Introduction
Pleural effusions are classically divided into
trasudate and exudate. Trasudate is a liquid that has accumulated
as a result of a systemic illness, such as heart failure or
cirrhosis, whereas exudate is generally associated with a localized
disorder, involving the pleural surfaces, such as inflammation, a
malignant process or an infection. This distinction was based on
classic Light's criteria (1). Since
Light criteria permit the classification of some pleural fluid,
although not always accurately (2–5), fluid
to serum protein ratio was suggested (5). The main importance of this
trasudate/exudate distinction is in determining the need for
subsequent diagnostic tests. If the effusion is trasudate, no other
pleural diagnostic action is required, but patients may require
other general diagnostic and, of course, therapeutic interventions.
If the effusion is exudate, further tests are required to determine
its cause.
Malignancy is one of the main causes of pleural
effusions and more than 90% of malignant pleural effusions are due
to metastatic disease (6). The most
frequent neoplasias that metastasize to the pleura are lung and
breast carcinomas and lymphomas, albeit less frequently, as well as
digestive and ovarian carcinomas (7). The differential diagnosis of the
various malignancies is a clinical and laboratory challenge.
Diagnosis is normally carried out by invasive techniques, such as
thoracoscopy, which show sensitivity but are not cost-effective and
induce physical and mental stress in the patient (8). The role of biochemical parameters or
tumour markers were previously studied in order to increase the
diagnostic capacity of pleural effusion analysis (9–13). The
detection of these parameters or markers in bodily fluids is the
result of a dynamic balance between the number of cells that
synthesize the tumour marker, its capacity for synthesis and the
amount eliminated by the organism relative to the nature, size and
metabolic mechanism of the marker. However, the exact role of
tumour marker assays in differentiating malignant from benign
pleural effusion has yet to be elucidated.
Another non-invasive method carried out for the
diagnosis of malignant effusion is the cytological examination,
which, however, has a sensitivity between 40 and 80% (14). This discrepancy is caused by various
factors, including the quality of the preparations, and the
presence of cellular materials of normal tissue and tumour cells,
which are very few. Notably, malignant cells will not always appear
in the effusions of cancer patients. This is due to the fact that
malignant disease produces pleural effusion through a series of
different mechanisms: lymphatic and capillary destruction,
resulting in a reduced absorbency of fluids and proteins; chemical
mediators increasing capillary permeability; or atelectasias or
erosion of the blood vessels producing malignant disorders
(15). In order to improve the
diagnostic sensitivity of cytological examination, new approaches
were proposed, including the individual or combined cellular
neoplastic markers by immunocytochemistry methods (15). However, this method requires an
antibody panel able to characterize or distinguish carcinoma cells.
The present study aimed to determine the optimal panel of tumour
markers in the supernatants and sediments of pleural fluids in
order to improve the diagnosis of malignant effusions, particularly
in cytologically negative effusions.
Materials and methods
Serum and pleural effusion samples
Between March 2007 to November 2008, 135 pleural
effusions were collected from patients with perfectly defined
aetiology from the Surgery Department of our Faculty, and examined.
Patient evaluation included anamnesis, physical examination, chest
X-ray and thoracocentesis with the biochemical, cytological and
bacteriological study of pleural fluid. When the result of the
cytological examination was negative or in doubt, patients
underwent blind pleural biopsies and/or thoracoscopic-guided
biopsies. Pleural effusions exhibited definite aetiologies. Fresh
pleural fluid was obtained by thoracocentesis, collected in sterile
tubes without anticoagulant and rapidly brought to our laboratory
with a blood sample of the same patient. Pleural fluids and blood
samples were immediately centrifuged. The supernatants were
aliquoted and stored at −80˚C and the sediments were partially used
immediately for cytological study. The remaining sediments were
then partially washed in 0.154 M NaCl on ice and resuspended in
lysis buffer (40 mM Hepes, 20% glycerol, 2% Triton, 2% Aprotinin
and 4 mM EDTA) and stored at −80˚C, until defrosted and tested for
tumour marker content.
Materials
Aprotinin, glycerol, Hepes and Triton were purchased
from Sigma Chemical Co. (St. Louis, MO, USA). The BioRad protein
assay reagent was from BioRad Laboratories. The LDH monotest and
Cholesterol assay reagents were from Diacron Laboratories (Italy).
The remaining reagents were available from commercial sources.
Measurement of proteins and tumour
markers
Proteins were measured by the Bradford procedure
(16). The amount of
carcinoembryonic antigen (CEA), carbohydrate antigen (Ca)125,
Ca19-9 and Ca15-3 in the blood samples, supernatants of pleural
effusions and the lysates of sediments were measured. Tumour
markers were determined using the Immulite analyzer, according to
the manufacturer's specifications and commercial kits (Diagnostic
Products, Los Angeles, CA, USA). Tumour marker contents in the
pleural fluids and serum were expressed as ng/ml for CEA, whereas
Ca15-3, Ca125 and Ca19-9 were expressed as U/ml. CEA was expressed
as ng/mg of protein in the cellular lysates and Ca15-3, Ca125 and
Ca19-9 as U/mg of protein.
Due to the non-normal distribution of the variables,
results are expressed as the median and interquartile (IQ)
range.
The optimal cut-off of tumour markers in pleural
fluids was determined by plotting the true-positive (sensitivity)
vs. the false-positive (1-specificity) results in receiver
operating characteristic (ROC) curves. An optimal cut-off point was
defined as a point on a ROC curve nearest to the point where
sensitivity and specificity were 1. P≤0.05 was considered to
indicate statistical significance. Statistical analyses were
performed using the MedCalc software v. 11 statistical program.
Results
During the 20-month period, a total of 135 patients
with pleural effusions were evaluated. Etiological diagnosis and
demographic data of patients are shown in Table I. Table
II shows the studied biochemical characteristics of the pleural
fluids. Using clinical criteria, pleural fluid protein, cholesterol
and LDH content were measured, and the pleural fluids were
classified into exudates and trasudates. Furthermore, fluid to
serum total protein ratio and fluid to serum LDH ratio analyses
were performed. For each parameter, a cut-off for distinguishing
exudates from trasudates was previously suggested (5). These cut-off values were also used in
this study (for fluid cholesterol 0.8 mmol/l, for fluid protein 28
g/l, for fluid LDH 380 U/l, for fluid to serum total protein ratio
0.4 and for fluid LDH to serum LDH ratio 0.9). The five parameters
allowed for the differentiation of exudates from trasudates.
Furthermore, the mean values (± SD) of fluid cholesterol, fluid
protein and the fluid protein/serum protein ratio were higher in
the malignant than in the benign fluids, whereas the fluid
LDH/serum LDH ratio was higher in the benign than in the malignant
fluids. The four tumour markers were measured in the serum and
pleural fluid of patients with benign and malignant disease.
Results were expressed as the median and IQ range (Table III).
 | Table IDemographic data and pleural fluids
etiology of patients with malignant and non-malignant
effusions. |
Table I
Demographic data and pleural fluids
etiology of patients with malignant and non-malignant
effusions.
| Cause | n | Gender (m/f) | Age range
(years) | Mean age (years) |
|---|
| Malignant | 103 | 41/62 | 22–84 | 61 |
| Breast cancer | 37 | 0/37 | 32–84 | 56 |
| Lung cancer | 29 | 23/6 | 43–78 | 64 |
| Ovarian cancer | 10 | 0/10 | 32–79 | 60 |
| Kidney cancer | 6 | 2/4 | 48–76 | 62 |
| Mesotheliomas | 11 | 8/3 | 57–76 | 64 |
| Lymphomas | 10 | 6/4 | 22–69 | 55 |
| Non-malignant | 32 | 26/6 | 37–80 | 62 |
| Trasudates | | | | |
| Liver
cirrhosis | 12 | 8/4 | 63–69 | 67 |
| Exudates | 20 | 14/6 | 42–80 | 63 |
| Pleuritis | 12 | 8/4 | 43–69 | 65 |
| Tuberculosis | 4 | 3/1 | 37–75 | 61 |
| Pancreatitis | 2 | 1/1 | 60–69 | 64 |
| Benign
asbestos | 1 | 1/0 | 64 | 64 |
| Pleural
amyloidosis | 1 | 1/0 | 66 | 66 |
 | Table IIBiochemical characteristics of pleural
fluids (mean ± SD). |
Table II
Biochemical characteristics of pleural
fluids (mean ± SD).
| Type of pleural
effusion | Pathology | Cholesterol
(mmol/l) | Protein (g/l) | LDH (U/l) | Protein pleural
effusion | LDH pleural
effusion |
|---|
| | | | |
|
|
|---|
| | | | | Protein serum | LDH serum |
|---|
| Trasudate | Cirrhosis | 0.37±0.21 | 22.6±7.2 | 153±52 | 0.37±0.17 | 0.65±0.14 |
| Benign exudate | Inflammation | 0.82±0.23 | 31±7.2 | 316±120 | 0.57±0.12 | 1.40±0.40 |
| Malignant
exudate | Breast cancer | 1.22±0.80 | 43±15 | 398±252 | 0.69±0.23 | 1.06±0.56 |
| Lung cancer | 1.33±0.87 | 37±12 | 450±266 | 0.71±0.45 | 0.97±18.0 |
| Ovarian cancer | 1.11±0.72 | 43±12 | 405±227 | 0.74±31.0 | 1.06±22.0 |
| Kidney cancer | 1.54±0.57 | 46±17 | 240±50 | 0.6±0.17 | 0.81±0.05 |
| Mesotheliomas | 1.15±76.0 | 42±18 | 272±190 | 0.9±70.0 | 0.97±29.0 |
| Lymphomas | 0.96±56.0 | 37±10 | 361±238 | 0.61±14.0 | 0.91±29.0 |
 | Table IIISerum and pleural effusion levels
expressed as median (IQ range) of CEA, Ca15-3, Ca125 and Ca19-9 in
patients with benign and malignant diseases. |
Table III
Serum and pleural effusion levels
expressed as median (IQ range) of CEA, Ca15-3, Ca125 and Ca19-9 in
patients with benign and malignant diseases.
| Pathology | Patient no. | CEA (ng/ml) | Ca15-3 (U/ml) | Ca125 (ng/ml) | Ca19-9 (U/ml) |
|---|
| |
|
|
|
|
|---|
| | Serum | Pleural effusion | Serum | Pleural effusion | Serum | Pleural effusion | Serum | Pleural effusion |
|---|
| Benign | 32 | 2 (1.5–2.2) | 1.5 (1.3–1.6) | 27 (22–32) | 20 (17–26) | 118 (96–138) | 282 (194–419) | 29 (22–34) | 20 (16–23) |
| Breast cancer | 37 | 17 (13–28) | 30 (28–38) | 1157 (828–1210) | 1277 (1159–1500) | 283 (197–387) | 1737
(1500–1981) | 30 (21–50) | 123 (113–136) |
| Lung cancer | 29 | 40 (32–49) | 163 (145–380) | 161 (110–215) | 368 (176–521) | 127 (62–141) | 1800
(1254–2136) | 83 (54–126) | 751 (562–860) |
| Ovarian cancer | 8 | 5.3 (4.6–6.6) | 84 (60–100) | 710 (654–728) | 757 (628–884) | 186 (177–207) | 682 (617–791) | 232 (217–248) | 229 (221–310) |
| Lymphomas | 10 | 3.2 (2.4–4.1) | 10 (8–11) | 129 (112–154) | 194 (123–347) | 146 (112–181) | 620 (505–818) | 92 (34–100) | 28 (21–33) |
| Mesotheliomas | 11 | 1.2 (1.1–1.4) | 1.5 (0.7–1.7) | 58 (33–82) | 68 (41–92) | 26 (12–61) | 500 (400–715) | 28 (19–30) | 4 (3.5–6.5) |
| Kidney cancer | 8 | 1.4 (0.8–2.5) | 1.3 (1.2–1.5) | 58 (46–66) | 57 (50–75) | 91 (79–151) | 302 (198–405) | 10 (10–13) | 4 (2.1–5.5) |
CEA levels in the serum of patients with breast and
lung cancers were 8- and 20-fold higher, respectively, than those
in the serum of patients with benign disease. In patients with
ovarian cancers and lymphomas, CEA serum levels were slightly
higher than those in patients with benign disease, but no
difference between CEA serum levels in subjects with kidney
cancers, mesotheliomas and benign disease was found. In patients
with breast, lung and ovarian cancers, the CEA pleural effusion
levels were much higher than those in the pleural fluids of
patients with benign disease and slightly higher in the pleural
effusions of patients with ovarian cancers and lymphomas. Values
similar in serum and pleural effusions of CEA levels in subjects
with kidney cancers, mesotheliomas and benign disease were
found.
In patients with breast, lung, ovarian cancers and
lymphomas, serum and pleural effusion Ca15-3 levels were much
higher than those in the pleural fluids of patients with benign
disease, but not so high in patients with kidney cancer and
mesotheliomas. Concerning Ca125, the serum levels were similar in
patients with benign and malignant disease. However, 6-fold higher
levels in the pleural effusions of patients with breast and lung
cancer related to those of patients with benign disease were
observed. Ca125 values in the pleural effusions of patients with
ovarian cancer and lymphomas were slightly higher than those in the
pleural fluids of patients with benign disease. On the other hand,
Ca125 values were similar to those of the patients with benign
disease in the pleural fluids of patients with mesotheliomas and
kidney cancer. No differences in the serum values of Ca19-9 in
patients with benign disease nor in patients with breast, lung,
kidney cancers, mesotheliomas and lymphomas were observed. However,
in the serum of patients with ovarian cancers, the Ca19-9 values
were 10-fold higher than those found in the pleural fluids of
patients with benign disease. However, these values were not noted
in the pleural effusions of patients with kidney cancer, lymphomas
and mesotheliomas. On the other hand, Ca19-9 values were between 6-
and 40-fold higher than those of the patients with benign disease
in the remaining pleural effusions of patients with malignant
disease (breast, lung and ovarian cancers). The percentage of
positivity for CEA, Ca15-3 and Ca125 in breast, lung and ovarian
cancer pleural fluids was also calculated, whereas any data
pertaining to Ca19-9 were no longer considered. The term ‘positive’
was considered for values equal or higher to the cut-off values.
Since normal pleural fluids were not available, the cut-off values
for the three markers were calculated using tumour marker values of
pleural fluids of patients with benign exudates. The optimal
cut-off points for pleural fluid were obtained by ROC-curve
analyses (Table IV). The optimal
cut-off values for pleural fluid were CEA 2.1 ng/ml, Ca15-3 41 U/ml
and Ca125 459 U/ml. Using these cut-off values, CEA and Ca15-3 were
found to be positive in all samples (37/37) of the pleural
effusions of breast cancer patients, and Ca125 was positive in
34/37 (90%) of the total samples of pleural effusions. In lung
cancers, CEA and Ca15-3 were positive in 23 (80%) and 20 (70%) of
the total samples of pleural effusions, respectively, and Ca125 was
positive in 20 (70%) of the total samples of pleural effusions. All
samples of pleural fluids analyzed were positive for CEA and Ca15-3
in ovarian cancers.
 | Table IVOptimal cut-off point of tumour
markers in pleural effusion. |
Table IV
Optimal cut-off point of tumour
markers in pleural effusion.
| Cut-off point | Sensitivity
(%) | Specificity
(%) |
|---|
| CEA | 2.1 ng/ml | 82 | 100 |
| Ca15-3 | 41 U/ml | 96 | 100 |
| Ca125 | 459 U/ml | 82 | 96 |
The pleural effusions were immediately centrifuged
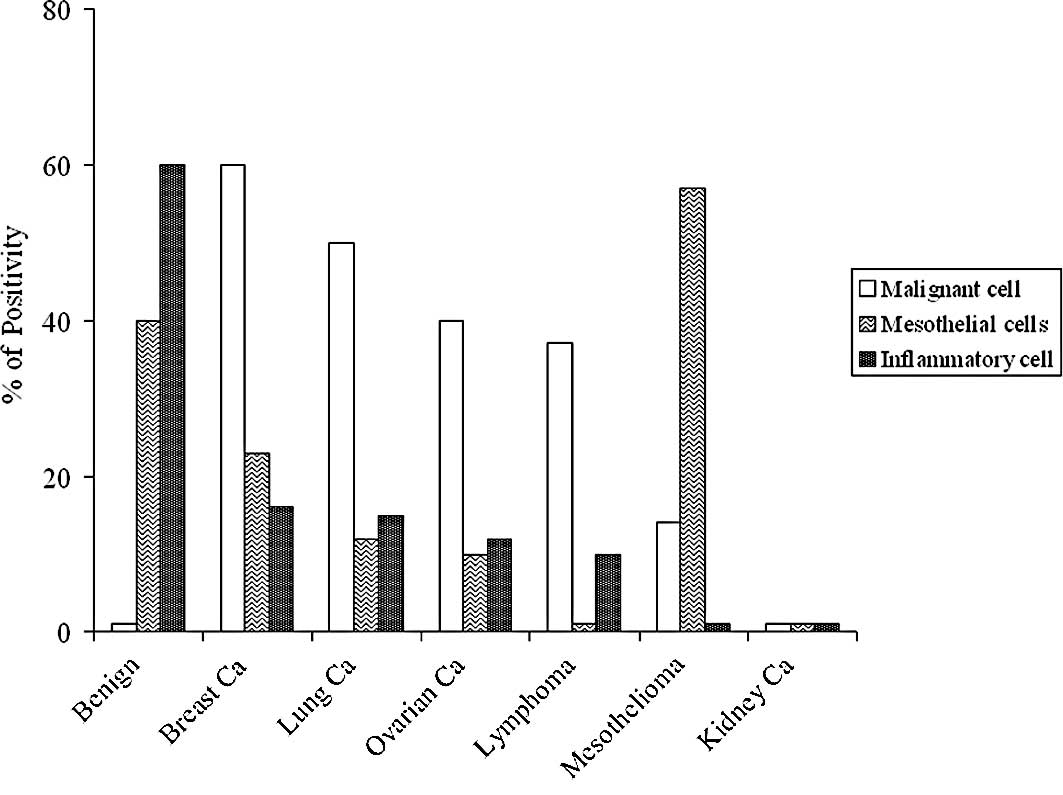
and a cytologic study was performed; Fig. 1 shows the results. The highest
percentage of malignant cells was found in the pleural effusions of
patients with breast cancers (60%), followed by those with lung
cancers (50%), ovarian cancers (40%) and lymphomas (37%). The
highest percentage of positivity for the inflammatory cells was
found in the samples of benign pleural effusions (60%). Low
percentage of positivity or negativity was observed for the
remaining samples. Mesotheliomas showed the highest percentage for
mesothelial cells (57%). However, 40% of samples from benign
pathologies were also positive for mesothelial cells. Low
percentages of positivity or negativity were found for the
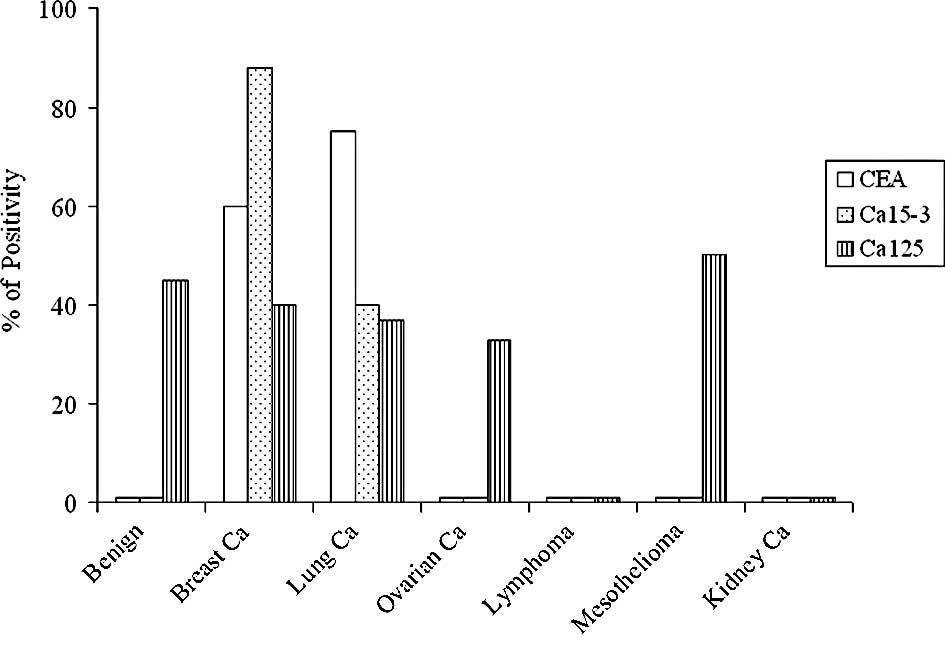
remaining samples. CEA, Ca15-3 and Ca125 were measured on all the
lysates obtained; Fig. 2 shows the
results. In these materials, a marker was considered positive when
it was detected (values higher than the analytical sensitivity of
the method). Ca125 was positive in 50% of samples from
mesotheliomas, followed by benign pathologies (45%), breast (40%),
lung (37%) and ovarian cancers (33%), negative kidney cancer and
lymphomas. CEA and Ca15-3 were undetectable in benign samples,
mesotheliomas, lymphomas and ovarian and kidney cancers. The
highest percentage of Ca15-3 positivity was observed in breast
cancers (88%), followed by lung cancers (40%), whereas CEA was very
high in lung cancers (75%), followed by breast cancers (60%). For
breast and lung cancers, the percentage of positivity of the three
markers was compared to the presence of malignant cells in
individual patients; Tables V and
VI show the results. The means ±
SE of the three markers were calculated using only the positive
samples and the data were expressed as ng or U/mg of proteins. In
breast cancer samples, the three markers values were: CEA, 131±40
ng/mg; Ca15-3, 305±77 U/mg and Ca125, 184±77 U/mg proteins. In lung
cancers samples, the following data were found: CEA, 348±71 ng/mg;
Ca15-3, 86±21 U/mg and Ca125, 204±96 U/mg proteins. On the other
hand, the three markers in lymphomas and kidney cancer samples were
undetectable, whereas Ca125 had high values in ovarian cancers
(339±124 U/mg proteins) and in mesotheliomas (219±61 U/mg
proteins).
 | Table VPresence of malignant cells and
expression of tumour markers in packed materials lysates of pleural
fluids of single patients with breast cancer. |
Table V
Presence of malignant cells and
expression of tumour markers in packed materials lysates of pleural
fluids of single patients with breast cancer.
| Patient no. | Malignant
cells | Expression of
tumour markers |
|---|
| |
|
|---|
| | CEA | Ca15-3 | Ca125 |
|---|
| 1 | − | + | + | − |
| 2 | + | − | + | − |
| 3 | + | + | + | + |
| 4 | + | + | + | − |
| 5 | − | − | + | − |
| 6 | + | + | + | + |
| 7 | − | − | + | − |
| 8 | − | − | − | + |
| 9 | + | + | + | + |
| 10 | − | − | + | − |
| 11 | − | − | + | − |
| 12 | − | + | + | − |
| 13 | − | − | − | + |
| 14 | + | + | + | − |
| 15 | + | − | + | + |
| 16 | + | + | + | − |
| 17 | − | + | − | + |
| 18 | + | + | + | − |
| 19 | − | − | + | − |
| 20 | − | − | − | + |
| 21 | + | + | + | − |
| 22 | + | + | + | − |
| 23 | + | + | + | − |
| 24 | + | + | + | + |
| 25 | + | + | + | − |
| 26 | + | + | + | + |
| 27 | − | − | + | − |
| 28 | + | + | + | − |
| 29 | − | − | − | + |
| 30 | + | + | + | − |
| 31 | − | − | + | − |
| 32 | + | + | + | + |
| 33 | + | − | + | + |
| 34 | + | + | + | − |
| 35 | + | + | + | + |
| 36 | + | + | + | − |
| 37 | − | − | + | + |
 | Table VIPresence of malignant cells and
expression of tumour markers in packed materials lysates of pleural
fluids of single patients with lung cancer. |
Table VI
Presence of malignant cells and
expression of tumour markers in packed materials lysates of pleural
fluids of single patients with lung cancer.
| Patient no. | Malignant
cells | Expression of
tumour markers |
|---|
| |
|
|---|
| | CEA | Ca15-3 | Ca125 |
|---|
| 1 | − | + | − | + |
| 2 | − | + | − | + |
| 3 | + | + | + | − |
| 4 | + | + | − | − |
| 5 | + | + | + | + |
| 6 | + | + | − | − |
| 7 | − | − | + | − |
| 8 | − | + | + | − |
| 9 | − | + | − | + |
| 10 | − | + | − | − |
| 11 | + | + | + | − |
| 12 | + | + | + | + |
| 13 | + | + | − | − |
| 14 | + | + | + | − |
| 15 | − | − | − | − |
| 16 | − | − | − | + |
| 17 | − | − | − | + |
| 18 | + | + | + | − |
| 19 | − | + | + | − |
| 20 | + | + | − | − |
| 21 | − | − | − | + |
| 22 | + | + | − | + |
| 23 | + | + | + | − |
| 24 | − | + | + | − |
| 25 | + | + | + | − |
| 26 | + | + | − | − |
| 27 | − | − | − | + |
| 28 | − | − | − | + |
| 29 | + | + | − | − |
Discussion
Pleural effusions are common complications of a wide
variety of diseases. To elucidate their precise etiologies and to
differentiate malignant from non-malignant effusions, the
laboratory plays an important role.
Cytological analysis (17) remains the main diagnostic approach.
Diagnosis of malignancy is well established when neoplastic cells
are found in pleural fluids. A point to be considered is whether
the cells are local or metastatic cancer cells. If the cells are
metastatic then the organ they originate from needs to be located.
Of note, however, is that in 40–80% of the malignant effusions the
cytological analysis depends on the investigator's experience
(18). Therefore, in order to
obtain more information from laboratory analyses, certain
parameters were measured in pleural fluid and serum in order to
determine trasudates and exudates. Furthermore, we measured a large
panel of tumour markers on lysate extracts of the sediments of the
pleural effusions, in the serum and in the supernatant of pleural
effusions, obtained from the same patients. Our study showed that
it is easy to differentiate exudates from trasudates by measuring
fluid choleseterol, protein and LDH and fluid to serum total
protein ratio, as well as fluid LDH to serum LDH ratio.
Additionally, we showed that fluid cholesterol, fluid protein and
the fluid protein/serum protein ratio were higher in the malignant
than in benign fluids, whereas fluid LDH/fluid serum ratio was
higher in the benign than in malignant fluids. In agreement with
other authors (19), pleural fluid
CEA levels in patients with lung cancers were significantly higher
than those in patients with benign disease, with 100% of positivity
rate at a cut-off level of 2.1 ng/ml. In addition, 75% of the
cytosolic materials of pleural effusion sediments were positive for
CEA, whereas the cytological analysis detected neoplastic cells in
only 50% of the samples. These results strongly suggest that the
combined use of CEA determination on pleural effusions and on
cytosolic materials of the pleural effusion sediments is more
useful than the cytological analysis. Our data showed the remaining
markers in pleural effusions and sediments to be less useful for
this malignancy. Pleural fluid Ca15-3 levels in patients with
breast cancers were significantly higher than those in patients
with benign disease, with 100% of positivity rate at a cut-off
level of 41 U/ml. In addition, 88% of the cytosolic materials of
pleural effusion sediments were positive for Ca15-3, whereas the
cytological analysis detected neoplastic cells in only 60% of the
samples. Our data indicated that the other markers are less useful
for breast cancer in pleural effusions and on cytological materials
of pleural effusion sediments. On the other hand, using cytological
analysis and tumour marker measurements, the percentage of
positivity was not higher than 50% of the samples for ovarian
cancers. Regarding the remaining malignant diseases (kidney cancer,
lymphomas and mesotheliomas), our results did not indicate any
analytical test to be useful for the diagnosis of these
diseases.
Furthermore, in agreement with other authors
(12,20), Ca125 is not recommended as a useful
diagnostic tool in malignant pleural effusion since
immunohistochemical studies have shown that Ca125 is released from
the pleura as well as from the peritoneum. In the present study,
when the percentage of positivity for Ca125 was considered in the
cytosolic materials of pleural effusion sediments, no significant
difference was observed between benign and malignant diseases.
Finally, Ca19-9, as well as Ca125, do not aid in the cytological
diagnosis of pleural effusions. Taking together these observations,
we suggest that: i) the tumour markers CEA and Ca15-3 measured in
pleural fluids may provide clinicians with additional information
on the nature of pleural fluids; ii) Ca15-3 and CEA appear to be
good indicators for breast and lung cancer, respectively, and
finally iii) the use of cytosols of pleural effusion sediments of
tumour markers is very useful, especially in cytologically negative
cases.
References
|
1
|
Light RW, Macgregor MI, Luchsinger PC and
Ball WC Jr: Pleural effusions: the diagnostic separation of
transudates and exudates. Ann Intern Med. 77:507–513. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gazquez I, Porcel JM, Vives M, Vicente de
Vera MC, Rubio M and Rivas MC: Comparative analysis of Light's
criteria and other biochemical parameters for distinguishing
transudates from exudates. Respir Med. 92:762–765. 1998.
|
|
3
|
Paramothayan NS and Barron J: New criteria
for the differentiation between transudates and exudates. J Clin
Pathol. 55:69–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heffner JE, Sahn SA and Brown LK:
Multilevel likelihood ratios for identifying exudative pleural
effusions (*). Chest. 121:1916–1920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terracciano D, Di Carlo A, Papa P,
Cicalese M, Maietta P, Cecere C, Mariano A and Macchia V: New
approaches in the diagnostic procedure of malignant pleural
effusions. Oncol Rep. 12:79–83. 2004.PubMed/NCBI
|
|
6
|
Fenton KN and Richardson JD: Diagnosis and
management of malignant pleural effusions. Am J Surg. 170:69–74.
1995. View Article : Google Scholar
|
|
7
|
Marel M, Stastny B, Melinova L, Svandova E
and Light RW: Diagnosis of pleural effusions. Experience with
clinical studies, 1986 to 1990. Chest. 107:1598–1603. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menzies R and Charbonneau M: Thoracoscopy
for the diagnosis of pleural disease. Ann Intern Med. 114:271–276.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
San Jose ME, Alvarez D, Valdes L,
Sarandeses A, Valle JM and Penela P: Utility of tumour markers in
the diagnosis of neoplastic pleural effusion. Clin Chim Acta.
265:193–205. 1997.PubMed/NCBI
|
|
10
|
Paganuzzi M, Onetto M, Marroni P,
Filiberti R, Tassara E, Parodi S and Felletti R: Diagnostic value
of CYFRA 21-1 tumor marker and CEA in pleural effusion due to
mesothelioma. Chest. 119:1138–1142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galbis Caravajal JM, Benlloch Carrion S,
Sanchez Paya J, Mafe Madueno JJ, Baschwitz Gomez B and Rodriguez
Paniagua JM: Prognostic value of the carcinoembryonic antigen found
in pleural lavage fluid from patients with lung carcinoma. Arch
Bronconeumol. 41:185–188. 2005.PubMed/NCBI
|
|
12
|
Ghayumi SM, Mehrabi S, Doroudchi M and
Ghaderi A: Diagnostic value of tumor markers for differentiating
malignant and benign pleural effusions of Iranian patients. Pathol
Oncol Res. 11:236–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonangelo L, Vargas FS, Seiscento M,
Bombarda S, Teixera L and Sales RK: Clinical and laboratory
parameters in the differential diagnosis of pleural effusion
secondary to tuberculosis or cancer. Clinics. 62:585–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fetsch PA and Abati A: Immunocytochemistry
in effusion cytology: a contemporary review. Cancer. 93:293–308.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kjeldsberg C and Knight J: Pleural and
Pericardial Fluids Body Fluids. 3rd edition. ASCP; Chicago: pp.
159–222. 1993
|
|
16
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bedrossian CW: Diagnostic problems in
serous effusions. Diagn Cytopathol. 19:131–137. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motherby H, Nadjari B, Friegel P, Kohaus
J, Ramp U and Bocking A: Diagnostic accuracy of effusion cytology.
Diagn Cytopathol. 20:350–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuralay F, Tokgoz Z and Comlekci A:
Diagnostic usefulness of tumour marker levels in pleural effusions
of malignant and benign origin. Clin Chim Acta. 300:43–55. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shitrit D, Zingerman B, Shitrit AB, Shlomi
D and Kramer MR: Diagnostic value of CYFRA 21-1, CEA, CA 19-9, CA
15-3 and CA 125 assays in pleural effusions: analysis of 116 cases
and review of the literature. Oncologist. 10:501–507. 2005.
View Article : Google Scholar : PubMed/NCBI
|