Introduction
Renal cell carcinoma (RCC) is known to metastasize
to distant organs such as the lung, lymph nodes, bone, liver and
adrenal glands (1). However,
gallbladder involvement in patients with RCC is extremely rare. The
gallbladder was identified as a metastatic site in only 4 out of
687 cases (0.58%) in large autopsy reviews (2). Patients with distant metastasis from
RCC have a poor prognosis, with a 5-year survival rate of <10%
(3). However, curative resection of
metastasis in selected patients may improve long-term survival
(4) in patients with gallbladder
and one-sided adrenal metastasis of RCC. We presented a case of
metachronous metastasis to the gallbladder and left adrenal gland
from clear cell-type RCC.
Case report
A 50-year-old man was diagnosed with a gallbladder
tumor during a postoperative follow-up by computed tomography (CT)
in January 2009. The patient had a history of radical nephrectomy
for right RCC in 2006. The pathological finding of renal tumor was
clear cell-type RCC, pT1aN0M0 according to the AJCC classification
of cancer staging (5). An enhanced
abdominal CT showed three enlarged polypoid masses with a mosaic
pattern and maximum tumor size of 11×8 mm in diameter in-filling
the gallbladder. The CT also showed an enhanced mass with a mosaic
pattern of 13×19 mm in diameter in the left adrenal gland. Magnetic
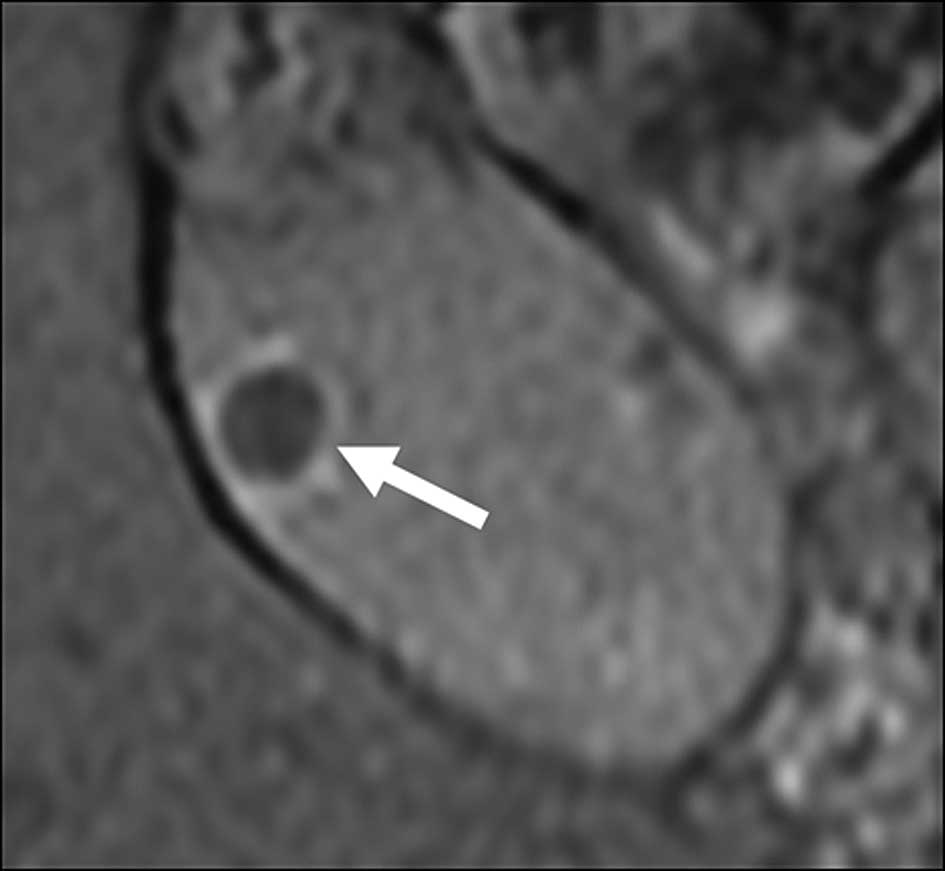
resonance imaging (MRI) revealed a polypoid lesion in the
gallbladder and a mass in the left adrenal gland. The polypoid
lesion in the gallbladder showed a low signal intensity on a
T1-weighted MRI image and an iso signal intensity on a T2-weighted
MRI image, but no suspicious findings with tumor invasion to the
gallbladder muscle layer (Fig. 1).
Similar to the polypoid lesion in the gallbladder, the mass in the
left adrenal gland showed a low signal intensity on a T1-weighted
MRI image and an iso signal intensity on a T2-weighted MRI image.
Blood biochemistry results were in the normal ranges, including
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9). The result of the systemic image screening was that
another metastatic lesion was not found.
Under the diagnosis of metastatic left adrenal tumor
and presumptive diagnosis of metastatic gallbladder tumor from RCC,
the patient underwent simple cholecystectomy and left
adrenalectomy. Macroscopically, the resected specimen of the
gallbladder tumor showed an 11×9 mm (Fig. 2), 7×9 mm and 8×8 mm-sized polypoid
lesion, and the adrenal mass showed a 12×16×13-diameter tumor.
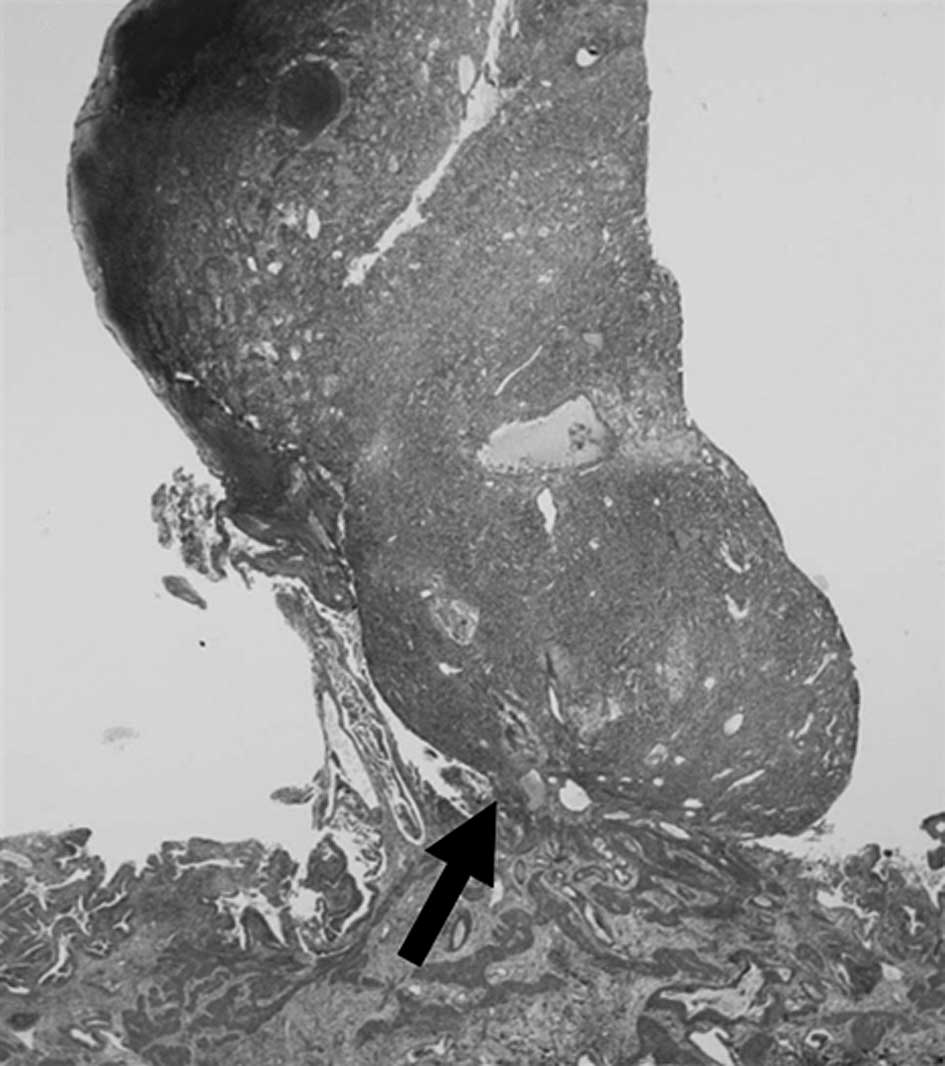
Microscopically, the gallbladder tumor was located in the
submucosal layer, lifting the surrounding mucosa of the gallbladder
(Fig. 3). The gallbladder mucosal
cells had no atypia.
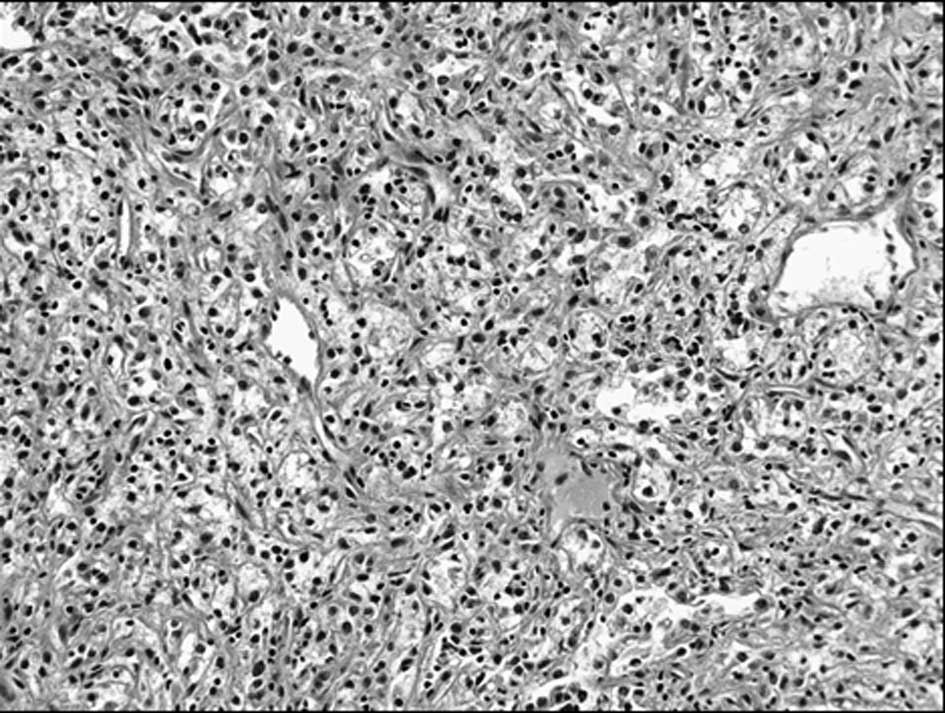
The tumor cells had round uniform nuclei, clear
cytoplasm and well-defined cytoplasmic borders, forming alveolar
patterns (Fig. 4). Neither foci of
mucin production nor ordinary adenocarcinoma patterns of digestive
organs were noted. Immunohistochemically, the tumor cells were
negative for cytokeratin 7 (CK7) and CEA, but positive for
vimentin. Adrenal tumor cell findings were similar to the renal
tumor microscopically. Therefore, the final diagnosis was
metastasis to the gallbladder and adrenal gland from clear
cell-type RCC. Pathological findings of the gallbladder tumor and
adrenal tumors showed clear cell carcinoma. The surgical specimen
of the gallbladder and adrenal tumor showed histological evidence
of clear cell carcinoma with the same characteristics as those of
the primary renal tumor. The patient showed no sign of recurrence 8
months after the last surgery and was without any additional
therapies.
Discussion
RCC is known to metastasize mainly to the lungs,
lymph nodes, bone, brain, liver, adrenal glands and the other
kidney, and rarely to organs such as the vertebrae, stomach,
spleen, pancreas and diaphragm (1).
However, gallbladder metastasis of RCC is seldom detected, being
present in 0.58% of autopsies (2).
In a review of the literature, we found 15 reported cases of
gallbladder metastasis of RCC (6–8).
Dynamic contrast-enhanced CT is useful in the
differential diagnosis of a metastatic gallbladder tumor from RCC
and a primary gallbladder carcinoma because the former is
hypervascular. In the present patient, contrast-enhanced CT showed
a high density on an arterial dominant phase image, and the MRI
showed a high signal intensity on a T2-weighted image. In the
literature, from macroscopic findings, 9 out of 15 cases (60%) were
polypoid type and 3 out of 15 cases (20%) were pedunculated type.
To determine the final differential diagnosis between primary and
secondary gallbladder tumors, an immunohistochemical evaluation was
required. Immunohistochemically, the primary clear cell carcinoma
of the gallbladder is strongly positive for CEA and CK7 and
moderately positive for CK10, but negative for vimentin. On the
other hand, metastatic RCC of the gallbladder is positive for
vimentin, but negative for CEA, CK7 and CK10 (9). Based on the immunohistochemical
findings, our final diagnosis was metastatic gallbladder tumor from
RCC as opposed to primary clear cell carcinoma of the
gallbladder.
Follow-up information on this series was
insufficient to demonstrate the efficacy of cholecystectomy for the
metastasis of RCC. However, cholecystectomy for curative resection
of metastatic RCC may be advocated since, following curative
resection of second and third metastasis, the survival rates have
not been found to be different from those after first
metastatectomy (4). Notably, no
patients who underwent simple cholecystectomy were reported to
develop local recurrence in the liver or the bile duct.
Hematogenous metastasis to the gallbladder occurs as small flat
nodules below the mucosal layer which grow as polyps. Therefore, we
consider that simple cholecystectomy may be sufficient for curative
resection of gallbladder metastasis from RCC if there is no
invasion of the muscle layer of the gallbladder. In our case, a
simple cholecystectomy was performed since no suspicious findings
of tumor invasion to the muscle layer of the gallbladder in
pre-operative imaging and intra-operative findings were noted.
In conclusion, RCC can metastasize to the
gallbladder, and this should be considered in patients with
hypervascular polypoid or pedunculated-type gallbladder tumors who
have a previous history of RCC. Pre-operative imaging and
intra-operative findings are important factors in deciding on
adequate treatment.
Abbreviations:
References
|
1
|
Saitoh H: Distant metastasis of renal
adenocarcinoma. Cancer. 48:1487–1491. 1981. View Article : Google Scholar
|
|
2
|
Weiss L, Harlos J, Torhorst J, et al:
Metastatic patterns of renal cell carcinoma: an analysis of 687
necropsies. Cancer Res Clin Oncol. 114:605–612. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelzang N and Stadler W: Kidney cancer.
Lancet. 352:1691–1696. 1998. View Article : Google Scholar
|
|
4
|
Kavolius J, Mastorakos D, Pavlovich C, et
al: Resection of metastasis renal cell carcinoma. J Clin Oncol.
16:2261–2266. 1998.
|
|
5
|
Greene F, Page D, Fleming I, et al: AJCC
Cancer Staging Manual. 6th edition. Springer-Verlag; New York:
2002, View Article : Google Scholar
|
|
6
|
Nojima H, Cho A, Yamamoto H, et al: Renal
cell carcinoma with unusual metastasis to the gallbladder. J
Hepatobiliary Pancreat Surg. 15:209–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci V, Carbone S, Testi W, et al: Single
gallbladder and multiple pancreatic metastases from renal cell
carcinoma sixteen years after nephrectomy. Chir Ital. 60:311–314.
2008.PubMed/NCBI
|
|
8
|
Sand M, Bechara FG, Kopp J, et al:
Gallbladder metastasis from renal cell carcinoma mimicking acute
cholecystitis. Eur J Med Res. 14:90–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bittinger A, Alterkrugar I and Barth P:
Clear cell carcinoma of the gallbladder. A histological and
immunohistochemical study. Pathol Res Pract. 191:1259–1265. 1995.
View Article : Google Scholar : PubMed/NCBI
|