Introduction
The prognosis of patients with glioblastoma, the
most common primary brain tumor, is very poor. Despite the
combination of surgery, radiotherapy and chemotherapy, the life
expectancy of patients with glioblastoma is approximately one year
from diagnosis (1,2). Therefore, developing a new therapeutic
strategy is imperative.
Resveratrol (trans-3,4′,5-trihydroxystilbene)
is a natural phytoalexin present in grapes, peanuts and red wine
(3). It has been reported to
possess anticancer activity based on its striking inhibition of
tumor initiation, promotion and progression (4). Furthermore, resveratrol has been shown
to induce apoptotic cell death in several human cancer cell lines,
such as MCF7, SW480, HCE7, Seg-1, HL60 and U251 glioma cells
(5,6). Although resveratrol inhibits different
stages of tumor growth depending on the cell type and cellular
environment, the molecular mechanism of its anticancer activity is
not entirely clear.
Autophagy has multiple physiological functions
including protein degradation, organelle turnover and the response
of cancer cells to chemotherapy. Autophagy is induced by a variety
of conditions, such as nutrient starvation, hormone treatment and
cellular stress. It has been reported that malignant glioma cells
are very resistant to apoptosis, but they undergo autophagy in
response to chemotherapeutic agents or treatment with γ-irradiation
(7,8). The role of autophagy in cancer therapy
is controversial in terms of whether it is protective of or
detrimental to cancer cells. However, the manipulation of autophagy
may lead to a novel therapeutic method for treating
apoptosis-resistant cancers. The regulation of autophagy by
signaling pathways overlaps with the control of cell growth,
proliferation, cell survival and death (9). Although it was established that
Akt/mTOR and ERK1/2 are two significant signaling pathways
regulating autophagy, several signaling pathways may modulate the
autophagic response in cancer cells depending, among other
criteria, on the cell type, the stage of cancer development and the
stromal and nutritional environment (10).
The present study investigated the anticancer effect
of resveratrol on U373 human glioma cells. Resveratrol was found to
inhibit cell growth in a dose-dependent manner. Resveratrol-induced
autophagy in glioma cells was also demonstrated. P38 and ERK1/2
inhibitors reduced the sensitivity of resveratrol-challenged glioma
cells to autophagy, indicating that P38 MAPK and ERK1/2 MAPK are
involved in resveratrol-induced autophagy in human glioma
cells.
Materials and methods
Reagents
Resveratrol, P38 inhibitor SB203580, ERK1/2
inhibitor PD98059 and PI3K inhibitor LY294002 were purchased from
Sigma (St. Louis, MO). These were dissolved in dimethyl sulfoxide
(DMSO, Sigma) to produce stock solutions.
Cell culture and cell viability
assay
U373 human glioma cells were grown in Dulbecco’s
modified Eagle’s medium (Sigma) supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (Sigma) at 37°C in 5%
CO2. The sensitivity of U373 cells to resveratrol was
determined in vitro by using the cell proliferation assay
reagent WST-1 (Roche Applied Science, Indianapolis, IN, USA). For
this purpose, 5×103 U373 cells were seeded on a
flat-bottomed 96-well microtiter plate in triplicate and cultured
overnight. After cells were treated with resveratrol (20–500 μM)
for 48 h, they were exposed to 10 μl of the WST-1 reagent for 3 h
at 37°C. The absorbance at 450 nm was measured using a Beckman
Coulter microplate reader. The viability of DMSO-treated cells was
considered to be 100%.
Apoptosis detection assay
U373 cells were cultured on coverslips overnight and
treated with 100 μM resveratrol for 48 h. The cells were stained
with the terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) assay using the In Situ Cell Death
Detection kit, TMR red (Roche Applied Science). Two hundred cells
were counted, and the percentage of TUNEL-positive cells was scored
under a microscope.
Analysis of autophagy
The pEGFP-LC3 plasmid was kindly provided by Dr N.
Mizushima (Tokyo Metropolitan Institute of Medical Science, Tokyo,
Japan). LC3, one of the mammalian homologues of Atg8, is widely
used as a specific marker of autophagosomes (11). Transfection of the plasmids into
U373 human glioma cells was performed using TransFectin Lipid
Reagent (BioRad Laboratories, Hercules, CA). Stable cell lines were
selected in 1 mg/ml geneticin (G418), and single clones were
selected in order to yield clonal cell lines. U373 cells expressing
GFP-LC3 were treated with resveratrol for 48 h, then fixed with 4%
paraformaldehyde. The number of GFP-LC3-labeled autophagosomes per
cell or the number of GFP-LC3-labeled autophagosome-positive cells
was counted under a confocal microscope (LSM5 Pascal, Zeiss,
Germany). The counting of GFP-LC3-labeled autophagosomes was
assisted by the Metamorph software (Molecular Devices, Sunnyvale,
CA).
To determine the effects of Akt/mTOR, P38 and ERK1/2
on resveratrol-stimulated autophagy in glioma cells, we evaluated
autophagy in cells pre-treated with or without 15 μM LY294002, 10
μM SB202190 and 20 μM PD98059 for 3 h. The cells were then treated
with resveratrol for 48 h, as described above.
Results
Resveratrol inhibits survival of U373
glioma cells
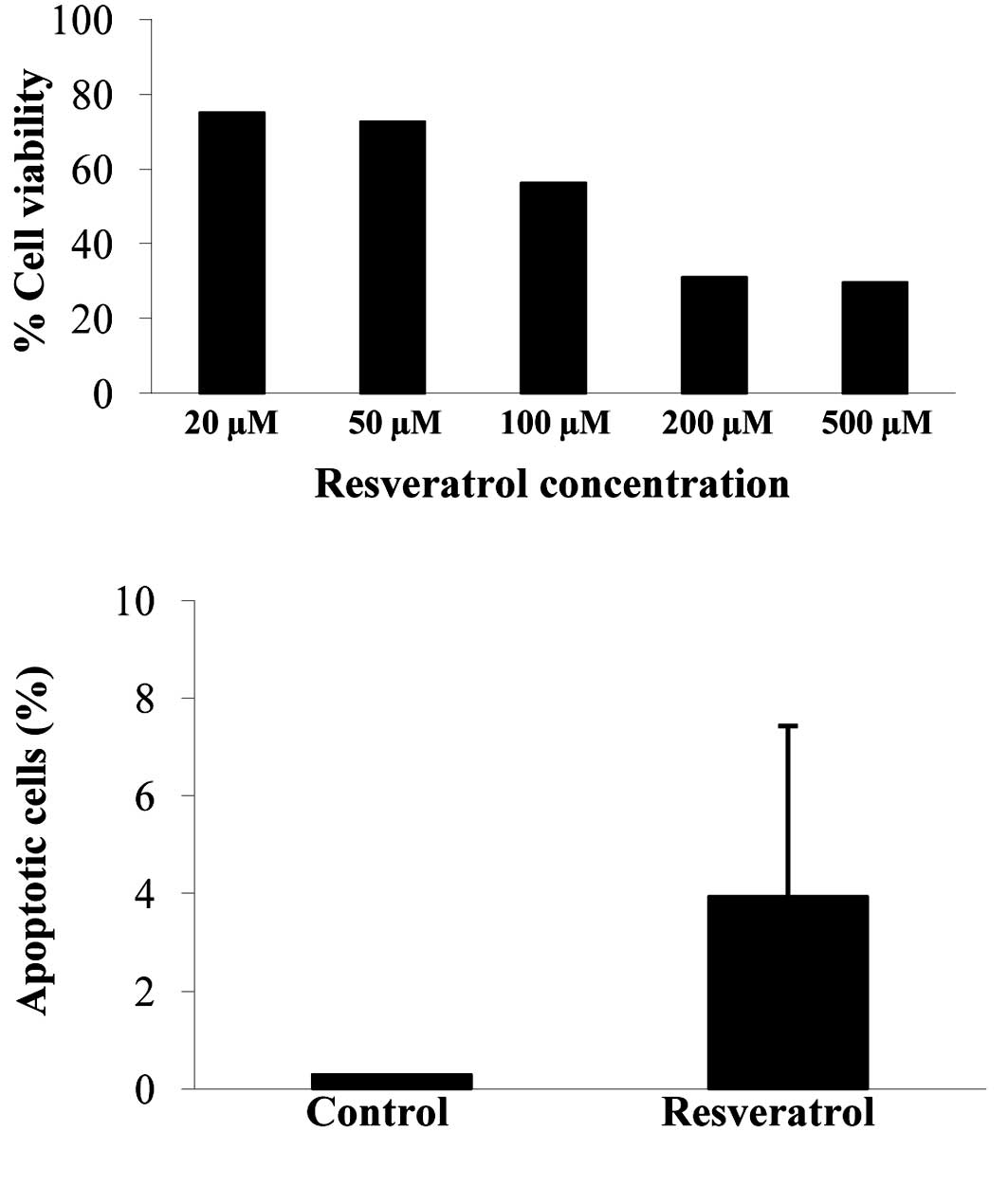
To examine the effect of resveratrol on cell
survival, we treated U373 cells with different doses of resveratrol
(0, 20, 50, 100, 200 and 500 μM). Cell viability was measured after
48 h by the WST-1 assay. Cell viability decreased in a
dose-dependent manner (Fig. 1A). To
examine whether resveratrol induces apoptosis, we performed the
TUNEL assay in U373 cells following treatment with resveratrol.
Treatment with 100 μM resveratrol increased the ratio of
TUNEL-positive cells compared with the control-treated cells
(Fig. 1B).
Resveratrol induces autophagy in U373
glioma cells
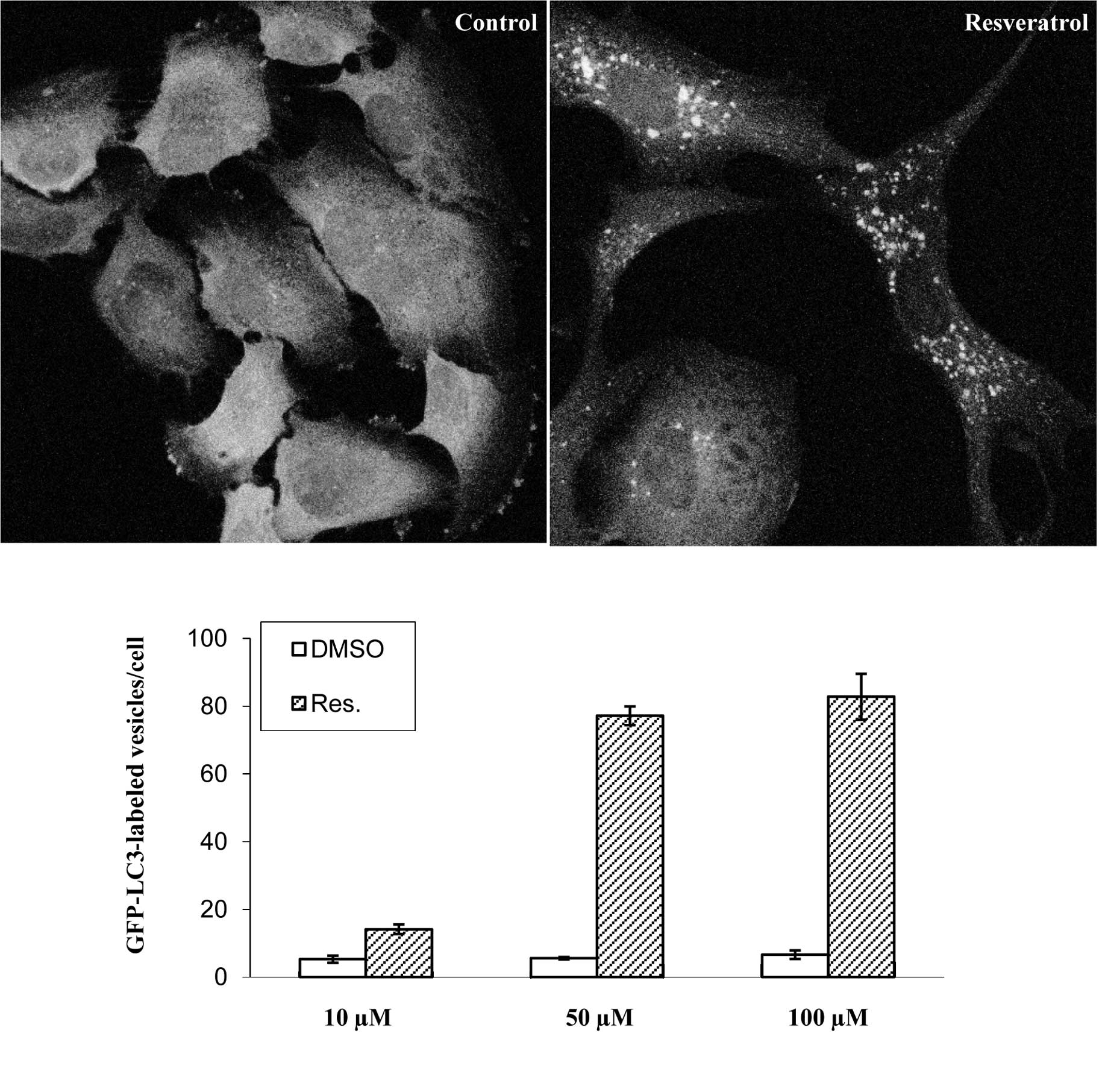
To visualize the induction of autophagy in human
glioma cells, a U373 cell line expressing GFP fused to the
N-terminus of LC3 was treated with 100 μM resveratrol for 48 h. In
control-treated cells, GFP-LC3 diffused throughout the cytosol
(Fig. 2A). When cells were treated
with resveratrol, GFP-LC3-labeled autophagosomes appeared in the
cytoplasm. The percentage of autophagic cells in GFP-positive cells
was ~90%. Furthermore, the number of GFP-positive vesicles per cell
dramatically increased in cells treated with 50 μM resveratrol
compared with those treated with 10 μM resveratrol (Fig. 2B).
P38 and ERK1/2 play a role in
resveratrol-induced autophagy
It has been reported that Akt/mTOR is the main
pathway, negatively regulating autophagy, whereas the ERK pathway
positively regulates autophagy in cancer cells upon starvation
(9). However, the molecular
mechanism regulating autophagy in response to anticancer agents
remains unclear, and may vary depending on the type of cancer cell
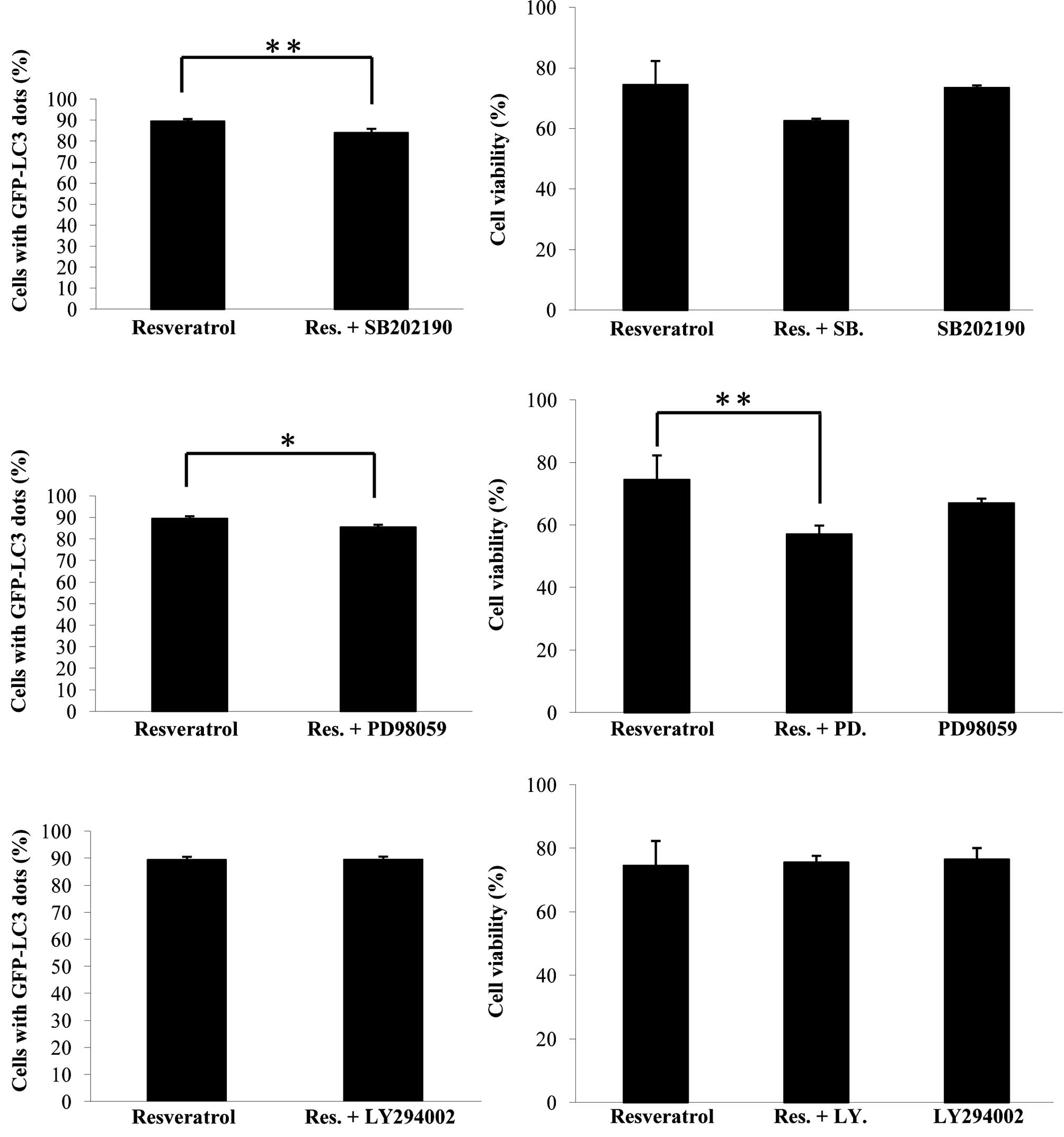
involved. To investigate the roles of PI3K, P38 and ERK1/2 kinases
on resveratrol-induced autophagy in U373 cells, inhibitors of these
kinases were used. As shown in Fig.
3A, the P38 SB203580 and ERK1/2 PD98059 inhibitors
down-regulated the autophagic process, suggesting that both P38 and
ERK1/2 promoted resveratrol-induced autophagy in glioma cells.
PD98059 reduced cell viability in the resveratrol-applied system,
indicating that ERK1/2 contributed to cell survival in this system
(Fig. 3B). The PI3K inhibitor,
LY294002, had no obvious effects on either resveratrol-induced
autophagy nor cell viability in glioma cells (Fig. 3).
Discussion
Resveratrol, a phytoalexin found in food products
such as nuts, grapes and wine, produces a variety of physiological
effects. It has been reported that a low dose of resveratrol has
cytoprotective activity, which is mostly attributed to its
antioxidant properties. On the other hand, a high dose of
resveratrol exhibits anticancer activity by interfering with
different cellular events associated with the initiation, promotion
and progression of multi-stage carcinogenesis (12,13).
The present study found that resveratrol elicited a dose-dependent
inhibition of glioma cell proliferation in the micromolar range.
The concentrations used were reported to result in anticancer
effects in different types of cancer cells (6,14).
Autophagy is a ubiquitous cellular process in
eukaryotic cells that results in the breakdown of cytoplasmic
organelles within the lysosomes following various types of cellular
stress. This breakdown allows for the cells to respond to
environmental changes or adapt to developmental processes. Opipari
et al (14) reported that
resveratrol inhibited cancer growth and induced autophagocytosis in
ovarian carcinoma cell lines. We found that resveratrol induced
autophagy in human glioma cells. Although there are multiple
mechanisms by which resveratrol may exert its actions on tumor
cells, autophagy may be a biological mechanism that accounts for
the anticancer effects of this compound. The implication of
autophagy in cancer therapy is controversial in terms of whether
autophagy plays a protective or detrimental role in cancer cells.
However, reduced autophagic activity has been reported in certain
types of cancer cells (15,16). Several natural compounds, such as
arsenic trioxide, soybean B-group triterpenoid saponins or
curcumin, have been shown to induce autophagy in a variety of tumor
cells (17–20). Thus, compounds that induce autophagy
may be of potential use as anticancer agents. Previous studies have
shown that resveratrol induces apoptosis in a variety of cancer
cells including glioma cells (5,6). In
this study, we also observed a certain degree of
resveratrol-induced apoptosis in U373 cells. Autophagy and
apoptosis may be triggered by common upstream signals occasionally
resulting in combined autophagy and apoptosis. In other instances,
however, the cell switches between the two responses in a mutually
exclusive manner (21). Further
research is necessary to identify the functional relationship
between autophagy and apoptosis in response to resveratrol.
In mammalian cells, several signaling pathways are
known to regulate autophagy in a cell type-specific and
signal-dependent manner, including the ERK, PI3K class I and II,
and mTOR pathways. Cancer often occurs following the deregulation
of these signaling pathways. Therefore, it is important to
investigate the interplay of their role in modulating autophagy,
cell survival and cell growth. This study showed that resveratrol
activated P38 and ERK, but not Akt/mTOR signaling, resulting in the
induction of autophagy and the reduction of cell viability.
Regarding the function of P38 MAPK in autophagy, abrogation of P38
by chemical inhibitors has been reported to be sufficient to
interfere with the normal autophagic maturation step (22,23).
Our study showed that P38 was a contributing factor to
resveratrol-induced autophagy. Oridonin, a herbal
ent-kaurane diterpenoid, has been shown to induce autophagy
by activating P38 signaling in HeLa cells, in accordance with our
findings (24). The ERK and Akt
pathways are known to regulate autophagy, but with opposite effects
in that the ERK pathway regulates autophagy positively, whereas the
Akt pathway regulates autophagy negatively (9). The natural products triterpenoid
B-group soysaponins and curcumin have been shown to induce
autophagy by inhibiting Akt signaling and enhancing ERK activity in
human colon cancer and human glioma cells, respectively (18,19).
Our data showed that the inhibition of the ERK pathway using
PD98059 inhibited resveratrol-induced autophagy, but the inhibition
of the Akt pathway had no effect. Cui et al (24) reported that ERK had no obvious role
in oridonin-induced autophagy in HeLa cells. Further studies are
needed to determine whether the combination of Akt inhibition and
ERK activation is essential for the autophagic process induced by
different anticancer agents in different cancer cell types.
In conclusion, our study showed that resveratrol
induces autophagy and that the ERK and P38 pathways are involved in
resveratrol-induced autophagy. This may offer novel therapeutic
options to explore the relationship between autophagy, cell
death/growth and response to anticancer agents in glioma cells.
Acknowledgements
We would like to thank Dr Noboru Mizushima for
providing the pEGFP-LC3 plasmid.
References
|
1
|
Mashaley MS Jr, Mettlin C, Natarajan N and
Laws ER Jr: National survey on patterns of care for brain-tumor
patients. J Neurosurg. 71:826–836. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H, Dessen P, Jourde B, et al:
Genetic pathways to glioblastoma: a population-based study. Cancer
Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gusman J, Malonne H and Atassi G: A
reappraisal of the potential chemopreventive and chemotherapeutic
properties of resveratrol. Carcinogenesis. 22:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
6
|
Jiang H, Zhang L, Kuo J, et al:
Resveratrol-induced apoptotic death in human U251 glioma cells. Mol
Cancer Ther. 4:554–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanazawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Diff.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santana P, Pena LA, Haimovitz-Friedman A,
Martin S, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z and
Kolesnick R: Acid sphingomyelinase-deficient human lymphoblasts and
mice are defective in radiation-induced apoptosis. Cell.
86:189–199. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Botti J, Djavaheri-Mergny M, Pilatte Y and
Codogno P: Autophagy signaling and the cogwheels of cancer.
Autophagy. 2:67–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meijer AJ and Codogno P: Regulation and
role of autophagy in mammalian cells. Int J Biochem Cell Biol.
36:2445–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Atg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fauconneau B, Waffo-Teguo P, Huguet F,
Barrier L, Decendit A and Merillon JM: Comparative study of radical
scavenger and anti-oxidant properties of phenolic compounds from
Vitis vinifera cell cultures using in vitro test. Life Sci.
61:2103–2110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, Moon RC and Pezzuto JM: Cancer chemopreventive activity of
resveratrol, a natural product derived from grapes. Science.
275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Opipari AW, Tan L, Boitano AE, Sorenson
DR, Aurora A and Liu JR: Resveratrol-induced autophagy in ovarian
cancer cells. Cancer Res. 64:696–703. 2004. View Article : Google Scholar
|
|
15
|
Knecht E, Hernandez-Yago J and Grisolia S:
Regulation of lysosomal autophagy in transformed and nontransformed
mouse fibroblasts under several growth conditions. Exp Cell Res.
154:224–232. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwarze PE and Seglen PO: Reduced
autophagic activity, improved protein balance and enhanced in vitro
survival of hepatocytes isolated from carcinogen-treated rats. Exp
Cell Res. 157:15–28. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanazawa T, Kondo Y, Ito H, Kondo S and
Germano I: Induction of autophagic cell death in malignant glioma
cells by arsenic trioxide. Cancer Res. 63:2103–2108.
2003.PubMed/NCBI
|
|
18
|
Ellington AA, Berhow M and Singletary KW:
Induction of macroautophagy in human colon cancer cells by soybean
B-group triterpenoid saponins. Carcinogenesis. 26:159–167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar
|
|
20
|
Rubinsztein D, Gestwicki JE, Murphy LO and
Klionsky DJ: Potential therapeutic applications of autophagy. Nat
Rev Drug Discov. 6:304–312. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Mol Cell Biol. 8:741–752. 2007.PubMed/NCBI
|
|
22
|
Corcelle E, Djerbi N, Mari M, Nebout M,
Fiorini C, Fenichel P, Hofman P, Poujeol P and Mograbi B: Control
of the autophagy maturation step by the MAPK ERK and P38: lessons
from environmental carcinogens. Autophagy. 3:57–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simone C: Signal-dependent control of
autophagy and cell death in colorectal cancer cell. Autophagy.
3:468–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Oridonin induced autophagy in human cervical carcinoma
HeLa cells through Ras, JNK and P38 regulation. J Pharmacol Sci.
105:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|