Introduction
Numerous studies have focused on the pathogenesis of
the HER [human epidermal growth factor receptor (EGFR)-related]
family in tumorigenesis. Moreover, HER-related signal pathways are
proving to be promising, effective molecular targets for anti-tumor
therapy including treatment for gastric cancer (GC) (1–4). The
HER family consists of four closely related members: EGFR/HER1,
HER2/neu, HER3 and HER4. HER receptors share a high degree of
structural and functional homology, including a glycosylated
extracellular ligand-binding domain, a hydrophobic transmembrane
domain and an intracellular domain with tyrosine kinase activity
(except HER3) (5,6). The formation of homodimerization or
heterodimerization induced by binding with neuregulins, β cellulin
and heparin-binding EGF-like growth factor triggers a complex
signal transduction cascade, predominantly through
phosphoinositide-3-kinase/Akt and extracellular signal-regulated
kinase 1/2, thereby regulating the proliferation, migration,
adhesion, angiogenesis and apoptosis of cancer cells (7,8).
The special importance of HER2/neu in tumorigenesis
implies that signaling pathways and downstream effectors have
evolved into key molecules in carcinoma-targeted therapy.
Herceptin, for example, a humanized monoclonal antibody targeting
the HER2/neu antigen, has been used as first-line cancer therapy in
breast cancer patients when tumors overexpress HER2/neu (9,10).
Therapy benefits in GC patients from the utility of herceptin were
also reported in pre-clinical trials (11,12).
Somatic mutations of the HER genes have drawn much
attention in cancer research, particularly after the notable
finding that EGFR gene mutations in non-small cell lung cancer
predict clinical responses to EGFR tyrosine kinase inhibitors
(13). The potential effect of HER
mutations, including HER2/neu, has become an important event in the
fields of cancer genetics and therapeutics (14).
According to published data, the incidence of
HER2/neu mutations in cancer tissues is modest (2.9–5%) (15). The gene mutation may vary depending
on the ethnicity of the cancer patients. To the best of our
knowledge, however, no reports on HER2/neu mutations in Chinese
patients with GC exist. In the present study, the somatic mutations
of HER2/neu and its expression in 72 Chinese patients with GC were
investigated concomitantly.
Materials and methods
Patients
Seventy-two patients diagnosed with GC and
identified from the pathology archives of the General Hospital of
PLA (from 2004 through 2006) were randomly enrolled in this study.
Patients that underwent curative surgery had no prior treatment
such as chemotherapy or radiation therapy. Of the 72 patients, 40
were men and 32 women with a mean age of 52 years (range 41–78). In
each case, GC tissues and adjacent non-tumorous tissues (non-tumor
group) were obtained for pathological examination. The detailed
pathological results were obtained from the Department of Pathology
of our hospital. The study protocol was approved by the Clinical
Research Ethics Committee of the Chinese PLA General Hospital.
Informed consent was obtained from each patient.
The histological tumor type of GC was defined
according to Lauren’s classification. In this study, the 72
patients with GC included 40 intestinal- and 32 diffuse-type. Tumor
node metastasis (TNM) staging was performed at the pathologic
diagnosis of GC according to the American Joint Committee on Cancer
staging system (Table I). Patients
were followed up for 8–30 months.
 | Table IOverexpression of HER2 and clinical
parameters. |
Table I
Overexpression of HER2 and clinical
parameters.
| n | HER2(+) |
|---|
| GC and non-tumor
tissue |
| GC | 72 | 13 (18.1%) |
| Non-tumor | 72 | 4 (5.6%) |
| P-value | | 0.038 |
| Lauren
classification |
| Intestinal type | 37 | 11 (29.7%) |
| Diffuse type | 35 | 2 (5.7%) |
| P-value | | 0.019 |
| TNM stage |
| I–II | 33 | 2 (6.6%) |
| III–IV | 39 | 11 (28.2%) |
| P-value | | 0.033 |
Microdissection
Tumor and normal cells from the same patients were
selectively procured from hematoxylin and eosin-stained slides,
using a 30G1/2 hypodermic needle (Becton Dickinson, Franklin Lakes,
NJ, USA). The needle was affixed to a micromanipulator by
microdissection. DNA extraction was performed by a modified
single-step DNA extraction method and then overnight digestion with
SDS and proteinase K at 37°C. This procedure was followed by
standard phenol-chloroform (1:1) extraction and ethanol
precipitation.
Immunohistochemistry (IHC)
Sections (5 μm) of formalin-fixed, paraffin-embedded
tissues were dewaxed in xylene and rehydrated through a series of
ethanol. Antigen retrieval was carried out at this stage in a
microwave oven. Sections were then blocked with 3% hydrogen
peroxidase followed by incubation with a 50% protein blocking
agent. Fetal bovine serum (10%), with or without the HER2/neu
antibody (1/100), was applied to each slide and incubated for 30
min. Slides were counterstained with hematoxylin and mounted.
Omission of the specific antibody was used as the negative control.
The anti-HER2 monoclonal antibody (Dako Herceptin Test kit) and
Dako EnVision™ kit (DakoCytomation Co., Denmark) were used for the
IHC staining.
After the IHC staining, slides were microscopically
interpreted in a blinded fashion by two pathologists. Membrane
staining for HER2/neu was taken into account. IHC expression of
HER2/neu was scored as: no staining or in <10% of the tumor
cells (score 0); faint/barely perceptible partial staining in
>10% of tumor cells (score 1+); weak to moderate staining of the
entire membrane and/or cytoplasma in >10% of tumor cells (score
2+) and strong staining in >10% of tumor cells (score 3+).
Scores 0 and 1+ were considered to be negative, and scores 2+ and
3+ positive for HER2/neu overexpression.
PCR and DNA sequencing
HER2 mutations in exons 18–23 encoding the kinase
domain were detected. Genomic DNA from each of the tumor and normal
cells was amplified with three primer pairs covering exon 18–23.
The primer sequences were (forward and reverse, respectively):
exons 18–19 (5-GACACCTAGCGGAGCGATGC-3 and 5-ATGGGGTC
CTTCCTGTCCTC-3), exons 20–21 (5-GTGATGGTTGGGA GGCTGTG-3 and
5-CTGCTCCTTGGTCCTTCAC-3), exons 22–23 (5-GGCCACCTCCCCACAACACA-3 and
5-GCTCAG CCACGCACATTTGAC-3). Calculations of cDNA of HER2 were
carried out with respect to the ATG start codon (NM_004448).
The PCR reaction mixture was denatured for 1 min at
94°C and incubated for 35 cycles (denaturing for 60 sec at 94°C,
annealing for 50 sec at 58–59°C and extension for 50 sec at 72°C).
Nest-PCR was performed when the amplification was not adequate for
direct sequencing. The primers designed for the nest-PCR are not
listed.
When the qualified PCR products were obtained, their
direct sequencing was carried out using a cyclic sequencing kit
(Perkin-Elmer, Foster City, CA, USA) according to the
manufacturer’s recommendation. When mutations were identified,
further confirmation using sequencing in both directions was
carried out.
Statistical analysis
The SPSS software package (SPSS, Inc., Chicago, IL,
USA) was used in the statistical analysis. Comparison of the
frequencies between the HER2/neu expression status and
clinicopathological variables was performed with Fisher’s exact
test (two-sided) by SPSS version 12.0. The log-rank test was used
for survival analysis. The Kaplan-Meier method was used to
calculate survival curves. Multivariate analysis was carried out
using Cox’s proportional hazards model in order to identify the
primary prognostic indicators that were independently associated
with survival. A probability of 0.05 was considered to be a
statistically significant difference.
Results
Overexpression and location of
HER2/neu
Overexpression for HER2/neu using IHC staining was
detected in 13 of the 72 GC patients and in 4 of the 72 tissue
samples in the non-tumor group. A significant difference for
HER2/neu overexpression between the GC and non-tumor group was
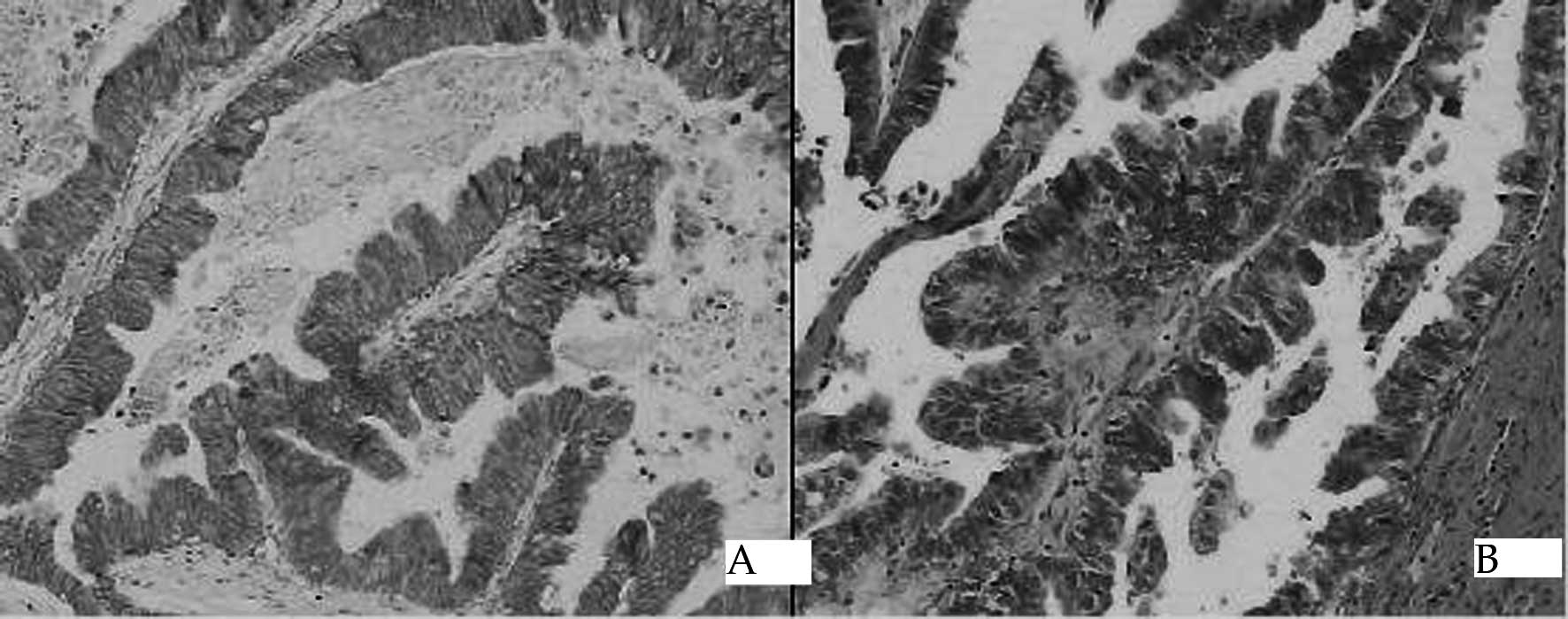
observed (18.1 vs. 5.6%, P<0.05) (Table I). The location of HER2/neu protein
was limited to the membrane (Fig.
1). No detectable immunoreactivity was noted in the negative
control (data not shown), indicating the specificity of IHC
staining.
As shown in Table I,
HER2/neu overexpression was detected in 11 of the 37
intestinal-type and in 2 of the 35 diffuse-type GC cases. The rate
of HER2/neu overexpression was higher in the intestinal-type GCs
than that of the diffuse-type (29.7 vs. 5.7%, P<0.05).
Relationship between HER2/neu
overexpression and clinicopathological parameters
No significant difference was noted in gender, age,
tumor location and tumor size with respect to the overexpression of
HER2/neu (data not shown). Table I
shows that 11 of the 39 stage III–IV cases were HER2-positive, with
a higher rate than that of stage I–II (28.2 vs. 6.6%,
P<0.05).
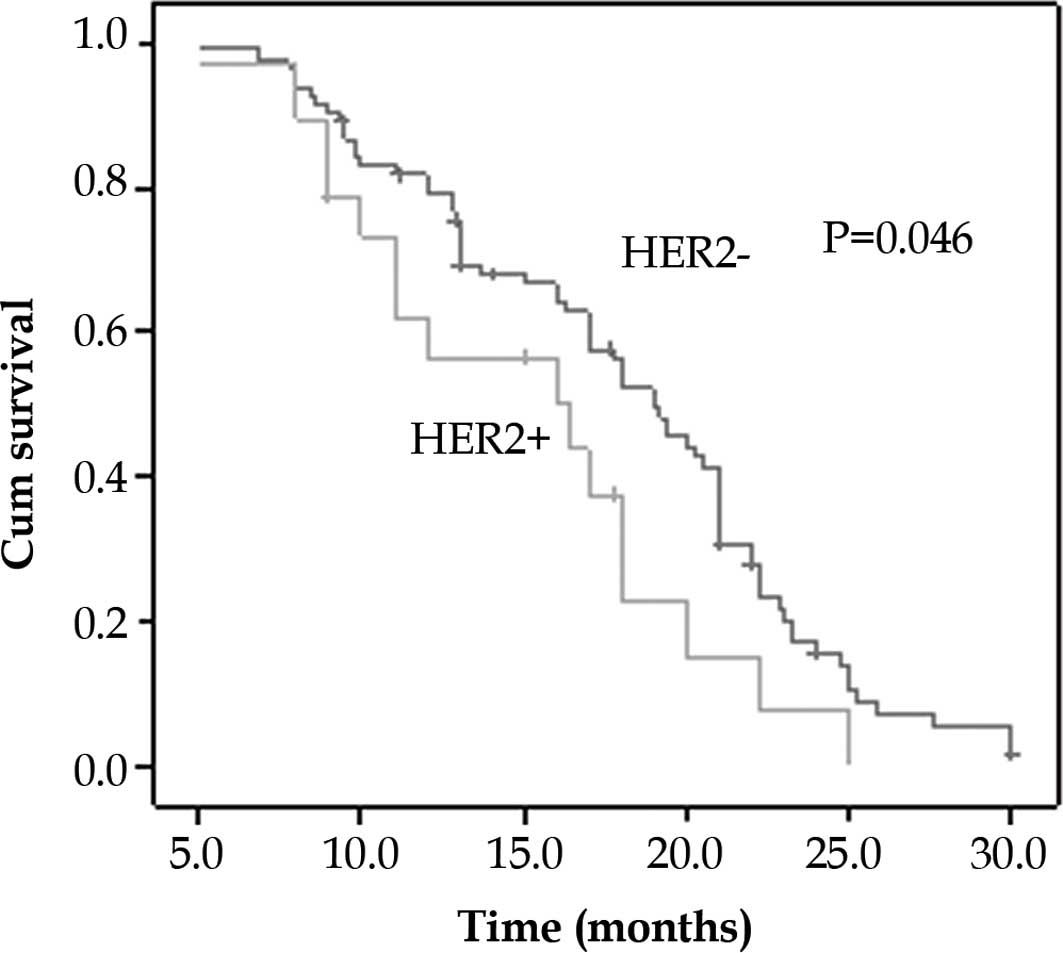
HER2/neu overexpression and survival
Univariate analysis (log-rank test) showed that age
(≥60 years), advanced TNM stage (III–IV) and the overexpression of
HER2/neu correlated with a less favorable patient survival
(Table II). Survival curves
computed according to the Kaplan-Meier method (Fig. 2) showed that overexpression of
HER2/neu correlated significantly with a decreased patient survival
(P=0.046). Although the TNM stage was the most dominant prognostic
factor, Cox’s proportional hazard model also identified HER2/neu
overexpression as an independent prognostic factor (Table III).
 | Table IIClinicopathological parameters and
patient survival (log-rank test). |
Table II
Clinicopathological parameters and
patient survival (log-rank test).
| n | 1-year survival
(%) | P-value |
|---|
| Age (years) | | | <0.05 |
| <60 | 34 | 61.3 | |
| ≥60 | 38 | 54.6 | |
| Gender | | | >0.05 |
| Male | 40 | 61.7 | |
| Female | 32 | 64.5 | |
| Lauren
classification | | | >0.05 |
| Intestinal type | 40 | 60.2 | |
| Diffuse type | 32 | 57.2 | |
| TNM stage | | | <0.01 |
| I–II | 33 | 70.2 | |
| III–IV | 39 | 53.6 | |
| HER2
overexpression | | | <0.05 |
| Absent | 59 | 71.2 | |
| Present | 13 | 59.7 | |
 | Table IIIMultivariate analysis (Cox’s
proportional hazards model). |
Table III
Multivariate analysis (Cox’s
proportional hazards model).
| HR (95% CI) | P-value |
|---|
| Age | 1.961
(1.386–2.446) | 0.047 |
| Diffuse vs.
intestinal type | 1.332
(0.974–2.543) | 0.615 |
| TNM stage I–II vs.
III–IV | 4.322
(3.116–11.024) | 0.000 |
| HER2 | 2.213
(0.992–3.217) | 0.040 |
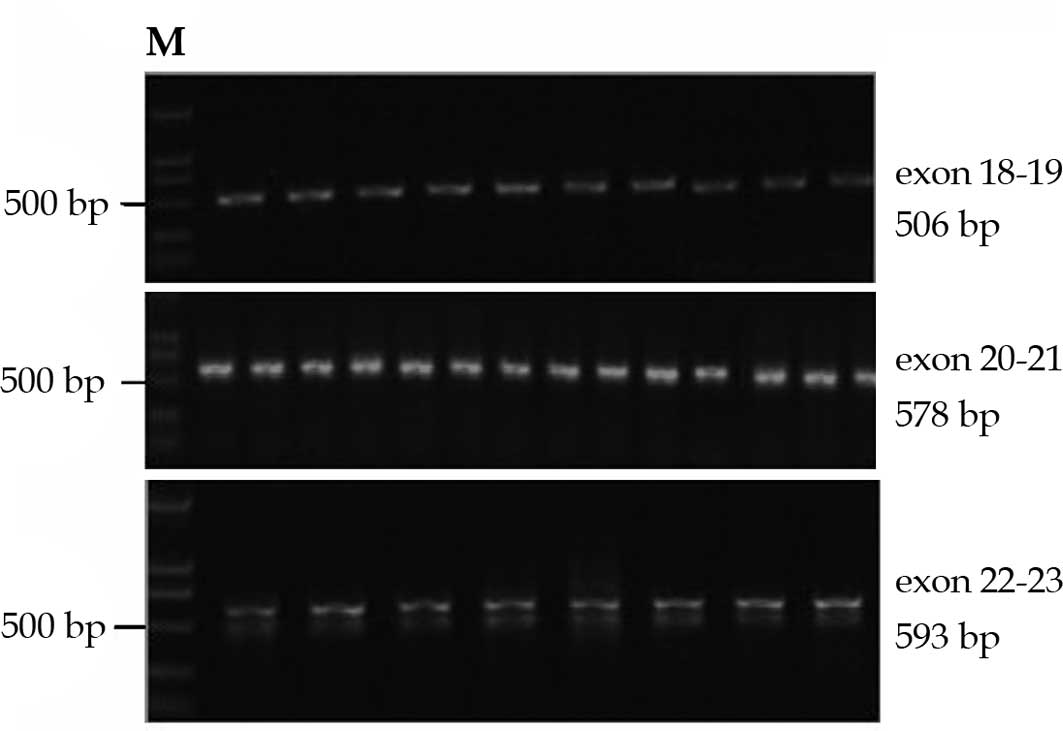
Detection of somatic mutations of
HER2
Each case in our study exhibited qualified DNA bands
comprising the kinase domain of HER2 for direct DNA sequencing
(Fig. 3). In the 72 cases examined,
no mutations were identified in the tumor or corresponding
tissues.
Discussion
HER2/neu functionally belongs to the tyrosine kinase
family. In carcinomas, HER2 acts as an oncogene, modulating the
proliferation, invasion and apoptosis of tumor cells.
Overexpression of HER2 protein in GC was first described in 1986
using IHC (16). The overexpression
rates in GC were reported to be 9–38%, and the staining location of
HER2/neu was found to be mainly in the cell membrane (17). The concordance between HER2/neu
protein overexpression and gene amplification was recently
elucidated in tumorigenesis, especially in the subgroup of cancers
that scored 3+ by IHC (18). The
present study showed a positive HER2/neu overexpression in 18.1% of
the GC patients and that the location of HER2/neu was largely
membranous, consistent with previously reported observations.
Intestinal and diffuse types of GC differ in their
epidemiology, pathogenesis, clinical outcome and genetic profiles
(19). A high correlation between
HER2/neu expression and the intestinal histologic type of GC was
reported by several investigators. Lordick et al reported
that HER2/neu positivity differed significantly according to the
histological subtype (intestinal 34% vs. diffuse 6%) (20). A similar distribution of HER2/neu in
the two histological types was also reported in another study
involving Chinese GC patients (25.4 vs. 4.7%, P<0.01) (21). Our results coincided with the
above-reported data (29.7 vs. 5.7%, P<0.05). However, the
reasons for the selective overexpression of HER2/neu in the
intestinal histological type remain complex and unclear.
The role of HER2/neu overexpression as a prognostic
factor in gastric cancer remains controversial (22,23).
However, increased evidence suggests a direct correlation between
HER2/neu overexpression and less favorable patient survival
(24). In a series of 260 gastric
cancers, Okegawa et al demonstrated that HER2/neu
overexpression was an independent factor and correlated with
serosal invasion and lymph node metastases (25). Our results demonstrated that
HER2/neu overexpression was statistically higher in stage III–IV
than in stage I–II cases (P<0.05) and was closely associated
with a less favorable patient outcome (P=0.046).
HER2 is encoded by a gene located on chromosome
17q21 (16), and its kinase domain
comprises 6 exons (exons 18–23). The discovery of somatic mutations
in the tyrosine kinase domain of the EGFR in non-small cell lung
cancer and their correlation with response to EGFR inhibitors has
raised concerns about the detection of mutations of other HER
genes, including HER2 and HER4 (26). Somatic mutations of the HER2 gene
have also been reported in gastric cancer, lung adenocarcinomas and
other types of human cancer. It has been reported that the
incidence of HER2 mutations in cancer tissues is low (2.9–5%)
(15). Of the total 6 exons of the
HER2 gene, the majority of identified mutations are predominantly
located in exons 19 and 20. Additionally, L755 and V777 appear to
be the most easily affected sites and target the identical
corresponding region as do EGFR insertions (15). Over 20 types of HER2 kinase domain
mutations, including insertion/duplication and missense mutations,
have been identified thus far (27,28).
However, the functional impact of the mutated HER2 gene in
tumorigenesis remains unknown.
The incidence of genetic alterations in certain
genes varies depending on ethnicity (13). Shigematsu et al investigated
394 adenocarcinoma cases and demonstrated that HER2 mutations
preferentially targeted individuals of Oriental ethnicity (3.9%)
compared to other ethnicities (0.7%) (28). Since data are limited regarding HER2
mutations in Chinese GC patients, we investigated the presence of
HER2 kinase domain mutations in 72 Chinese gastric carcinomas, and
no mutations were identified. Although the number of GC patients
enrolled in the present study was small, our results suggest that
in Chinese GC patients, the incidence of somatic mutations in the
HER2 kinase domain is anticipated to be a low-frequency rather than
a high-frequency event.
References
|
1
|
Ahmed KM, Cao N and Li JJ: HER-2 and
NF-kappaB as the targets for therapy-resistant breast cancer.
Anticancer Res. 26:4235–4243. 2006.PubMed/NCBI
|
|
2
|
Jin Q and Esteva FJ: Cross-talk between
the ErbB/HER family and the type I insulin-like growth factor
receptor signaling pathway in breast cancer. J Mammary Gland Biol
Neoplasia. 13:485–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Derin D, Eralp Y, Ozluk Y, Yavuz E, Guney
N, Saip P, Igci A, Ozmen V, Kücücük S, Aslay I, Aydiner A and Topuz
E: Lower level of MAPK expression is associated with anthracycline
resistance and decreased survival in patients with hormone receptor
negative breast cancer. Cancer Invest. 26:671–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burris H and Rocha-Lima C: New therapeutic
directions for advanced pancreatic cancer: targeting the epidermal
growth factor and vascular endothelial growth factor pathways.
Oncologist. 13:289–298. 2008. View Article : Google Scholar
|
|
5
|
Duneau JP, Vegh AP and Sturgis JN: A
dimerization hierarchy in the transmembrane domains of the HER
receptor family. Biochemistry. 46:2010–2019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaczek A, Brandt B and Bielawski KP: The
diverse signaling network of EGFR, HER2, HER3 and HER4 tyrosine
kinase receptors and the consequences for therapeutic approaches.
Histol Histopathol. 20:1005–1015. 2005.PubMed/NCBI
|
|
7
|
Yokoyama H, Ikehara Y, Kodera Y, Ikehara
S, Yatabe Y, Mochizuki Y, Koike M, Fujiwara M, Nakao A, Tatematsu M
and Nakanishi H: Molecular basis for sensitivity and acquired
resistance to gefitinib in HER2-overexpressing human gastric cancer
cell lines derived from liver metastasis. Br J Cancer.
95:1504–1513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pratilas CA, Hanrahan AJ, Halilovic E, et
al: Genetic predictors of MEK dependence in non-small cell lung
cancer. Cancer Res. 68:9375–9383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Graeff P, Crijns AP, ten Hoor KA, Klip
HG, Hollema H, Oien K, Bartlett JM, Wisman GB, de Bock GH, de Vries
EG, de Jong S and van der Zee AG: The ErbB signalling pathway:
protein expression and prognostic value in epithelial ovarian
cancer. Br J Cancer. 99:341–349. 2008.PubMed/NCBI
|
|
10
|
Kim KK, Lee JJ, Yang Y and Lee JH:
Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the
transactivation of ErbB2 in human breast and gastric cancer cells.
Carcinogenesis. 29:704–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SY, Kim HP, Kim YJ, Oh do Y, Im SA,
Lee D, Jong HS, Kim TY and Bang YJ: Trastuzumab inhibits the growth
of human gastric cancer cell lines with HER2 amplification
synergistically with cisplatin. Int J Oncol. 32:89–95.
2008.PubMed/NCBI
|
|
12
|
Inui T, Asakawa A, Morita Y, Mizuno S,
Natori T, Kawaguchi A, Murakami M, Hishikawa Y and Inui A: HER-2
overexpression and targeted treatment by trastuzumab in a very old
patient with gastric cancer. J Intern Med. 260:484–487. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ,
Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE and
Meyerson M: EGFR mutations in lung cancer: correlation with
clinical response to gefitinib therapy. Science. 304:1497–1500.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J and
Haber DA: Activating mutations in the epidermal growth factor
receptor underlying responsiveness of non-small cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JW, Soung YH, Seo SH, Kim SY, Park CH,
Wang YP, Park K, Nam SW, Park WS, Kim SH, Lee JY, Yoo NJ and Lee
SH: Somatic mutations of ERBB2 kinase domain in gastric, colorectal
and breast carcinomas. Clin Cancer Res. 12:57–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akiyama T, Sudo C and Ogawara H: The
product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein
with tyrosine kinase activity. Science. 232:1644–1646. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokunaga A, Onda M, Okuda T, Teramoto T,
Fujita I, Mizutani T, Kiyama T, Yoshiyuki T, Nishi K and Matsukura
N: Clinical significance of epidermal growth factor (EGF), EGF
receptor and c-erbB-2 in human gastric cancer. Cancer.
75:1418–1425. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2
protein expression assessed by immunohistochemistry in gastric
cancer. Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
19
|
Bulanov D: Gastric cancer – current state
of the problem. Part I. Epidemiology. Pathology. Classification.
Staging. Khirurgiia (Sofia). 4:48–59. 2007.
|
|
20
|
Lordick F, Bang YJ and Kang YK:
HER2-positive advanced gastric cancer: similar HER2-positivity
levels to breast cancer. Eur J Cancer. 5:2712007. View Article : Google Scholar
|
|
21
|
Cooperative Study Group of HER2 in China.
Multicenter study on HER-2/neu gene amplification and protein
expression in patients with gastric cancer. Chin J Dig. 26:657–660.
2006.
|
|
22
|
Tateishi M, Toda T, Minamisono Y and
Nagasaki S: Clinicopathological significance of c-erbB-2 protein
expression in human gastric carcinoma. Surg Oncol. 49:209–212.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasano H, Date F, Imatani A, Asaki S and
Nagura H: Double immunostaining for c-erbB-2 and p53 in human
stomach cancer cells. Hum Pathol. 24:584–589. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chia S, Norris B, Speers C, Cheang M,
Gilks B, Gown AM, Huntsman D, Olivotto IA, Nielsen TO and Gelmon K:
Human epidermal growth factor receptor 2 overexpression as a
prognostic factor in a large tissue microarray series of
node-negative breast cancers. J Clin Oncol. 26:5697–5704. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okegawa T, Kinjo M, Nutahara K and
Higashihara E: Pretreatment serum level of HER2/nue as a prognostic
factor in metastatic prostate cancer patients about to undergo
endocrine therapy. Int J Urol. 13:1197–1201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soung YH, Lee JW, Kim SY, Wang YP, Jo KH,
Moon SW, Park WS, Nam SW, Lee JY, Yoo NJ and Lee SH: Somatic
mutations of the ERBB4 kinase domain in human cancers. Int J
Cancer. 118:1426–1429. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stephens P, Hunter C, Bignell G, et al:
Lung cancer: intragenic ERBB2 kinase mutations in tumors. Nature.
431:525–526. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shigematsu H, Takahashi T, Nomura M,
Majmudar K, Suzuki M, Lee H, Wistuba II, Fong KM, Toyooka S,
Shimizu N, Fujisawa T, Minna JD and Gazdar AF: Somatic mutations of
the HER2 kinase domain in lung adenocarcinomas. Cancer Res.
65:1642–1626. 2005. View Article : Google Scholar : PubMed/NCBI
|