Introduction
Therapy in Japan for stage Ib2-IIb cancer of the
uterine cervix with a bulky mass differs from that in Europe and
the US. Based on the results of numerous large-scale randomized
clinical studies and meta-analyses of the data, concurrent
chemoradiation (CCRT) is recommended as a standard therapy in
Europe and the US (1–7). An approach to neoadjuvant chemotherapy
(NAC) was introduced in Japan, as well as in other countries, such
as Korea and Italy (8). The
clinical significance of NAC involves: i) contraction of the tumor
size in an attempt to improve permanent curability and safety of
surgery, and ii) an anticipated systemic effect on
latent/micro-lymph node metastasis. However, this approach has the
disadvantage of causing a delay in the initiation of the primary
treatment. Thus, it is necessary to complete NAC as an adjunctive
therapy in a short period period of time.
Irinotecan hydrochloride, an inhibitor of DNA
topoisomerase I, has received considerable attention as an
anticancer drug, which, in the active metabolite form, exerts an
antitumor effect by inhibiting nucleic acid synthesis. Irinotecan
administered alone or in combination with cisplatin is useful in
the treatment of recurrent cervical cancer (9,10).
Cisplatin and irinotecan chemotherapy with a schedule consisting of
cisplatin administered on Day 1 and irinotecan on Days 1, 8 and 15,
followed by a 2-week withdrawal, is recommended as a standard
regimen (11). The present study
was conducted to assess the toxicity and efficacy of a tri-weekly
cisplatin and irinotecan combination therapy regimen with an
increased cisplatin dose intensity and concurrent irinotecan, in
order to reduce the duration of the therapy regimen to the primary
surgical treatment.
Patients and methods
Patients
The study population comprised 20 patients with
locally advanced squamous cell carcinoma of the uterine cervix at
FIGO stage Ib2-IIIb, who were scheduled for radical hysterectomy.
The patients gave informed consent. The study was conducted June
2002 and March 2008.
Inclusion criteria
Inclusion criteria for the study included: i)
histologically verified squamous cell carcinoma of the uterine
cervix; ii) locally advanced stage Ib2-IIIb disease; iii) ≥20 years
and <70 years of age (or <45 years in the case of patients
with stage III disease who selected conservation of the ovary); iv)
Eastern Cooperative Oncology Group (ECOG) performance status (PS)
0–2; v) initially treated case; vi) presence of a magnetic
resonance imaging (MRI)-measurable bulky mass in the uterine
cervix; vii) hematological and blood biochemical findings meeting
the following criteria: WBC count ≥4,000/mm3, neutrophil
count ≥2,000/mm3, platelet count
≥100,000/mm3, hemoglobin ≥10.0 g/dl, AST and ALT levels
≤2 times the upper limit of the normal reference range at the study
site, serum total bilirubin level ≤1.5 mg/dl, serum creatinine ≤1.5
mg/dl, and creatinine clearance ≥60 ml/min; viii) life expectancy
≥6 months; and ix) written informed consent personally provided by
the subject.
Exclusion criteria
Exclusion criteria included: i) patients with overt
infection; ii) patients with a serious complication(s) (e.g.,
cardiac disease, poorly controlled diabetes mellitus, malignant
hypertension and bleeding tendency); iii) patients with active
multiple cancer; iv) patients with interstitial pneumonia or
pulmonary fibrosis; v) patients with effusions; vi) patients with a
history of unstable angina or myocardial infarction within 6 months
after registration, or with a concurrent serious arrhythmia
requiring treatment; vii) patients for whom treatment with
cisplatin and irinotecan is contraindicated; viii) patients with
(watery) diarrhea; ix) patients with intestinal paralysis or ileus;
x) pregnant women, nursing mothers or women wishing to become
pregnant; xi) patients with a history of serious drug
hypersensitivity or allergy; and xii) patients who were inadequate
for safe conduct of this study as judged by the attending
physician.
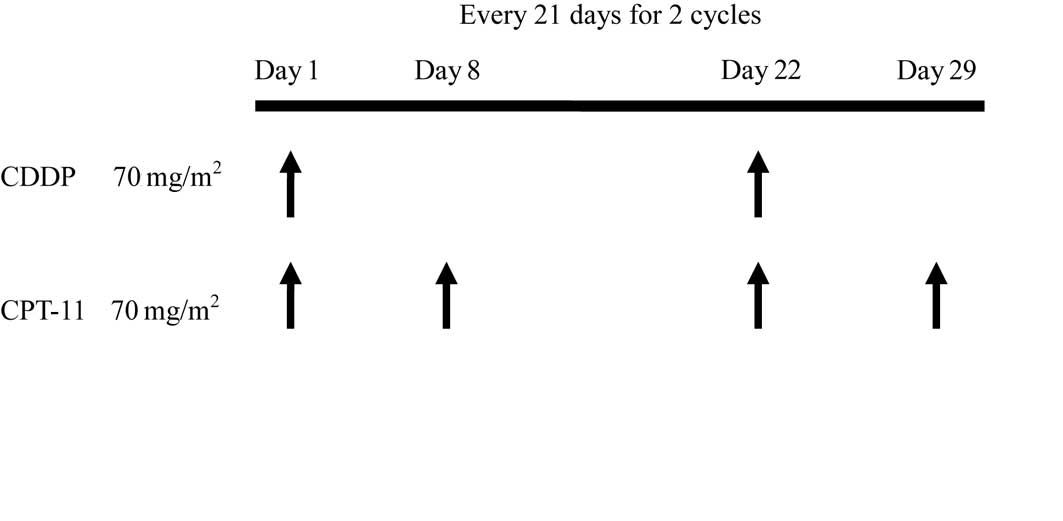
Treatment protocol
We designed the treatment schedule on the basis of a
phase II clinical study in patients with small-cell lung carcinoma.
Two 21-day cycles consisting of intravenous administration of
cisplatin (CDDP) at 70 mg/m2 on Day 1 and irinotecan
(CPT-11) at 70 mg/m2 on Days 1 and 8 were performed
(Fig. 1).
Dose modification criteria
Criteria for CPT-11 dose skip
The irinotecan dose on Day 8 was skipped when
hematological test values within 2 days before Day 8 failed to
fulfill the following criteria: a) neutrophil count
≥1,000/mm3 and b) platelet count
≥75,000/mm3.
Criteria for the initiation of the
second cycle
Initiation of the second cycle was postponed up to a
maximum of 2 weeks when the hematologic test values within 2 days
before the scheduled second cycle initiation day failed to fulfill
the following criteria: a) neutrophil count ≥1,500/mm3,
b) platelet count ≥75,000/mm3 and c) serum creatinine
≤1.5 mg/dl.
Dose reduction criteria
The doses of cisplatin and irinotecan were reduced
to 70 and 60 mg/m2, respectively, in the second course
for patients who presented any of the following signs of toxicity
in the first cycle: i) grade 4 neutropenia persisting for ≥7 days,
ii) febrile neutropenia persisting for ≥4 days, iii) grade 4
thrombocytopenia, iv) grade 3 thrombocytopenia with hemorrhage and
v) grade ≥3 non-hematologic toxicity excluding nausea, vomiting,
appetite loss, fatigue and hair loss.
Supportive therapy
Therapeutic administration of G-CSF preparations was
undertaken in the case where grade 4 neutropenia was noted in the
first cycle. In the second and subsequent cycles, prophylactic use
of the preparation in patients with grade 3 neutropenia was
acceptable when grade 4 neutropenia was noted in the first cycle.
Antiemetics were used for preventive purposes.
Endpoints/variables
The primary endpoint was an antitumor response and
the secondary endpoints comprised adverse events and surgery
completion rate. The antitumor response and surgery completion rate
were calculated for the subgroups of FIGO stage I–II and III. As a
rule, hematological tests and urinalysis were performed prior to
the subjected chemotherapy and at weekly intervals after
chemotherapy commenced. Electrocardiography and a chest X-ray
examination were conducted prior to commencing and at completion of
the chemotherapy.
Evaluation of antitumor response
Pre-treatment MRI was regarded as baseline data.
MRIs were therefore obtained at completion of the first and second
cycles, and the antitumor response was rated according to the
Response Evaluation Criteria in Solid Tumors. Assessments of the
duration of response were not taken into account in this
evaluation.
Method of assessing adverse
events
The National Cancer Institute Common Toxicity
Criteria (NCI-CTCAE) version 3.0 was used to assess adverse
events.
Main treatment
Stage Ib2-IIb patients were subjected to a radical
hysterectomy except when the antitumor response was progressive
disease (PD) or up-stage progression. A radical hysterectomy was
performed on stage IIIb patients with down-stage progression. CCRT
was carried out on patients whose conditions were inoperable.
Postoperative therapy
Postoperative radio-or chemotherapy was undertaken
in patients with positive vaginal stump, positive lymphadenopathy,
positive invasion of the cardinal ligament or evident invasion of
the vasculature.
Results
Demographic and baseline clinical
characteristics
Regarding the age distribution of the 20 enrolled
patients, stage I–II patients ranged from 25 to 56 years of age
(median 40) and stage III patients from 40 to 44 years of age
(median 44). The PS was 0 in 17 patients (85%) and 1 in 3 patients
(15%). The FIGO stage at initial diagnosis was stage Ib2 in 3
patients (15.8%), stage IIa in 2 (10.5%), stage IIb in 9 (47.4%)
and stage IIIb in 5 patients (26.3%) (Table I).
 | Table IPatient characteristics (n=20). |
Table I
Patient characteristics (n=20).
| Median age, in years
(range) |
| Stage I–II | 40 (25–56) |
| Stage III | 44 (44–46) |
| Performance status at
entry |
| 0 | 17 (85%) |
| 1 | 3 (15%) |
| 2 | 0 (0%) |
| FIGO stage at initial
diagnosis |
| Ib | 3 (15%) |
| IIa | 2 (10%) |
| IIb | 10 (50%) |
| IIIb | 5 (25%) |
| Tumor
diametera |
| <5 cm | 10 (50%) |
| ≥5 cm | 10 (50%) |
Antitumor response
Of the 15 stage I–II patients, the antitumor
response was complete response (CR) in 4 patients (26.7%), partial
response (PR) in 9 (60%), stable disease (SD) in 1 (6.7%) and PD in
1 patient (6.7%). Of the 5 stage III patients, the response was PR
in 1 patient (20%) and SD in 4 patients (80%). The overall response
rates of stage I–II and III patients were 86.7 and 20%,
respectively (Table II).
 | Table IIAntitumor response. |
Table II
Antitumor response.
| CR (%) | PR (%) | SD (%) | PD (%) | Overall response rate
(%) |
|---|
| FIGO stage |
| I–II (n=15) | 3 (20.0) | 10 (66.7) | 1 (6.7) | 1 (6.7) | 13 (86.7) |
| III (n=5) | 0 (0.0) | 1 (20.0) | 4 (80.0) | 0 (0.0) | 1 (20.0) |
| Tumor diameter |
| <5 cm (n=10) | 4 (40.0) | 4 (40.0) | 1 (10.0) | 1 (10.0) | 8 (80.0) |
| ≥5 cm (n=10) | 0 (0.0) | 6 (60.0) | 4 (40.0) | 0 (0.0) | 6 (60.0) |
Toxicity and treatment received
Grade 3 and 4 neutropenia was noted in 6 patients
(30%) each, while 2 patients (10%) experienced febrile neutropenia.
G-CSF preparations were administered to 11 (55%) of the 20 patients
during 17 (42.5%) of a total of 40 cycles. The mean duration of the
use of G-CSF preparations was 3.4 days per cycle. Three patients
(15%) had grade ≥3 anemia, and 1 patient with grade 4 anemia
received a blood transfusion. No patients developed grade ≥2
thrombocytopenia. Queasiness and vomiting, as grade ≥3
non-hematotoxic events, occurred in 1 patient (5%) each, and both
patients were administered parenteral fluid infusions (Table III). Chemotherapy was completed as
scheduled by 18 (90%) of the 20 patients. The irinotecan dose on
Day 8 in the second cycle was skipped in the remaining 2 patients.
These skips were made due to persistent queasiness, based on the
judgment of the attending physician. Although the second cycle was
started within 7 days of the first in 3 patients, this second cycle
of postoperative chemotherapy was postponed in all 3 (15%) patients
due to failure of the neutrophil count to adhere to the initiation
criterion. The cisplatin and irinotecan dose was reduced in the
second cycle in 2 patients (10%) with febrile neutropenia lasting
for ≥4 days.
 | Table IIIToxicity. |
Table III
Toxicity.
| Grade |
|---|
|
|
|---|
| 1 | 2 | 3 | 4 | ≥3 (%) |
|---|
| Leukopenia | 4 | 9 | 5 | 2 | 7 (35) |
| Neutropenia | 1 | 7 | 6 | 6 | 12 (60) |
| Thrombocytopenia | 4 | 0 | 0 | 0 | 0 (0) |
| Anemia | 5 | 11 | 3 | 1 | 4 (20) |
| Nausea | 14 | 5 | 1 | 0 | 1 (5) |
| Vomiting | 12 | 7 | 1 | 0 | 1 (5) |
| Diarrhea | 0 | 1 | 0 | 0 | 0 (0) |
| Neurotoxicity | 0 | 0 | 0 | 0 | 0 (0) |
| Renal toxicity | 0 | 0 | 0 | 0 | 0 (0) |
| Febrile
neutropenia | 0 | 0 | 2 | 0 | 2 (10) |
Surgery completion rate
Of the total of 20 patients, 18 (90%) underwent
surgery for the disease. A radical hysterectomy was completed in 15
of these patients; thus, the overall surgery completion rate was
75%. For the 15 stage I–II patients, the radical hysterectomy
completion rate was 93.3%, since the surgical operation was
incomplete in a patient with PD. Surgery was performed in three
stage III patients, with a radical hysterectomy being completed in
1 patient with PR; thus, the completion rate was 33.3%.
Discussion
The key drug for the treatment of advanced/recurrent
cervical cancer is cisplatin (50 mg/m2); thus, a variety
of combination therapy regimens with cisplatin were investigated
(12–14). Results of randomized studies by the
Gynecologic Oncology Group (GOG) demonstrated the usefulness of
cisplatin in combination with paclitaxel or topotecan, and the use
of cisplatin along with another antineoplastic agent was
recommended for the treatment of advanced/recurrent cervical cancer
(13,14). At the 2008 Annual Conference of the
American Society of Clinical Oncology (ASCO), GOG reported that
cisplatin and paclitaxel regimens would be used as a control arm in
subsequent randomized clinical studies (GOG Protocol 204).
Irinotecan, an inhibitor of DNA topoisomerase I, as in the case of
topotecan, is widely used in Japan. It has been reported that in
the treatment of recurrent/advanced cervical cancer, the response
rate was 23.6% with irinotecan (100 mg/m2, Days 1, 8 and
15) alone (15) and 59.9% with
combination chemotherapy consisting of cisplatin at 60
mg/m2 (Day 1) and irinotecan at 60 mg/m2
(Days 1, 8 and 15) (11). A
response rate of 78.8% was reported with cisplatin and irinotecan
combination chemotherapy as NAC (8). In these cisplatin and irinotecan
therapies with doses administered at 4-week intervals, irinotecan
doses on Days 8 and 15 were skipped in approximately 30% of
patients. However, the efficacy of the therapies was confirmed even
in patients with dose skips, suggesting the need for reassessment
of the appropriate method for the dosing of cisplatin and
irinotecan chemotherapy.
On the other hand, a meta-analysis of data
concerning NAC yielded results that negated the efficacy of
treatment with radiation therapy as a primary regimen. Results of
the meta-analysis, nevertheless, suggested that NAC is useful as
treatment when i) each cycle extends to 14 days or less, and ii)
the cisplatin dose intensity is ≥25 mg/m2/week (16). Consequently, the present study
examined the efficacy and safety of cisplatin and irinotecan
chemotherapy, documented to be efficacious as NAC, with
modifications of the dose and dosing schedule. Doses of cisplatin
and irinotecan were set at 70 mg/m2; the former was
administered on Day 1 of each cycle and the latter on Days 1 and 8,
and each patient received 2 tri-weekly cycles of the regimens.
Thus, the dose intensity of cisplatin was increased to 23.3
mg/m2/week, leading to a decrease in the dose skip rate
according to this dosing schedule. In turn, 2-week reduction in
duration to surgery occurred.
The overall response rate was 70% in the study
regimen, while the response rate for stage I–II patients was 86.7%,
which appeared to be comparable to or even greater than the monthly
regimen. In contrast, of the 5 stage III patients requiring
conservation of ovarian function, NAC proved effective only in 1
patient, enabling a radical hysterectomy, and indicating the
difficulty of NAC in stage III cases. The response rate according
to tumor size (diameter) was 80% for the 10 patients with a tumor
size <5 cm and 60% for the 10 patients with a tumor size ≥5 cm
(Table II). The results were
consistent with reports that the smaller the size of the tumor
mass, the greater the efficacy of NAC (17,18).
Our findings suggest that a tumor size ≥5 cm may constitute a risk
factor for NAC as described by Huang and co-researchers (19).
Regarding adverse events, a high incidence of
neutropenia was noted, which decreased in response to brief G-CSF
treatment (mean duration 3.4 days). Severe diarrhea characteristic
of irinotecan chemotherapy did not occur at the dose level of 70
mg/m2; thus, quality of life appeared to be maintained.
Patients completed the first cycle as scheduled, whereas in the
second cycle, the initiation of the treatment was postponed within
a period of 1 week for 3 patients, and the Day 8 dose of irinotecan
was skipped by 2 patients. The dose was reduced during the second
cycle for only 2 patients; thus, the present regimen was considered
to confer no increased toxicity as compared to the monthly
regimen.
In conclusion, the present study demonstrated that
the tri-weekly cisplatin and irinotecan combination chemotherapy
with an increased dose intensity of cisplatin at 70
mg/m2 was safely conducted, yielded a high response rate
and proved useful as a NAC regimen.
References
|
1
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitney CW, Sause W, Bundy BN, Malfetano
JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL and Liao SY:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.
|
|
4
|
Pearcey R, Brundage M, Drouin P, Jeffrey
J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G and Tu D:
Phase III trial comparing radical radiotherapy with and without
cisplatin chemotherapy in patients with advanced squamous cell
cancer of the cervix. J Clin Oncol. 20:966–972. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: an update of
Radiation Therapy Oncology Group trial (RTOG) 90–01. J Clin Oncol.
22:872–880. 2004.PubMed/NCBI
|
|
6
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: a systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lukka H, Hirte H, Fyles A, Thomas G, Elit
L, Johnston M, Fung MF and Browman G: Concurrent cisplatin-based
chemotherapy plus radiotherapy for cervical cancer – a
meta-analysis. J Clin Oncol. 14:203–212. 2002.
|
|
8
|
Sugiyama T, Nishida T, Kumagai S, Nishino
S, Fujivoshi K, Okura N, Yakushiji M, Hiura M and Umesaki N:
Combination chemotherapy with irinotecan and cisplatin as
neoadjuvant in locally advanced cervical cancer. Br J Cancer.
81:95–98. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiyama T, Nishida T, Kataoka A, Imaishi
K, Komai K, Ushijima K, Hasuo Y, Ookura N and Yakushiji M:
Combination of irinotecan hydrochloride (CPT-11) and cisplatin as a
new regimen for patients with advanced ovarian cancer. Acta Obstet
Gynecol Japan. 48:827–834. 1996.PubMed/NCBI
|
|
10
|
Takeuchi S, Dobashi K, Fujimoto S, et al:
A late phase II study of CPT-11 on uterine cervical cancer and
ovarian cancer. Research Groups of CPT-11 in Gynecologic Cancers.
Gan To Kagaku Ryoho. 18:1861–1689. 1991.PubMed/NCBI
|
|
11
|
Sugiyama T, Yakushiji M, Noda K, Ikeda M,
Kudoh R, Yajima A, Tomoda Y, Terashima Y, Takeuchi S, Hiura M, Saji
F, Takahashi T, Umesaki N, Sato S, Hatae M and Ohashi Y: Phase II
study of irinotecan and cisplatin as first-line chemotherapy in
advanced or recurrent cervical cancer. Oncology. 58:31–37. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonomi P, Blessing JA, Stehman FB, DiSaia
PJ, Walton L and Major FJ: Randomized trial of three cisplatin dose
schedules in squamous-cell carcinoma of the cervix: a Gynecologic
Oncology Group study. J Clin Oncol. 3:1079–1085. 1985.PubMed/NCBI
|
|
13
|
Rose PG, Blessing JA, Gershenson DM and
McGehee R: Paclitaxel and cisplatin as first-line therapy in
recurrent or advanced squamous cell carcinoma of the cervix: a
Gynecologic Oncology Group study. J Clin Oncol. 17:2676–2680.
1999.
|
|
14
|
Moore DH, Blessing JA, McQuellon RP,
Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF
and Rocereto TF: Phase III study of cisplatin with or without
paclitaxel in stage IVB, recurrent, or persistent squamous cell
carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin
Oncol. 22:3113–3119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeuchi S, Noda K and Yakushiji M; CPT-11
Study Group on Gynecologic Malignancy. Late phase II study of
CPT-11, topoisomerase I inhibitor, in advance cervical carcinoma
(CC). Proc Am Soc Clin Oncol. 11:2241992.
|
|
16
|
Tierney J: Neoadjuvant chemotherapy for
locally advanced cervical cancer: a systematic review and
meta-analysis of individual patient data from 21 randomised trials.
Eur J Cancer. 39:2470–2486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benedetti-Panici P, Greggi S, Colombo A,
Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F,
Zola P, Mangioni C and Landoni F: Neoadjuvant chemotherapy and
radical surgery versus exclusive radiotherapy in locally advanced
squamous cell cervical cancer: results from the Italian Multicenter
Randomized study. J Clin Oncol. 20:179–188. 2002. View Article : Google Scholar
|
|
18
|
Napolitano C, Imperato F, Mossa B,
Framarino ML, Marziani R and Marzetti L: The role of neoadjuvant
chemotherapy for squamous cell cervical cancer (Ib-IIIb): a
long-term randomized trial. Eur J Gynaec Oncol. 14:51–59.
2002.PubMed/NCBI
|
|
19
|
Huang HJ, Chang TC, Hong JH, Tseng CJ,
Chou HH, Huang KG and Lai CH: Prognostic value of age and
histologic type in neoadjuvant chemotherapy plus radical surgery
for bulky (>4 cm) stage IB and IIA cervical carcinoma. Int J
Gynecol Cancer. 13:204–211. 2003. View Article : Google Scholar : PubMed/NCBI
|