Introduction
Vascular endothelial growth factor receptor
(VEGFR)-3 (also known as Flt-4) is a tyrosine kinase (TK) receptor
of vascular endothelial growth factor (VEGF)-C and VEGF-D. It is
mainly expressed in lymphatic endothelial cells, but also in
neovascular endothelial cells of the liver, splenic sinusoid,
trauma repair tissues and tumor tissues. Studies showed that cancer
cells express VEGFR-3/Flt-4, which contributes to malignant tumor
progression in various ways (1). In
this study, an immunohistochemical streptavidin-peroxidase (SP)
method was used to detect the expression of VEGF-C, VEGF-D and
VEGFR-3/Flt-4 in early-stage cervical cancer tissues. The
VEGFR-3/Flt-4-marked vascular density (MVD) was also determined.
The relationship of these factors with clinicopathological factors
was analyzed, as well as the influence of VEGFR-3/Flt-4 in the
early stages of cervical cancer.
Materials and methods
Subjects
This study was carried out following international
and national regulations, and informed consent was obtained from
all of the patients. Cervical cancer tissues were extracted from
the 41 patients included in the study by gynecologic surgery
between September 2007 and December 2008 at the Qilu Hospital of
Shandong University. The tumor tissues were fixed in 4%
paraformaldehyde and embedded in paraffin. The patients did not
undergo any pre-operative chemotherapy, but a definite diagnosis of
cervical cancer was established by postoperative pathology.
Clinical staging was performed according to the International
Federation of Gynecology and Obstetrics (FIGO, 2000) staging
system: stage Ia was found in 7 cases, stage Ib in 14 cases and
stage IIa in 20 cases. The histological grading revealed G1 in 7
cases, G2 in 13 cases and G3 in 21 cases. The histological
classification showed 37 tumors to be squamous cell carcinomas and
4, adenocarcinomas. Lymph node metastasis was found in 15 patients
and non-lymph node metastasis in 26 patients. The patients were
26–70 years of age (median 42). Additionally, 12 patients had
developed cancer at menopause and 29 prior to menopause.
Microscopic examination of the cancer cells from the lymphatic
lumen confirmed lymphatic invasion in 6 cases and non-lymphatic
invasion in 35 cases.
Immunohistochemical SP staining
The immunohistochemical SP staining kit (LHK612;
Jingmei Bioengineering Co., Ltd.) was used in this study strictly
according to the instructions provided by the manufacturer. The
paraffin-embedded tissues were sliced into 5-μm sections and
treated. After conventional dewaxing with xylene and rehydration,
microwave antigen retrieval was performed at 95°C for 10 min using
a citrate buffer (pH 6.0) as the antigen retrieval buffer. This
solution was incubated with a hydrogen peroxide (3%) and methanol
solution for 10 min to block the endogenous peroxidase. A blocking
solution containing 10% goat serum was used to block non-specific
antibodies. Certain polyclonal antibodies were used as the first
antibody, including rabbit anti-human VEGF-C (anti-VEGF-C, ZA-0266;
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) at a dilution of
1:50, rabbit anti-human VEGF-D (anti-VEGF-D, BA1461; Wuhan Boster
Co., Ltd.) at a dilution of 1:100 and rabbit anti-human
VEGFR-3/Flt-4 (anti-VEGFR-3/Flt-4, ab27278; Abcam Ltd., UK) at a
dilution of 1:200. After incubation for 60 min, anti-rabbit
biotinylated secondary antibody was added, and the mixture was
incubated for 30 min. Horseradish peroxidase-labeled streptavidin
was added, and the mixture was incubated for another 30 min.
Staining with 3,3′-diaminobenzidine substrate and restaining with
hematoxylin were performed. After the slides containing the
sections with neutral gum were sealed, the stained sections were
microscopically examined. In this experiment, the control was
treated in the same manner, except that PBS buffer was used instead
of the first antibody as a negative control, and the sections of
breast cancer tissue were used as a positive control.
Determinations
The appearance of yellowish-brown granules in the
cytoplasm indicated positive results for VEGF-C, VEGF-D and
VEGFR-3/Flt-4 staining. On the basis of the method reported by
Jüttner et al (2) and the
proportion of positive cells, the results were classified as: −, no
positive cells; +, 0–5% positive cells; ++, 5–50% positive cells;
+++, >50% positive cells), with ++ and +++ being considered a
positive expression. MVD was determined according to the method
reported by Weidner et al (3). Briefly, the dense-staining zones (‘hot
spots’) of the marker-positive vessel lumens were microscopically
observed. The number of positive lumens in the scope of one viewing
field were counted at a high magnification, and the mean value of
the five numbers (from five viewing fields) of the marker-positive
lumens at high magnification represented MVD.
Measurement data were analyzed using the statistical
software SPSS 13.0. MVD is expressed as the mean ± SD. P<0.05
indicates that the differences were statistically significant.
Results
Expression of VEGFR-3/Flt-4 in
early-stage cervical cancer tissues
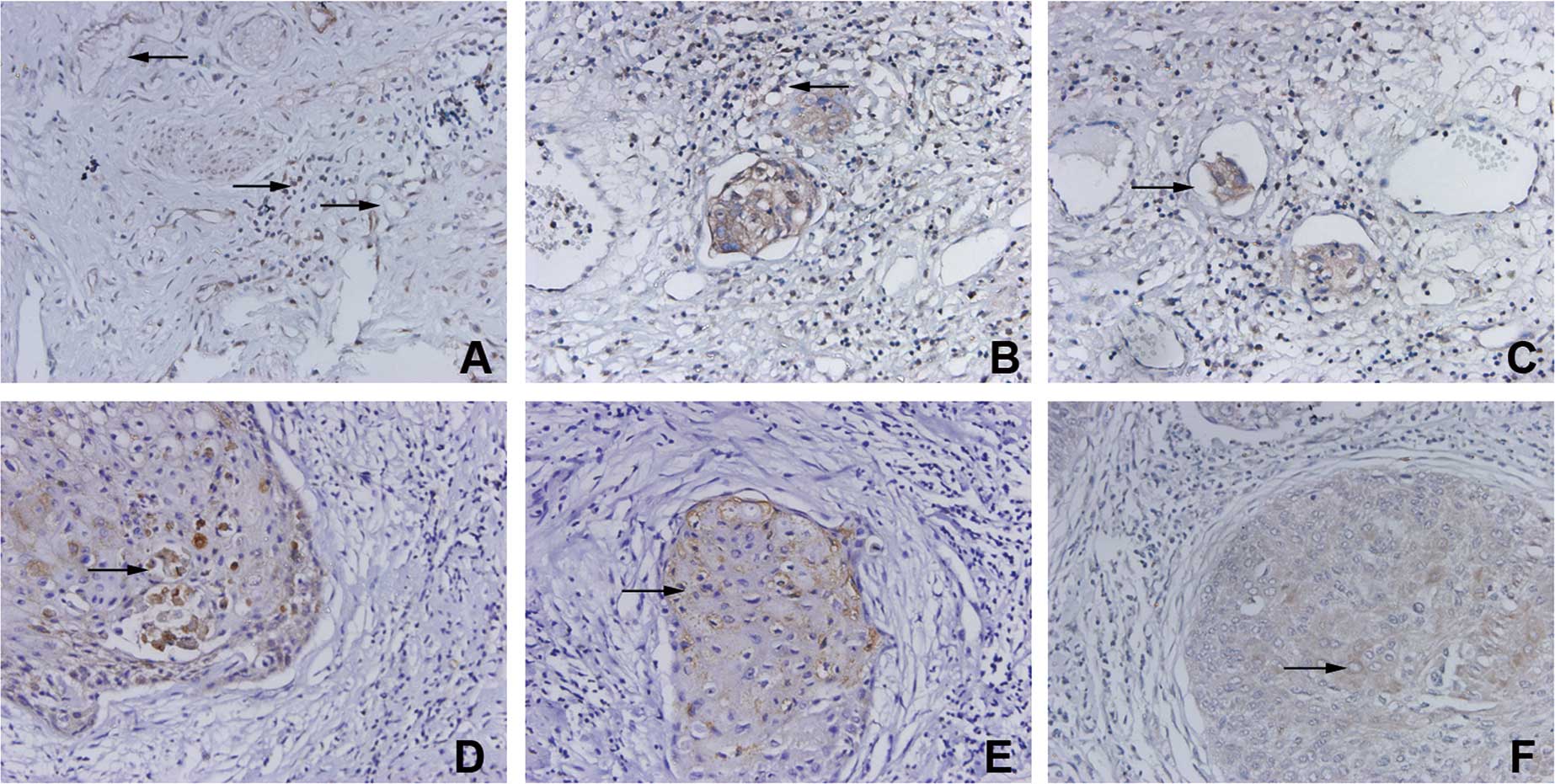
VEGFR-3/Flt-4 was found to be expressed in lymphatic
endothelial cells and in certain vascular endothelial cells.
VEGFR-3/Flt-4-positive vessels were morphologically divided into
blood and lymphatic vessels. VEGFR-3/Flt-4 was also expressed in
certain inflammatory cells around the VEGFR-3/Flt-4-positive
vessels (Fig. 1A). Some
VEGFR-3/Flt-4-positive vessels were mainly distributed in the
stroma surrounding the tumor tissues (Fig. 1B). Cancer cells were found in some
VEGFR-positive vessels (Fig. 1C).
Cancer cells were found to express VEGF-C, VEGF-D and
VEGFR-3/Flt-4, and their positive expression rate was 48.7%
(20/41), 58.5% (24/41) and 63.4% (26/41), respectively (Fig. 1D-F).
Respective correlation of VEGFR-3/Flt-4
and MVD with clinicopathological factors, VEGF-C and VEGF-D in
early-stage cervical cancer tissues
VEGFR-3/Flt-4 expression in the cancer cells of
patients with cervical cancer was found to be correlated to the
clinical stage, lymph node metastasis, lymphatic invasion and the
expression of VEGF-C and VEGF-D. The expression, however, was
unrelated to menstrual status, histological grade and histological
classification. MVD was correlated to the clinical stage and the
expression of VEGF-C and VEGF-D, but was unrelated to menstrual
status, histological grade, histological classification, lymph node
metastasis and lymphatic invasion (Table I).
 | Table IRespective correlation of
VEGFR-3/Flt-4 and marked vascular density (MVD) with
clinicopathological factors, VEGF-C and VEGF-D in early-stage
cervical cancer tissues. |
Table I
Respective correlation of
VEGFR-3/Flt-4 and marked vascular density (MVD) with
clinicopathological factors, VEGF-C and VEGF-D in early-stage
cervical cancer tissues.
| Clinicopathological
factors | n | VEGFR-3/Flt-4 | MVD [mean (SD)] | P-value |
|---|
| |
| | |
|---|
| | (+) | (−) | P-value | | |
|---|
| Menstrual status |
| Pre-menopausal | 29 | 19 | 10 | NS | 25.97 (1.48) | NS |
| Menopausal | 12 | 7 | 5 | | 25.41 (1.83) | |
| Clinical stages |
| Ia | 7 | 3 | 4 | <0.01 | 23.29 (0.49) | <0.01 |
| Ib | 14 | 5 | 9 | | 25.21 (0.43) | |
| IIa | 20 | 18 | 2 | | 27.10 (0.85) | |
| Histological
grade |
| G1 | 7 | 4 | 3 | NS | 25.43 (1.98) | NS |
| G2 | 13 | 8 | 5 | | 26.08 (1.75) | |
| G3 | 21 | 12 | 9 | | 25.76 (1.37) | |
| Lymph node
metastasis |
| No | 26 | 12 | 14 | <0.05 | 25.50 (1.84) | NS |
| Yes | 15 | 14 | 1 | | 26.33 (0.82) | |
| Lymphatic
invasion |
| No | 35 | 20 | 15 | <0.05 | 25.69 (1.66) | NS |
| Yes | 6 | 6 | 0 | | 26.50 (0.84) | |
| Histological
classification |
| Squamous cell
carcinoma | 37 | 23 | 14 | NS | 25.78 (1.64) | NS |
| Adenocarcinoma | 4 | 3 | 1 | | 26.00 (1.15) | |
| VEGF-C |
| (+) | 20 | 17 | 3 | <0.01 | 24.50 (1.00) | <0.01 |
| (−) | 21 | 9 | 12 | | 27.05 (0.86) | |
| VEGF-D |
| (+) | 24 | 20 | 4 | <0.01 | 25.08 (1.38) | <0.01 |
| (−) | 17 | 6 | 11 | | 26.82 (1.28) | |
Discussion
Members of the vascular endothelial growth factor
families (VEGF-A-E) and their receptors (VEGFR-1–3) have been the
focus of research owing to their ability to promote angiogenesis or
lymphangiogenesis. In particular, this study involved the analysis
of the mechanisms of the growth factor family in the promotion of
malignant tumor growth and metastasis, and the corresponding gene
therapy strategies. Current research has shown that tumor
angiogenesis is mainly regulated by the VEGF-A/VEGFR-2 system and
that tumor lymphangiogenesis is mainly regulated by the VEGF-C,
-D/VEGFR-3 (Flt-4) system (4). The
results of our study showed that VEGFR-3/Flt-4 was expressed in
lymphatic endothelial cells and, to a certain extent, in vascular
endothelial cells with VEGFR-3/Flt-4-positive vessels being divided
into blood and lymphatic vessels. VEGFR-3/Flt-4-positive vascular
density (MVD) was found to be correlated to the clinical stage of
the cancers and the expression of VEGF-C and VEGF-D, but was
unrelated to menstrual status, histological grade and other
clinicopathological factors. These results differed from Yasuoka
et al (5) due to the fact
that VEGFR-3/Flt-4 plays an important role in the regulation of
tumor lymphangiogenesis and angiogenesis. VEGF-C and VEGF-D are
secreted by cancer cells and show a paracrine action on the TK
receptor (VEGFR-3/Flt-4), which is expressed in lymphatic and
vascular endothelial cells to mediate proliferation and
differentiation of endothelial cells and lumen formation. The
increase in the number of blood vessels that supply tumor tissues
provides essential nutrients for cancer cell growth, while an
increase in the number of lymphatic vessels in tumor tissues
provides channels for lymphatic invasion and metastasis of cancer
cells and facilitates their transfer and spread. However, there is
a lack of accurate lymphatic endothelial markers which differ
according to the type, position and stages of cancers for lymphatic
vessels (6). Therefore, the exact
mechanism underlying VEGFR-3/Flt-4 action on tumor angiogenesis and
lymphangiogenesis warrants further investigation.
VEGFR-3/Flt-4 is mainly expressed in endothelial
cells as a TK receptor of VEGF-C and VEGF-D. In addition, studies
have shown that numerous cancer cells express Flt-4 (i.e.,
VEGFR-3), which plays an important role in tumor progression.
Clinical trials in many human malignant tumors have shown that
VEGFR-3 expression in cancer cells is correlated to the clinical
stage of cancer in patients, the degree of cell differentiation,
lymph node metastasis and patient prognosis (7–9). Van
Trappen et al reported that VEGFR-3/Flt-4 expression levels
changed with the progression of cervical intraepithelial carcinoma
into cervical cancer (10).
Therefore, it is speculated that VEGFRs may be involved in the
phenotypic transformation of carcinoma cells to promote
lymphangiogenesis in cervical cancer. The results obtained by in
vitro migration and cervical mucus invasion tests (11,12)
indicated that strong invasive cancer cell lines, such as SiHa and
breast cancer cell lines MDA-MB-231 and Hs578T, express Flt-4
(i.e., VEGFR-3) and VEGF-C. Recombinant human VEGF-C (Cys156Ser)
facilitates the migration and invasion of cancer cells. However,
cellular migration and invasion were greatly reduced when
recombinant Flt-4/Fc was used to block the VEGF-C receptor
(VEGFR-3/Flt-4). Masood et al reported that VEGFR-3/Flt-4
promotes the growth of malignant mesothelioma (13). Dias et al reported that
VEGFR-3/Flt-4 may be involved in leukemic cell proliferation,
survival and chemical drug resistance (14). The results of this study showed that
the VEGFR-3/Flt-4 expression of cancer cells in cervical cancer
tissues was correlated to lymph node metastasis and lymphatic
invasion, and the clinical stage was correlated to the expression
of the VEGFR-3/Flt-4 ligands (VEGF-C and VEGF-D). The possible
mechanism involved is expressed by cancer cells that secrete VEGF-C
and VEGF-D which, in turn, act in an autocrine manner on
VEGFR-3/Flt-4 in cancer cells to facilitate migration and invasion,
thereby promoting tumor lymphatic invasion and lymph node
metastasis. However, the receptors of VEGF-C and VEGF-D have been
reported to be heterogeneous in different types of cancer cells,
e.g., VEGF-C or VEGF-D receptors also interacted with VEGFR-2,
NRP-1 and NRP-2 besides VEGFR-3/Flt-4 (12), which has led to inconsistencies in
the results. For example, Jüttner et al (2) reported that the VEGFR-3/Flt-4
expression of gastric cancer cells was unrelated to lymph node
metastasis in patients with gastric cancer.
The results of the present study showed that
VEGFR-3/Flt-4 was expressed in certain inflammatory cells in the
stroma surrounding tumor tissues, but its role remains unknown. A
previous study showed (15) that
the inflammatory cells of tumor stroma may also play an important
role in tumor lymphangiogenesis. In conclusion, VEGFR-3/Flt-4 may
play several roles in malignant tumor progression, and its effect
may vary according to the type of cancer. However, the exact
mechanism underlying these roles requires further
investigation.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shandong Province (no. Y2008C70).
References
|
1
|
Su JL, Yen CJ, Chen PS, et al: The role of
the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer.
96:541–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jüttner S, Wissmann C, Jons T, et al:
Vascular endothelial growth factor-D and its receptor VEGFR-3: two
novel independent prognostic markers in gastric adenocarcinoma. J
Clin Oncol. 24:228–240. 2006.PubMed/NCBI
|
|
3
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis – correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
4
|
Rouzaut A, Irigoyen M and Montuenga LM:
Lymphangiogenesis and lung cancer. J Thorac Oncol. 2:384–386. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasuoka H, Nakamura Y, Zuo H, et al:
VEGF-D expression and lymph vessels play an important role for
lymph node metastasis in papillary thyroid carcinoma. Mod Pathol.
18:1127–1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawai Y, Minami T, Fujimori M, et al:
Characterization and microarray analysis of genes in human
lymphatic endothelial cells from patients with breast cancer.
Lymphat Res Biol. 5:115–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Wu HF, Qian LX, et al: Increased
expression of vascular endothelial growth factor (VEGF), VEGF-C and
VEGF receptor-3 in prostate cancer tissue are associated with tumor
progression. Asian J Androl. 8:169–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Filho AL, Baltazar F, Bedrossian C,
Michael C and Schmitt FC: Immunohistochemical expression and
distribution of VEGFR-3 in malignant mesothelioma. Diagn
Cytopathol. 35:786–791. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saintigny P, Kambouchner M, Ly M, et al:
Vascular endothelial growth factor-C and its receptor VEGFR-3 in
non-small cell lung cancer: concurrent expression in cancer cells
from primary tumour and metastatic lymph node. Lung Cancer.
58:205–213. 2007. View Article : Google Scholar
|
|
10
|
Van Trappen PO, Steele D, Lowe DG, et al:
Expression of vascular endothelial growth factor (VEGF)-C and
VEGF-D, and their receptor VEGFR-3, during different stages of
cervical carcinogenesis. J Pathol. 201:544–554. 2003.PubMed/NCBI
|
|
11
|
Su JL, Yang PC, Shih JY, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Timoshenko AV, Rastogi S and Lala PK:
Migration-promoting role of VEGF-C and VEGF-C binding receptors in
human breast cancer cells. Br J Cancer. 97:1090–1098. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masood R, Kundra A, Zhu S, Xia G, Scalia
P, Smith DL and Gill PS: Malignant mesothelioma growth inhibition
by agents that target the VEGF and VEGF-C autocrine loops. Int J
Cancer. 104:603–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dias S, Choy M, Alitalo K and Rafii S:
Vascular endothelial growth factor (VEGF)-C signaling through FLT-4
(VEGFR-3) mediates leukemic cell proliferation, survival and
resistance to chemotherapy. Blood. 99:2179–2184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong SY and Hynes RO: Tumor-lymphatic
interactions in an activated stromal microenvironment. J Cell
Biochem. 101:840–850. 2007. View Article : Google Scholar : PubMed/NCBI
|