Introduction
Cancer is the second leading cause of human
mortality worldwide, and conventional therapies are known for their
serious side effects. Therefore, many efforts have been made to
determine novel alternative approaches for the prevention or
treatment of cancer. Advances in cancer research have shown that
alterations in the expression or function of genes that control the
cell cycle and apoptosis enhance tumor survival through the
disruption of the balance between these processes. Plants have been
used as a source of anti-cancer agents since the 1950s, when the
alkaloids vinblastine and vincristine were isolated from
Catharanthus roseus G. Don. Chemotherapeutic agents in
clinical use such as paclitaxel and flavopiridol (1) are also derived from plants.
Pterodon pubescens Benth.
(Leguminosae-Papilionoidea) is popularly known as ‘Sucupira
branca’, and its seeds are used in folk medicine to treat rheumatic
and inflammatory diseases. Scientific data have confirmed the
anti-arthritic effects of the hydroalcoholic extract of Pterodon
pubescens seeds in type II collagen-induced arthritis in mice
without subacute toxic effects (2–4).
Although anti-inflammatory action has been noted for the seed
ethanolic extract (EEPp) (5),
anti-proliferative effects on leukemic cells have yet to be
demonstrated. The present study showed that EEPp deregulates cyclin
D1 and E2 mRNA expression by inducing cell cycle arrest in the
G1 phase and apoptosis in the chronic myelogenous
leukemia K562.
Materials and methods
Extract preparation
The seeds of Pterodon pubescens Benth. were
collected by Luciana Pontes Coelho in Goiás, Brazil, and identified
by Haroldo Cavalcante de Lima at the Jardim Botânico do Estado do
Rio de Janeiro, Brazil, where a voucher was deposited (RB 350279).
The powdered seeds were submitted to 100% ethanol (15 g/100 ml) for
15 days. Following ethanol evaporation, the viscous oil (EEPp) was
obtained, yielding 50% (w/w). EEPp was dissolved in ethanol and
then diluted with a supplemented medium consisting of RPMI-1640
with 10% fetal bovine serum (Cultilab, Brazil), penicillin (70
mg/l) and streptomycin (100 mg/l) to a final ethanol concentration
of 0.01%. Control cultures received only 0.01% ethanol in the
supplemented medium.
Cell growth
The human chronic myelogenous leukemia cell line
K562 (CCL-243), purchased from the American Type Culture
Collection, was always cultured (2.5×105 cells/ml) in
the supplemented medium. For cell growth, the cell line was treated
with 10, 30 or 50 μg/ml EEPp for 72 h at 37°C and 5%
CO2. Viable cells (by trypan blue dye exclusion) were
counted at a 12-h interval.
Cell proliferation assay
The cells were cultured in 96-well plates (200 μl)
with different EEPp concentrations and 0.25 μCi/well
[3H]-methyl-thymidine (Amersham Biosciences, Brazil) for
24 h at 37°C in 5% CO2. Subsequently, the cells were
harvested on filter papers and processed for the determination of
3H-Tdr radioactivity using liquid scintillation.
Cell cycle analysis
Cells were cultured with 30 μg/ml EEPp for 36 h at
37°C in 5% CO2. After centrifugation (400 × g),
1×106 viable cells were treated with 0.3% Triton X-100
containing 50 μg/ml propidium iodide (PI) in 43 mM citrate buffer
solution (pH 8.2) for 15 min in the dark, and then with 500 μl of
100 μg/ml ribonuclease A (Sigma Chemical Co., USA) for 15 min. PI
fluorescence (585±15 nm) was measured (100,000 events) using a
FACSCalibur cytometer (Beckton-Dickinson, USA), and data were
analyzed using the WinMdi 2.8 software.
Analysis of apoptosis using flow
cytometry
Cells were treated with 50 μg/ml EEPp for 36 h at
37°C in 5% CO2. Cell death was evaluated by the loss of
membrane integrity (high PI fluorescence at 485 nm) after treatment
with PI solution (final concentration 2 μg/ml). Apoptosis was
determined by cell shrinkage (size reduction) and evaluated by the
forward-scatter (FSC) parameter. Phosphatidylserine exposure was
then determined using the Annexin V-FITC/PI double staining kit (BD
Pharmingen, USA). Briefly, cells (1×105) were washed
with PBS, suspended in binding buffer (10 mM HEPES pH 7.4, 140 mM
NaCl and 2.5 mM CaCl2) and treated with Annexin V (5 μl)
and 50 μg/ml PI solution (10 μl) for 15 min at room temperature.
Annexin binding was determined by FITC fluorescence (535±15 nm).
Cells (105/assay) were obtained using Cell Quest
software and analyzed using WinMdi 2.8 software.
Analysis of mRNA expression using
RT-PCR
Total RNA was extracted using TRIzol (Invitrogen).
The reverse transcription reaction was performed by adding MMLV
reverse transcriptase (Invitrogen), RNA and random primers to the
reaction mixture. PCR was performed in a Perkin Elmer GeneAmp PCR
System 9600. cDNA was added to a 25-μl PCR mixture containing dNTP,
the specific primers and Platinum Taq DNA polymerase (Invitrogen).
Each cycle consisted of 30 sec at 94°C, 30 sec at annealing
temperature, and 1 min at 72°C. The PCR products were resolved on a
2% agarose-ethidium bromide gel and quantified by Lab Image
software (Germany). The primers used were: GAPDH (housekeeping
gene), forward 5′-TGTGAACGGATTTGGCCGTA-3′ and reverse
5′-TCGCTCCTGGAAGATGGTGA-3′ (58°C, 30 cycles, 200 bp); cyclin D1,
forward 5′-CTGGCCATGAACTACCT GGA-3′ and reverse
5′-GTCACACTTGATCACTCTGG-3′ (59°C, 30 cycles, 482 bp) and cyclin E2,
forward 5′-ATCCAGG CCAAGAAGAGGAAA-3′ and reverse 5′-GCACAAGGCAG
CAGCAGTC-3′ (63°C, 32 cycles, 612 bp).
Statistical analysis
Significant differences between the two groups were
assessed using Student’s t-test, with a level of significance set
at p<0.05.
Results
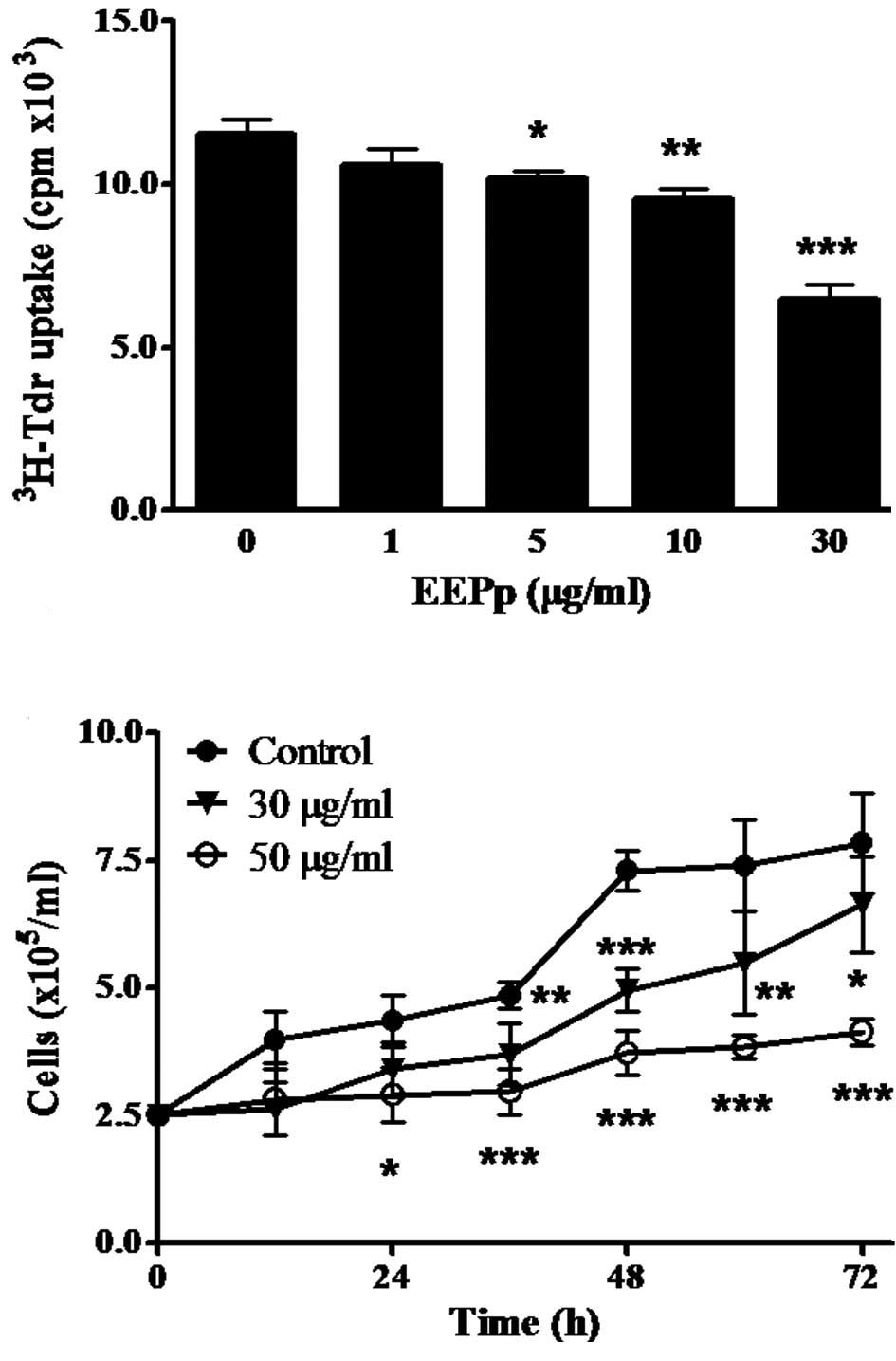
This study reports on the effect of EEPp on the
proliferation of K562 leukemic cells using different methods. The
incorporation of 3H-Tdr to DNA was significantly
inhibited after a 24-h incubation (Fig.
1A). This effect began at 5 μg/ml EEPp (p<0.05), reaching
44% inhibition at 30 μg/ml (p<0.001). EEPp also induced a
concentration- and time-dependent cell growth inhibition (Fig. 1B). When cultures were treated with
EEPp at 30 μg/ml, cell growth was only slightly inhibited, while
intense and continuous inhibition levels (70–80%) were observed at
50 μg/ml.
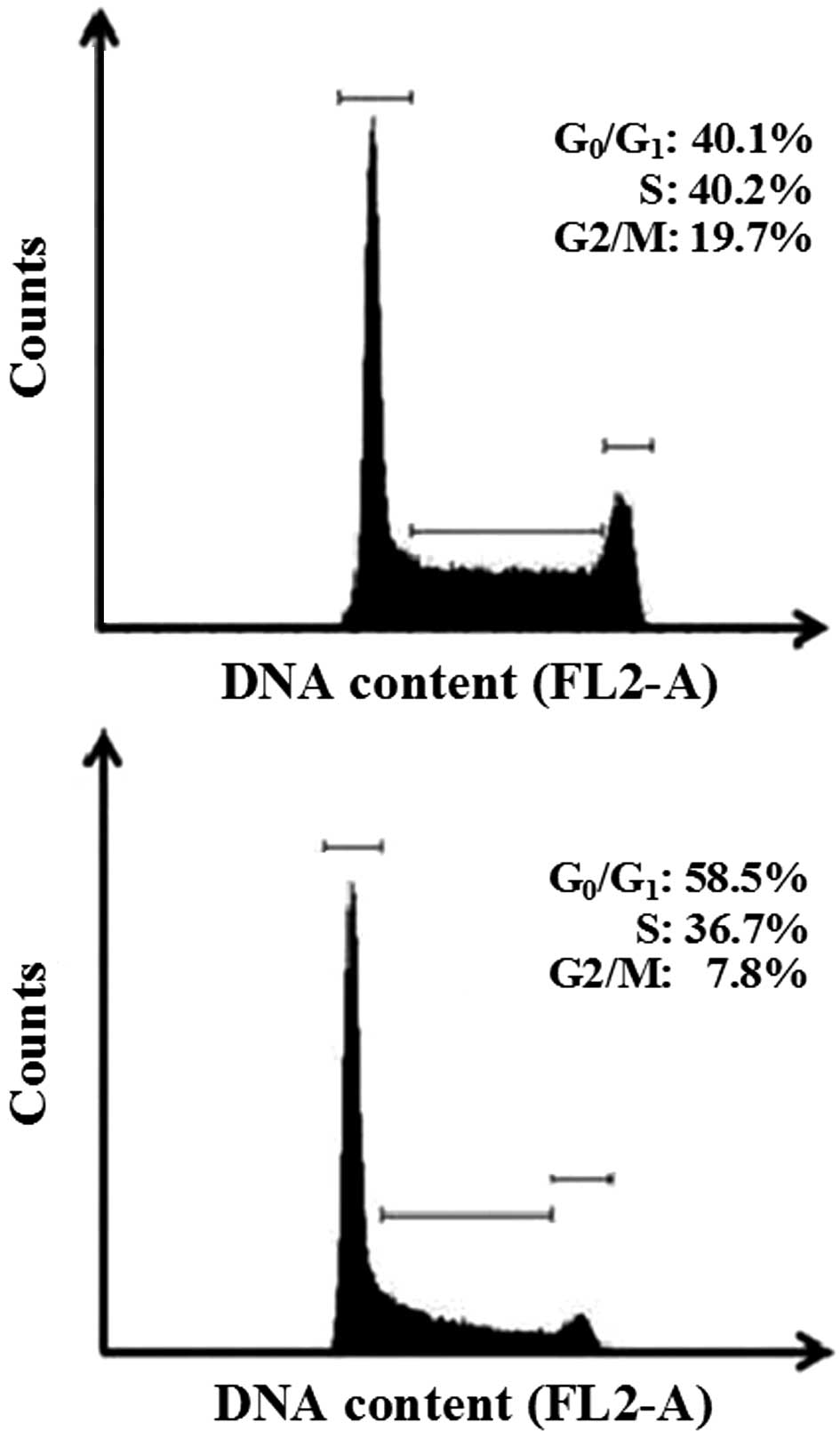
The anti-proliferative effects of EEPp with regard
to other cell cycle phases were also studied. The histograms in
Fig. 2 show a nuclear PI
fluorescence distribution that is proportional to the DNA content
(FL2-A). EEPp at 50 μg/ml effectively arrested K562 cell cycle
progression from the G1 to the S phase (Fig. 2, lower panel). Apoptotic bodies,
nuclear fragments or nuclear doublets were eliminated from the
analysis. In this representative experiment, 50 μg/ml EEPp induced
a 45% increase in cells at the G1 phase, while cell
numbers at the S and G2/M phases reciprocally decreased
by 16 and 60%, respectively. These effects were confirmed by
analyzing the mean of three independent experiments (data not
shown). The relative number of cells at
G0/G1, S and G2/M phases in the
control cultures was 40.1±1.2, 40.2±1.1 and 18.7±0.9%,
respectively. In the presence of 50 μg/ml EEPp these numbers
changed to 60.2±2.5% (50% increase, p<0.01), 31.8±2.6% (21%
reduction, p=0.054) and 7.9±0.1% (58% reduction, p<0.01),
respectively.
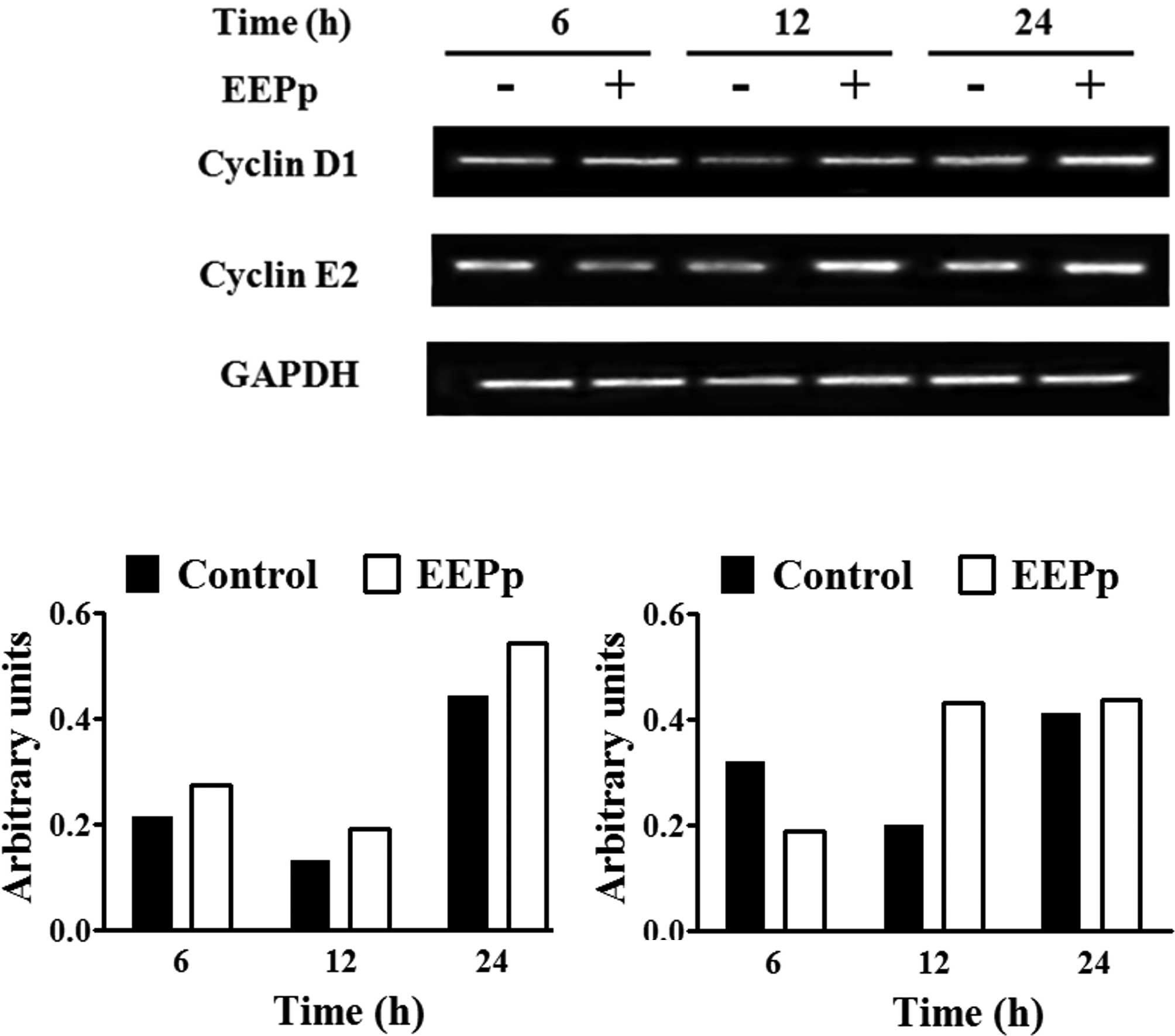
Analysis of cyclin mRNA expression using RT-PCR is
shown in Fig. 3A. Control cultures
exhibited a reduction in cyclin D1 mRNA levels at 12 h and an
increase at 24 h (Fig. 3B), similar
to cyclin E2 mRNA levels (Fig. 3C).
EEPp at 30 μg/ml induced a higher cyclin D1 mRNA expression at all
of the times analyzed (Fig. 3B).
This concentration caused a reduction in cyclin E2 expression at 6
h and an increase at 12 h (Fig.
3C).
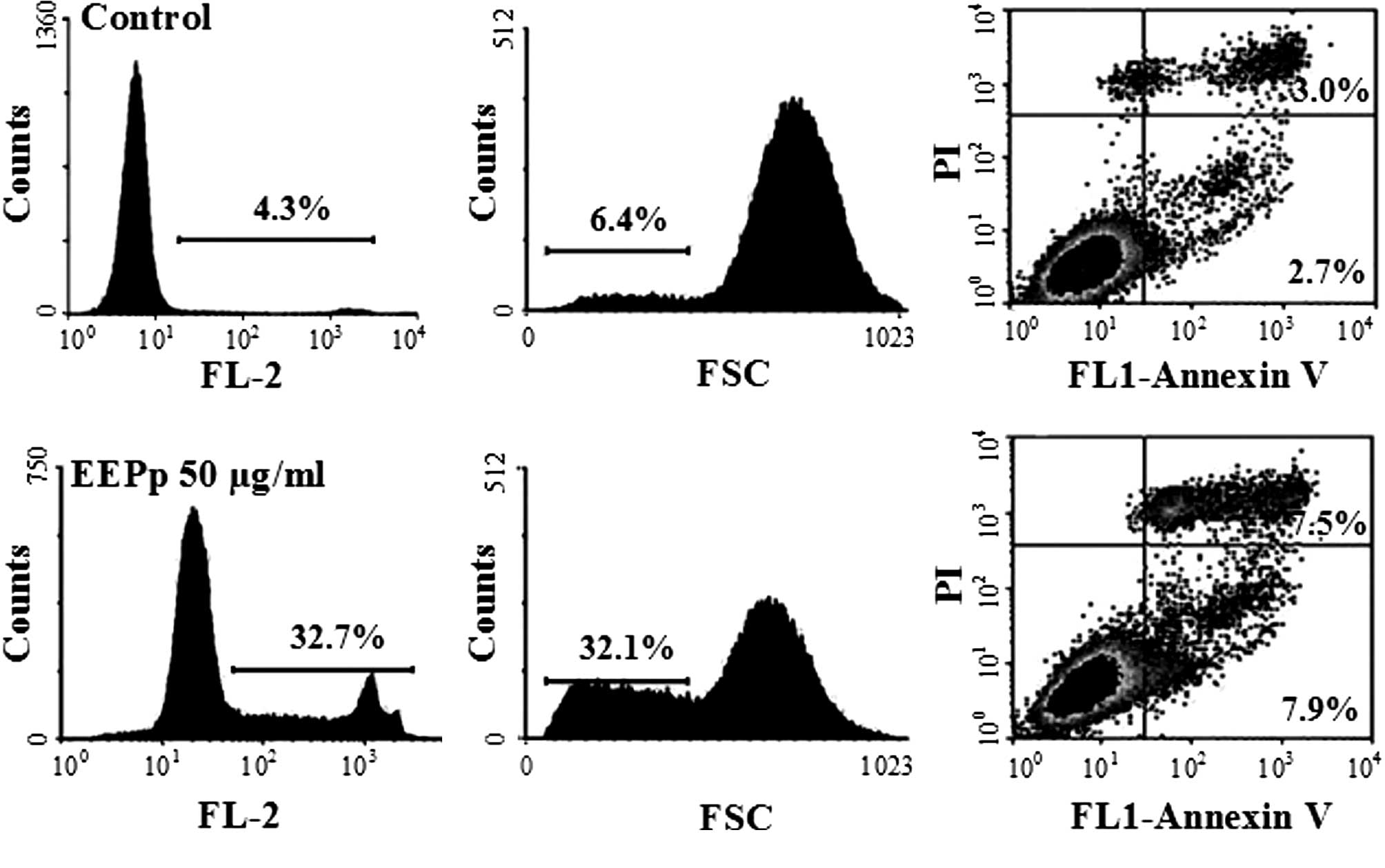
Apart from inhibiting cell proliferation and
inducing cell cycle arrest at the G1 phase, EEPp at 50
μg/ml also induced K562 cell death (Fig. 4A). An increase in the relative
number of shrunken cells from 6.4 to 32% (Fig. 4B) indicated that EEPp induces
leukemic cells into apoptosis, which was confirmed by the increase
in Annexin V-positive cells from 5.7 to 15.4% (Fig. 4C).
Discussion
Many plant products have been found to possess
chemotherapeutic activities both in vitro and in vivo
(6). This study noted the
anti-proliferative activity of EEPp on chronic myelogenous
leukemia-derived K562 cells. EEPp inhibited DNA synthesis, cell
growth and arrested the cell cycle at the G1 phase,
similar to other plant extracts with anti-tumoral activity
(6). This effect was not due to
ethanol, since ethanol treatment of the control cultures did not
alter the responses (data not shown). Vieira and collaborators
reported the anti-proliferative activity of a crude ethanolic
extract of Pterodon pubescens seeds against the human
melanoma cell line SK MEL 37, but data were not presented (7).
Cyclins D1 and E2 activate specific cyclin-dependent
kinases, inducing the cell cycle progression from the G1
to S phase. In contrast to conventional drugs which inhibit tumor
cell line proliferation by reducing cyclin D1 mRNA levels, EEPp
treatment increased it. Similar results have been reported for
other cell lines (8). The reduction
in cyclin E2 mRNA expression in tumor cells by EEEp treatment (6 h)
has also been described for several anti-cancer agents (9).
Traditional chemotherapeutic agents and potential
anti-cancer drugs (6) deregulate
cell cycle components, triggering apoptosis in tumor cells. EEPp
induced K562 cells into apoptosis despite their mutated TP53
tumor suppressor gene and resistance to several anti-cancer drugs
(10), suggesting that this effect
occurs through a p53-independent mechanism.
EEPp is a viscous, brown and fragrant oil,
containing geranylgeraniol, farnesol, naphthalene,
dimethyldodecatrienol and vouacapan diterpene derivatives (5,7). A
vouacapan diterpene, isolated from a Pterodon pubescens
extract, was shown to reduce proliferation and induce apoptosis of
melanoma cells (7). The compounds
geranylgeraniol and farnesol were shown to induce tumor cell
anti-proliferative effects (11)
and apoptosis (12,13). Thus, different substances present in
EEPp may be involved in its anti-leukemic effects.
This study showed that the ethanolic extract of
Pterodon pubescens seeds induced the cell cycle arrest and
apoptosis of a resistant leukemic cell line and that the
deregulation of D1 and E2 cyclin mRNA expression may be related to
these anti-proliferative effects. The fractionation of this extract
as well as experiments to clarify other mechanisms involved in the
anti-leukemic effects of EEPp are currently in progress.
Acknowledgements
We thank the LIA-BPPN Laboratory personnel for their
technical assistance. This study was supported by FAPERJ
(E-26/171.330/2006).
References
|
1
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.PubMed/NCBI
|
|
2
|
Sabino KCC, Castro FA, Oliveira JCR,
Dalmau SRA and Coelho MGP: Successful therapy of collagen-induced
arthritis in mice with a hydroalcoholic extract of seeds of
Pterodon pubescens. Phytother Res. 13:613–615. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coelho MGP, Marques PR, Gayer CRM, Vaz
LCA, Nogueira Neto JF and Sabino KCC: Subacute toxicity evaluation
of a hydroalcoholic extract of Pterodon pubescens seeds in
mice with collagen-induced arthritis. J Ethnopharmacol. 77:159–164.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coelho MGP, Sabino KCC and Dalmau SR:
Immunomodulatory effects of sucupira (Pterodon pubescens)
seed infusion on collagen-induced arthritis. Clin Exp Rheumatol.
22:213–218. 2004.
|
|
5
|
Silva MC, Gayer CR, Lopes CS, et al: Acute
and topic anti-edematogenic fractions isolated from the seeds of
Pterodon pubescens. J Pharm Pharmacol. 56:135–141. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: a global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vieira CR, Marques MF, Soares PR, et al:
Antiproliferative activity of Pterodon pubescens Benth. seed
oil and its active principle on human melanoma cells.
Phytomedicine. 15:528–532. 2008.
|
|
8
|
Okabe H, Lee SH, Phuchareon J, Albertson
DG, McCormick F and Tetsu O: A critical role for FBXW8 and MAPK in
cyclin D1 degradation and cancer cell proliferation. PLoS ONE.
1:e1282006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diaz-Carballo D, Malak S, Freistühler M,
Elmaagacli A, Bardenheuer W and Reusch HP: Nemorosone blocks
proliferation and induces apoptosis in leukemia cells. Int J Clin
Pharmacol Ther. 46:428–439. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pytel D, Wysocki T and Majsterek I:
Comparative study of DNA damage, cell cycle and apoptosis in human
K562 and CCRF-CEM leukemia cells: role of BCR/ABL I therapeutic
resistance. Comp Biochem Physiol. 144:85–92. 2006.PubMed/NCBI
|
|
11
|
Miquel K, Pradines A, Tercé F, Selmi S and
Favre G: Competitive inhibition of choline phosphotransferase by
geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis
and induces apoptosis in human lung adenocarcinoma A549 cells. J
Biol Chem. 40:26179–26186. 1998. View Article : Google Scholar
|
|
12
|
Masuda Y, Nakaya M, Aiuchi T, Hashimoto S,
Nakajo S and Nakaya K: The mechanism of geranylgeraniol-induced
apoptosis involves activation, by a caspase-3-like protease, of a
c-Jun N-terminal kinase signaling cascade and differs from
mechanisms of apoptosis induced by conventional chemotherapeutic
drugs. Leuk Res. 24:937–950. 2000. View Article : Google Scholar
|
|
13
|
Rioja A, Pizzey AR, Marson CM and Thomas
NS: Preferential induction of apoptosis of leukaemic cells by
farnesol. FEBS Lett. 467:291–295. 2000. View Article : Google Scholar : PubMed/NCBI
|