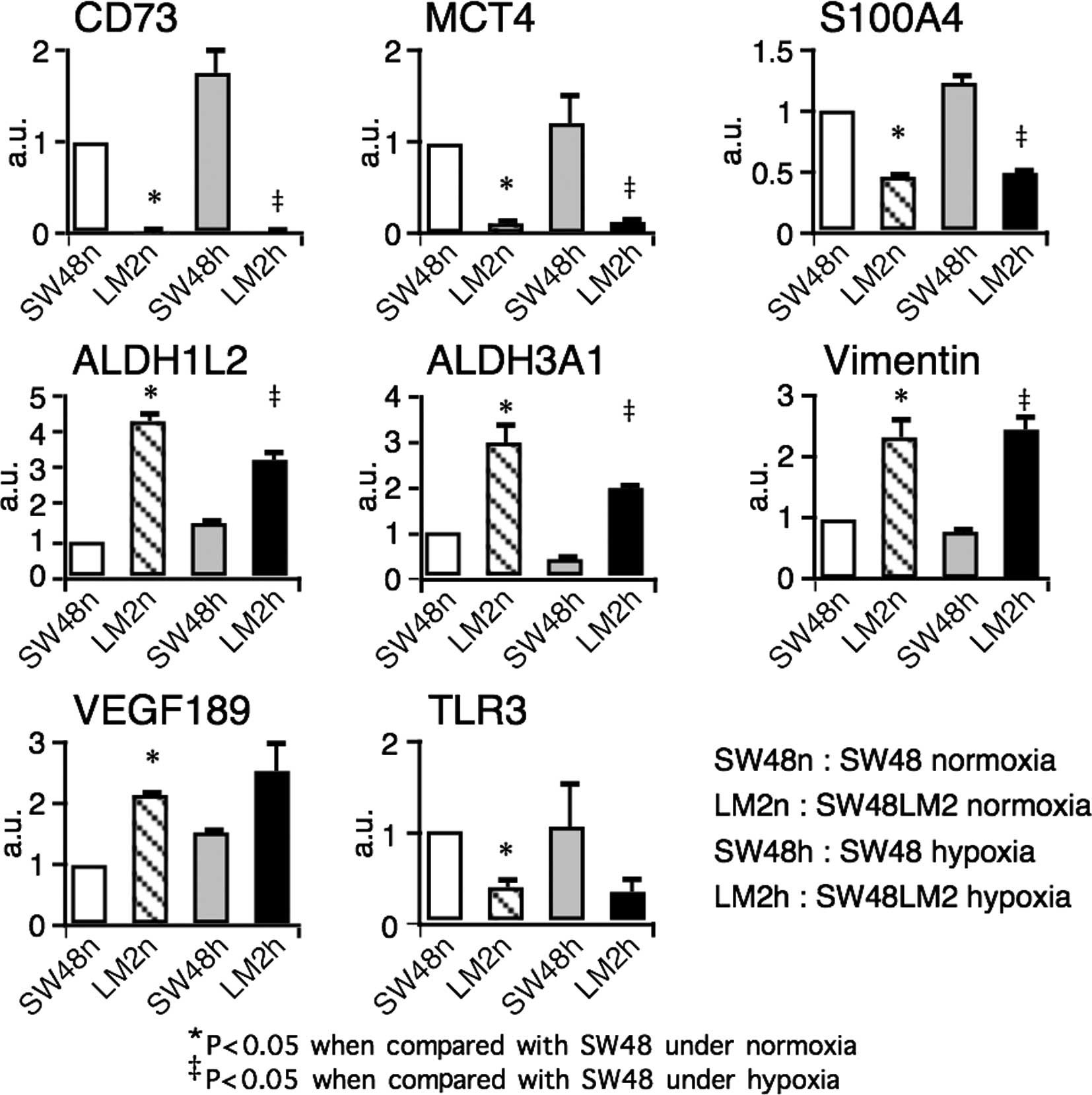
Gene transcripts of 41 molecules reportedly
associated positively or negatively with tumor growth, invasion or
metastases were analyzed by real-time RT-PCR (2,9–16).
Four overexpressed and four underexpressed genes were detected in
the SW48LM2 cells (Fig. 3).
Notably, an ~50-fold reduction under normoxia and an ~100-fold
reduction under hypoxia were observed with respect to the
ecto-5′-nucleotidase CD73 (also known as NT5E) gene transcripts. Of
note was that the gene transcripts of CD73, the monocarboxylate
transporter MCT4, and S100A4 were reduced in the liver-metastatic
cell line, conflicting with previous reports showing a positive
association with metastatic cancer phenotypes (2,9–12). In
contrast, a lower expression of the TLR3 gene as well as a higher
expression of aldehyde dehydrogenase ALDH1L2 and ALDH3A1 genes and
a splicing variant of VEGF-A, VEGF189, corroborated reported
findings (13–15). It appeared plausible that an
epithelial mesenchymal transition-associated molecule, vimentin,
was highly expressed in the SW48LM2 cells (16). Differential expression of genes
encoding metabolic enzymes and transporter prompted us to examine
the metabolomic profiles of the SW48 and SW48LM2 cells.
Profiles of metabolites in the SW48 and
SW48LM2 cells
Metabolites of glycolysis, the pentose phosphate
pathway, TCA cycle and anabolic and catabolic reactions of amino
acids, as well as nucleotides in the SW48 and SW48LM2 cells were
analyzed by CE-MS. As a result, a decrease in purine but not
pyrimidine nucleosides and a reciprocal increase in purine
nucleotides were observed in the SW48LM2 cells under normoxia and
hypoxia, suggesting a possible association with a reduced CD73
expression (Fig. 4A).
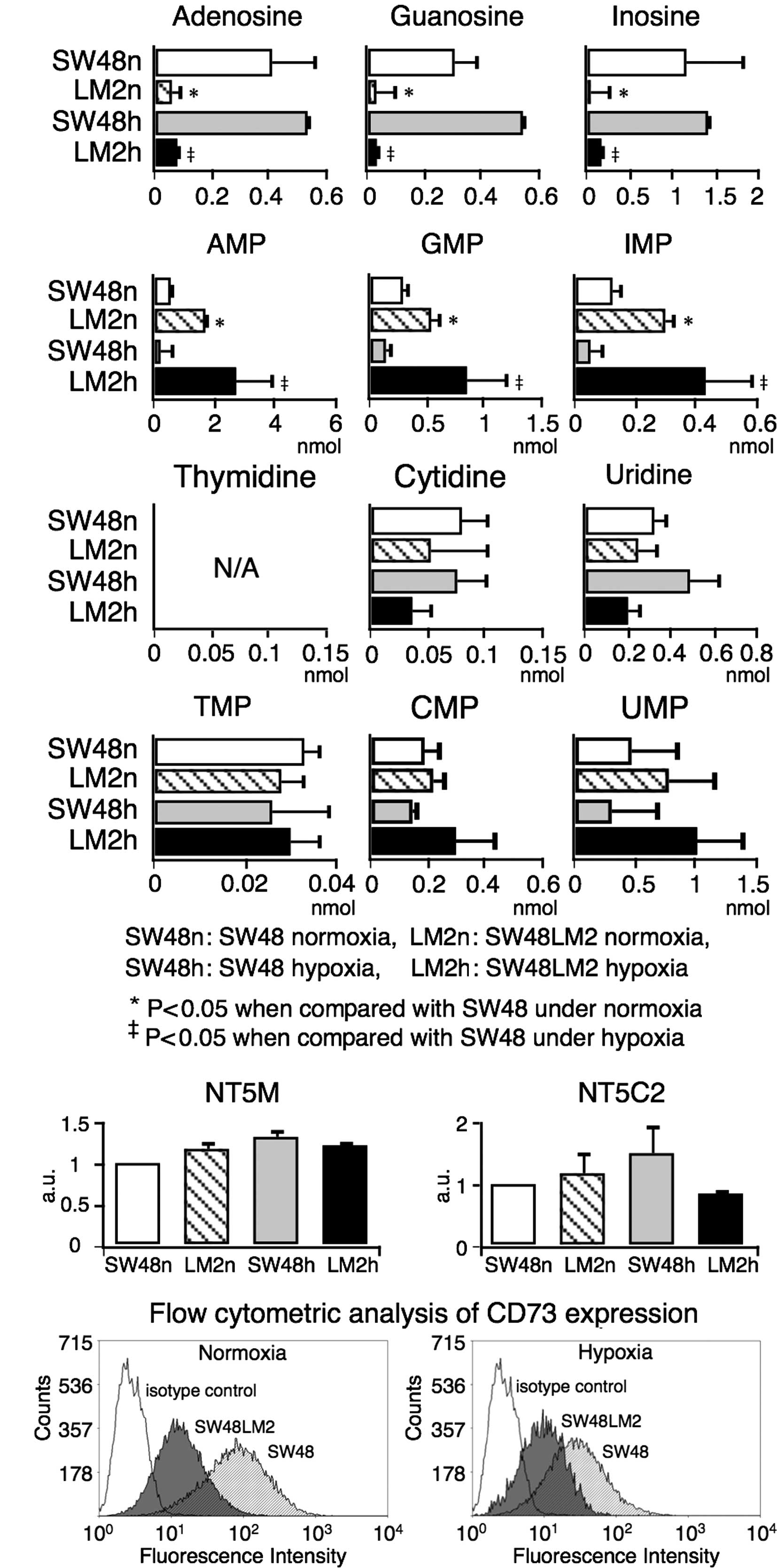 | Figure 4(A) The profiles of nucleotide
metabolites in SW48 and SW48LM2 cells cultured under normoxia and
hypoxia. In SW48LM2 cells cultured under both normoxia and hypoxia,
purine nucleosides (adenosine, guanosine and inosine) were
significantly decreased, while purine nucleotides (AMP, GMP and
IMP) were reciprocally increased. There were no significant
differences in the amounts of pyrimidine nucleosides (thymidine,
cytidine and uridine) or nucleotides (TMP, CMP and UMP) between
SW48 and SW48LM2 cells. (B) Gene expression profiles of purine
nucleotidases other than CD73 in SW48 and SW48LM2 cells cultured
under normoxia and hypoxia. Gene expression of NT5C2 and NT5M was
not significantly different between the two cell lines. SW48n,
LM2n, SW48h and LM2h indicate SW48 cells under normoxia, SW48LM2
cells under normoxia, SW48 cells under hypoxia and SW48LM2 cells
under hypoxia, respectively. (C) Flow cytometric analysis of CD73
expression in SW48 and SW48LM2 cells. Representative results of
three or more experiments are shown. |
Expression of nucleotidases converting
purine nucleotides to nucleosides in the SW48 and SW48LM2
cells
To rule out the contribution of four purine
nucleotidases other than CD73 to the altered metabolism, the
expression levels of the nucleotisades were analyzed by real-time
RT-PCR (Fig. 4B). No significant
differences were noted in the expression levels of either the NT5M
or NT5C2 gene between the SW48 and SW48LM2 cells cultured under
normoxia and hypoxia (Fig 4B). Gene
transcript levels of NT5CA1 and NT5CB1 were undetectable within the
evaluable cycle numbers of real-time RT-PCR. The flow cytometric
analysis showed an evident reduction in CD73 expression in the
SW48LM2 cells (Figs. 3 and 4C). Purine nucleosides generated
extracellularly by CD73 can be transferred into tumor cells via
their nucleoside transporters, such as ENT1 and ENT2 (17). Collectively, decreased purine
nucleosides and reciprocally increased purine nucleotides in the
SW48LM2 cells appeared to be associated with a reduced CD73
expression.
Discussion
It was previously reported that tumor cells
metastasizing to lymph nodes highly express CD73 (9,10). As
adenosine generated by CD73 restricts lymphocyte migration, it is
plausible that CD73 expression in tumor cells leads to prevention
against lymphocyte attacks (18).
On the other hand, in the liver metastasis model using NOG mice,
hepatic resident macrophages likely play a role in the first line
of defense against circulating tumor cells in the absence of T, B
and NK cells. Adenosine enhances macrophages to produce nitric
oxide (NO) (19). The reduction in
CD73 expression in tumor cells may negatively control hepatic
tumoricidal macrophages via the loss of effects of adenosine on NO
production. Experimental efforts are underway to functionally
examine whether such mechanisms result in SW48LM2 cells being
highly metastatic to the liver.
As suggested by the morphological differences in the
spheroid formation between SW48 and SW48LM2 cells, potential
mechanisms other than the negative control of macrophages should
also be investigated to explain the characteristics of SW48LM2
cells. It is possible that adenosine extracellularly generated by
CD73 in tumor cells influences, not only adjacent non-tumor cells,
but also the tumor cells themselves in an autocrine and/or
paracrine manner (17). In a
previous study, the chemical inhibition of CD73 enzymic activities
led to the decrease in both proliferation and apoptosis of breast
cancer cells (20). Similarly, a
reduction in CD73 expression may not only slow cell cycling, but
also lead to resistance to apoptosis, potentially contributing to
tumor progression.
In addition to CD73, it is important to ascertain
whether other molecules, highly or poorly expressed, are also
functionally associated with the distinct features of SW48LM2
cells. The increased expression of ALDH1L2, ALDH3A1 and VEGF189 by
SW48LM2 cells appears to be compatible with previous studies which
found a positive association of their expression with tumor growth
and/or metastases (14,15). In contrast, a decreased expression
of the S100A4 and MCT4 genes conflicts with previous findings
suggesting that there is a positive association of their expression
with tumor progression (2,11,12).
In particular, S100A4 has been identified as a key molecular
regulator of liver metastases through the establishment of a highly
metastatic pancreatic cancer cell subline in a manner similar to
the present study (2). Our results
highlight the need for interpretative caution when investigating
metastasis-associated molecules for diagnostic and therapeutic
applications and provide insights into the mechanisms of liver
metastases.
Acknowledgements
The present study was supported, in part, by the
Keio University Global Center of Excellence (G-COE) Program ‘Center
for Human Metabolomic Systems Biology’ funded by the Ministry of
Education, Culture, Sport, Science and Technology, Japan, as well
as the Prototype Validation/Practical Realization Program for
Advanced Measurement and Analysis (Program-P) funded by the Japan
Science and Technology Agency, Japan. We thank Dr Toshihide
Imaizumi for his consideration in completing the present study. We
also thank Ms. Yoshiko Nagahata and Ms. Tomomi Matsuura for the
help in running CE-MS.
References
|
1
|
Ito M, Hiramatsu H, Kobayashi K, Suzue K,
Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K,
Heike T and Nakahata T: NOD/SCID/gamma(c)(null) mouse: an excellent
recipient mouse model for engraftment of human cells. Blood.
100:3175–3182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suemizu H, Monnai M, Ohnishi Y, Ito M,
Tamaoki N and Nakamura M: Identification of a key molecular
regulator of liver metastasis in human pancreatic carcinoma using a
novel quantitative model of metastasis in NOD/SCID/γcnull (NOG)
mice. Int J Oncol. 31:741–751. 2007.PubMed/NCBI
|
|
3
|
Ruers T and Bleichrodt RP: Treatment of
liver metastases, an update on the possibilities and results. Eur J
Cancer. 38:1023–1033. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khatib AM, Fallavollita L, Wancewicz EV,
Monia BP and Brodt P: Inhibition of hepatic endothelial E-selectin
expression by C-raf antisense oligonucleotides blocks colorectal
carcinoma liver metastasis. Cancer Res. 62:5393–5398. 2002.
|
|
5
|
Hamada K, Monnai M, Kawai K, Nishime C,
Miyazaki N, Ohnishi Y, Nakamura M and Suemizu H: Liver metastasis
models of colon cancer for evaluation of drug efficacy using
NOD/Shi-scid IL2Rγnull (NOG) mice. Int J Oncol. 32:153–159.
2008.PubMed/NCBI
|
|
6
|
Abu-Eid R and Landini G: Morphometrical
differences between pseudo-epitheliomatous hyperplasia in granular
cell tumors and squamous cell carcinomas. Histopathology.
48:407–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soga T, Baran R, Suematsu M, Ueno Y, Ikeda
S, Sakurakawa T, Kakazu Y, Ishikawa T, Robert M, Nishioka T and
Tomita M: Differential metabolomics reveals ophthalmic acid as an
oxidative stress biomarker indicating hepatic glutathione
consumption. J Biol Chem. 281:16768–16776. 2006. View Article : Google Scholar
|
|
8
|
Frieboes HB, Zheng X, Sun CH, Tromberg B,
Gatenby R and Cristini V: An integrated computational/experimental
model of tumor invasion. Cancer Res. 66:1597–1604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee H, Lin EC, Liu L and Smith JW: Gene
expression profiling of tumor xenografts: in vivo analysis of
organ-specific metastasis. Int J Cancer. 107:528–534. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leth-Larsen R, Lund R, Hansen HV,
Laenkholm AV, Tarin D, Jensen ON and Ditzel HJ: Metastasis-related
plasma membrane proteins of human breast cancer cells identified by
comparative quantitative mass spectrometry. Mol Cell Proteomics.
8:1436–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gallagher SM, Castorino JJ, Wang D and
Philp NJ: Monocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231. Cancer Res. 67:4182–4189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saleem M, Kweon MH, Johnson JJ, Adhami VM,
Elcheva I, Khan N, Bin Hafeez B, Bhat KM, Sarfaraz S, Reagan-Shaw
S, Spiegelman VS, Setaluri V and Mukhtar H: S100A4 accelerates
tumorigenesis and invasion of human prostate cancer through the
transcriptional regulation of matrix metalloproteinase 9. Proc Natl
Acad Sci USA. 103:14825–14830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salaun B, Coste I, Rissoan MC, Lebecque SJ
and Renno T: TLR3 can directly trigger apoptosis in human cancer
cells. J Immunol. 176:4894–4901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreb JS, Baker HV, Chang LJ, Amaya M,
Lopez MC, Ostmark B and Chou W: ALDH isozymes downregulation
affects cell growth, cell motility and gene expression in lung
cancer cells. Mol Cancer. 7:872008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tokunaga T, Oshika Y, Abe Y, Ozeki Y,
Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Ueyama Y, Tamaoki N
and Nakamura M: Vascular endothelial growth factor (VEGF) mRNA
isoform expression pattern is correlated with liver metastasis and
poor prognosis in colon cancer. Br J Cancer. 77:998–1002. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells – an
integrated concept of malignant tumour progression. Nat Rev Cancer.
5:744–749. 2005.PubMed/NCBI
|
|
17
|
Cho SY, Polster L, Engles JM, Hilton J,
Abraham EH and Wahl RL: In vitro evaluation of adenosine
5′-monophosphate as an imaging agent of tumor metabolism. J Nucl
Med. 47:837–845. 2006.
|
|
18
|
Takedachi M, Qu D, Ebisuno Y, Oohara H,
Joachims ML, McGee ST, Maeda E, McEver RP, Tanaka T, Miyasaka M,
Murakami S, Krahn T, Blackbum MR and Thompson LF: CD73-generated
adenosine restricts lymphocyte migration into draining lymph nodes.
J Immunol. 180:6288–6296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasko G, Szabo C, Nemeth ZH, Kvetan V,
Pastores SM and Vizi ES: Adenosine receptor agonists differentially
regulate IL-10, TNF-α and nitric oxide production in RAW 264.7
macrophages and in endotoxemic mice. J Immunol. 157:4634–4640.
1996.PubMed/NCBI
|
|
20
|
Zhou X, Zhi X, Zhou P, Chen S, Zhao F,
Shao Z, Ou Z and Yin L: Effects of ecto-5′-nucleotidase on human
breast cancer cell growth in vitro and in vivo. Oncol
Rep. 17:1341–1346. 2007.
|